Preeclampsia affects ≈7% of first pregnancies and is one of the leading causes of maternal and neonatal mortality and morbidity in the United States and the world.1–3 The clinical hallmarks of the disorder include hypertension, proteinuria, hypercoagulability, edema, and placental abnormalities. In advanced stages, clinical symptoms include cerebral edema, renal failure, and the hemolysis, elevated liver enzymes, low platelets (HELLP) syndrome. The clinical management of preeclampsia is hampered by the lack of reliable diagnostic tests and effective therapy for the disorder. In some cases, termination of pregnancy is the only available option to prevent further deterioration of the fetus and mother. Fifteen percent of all preterm births are indicated early deliveries for preeclampsia.3 The resulting preterm births and the associated increased infant morbidity and mortality are especially disheartening consequences of preeclampsia.
Despite being one of the leading causes of maternal death and a major contributor to maternal and perinatal morbidity, the mechanisms responsible for the pathogenesis of preeclampsia are poorly understood. Roberts and colleagues4,5 were among the first to propose that alterations in endothelial cell function by activating agents produced by the placenta initiate the clinical syndromes of preeclampsia. Circulating factors, such as inflammatory cytokines, endothelin, and soluble vascular endothelial growth factor receptor termed soluble fms-like tyrosine kinase-1 (sFlt-1), are elevated in preeclamptic women and are proposed to be important links between placental ischemia and endothelial dysfunction.5–9 In particular, recent studies have shown that preeclampsia is associated with the presence of maternal autoantibodies capable of binding to and activating the angiotensin (Ang) receptor type-1 (AT1).10–15 AT1 receptor agonistic antibodies, herein termed AT1-AA, are rarely seen in normotensive pregnant women.10,11 Since the initial discovery of these autoantibodies, considerable evidence supporting a pathophysiological role of AT1-AA in preeclampsia has accumulated.
Initial Identification of Agonistic Autoantibodies Activating the AT1 Receptor in Preeclampsia
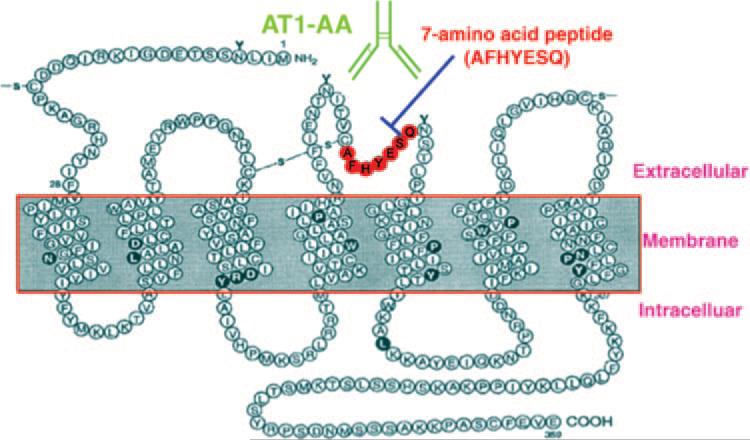
A major advance in our understanding of preeclampsia was made by Wallukat et al,10 who reported that sera from preeclamptic women contain an IgG autoantibody that reacts with the AT1 receptor, a 7 transmembrane G protein coupling receptor, in a stimulatory fashion (Figure 1). They relied on a bioassay for AT1 agonistic autoantibodies (termed AT1-AA) that consists of spontaneously beating neonatal rat cardiomyocytes. They showed that AT1-AA increase the spontaneous beating rate of the cultured cardiomyocytes, a feature that is blocked by AT1 receptor antagonists but not AT2 receptor antagonists or antagonists of adrenergic receptors. With affinity purification and peptide competition experiments they showed that the AT1-AA bind to a 7 amino acid sequence present on the second extracellular loop of the AT1 receptor (Figure 1). The presence of this sequence, AFHYESQ, in the cardiomyocyte contraction assay blocked antibody-induced stimulation of cardiomyocyte contraction. These remarkable findings were the first to show that preeclamptic women develop stimulatory autoantibodies against the AT1 receptor and that these autoantibodies are directed to a common epitope associated with the second extracellular loop.
Figure 1.
Schematic diagrams of AT1 receptor and the sequence of 7 amino acid antibody blocking epitope peptide. AT1-AA interacts with second extracellular loop of AT1 receptor. This interaction can be blocked by a specific 7 amino acid peptide.
Pathophysiological Role of AT1-AA in Preeclampsia
Immune mechanisms and the renin-angiotensin system are implicated in preeclampsia.16,17 These 2 concepts were united by Wallukat et al,10 who reported that sera from preeclamptic women contain an autoantibody that reacts with AT1 receptors in a stimulatory fashion. Subsequent to these findings, multiple other groups, including our own, showed that many features of preeclampsia could be explained by the ability of these autoantibodies to activate AT1receptors on a variety of cells.10–14,18,19 Examples of these possibilities are reviewed below and summarized in the Table.
Pathophysiological Role of AT1-AA in Preeclampsia
| Cell Types | Factors Changed | Features Associated With Preeclampsia |
|---|---|---|
| Cardiac myocytes | Increased contraction rate | Cardiac hypertrophy10,11 |
| Mesangial cells | Increased PAI-1 and IL-6 | Proteinuria and hypercoagulation18 |
| Increased inflammatory response | ||
| Human trophoblasts | Increased PAI-1 | Shallow invasion and hypercoagulation11 |
| sFlt-1 | Maternal endothelial dysfunction41 | |
| NADPH oxidase | Increased reactive oxygen species13 | |
| CHO-AT1R cell line | Increased Ca2+ | Increased contractility14 |
| Platelet abnormal function | ||
| Vascular smooth muscle cells | Increased TF | Hypercoagulation12 |
| Increased NADPH oxidase | Increased reactive oxygen species13 | |
| Monocytes | Increased TF | Hypercoagulation20 |
Role of AT1-AA on Hypercoagulation in Preeclampsia
Severe preeclampsia may be accompanied by disseminated intravascular coagulation and reduced fibrinolysis. These changes may be due to alterations in components of the coagulation and fibrinolytic systems, including tissue factor and plasminogen activator inhibitor (PAI)-1. These possibilities are considered below.
Coagulation System
Tissue factor (TF) is a 47-kDa transmembrane protein that initiates the extrinsic pathway of coagulation via formation of an enzymatic complex with factor VII/factor VIIa. Increased expression of TF is associated with placentas from women with preeclampsia. Dechend et al12 showed that AT1-AA stimulated increased TF expression in human vascular smooth muscle cells. They also showed that AT1-AA activate TF promoter/luciferase constructs after transfection into cells that contain AT1 receptors and that this activation required the presence of activating protein-1 binding sites. Increased TF synthesis by vascular smooth muscle cells and the activation of the TF/luciferase reporter were blocked by losartan. IgG from normotensive pregnant women had no effect in either assay. Dechend et al12 also confirmed earlier reports showing that preeclamptic placentas exhibit increased TF expression and activity compared with placentas from normotensive pregnant women. In subsequent studies, Dorffel et al20 reported that AT1-AA stimulated monocytes to produce increased amounts of TF, a feature that could contribute to increased endothelial cell adherence. Thus, the studies of Dechend et al12 and Dorffel et al20 show that AT1-AA activates AT1 receptors, initiating a signaling cascade resulting in increased TF expression. Together these studies suggest that the action of AT1-AA on human vascular smooth muscle cells and monocytes may contribute to the hypercoagulability associated with preeclampsia.
Fibrinolytic System
The fibrinolytic system is best known for its role in the regulated digestion of fibrin clots.10,11 A key component of this system is plasminogen, an inactive zymogen, which is converted into the active protease plasmin by the action of plasminogen activators. Plasminogen activator activity is controlled by PAIs, of which PAI-1 is the predominant physiological inhibitor of its class.21,22 PAI-1 is upregulated in placentas of preeclamptic women, leading to a reduced fibrinolytic activity.14–16 Elevated PAI-1, which occurs in the maternal circulation in preeclampsia, is believed to contribute to the hypercoagulation and fibrinolytic imbalance associated with this condition.23 Our studies11 originally showed that Ang II stimulates PAI-1 synthesis and secretion by human trophoblasts in a time- and concentration-dependent manner. In subsequent experiments, we found that AT1-AA, like Ang II, stimulates PAI-1 synthesis and secretion by activating AT1 receptors on human trophoblasts.18 Antibody-induced PAI-1 induction was blocked by losartan and the AT1 receptor epitope peptide, thereby providing strong evidence that the antibody effect is mediated through AT1 receptor activation. In several other cell types, PAI-1 production is also controlled by the action of Ang II on AT1 receptors. Such cells include mesangial cells,24,25 myocardial cells,26 vascular smooth muscle cells, and endothelial cells.27 We also showed that AT1-AA activates AT1 receptors on human mesangial cells and induces PAI-1 secretion.18 Thus, action of maternal AT1-AA on the AT1 receptors of trophoblast cells, mesangial cells, and other cell types in the maternal system is likely to contribute to the increased circulating PAI-1 and reduced fibrinolysis associated with preeclampsia.
Role of AT1-AA on Increased Production of Reactive Oxygen Species in Preeclampsia
Reactive oxygen species (ROS) production by the placenta and maternal tissues is increased in preeclamptic women and likely contributes to the oxidative stress associated with preeclampsia.28 The identity of the ROS-producing enzymes in preeclampsia has been investigated by Dechend et al,13 who recognized that reduced nicotinamide-adenine dinucleotide phosphate (NADPH) oxidase is a major source of ROS in arteriosclerosis and reperfusion injury. Several important findings emerged from their studies. First, IgG from preeclamptic women induces intracellular ROS in vascular smooth muscle cells and trophoblasts, an induction that is mediated by NADPH oxidase. Second, AT1-AA activates nuclear factor κB (NFκB) as a downstream target, resulting in the marked upregulation of nuclear factor κB. Third, ROS production is increased in preeclamptic placentas, especially in and around the blood vessels. Finally, NADPH oxidases are present in the placenta and are massively elevated in preeclampsia. They suggest that AT1-AA, through activation of NADPH oxidase, could contribute to ROS production and the inflammatory responses associated with preeclampsia.
Role of AT1-AA in Abnormalities of Calcium Metabolism Associated With Preeclampsia
Preeclampsia is associated with abnormalities in Ca2+ metabolism and increased intracellular Ca2+ levels in platelets, erythrocytes, and lymphocytes.29,30 Haller et al29 showed that basal intracellular free Ca2+ in platelets is substantially elevated in preeclamptic patients compared with women with uncomplicated pregnancy. This phenomenon completely disappears 6 weeks after delivery, which suggests a relevant relationship to preeclampsia. Similar studies using lymphocytes and erythrocytes showed that the intracellular free Ca2+ concentration is increased in these cells of preeclamptic patients.30,31 In addition, a more widespread dysregulation of cellular Ca2+ metabolism is also implicated in preeclampsia.29 The underlying mechanism dictating changes in intracellular free Ca2+ levels and Ca2+ metabolism in preeclampsia was investigated by Thway et al14 from our laboratory. We found that IgG from preeclamptic patients activated AT1 receptors and increased intracellular free calcium. In contrast, IgG from normotensive individuals was incapable of activating AT1 receptors and calcium mobilization. The specific mobilization of intracellular Ca2+ by AT1-AA from women with preeclampsia was blocked by losartan and by the 7-amino acid epitope peptide that corresponds with a site on the second extracellular loop of the AT1 receptor. Overall, our studies suggest that AT1-AA may account for increased intracellular free Ca2+ concentrations and changes in gene expression associated with preeclampsia.14
Role of AT1-AA on Excess sFlt-1 Secretion in Preeclampsia
Pregnancy is characterized by significant changes in the abundance of angiogenic factors, such as vascular endothelial growth factor and placental growth factor, and their antagonist, sFlt-1.32 The levels of sFlt-1 in the maternal circulation increase in the third trimester of normal pregnancy.33–35 Numerous recent reports provide convincing evidence that the level of sFlt-1 in plasma of women with preeclampsia is elevated in comparison with women with normal pregnancy.33,34,36–38 Circulating sFlt-1 binds circulating vascular endothelial growth factor and placental growth factor and prevents their interactions with their proangiogenic receptors. Administration of sFlt-1 to rats resulted in elevated blood pressure, proteinuria, and renal changes, classic features of preeclampsia.9 Thus, excessive placental-derived sFlt-1 may contribute to endothelial dys-function, hypertension, and proteinuria associated with preeclampsia. However, the potential mechanism for increased sFlt-1 production in preeclampsia has not been identified. We have reported recently that Ang II stimulates increased sFlt-1 by human trophoblast cells, villous placental explants, and pregnant mice.39 Subsequent to these studies, we have shown that IgG from women with preeclampsia induces the synthesis and secretion of sFlt-1 by human placental villous ex-plants and by human trophoblast cells.40 The secreted sFlt-1 has significant antiangiogenic properties as judged by its impact on in vitro endothelial cell migration and tube formation assays. The introduction of IgG from preeclamptic patients into pregnant mice resulted in increased synthesis and secretion of sFlt-1. We also found that Ang II and AT1-AA can function additively to induce sFlt-1 secretion through AT1 receptor activation.40 Overall, our findings support the view that Ang II is a key regulator of sFlt-1 synthesis and secretion during normal pregnancy39 and that the excessive accumulation of sFlt-1 observed in women with preeclampsia is because of additional activation of AT1 receptors mediated by AT1-AA.41
Role of AT1-AA in Shallow Trophoblast Invasion in Preeclampsia
Impaired placental development resulting from shallow trophoblast invasion is a well-recognized feature of preeclampsia.1,8,41–44 This placental abnormality results in reduced placenta perfusion, a feature that can be readily detected by Doppler sonography before 20 weeks. A recent report shows that AT1-AA is associated with pregnant women who display impaired placental development as judged by abnormal uterine perfusion detected by Doppler sonography. Using the cardiomyocyte contraction assay, Walther et al15 found that the AT1-AA was detectable between 18 and 22 weeks in women with abnormal uterine perfusion. When followed to term, these women fell into 3 groups: those who developed preeclampsia, those characterized by fetuses with intrauterine growth retardation, and those with otherwise normal outcomes. AT1-AA was not observed in second-trimester women with normal Doppler ultrasound. Thus, AT1-AA tracks with abnormal placental development and may serve to identify women at risk for intrauterine growth retardation and/or preeclampsia. The authors of this study suggested, as we had done earlier,11 that AT1-AAs may be responsible for reduced trophoblast invasion and impaired placental development.
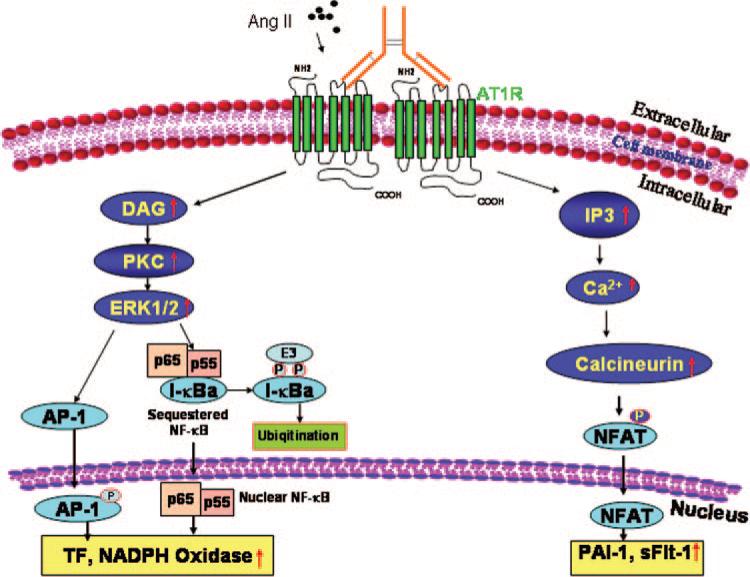
Placental development depends on the regulated production of proteolytic enzymes such as plasmin, a key enzyme in the fibrinolytic system. This system plays a critical role in extracellular matrix degradation to facilitate trophoblast invasion of the maternal uterus. Of particular importance in the regulation of plasmin production is the urokinase-type plasminogen activator that is synthesized by trophoblasts and is activated on association with specific receptors, urokinase-type plasminogen activator receptors, located on the surface of trophoblasts. We used an in vitro Matrigel invasion assay to show that Ang II–induced synthesis and secretion of PAI-1 in response to AT1 receptor activation resulted in reduced trophoblast invasion.17 A significant stimulation of PAI-1 secretion from human trophoblasts was also observed with IgG from patients with preeclampsia.11 IgG obtained from normotensive pregnant patients did not stimulate PAI-1 secretion. Activation of AT1 receptors by AT1-AA was blocked by losartan, the 7 amino epitope peptide, and FK506, a calcineurin-specific inhibitor,45 suggesting that calcineurin signaling functions downstream of AT1 receptor activation to mediate PAI-1 induction (Figure 2). Thus, activation of AT1 receptors by AT1-AA on human trophoblasts may contribute to increased PAI-1 production and shallow trophoblast invasion.
Figure 2.
Molecular mechanism of AT1-AA–mediated AT1 receptor activation. Multiple signaling pathways function downstream of AT1-AA–mediated AT1 receptor activation. AT1-AA–mediated AT1 receptor activation leads to increased PKC and calcineurin activity. As a result, downstream transcription factors, such as activating protein-1, nuclear factor κB (NF-κB), and nuclear factor activating T cell (NFAT), are activated and translocate from cytosol to nucleus, leading to increased gene expression. Because AT1 activating autoanti-bodies are bivalent IgG protein complexes, we propose that they exert their agonistic effect by cross-linking and thereby stabilizing AT1 receptor homodimers. DAG indicates diacylglycerol; IP3, inositol triphosphate; PKC, protein kinase C.
Mechanism of Antibody-Induced Receptor Activation
Multiple signaling pathways have been reported to be involved in AT1 receptor activation mediated by AT1-AA (Figure 2). Wallukat et al10 initially showed that AT1-AA– mediated AT1 receptor activation results in downstream activation of protein kinase C pathways in vascular smooth muscle cells. Dechend et al12 found that AT1-AA increased extracellular signal–regulated kinase 1/2 phosphorylation and led to AP-1 and nuclear factor κB activation. These activated transcription factors are required for TF and NADPH oxidase induction mediated by AT1-AA in vascular smooth muscle cells, trophoblast cells, and placenta.12 We have shown that the calcineurin-nuclear factor activating T-cell signaling pathway is involved in PAI-1 induction and trophoblast shallow invasion in human trophoblast cells mediated by AT1-AA.11 More recently, we revealed that calcineurin-nuclear factor activating T-cell signaling pathway is also essential for both Ang II- and AT1-AA–mediated sFlt-1 induction at the transcriptional level.39,40 In addition, Ang II and AT1-AA additively induced nuclear factor activating T-cell activity in human trophoblast cells resulting in excess sFlt-1 secretion, suggesting that additional activation of AT1 receptor may be responsible for increased sFlt-1 secretion in preeclampsia.40 Thus, multiple studies imply that downstream signaling pathways involved in autoantibody-mediated AT1 receptor activation are similar to those induced by Ang II.
Physiological Basis for Autoantibody Production-Rodent Models of Preeclampsia
The etiology of autoimmune disease remains largely unknown. Multiple factors, including genetic predisposition, maladaptive immune system, and environment challenge, have been proposed to be involved in autoantibody production.46 – 48 Evidence presented below suggests that the generation of AT1-AA is secondary to reduced placental perfusion and the increased maternal inflammatory response that is associated with preeclampsia.1,3,8,41,42
A Transgenic Model of Preeclampsia
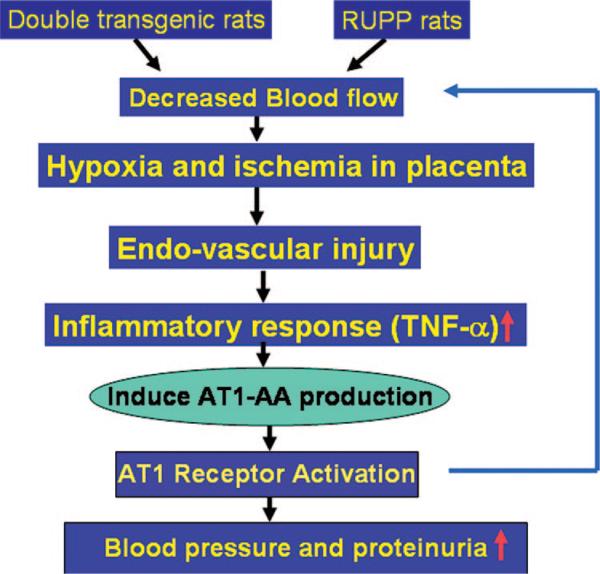
Takimoto et al49 created lines of transgenic mice carrying either the human renin gene or the human angiotensinogen gene. They observed that female transgenic mice carrying the human angiotensinogen gene, which displayed normal blood pressure in the nonpregnant state, developed hypertension late in gestation, but only when mated with transgenic males carrying the human renin gene. Blood pressure returned to normal after birth of the pups. The rise in maternal blood pressure correlated with an increase in secretion of human renin from the transgenic placentas late in pregnancy and an associated increase in circulating Ang II. Histopathologic examination revealed uniform enlargement of glomeruli associated with an increase in urinary protein excretion, myocardial hypertrophy, and necrosis and edema in the placenta. Dechend et al50 examined comparable lines of transgenic rats and also observed an increase in Ang II late in pregnancy and associated proteinuria, renal pathology, placental abnormalities, and hypertension that resolved with delivery. Dechend et al50 used the cardiomyocyte contraction assay to detect the presence of AT1 agonistic antibody in pregnant transgenic rats at day 18 of gestation. Peptide competition experiments showed that the antibody interacted with the same 7 amino acid epitope on the second extracellular loop of the AT1 receptor defined by AT1-AA obtained from women with preeclampsia. Thus, this study suggests that increased blood pressure by overproduction of Ang II in double transgenic pregnant rats may lead to decreased blood flow to placenta and is associated with the production of AT1-AA (Figure 3).
Figure 3.
Molecular basis of AT1-AA production in rat models of preeclampsia: generation of AT1-AA may be secondary to placental ischemia, vascular damage, and enhanced inflammatory response. The decreased blood flow to placenta in double transgenic rats and RUPP rats leads to placental ischemia and hypoxia. This will result in endovascular damage and the enhanced inflammatory response with increased secretion of inflammatory cytokines (eg, TNF-α). The resulting inflammatory cytokine secretion contributes to AT1 receptor agonistic antibody production. AT1-AA will directly induce higher blood pressure and proteinuria via AT1 receptor activation. This may eventually lead more hypoxia, endovascular damage, and enhanced inflammatory response. Thus, we speculate that hypoxia because of reduced placental perfusion may lead to an inflammatory response that contributes to the generation of AT1-AA. The resulting AT1-AA may further contribute to decreased trophoblast invasion, increased hypoxia, and an enhanced inflammatory response, leading to more AT1-AA production. Therefore, reduced placental perfusion, hypoxia, inflammatory response, and AT1-AA production act as a detrimental cycle to contribute to pathophysiology of preeclampsia.
Experimentally Induced Models of Preeclampsia in Rats
It is a widely held view that the maternal syndrome of preeclampsia is secondary to placental abnormalities, especially those resulting from placental ischemia.43,44 In this regard, Granger and colleagues51–53 have developed a rat model of preeclampsia resulting from experimentally induced placental ischemia resulting from reduced uterine perfusion pressure (RUPP). In these rats, they observed increased maternal blood pressure, proteinuria, and other features associated with preeclampsia.52 They also investigated the placentas and maternal circulation of RUPP-manipulated rats and found tumor necrosis factor (TNF)-[H9251] expression up several fold.54 More recently, they found that sera from RUPP manipulated pregnant rats enhanced endothelin synthesis by endothelial cells through AT1 receptor activation.54 Llinas and colleagues, in collaboration with Granger and colleagues,55,56 examined these rat models of preeclampsia for the presence of AT1-AA. They observed the presence of AT1 receptor agonistic autoantibody as judged by the cardiomyocyte contraction assay only in RUPP pregnant rats but not normal pregnant rats.55,56 TNF-α, an inflammatory cytokine, is induced in RUPP pregnant rats. They found that low-dose TNF-α infusion in pregnant rats also resulted in increased blood pressure and the appearance of AT1 receptor agonistic autoantibodies.56 Neither feature was induced by TNF-α infusion into nonpregnant rats, suggesting that hypoxia and ischemia in the placenta may lead to enhanced inflammatory response and result in AT1-AA production.
In summary, AT1-AA is present in 3 different animal models of preeclampsia: double transgenic rats, chronic RUPP, and low-dose TNF-α infusion during pregnancy. These findings suggest that the generation of AT1-AA is a secondary event to reduction in placental perfusion leading to chronic inflammation (Figure 3). The decreased blood flow to placenta leads to placental ischemia and hypoxia. This will result in endovascular damage and the enhanced inflammatory response with increased secretion of inflammatory cytokines (eg, TNF-α). The resulting inflammatory cytokine secretion may lead to AT1 receptor–agonistic antibody production in ways that are not yet understood. The resulting AT1-AA will contribute to higher blood pressure and proteinuria via AT1 receptor activation. In addition, increased AT1-AA may also further decrease trophoblast invasion and spiral artery remolding. This eventually leads to more hypoxia, endovascular damage, and potent inflammatory response. Thus, we speculate that hypoxia because of reduced placental perfusion may lead to an inflammatory response that contributes to the generation of AT1-AA. The resulting AT1-AA may further contribute to decreased trophoblast invasion, increased hypoxia, and an enhanced inflammatory response, leading to more AT1-AA production. Therefore, reduced placental perfusion, hypoxia, inflammatory response, and AT1-AA production act as a detrimental cycle to contribute to the pathophysiology of preeclampsia. These experimentally induced models of preeclampsia in the pregnant rat may provide a valuable model system to determine the physiological basis for autoantibody production.
Potential Therapeutic Effects of Small Antibody Blocking Peptides
Currently there is no effective treatment for preeclampsia, and severe cases often require premature delivery of the infant. If maternal circulating AT1-AA contributes to the pathophysiology of preeclampsia, as the multiple studies from us and others suggest, blocking the action of these autoantibodies with the 7 amino acid epitope peptide may provide significant therapeutic benefit. This expectation is supported by previous in vitro studies showing that AT1-AA typically recognizes a 7 amino acid sequence present on the second extracellular loop of the AT1 receptor (Figure 1), and the ability of AT1-AA to activate AT1 receptors on various cell types can be blocked by the 7 amino acid antibody blocking epitope peptide.10–14,18 Our current studies show that this peptide can neutralize AT1-AA in vivo and thereby prevent autoantibody-induced sFlt-1 production in pregnant mice.41 Thus, the use of epitope peptide therapy to block the action of Ang receptor–activating autoantibodies has the potential of being a safe and effective treatment of preeclampsia (Figure 1).
Conclusion
In 1999, Wallukat et al10 reported their remarkable findings that sera from women with preeclampsia contain autoanti-bodies that react with the AT1 receptor in a stimulatory fashion. These findings provided a new way of thinking about the abnormalities associated with preeclampsia. As described here, these important findings have been extended in numerous ways showing that these autoantibodies activate AT1 receptors on cardiac myocytes, trophoblast cells, endothelial cells, mesangial cells, and vascular smooth muscle cells, among others. Although the studies show that the AT1-AA activates AT1 receptors on a variety of cell types and provokes biological responses that are relevant to the patho-physiology of preeclampsia, the etiology or causality of the disease still remains elusive.
Acknowledgments
Sources of Funding
This work was supported by National Institutes of Health grants HL076558 (to Y.X.) and HD34130 (to R.E.K.).
Footnotes
Disclosures
None.
References
- 1.Walker JJ. Pre-eclampsia. Lancet. 2000;356:1260–1265. doi: 10.1016/S0140-6736(00)02800-2. [DOI] [PubMed] [Google Scholar]
- 2.Roberts JM. Endothelial dysfunction in preeclampsia. Semin Reprod Endocrinol. 1998;16:5–15. doi: 10.1055/s-2007-1016248. [DOI] [PubMed] [Google Scholar]
- 3.Roberts JM, Cooper DW. Pathogenesis and genetics of pre-eclampsia. Lancet. 2001;357:53–56. doi: 10.1016/s0140-6736(00)03577-7. [DOI] [PubMed] [Google Scholar]
- 4.Roberts JM, Lain KY. Recent insights into the pathogenesis of preeclampsia. Placenta. 2002;23:359–372. doi: 10.1053/plac.2002.0819. [DOI] [PubMed] [Google Scholar]
- 5.Roberts JM, Edep ME, Goldfien A, Taylor RN. Sera from preeclamptic women specifically activate human umbilical vein endothelial cells in vitro: morphological and biochemical evidence. Am J Reprod Immunol. 1992;27:101–108. doi: 10.1111/j.1600-0897.1992.tb00735.x. [DOI] [PubMed] [Google Scholar]
- 6.Taylor RN, Musci TJ, Rodgers GM, Roberts JM. Preeclamptic sera stimulate increased platelet-derived growth factor mRNA and protein expression by cultured human endothelial cells. Am J Reprod Immunol. 1991;25:105–108. doi: 10.1111/j.1600-0897.1991.tb01075.x. [DOI] [PubMed] [Google Scholar]
- 7.Conrad KP, Benyo DF. Placental cytokines and the pathogenesis of preeclampsia. Am J Reprod Immunol. 1997;37:240–249. doi: 10.1111/j.1600-0897.1997.tb00222.x. [DOI] [PubMed] [Google Scholar]
- 8.Granger JP, Alexander BT, Bennett WA, Khalil RA. Pathophysiology of pregnancy-induced hypertension. Am J Hypertens. 2001;14:178S–185S. doi: 10.1016/s0895-7061(01)02086-6. [DOI] [PubMed] [Google Scholar]
- 9.Maynard SE, Min JY, Merchan J, Lim KH, Li J, Mondal S, Libermann TA, Morgan JP, Sellke FW, Stillman IE, Epstein FH, Sukhatme VP, Karumanchi SA. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest. 2003;111:649–658. doi: 10.1172/JCI17189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wallukat G, Homuth V, Fischer T, Lindschau C, Horstkamp B, Jupner A, Baur E, Nissen E, Vetter K, Neichel D, Dudenhausen JW, Haller H, Luft FC. Patients with preeclampsia develop agonistic autoantibodies against the angiotensin AT1 receptor. J Clin Invest. 1999;103:945–952. doi: 10.1172/JCI4106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xia Y, Wen H, Bobst S, Day MC, Kellems RE. Maternal autoantibodies from preeclamptic patients activate angiotensin receptors on human trophoblast cells. J Soc Gynecol Investig. 2003;10:82–93. doi: 10.1016/s1071-5576(02)00259-9. [DOI] [PubMed] [Google Scholar]
- 12.Dechend R, Homuth V, Wallukat G, Kreuzer J, Park JK, Theuer J, Juepner A, Gulba DC, Mackman N, Haller H, Luft FC. AT(1) receptor agonistic antibodies from preeclamptic patients cause vascular cells to express tissue factor [see comment]. Circulation. 2000;101:2382–2387. doi: 10.1161/01.cir.101.20.2382. [DOI] [PubMed] [Google Scholar]
- 13.Dechend R, Viedt C, Muller DN, Ugele B, Brandes RP, Wallukat G, Park JK, Janke J, Barta P, Theuer J, Fiebeler A, Homuth V, Dietz R, Haller H, Kreuzer J, Luft FC. AT1 receptor agonistic antibodies from preeclamptic patients stimulate NADPH oxidase. Circulation. 2003;107:1632–1639. doi: 10.1161/01.CIR.0000058200.90059.B1. [DOI] [PubMed] [Google Scholar]
- 14.Thway TM, Shlykov SG, Day MC, Sanborn BM, Gilstrap LC, 3rd, Xia Y, Kellems RE. Antibodies from preeclamptic patients stimulate increased intracellular Ca2+ mobilization through angiotensin receptor activation. Circulation. 2004;110:1612–1619. doi: 10.1161/01.CIR.0000142855.68398.3A. [DOI] [PubMed] [Google Scholar]
- 15.Walther T, Wallukat G, Jank A, Bartel S, Schultheiss HP, Faber R, Stepan H. Angiotensin II type 1 receptor agonistic antibodies reflect fundamental alterations in the uteroplacental vasculature. Hypertension. 2005;46:1275–1279. doi: 10.1161/01.HYP.0000190040.66563.04. [DOI] [PubMed] [Google Scholar]
- 16.de Jong CL, Dekker GA, Sibai BM. The renin-angiotensin-aldosterone system in preeclampsia. A review. Clin Perinatol. 1991;18:683–711. [PubMed] [Google Scholar]
- 17.Alhenc-Gelas F, Tache A, Saint-Andre JP, Milliez J, Sureau C, Corvol P, Menard J. The renin-angiotensin system in pregnancy and parturition. Adv Nephrol Necker Hosp. 1986;15:25–33. [PubMed] [Google Scholar]
- 18.Bobst SM, Day MC, Gilstrap LC, 3rd, Xia Y, Kellems RE. Maternal autoantibodies from preeclamptic patients activate angiotensin receptors on human mesangial cells and induce interleukin-6 and plasminogen activator inhibitor-1 secretion. Am J Hypertens. 2005;18:330–336. doi: 10.1016/j.amjhyper.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 19.Dragun D, Muller DN, Brasen JH, Fritsche L, Nieminen-Kelha M, Dechend R, Kintscher U, Rudolph B, Hoebeke J, Eckert D, Mazak I, Plehm R, Schonemann C, Unger T, Budde K, Neumayer HH, Luft FC, Wallukat G. Angiotensin II type 1-receptor activating antibodies in renal-allograft rejection [see comment]. N Engl J Med. 2005;352:558–569. doi: 10.1056/NEJMoa035717. [DOI] [PubMed] [Google Scholar]
- 20.Dorffel Y, Wallukat G, Bochnig N, Homuth V, Herberg M, Dorffel W, Pruss A, Chaoui R, Scholze J. Agonistic AT(1) receptor autoantibodies and monocyte stimulation in hypertensive patients. Am J Hypertens. 2003;16:827–833. doi: 10.1016/s0895-7061(03)00982-8. [DOI] [PubMed] [Google Scholar]
- 21.Chapman HA. Plasminogen activators, integrins, and the coordinated regulation of cell adhesion and migration. Curr Opin Cell Biol. 1997;9:714–724. doi: 10.1016/s0955-0674(97)80126-3. [DOI] [PubMed] [Google Scholar]
- 22.Waltz DA, Natkin LR, Fujita RM, Wei Y, Chapman HA. Plasmin and plasminogen activator inhibitor type 1 promote cellular motility by regulating the interaction between the urokinase receptor and vitronectin. J Clin Invest. 1997;100:58–67. doi: 10.1172/JCI119521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shaarawy M, Didy HE. Thrombomodulin, plasminogen activator inhibitor type 1 (PAI-1) and fibronectin as biomarkers of endothelial damage in preeclampsia and eclampsia. Int J Gynaecol Obstet. 1996;55:135–139. doi: 10.1016/s0020-7292(96)02755-5. [DOI] [PubMed] [Google Scholar]
- 24.Nakamura S, Nakamura I, Ma L, Vaughan DE, Fogo AB. Plasminogen activator inhibitor-1 expression is regulated by the angiotensin type 1 receptor in vivo. Kidney Int. 2000;58:251–259. doi: 10.1046/j.1523-1755.2000.00160.x. [DOI] [PubMed] [Google Scholar]
- 25.Fogo AB. The role of angiotensin II and plasminogen activator inhibitor-1 in progressive glomerulosclerosis. Am J Kidney Dis. 2000;35:179–188. doi: 10.1016/s0272-6386(00)70324-6. [DOI] [PubMed] [Google Scholar]
- 26.Chen HC, Bouchie JL, Perez AS, Clermont AC, Izumo S, Hampe J, Feener EP. Role of the angiotensin AT(1) receptor in rat aortic and cardiac PAI-1 gene expression. Arterioscler Thromb Vasc Biol. 2000;20:2297–2302. doi: 10.1161/01.atv.20.10.2297. [DOI] [PubMed] [Google Scholar]
- 27.Feener EP, Northrup JM, Aiello LP, King GL. Angiotensin II induces plasminogen activator inhibitor-1 and -2 expression in vascular endothelial and smooth muscle cells. J Clin Invest. 1995;95:1353–1362. doi: 10.1172/JCI117786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hubel CA. Oxidative stress in the pathogenesis of preeclampsia. Proc Soc Exp Biol Med. 1999;222:222–235. doi: 10.1177/153537029922200305. [DOI] [PubMed] [Google Scholar]
- 29.Haller HOT, Hauck U, Distler A, Philipp T. Increased intracellular free calcium and sensitivity to angiotensin II in platelets of preeclamptic women. Am J Hypertens. 1989;2:238–243. doi: 10.1093/ajh/2.4.238. [DOI] [PubMed] [Google Scholar]
- 30.Hojo M, Suthanthiran M, Helseth G, August P. Lymphocyte intracellular free calcium concentration is increased in preeclampsia. Am J Obstet Gynecol. 1999;180:1209–1214. doi: 10.1016/s0002-9378(99)70618-6. [DOI] [PubMed] [Google Scholar]
- 31.Sowers JR, Zemel MB, Bronsteen RA, Zemel PC, Walsh MF, Standley PR, Sokol RJ. Erythrocyte cation metabolism in preeclampsia. Am J Obstet Gynecol. 1989;161:441–445. doi: 10.1016/0002-9378(89)90539-5. [DOI] [PubMed] [Google Scholar]
- 32.Ahmed A, Dunk C, Ahmad S, Khaliq A. Regulation of placental vascular endothelial growth factor (VEGF) and placenta growth factor (PIGF) and soluble Flt-1 by oxygen–a review. Placenta. 2000;21(suppl A):S16–S24. doi: 10.1053/plac.1999.0524. [DOI] [PubMed] [Google Scholar]
- 33.Chaiworapongsa T, Romero R, Espinoza J, Bujold E, Mee Kim Y, Goncalves LF, Gomez R, Edwin S. Evidence supporting a role for blockade of the vascular endothelial growth factor system in the pathophysiology of preeclampsia. Young Investigator Award. Am J Obstet Gynecol. 2004;190:1541–1550. doi: 10.1016/j.ajog.2004.03.043. [DOI] [PubMed] [Google Scholar]
- 34.Chaiworapongsa T, Romero R, Kim YM, Kim GJ, Kim MR, Espinoza J, Bujold E, Goncalves L, Gomez R, Edwin S, Mazor M. Plasma soluble vascular endothelial growth factor receptor-1 concentration is elevated prior to the clinical diagnosis of pre-eclampsia. J Matern Fetal Neonatal Med. 2005;17:3–18. doi: 10.1080/14767050400028816. [DOI] [PubMed] [Google Scholar]
- 35.Levine RJ, Maynard SE, Qian C, Lim KH, England LJ, Yu KF, Schisterman EF, Thadhani R, Sachs BP, Epstein FH, Sibai BM, Sukhatme VP, Karumanchi SA. Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med. 2004;350:672–683. doi: 10.1056/NEJMoa031884. [DOI] [PubMed] [Google Scholar]
- 36.Koga K, Osuga Y, Yoshino O, Hirota Y, Ruimeng X, Hirata T, Takeda S, Yano T, Tsutsumi O, Taketani Y. Elevated serum soluble vascular endothelial growth factor receptor 1 (sVEGFR-1) levels in women with preeclampsia. J Clin Endocrinol Metab. 2003;88:2348–2351. doi: 10.1210/jc.2002-021942. [DOI] [PubMed] [Google Scholar]
- 37.Tsatsaris V, Goffin F, Munaut C, Brichant J-F, Pignon M-R, Noel A, Schaaps J-P, Cabrol D, Frankenne F, Foidart J-M. Overexpression of the soluble vascular endothelial growth factor receptor in preeclamptic patients: pathophysiological consequences. J Clin Endocrinol Metab. 2003;88:5555–5563. doi: 10.1210/jc.2003-030528. [DOI] [PubMed] [Google Scholar]
- 38.Nagamatsu T, Fujii T, Kusumi M, Zou L, Yamashita T, Osuga Y, Momoeda M, Kozuma S, Taketani Y. Cytotrophoblasts up-regulate soluble fms-like tyrosine kinase-1 expression under reduced oxygen: an implication for the placental vascular development and the pathophysiology of preeclampsia. Endocrinology. 2004;145:4838–4845. doi: 10.1210/en.2004-0533. [DOI] [PubMed] [Google Scholar]
- 39.Zhou CC, Ahmad S, Mi T, Xia L, Abbasi S, Hewett PW, Sun CX, Ahmed A, Kellems RE, Xia Y. Angiotensin II induces soluble fms-like tyrosine kinase-1 (sFlt-1) release via calcineurin signaling pathway in pregnancy. Circ Res. 2007;100:88–95. doi: 10.1161/01.RES.0000254703.11154.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhou CC, Abbasi S, Xia Lingw, Day M-C, Ramin SM, Kellems RE, Xia Y. Upregulation of placental soluble fms-like tyrosine kinase 1 (sFlt-1) by AT1 receptor agonistic autoantibodies in preeclampsia. Hypertens Pregnancy. 2006;25(suppl 1):38. Abstract. [Google Scholar]
- 41.Redman CW, Sargent IL. Latest advances in understanding preeclampsia. Science. 2005;308:1592–1594. doi: 10.1126/science.1111726. [DOI] [PubMed] [Google Scholar]
- 42.Roberts JM. Preeclampsia: what we know and what we do not know. Semin Perinatol. 2000;24:24–28. doi: 10.1016/s0146-0005(00)80050-6. [DOI] [PubMed] [Google Scholar]
- 43.Granger JP, Alexander BT, Llinas MT, Bennett WA, Khalil RA. Pathophysiology of hypertension during preeclampsia linking placental ischemia with endothelial dysfunction. Hypertension. 2001;38:718–722. doi: 10.1161/01.hyp.38.3.718. [DOI] [PubMed] [Google Scholar]
- 44.Granger JP, Alexander BT, Llinas MT, Bennett WA, Khalil RA. Pathophysiology of preeclampsia: linking placental ischemia/hypoxia with microvascular dysfunction. Microcirculation. 2002;9:147–160. doi: 10.1038/sj.mn.7800137. [DOI] [PubMed] [Google Scholar]
- 45.Decker EL, Nehmann N, Kampen E, Eibel H, Zipfel PF, Skerka C. Early growth response proteins (EGR) and nuclear factors of activated T cells (NFAT) form heterodimers and regulate proinflammatory cytokine gene expression. Nucleic Acids Res. 2003;31:911–921. doi: 10.1093/nar/gkg186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pearce SH, Merriman TR. Genetic progress towards the molecular basis of autoimmunity. Trends Mol Med. 2006;12:90–98. doi: 10.1016/j.molmed.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 47.Fujinami RS, von Herrath MG, Christen U, Whitton JL. Molecular mimicry, bystander activation, or viral persistence: infections and autoimmune disease. Clin Microbiol Rev. 2006;19:80–94. doi: 10.1128/CMR.19.1.80-94.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Barak Y. The immune system and happiness. Autoimmun Rev. 2006;5:523–527. doi: 10.1016/j.autrev.2006.02.010. [DOI] [PubMed] [Google Scholar]
- 49.Takimoto E, Ishida J, Sugiyama F, Horiguchi H, Murakami K, Fukamizu A. Hypertension induced in pregnant mice by placental renin and maternal angiotensinogen. Science. 1996;274:995–998. doi: 10.1126/science.274.5289.995. [DOI] [PubMed] [Google Scholar]
- 50.Dechend R, Gratze P, Wallukat G, Shagdarsuren E, Plehm R, Brasen JH, Fiebeler A, Schneider W, Caluwaerts S, Vercruysse L, Pijnenborg R, Luft FC, Muller DN. Agonistic autoantibodies to the AT1 receptor in a transgenic rat model of preeclampsia. Hypertension. 2005;45:742–746. doi: 10.1161/01.HYP.0000154785.50570.63. [DOI] [PubMed] [Google Scholar]
- 51.Gadonski G, LaMarca BB, Sullivan E, Bennett W, Chandler D, Granger JP. Hypertension produced by reductions in uterine perfusion in the pregnant rat: role of interleukin 6. Hypertension. 2006;48:711–716. doi: 10.1161/01.HYP.0000238442.33463.94. [DOI] [PubMed] [Google Scholar]
- 52.Granger JP, LaMarca BB, Cockrell K, Sedeek M, Balzi C, Chandler D, Bennett W. Reduced uterine perfusion pressure (RUPP) model for studying cardiovascular-renal dysfunction in response to placental ischemia. Methods Mol Med. 2006;122:383–392. doi: 10.1385/1-59259-989-3:381. [DOI] [PubMed] [Google Scholar]
- 53.Roberts L, LaMarca BB, Fournier L, Bain J, Cockrell K, Granger JP. Enhanced endothelin synthesis by endothelial cells exposed to sera from pregnant rats with decreased uterine perfusion. Hypertension. 2006;47:615–618. doi: 10.1161/01.HYP.0000197950.42301.dd. [DOI] [PubMed] [Google Scholar]
- 54.LaMarca BB, Bennett WA, Alexander BT, Cockrell K, Granger JP. Hypertension produced by reductions in uterine perfusion in the pregnant rat: role of tumor necrosis factor-alpha. Hypertension. 2005;46:1022–1025. doi: 10.1161/01.HYP.0000175476.26719.36. [DOI] [PubMed] [Google Scholar]
- 55.Llinas M, Wallukat GDR, Mueller DN, Luft FC, Alexander BT, Lamarca BB, Granger JP. Agonistic autoantibodies to the AT1 receptor in a rat model of preeclampsia induced by chronic reductions in uterine perfrusion pressure (RUPP) [abstract]. Hypertension. 2005;46:883. [Google Scholar]
- 56.Dechend R, Llinas M, Caluwaerts S, Herse F, Lamarca B, Mueller DN, Luft FC, Pijnenborg R, Wallukat G, Granger JP. Agonistic autoantibodies to the AT1 receptor in rat models of preeclampsia: induced by chronic reduction in uterine perfusion pressure (RUPP) and low dose TNF-a infusion [abstract]. Hypertens Pregnancy. 2006;25:70. [Google Scholar]