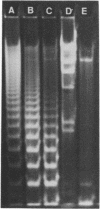
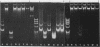
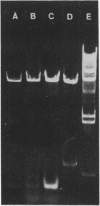
Abstract
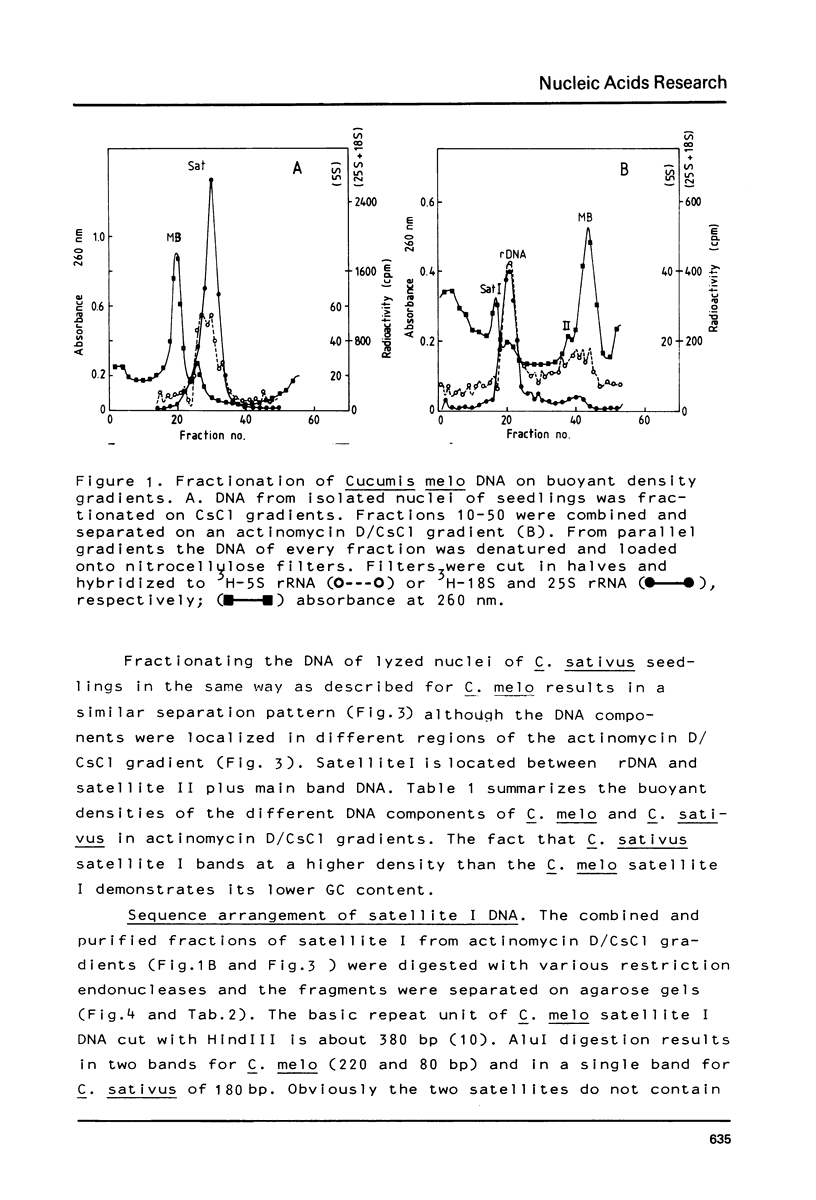
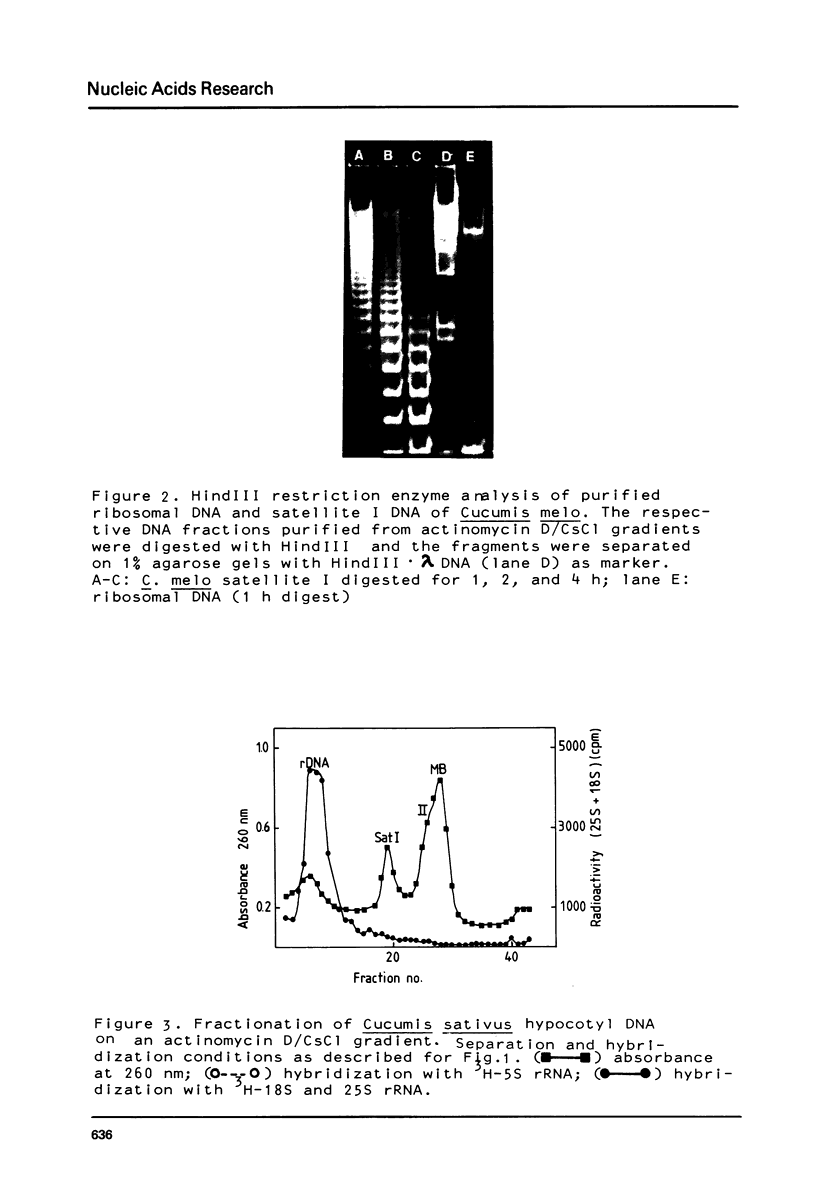
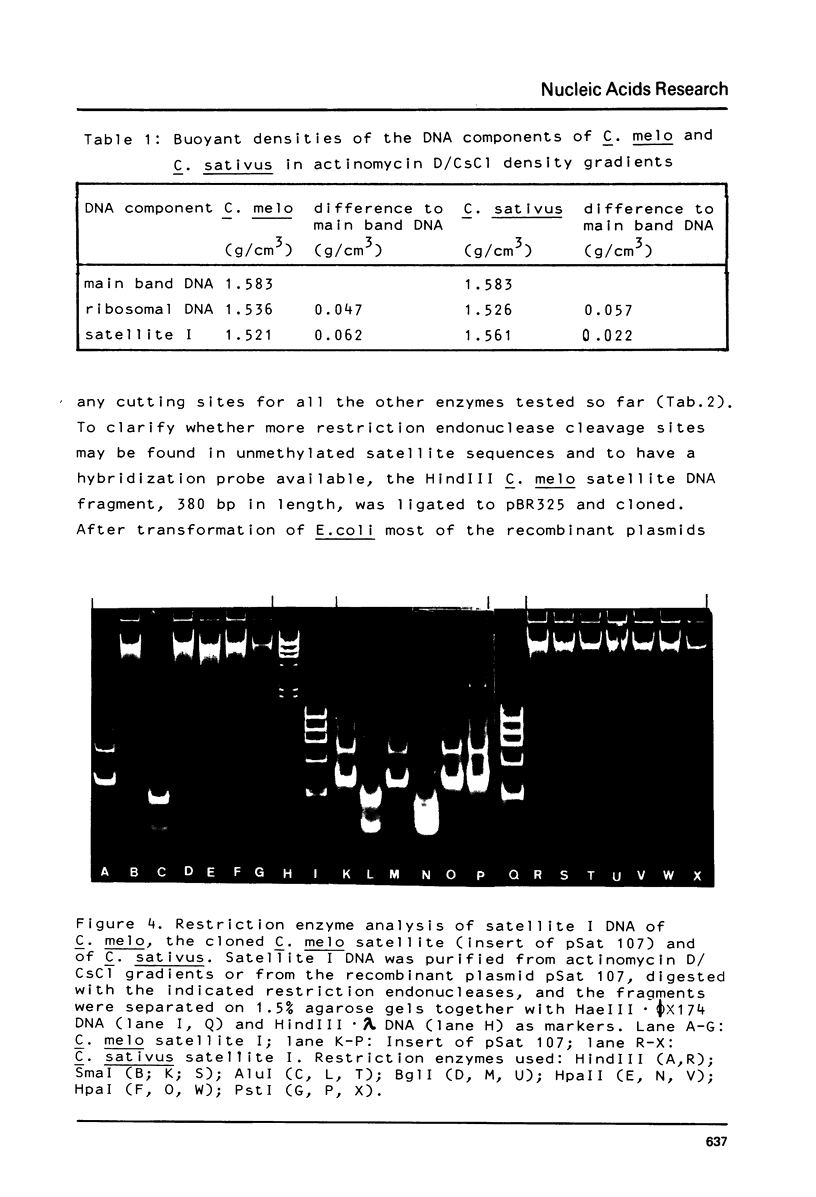
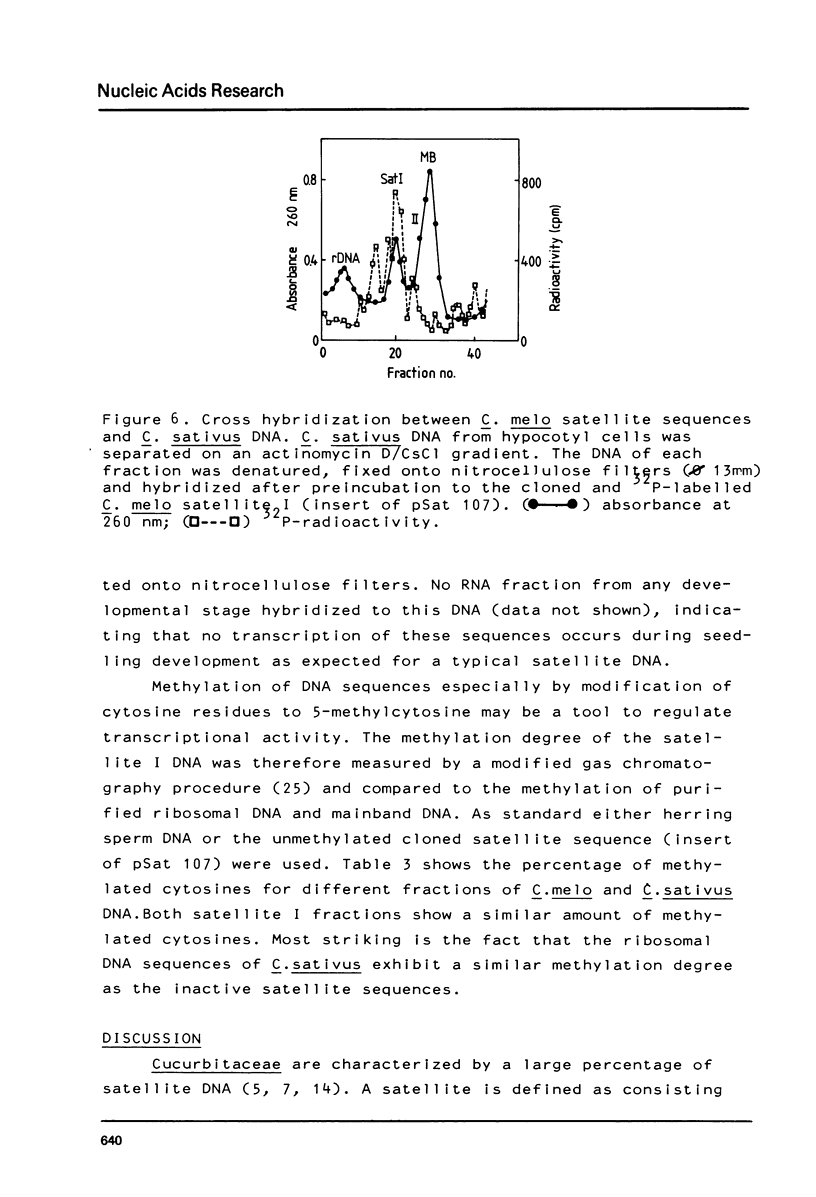
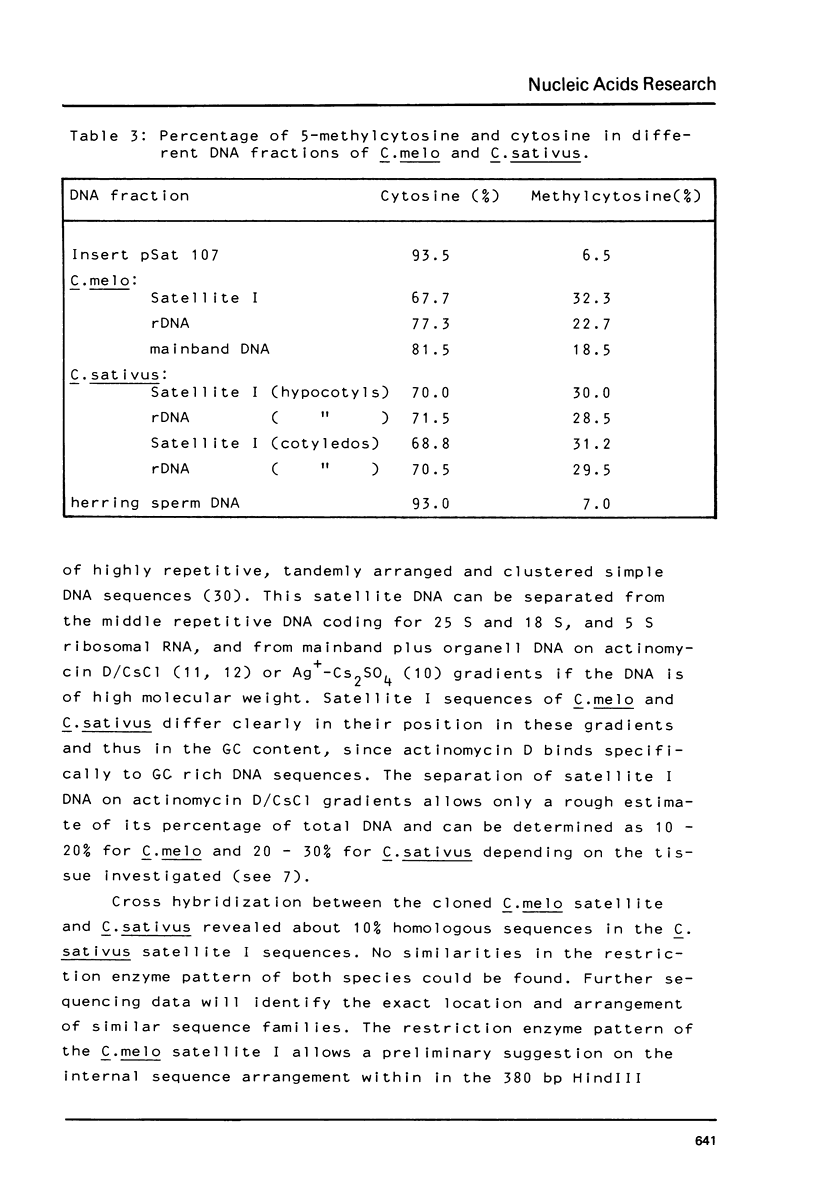
The prominent satellites of the Cucurbitaceae Cucumis melo (melon) and Cucumis sativus (cucumber) have been characterized, in actinomycin/CsCl gradients where the satellite sequences can be separated from ribosomal, organelle, and main band DNA the location of the satellites is different indicating a different GC content. The purified satellite of C. melo is cut by HindIII into a repeat unit of 380 bp; AluI digestion gives rise to two bands (about 80 and 220 bp in size). The HindIII repeat unit if cloned into pBR325 exhibits new recognition sites for HpaII leaving two bands with 150 and 80 bp suggesting methylation of the C/CGG cutting site in the uncloned material. The restriction pattern indicates an internal sequence repeat within the 380 bp HindIII fragment. The C. sativus satellite is cut by AluI to a repeat unit of 180 bp showing no other recognition site for the restriction enzymes tested so far. About 10% sequence homology has been determined between the C. melo and C. sativus satellites by cross hybridization studies. A high methylation degree of cytosines has been measured for both satellites and the ribosomal DNA of C. sativus (about 30%). No transcription products of the C. melo satellite were found during seedling development.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appels R., Peacock W. J. The arrangement and evolution of highly repeated (satellite) DNA sequences with special reference to Drosophila. Int Rev Cytol Suppl. 1978;Suppl 8:69–126. doi: 10.1016/s0074-7696(08)60472-6. [DOI] [PubMed] [Google Scholar]
- Bazaral M., Helinski D. R. Circular DNA forms of colicinogenic factors E1, E2 and E3 from Escherichia coli. J Mol Biol. 1968 Sep 14;36(2):185–194. doi: 10.1016/0022-2836(68)90374-4. [DOI] [PubMed] [Google Scholar]
- Bedbrook J. R., Jones J., O'Dell M., Thompson R. D., Flavell R. B. A molecular description of telometic heterochromatin in secale species. Cell. 1980 Feb;19(2):545–560. doi: 10.1016/0092-8674(80)90529-2. [DOI] [PubMed] [Google Scholar]
- Bendich A. J., Anderson R. S. Novel properties of satellite DNA from muskmelon. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1511–1515. doi: 10.1073/pnas.71.4.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. N., Chang A. C., Hsu L. Nonchromosomal antibiotic resistance in bacteria: genetic transformation of Escherichia coli by R-factor DNA. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2110–2114. doi: 10.1073/pnas.69.8.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deumling B. Sequence arrangement of a highly methylated satellite DNA of a plant, Scilla: A tandemly repeated inverted repeat. Proc Natl Acad Sci U S A. 1981 Jan;78(1):338–342. doi: 10.1073/pnas.78.1.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Getz M. J., Altenburg L. C., Saunders G. F. The use of RNA labeled in vitro with iodine-125 in molecular hybridization experiments. Biochim Biophys Acta. 1972 Dec 22;287(3):485–494. doi: 10.1016/0005-2787(72)90293-6. [DOI] [PubMed] [Google Scholar]
- Grierson D. Characterization of ribonucleic acid components from leaves of Phaseolus aureus. Eur J Biochem. 1974 May 15;44(2):509–515. doi: 10.1111/j.1432-1033.1974.tb03509.x. [DOI] [PubMed] [Google Scholar]
- Ingle J., Pearson G. G., Sinclair J. Species distribution and properties of nuclear satellite DNA in higher plants. Nat New Biol. 1973 Apr 18;242(120):193–197. doi: 10.1038/newbio242193a0. [DOI] [PubMed] [Google Scholar]
- Ingle J., Timmis J. N., Sinclair J. The Relationship between Satellite Deoxyribonucleic Acid, Ribosomal Ribonucleic Acid Gene Redundancy, and Genome Size in Plants. Plant Physiol. 1975 Mar;55(3):496–501. doi: 10.1104/pp.55.3.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John B., Miklos G. L. Functional aspects of satellite DNA and heterochromatin. Int Rev Cytol. 1979;58:1–114. doi: 10.1016/s0074-7696(08)61473-4. [DOI] [PubMed] [Google Scholar]
- Kadouri A., Atsmon D., Edelman M. Satellite-rich DNA in cucumber: hormonal enhancement of synthesis and subcellular identification. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2260–2264. doi: 10.1073/pnas.72.6.2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leber B., Hemleben V. Structure of plant nuclear and ribosomal DNA containing chromatin. Nucleic Acids Res. 1979 Nov 10;7(5):1263–1281. doi: 10.1093/nar/7.5.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniatis T., Jeffrey A., Kleid D. G. Nucleotide sequence of the rightward operator of phage lambda. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1184–1188. doi: 10.1073/pnas.72.3.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orosz J. M., Wetmur J. G. In vitro iodination of DNA. Maximizing iodination while minimizing degradation; use of buoyant density shifts for DNA-DNA hybrid isolation. Biochemistry. 1974 Dec 31;13(27):5467–5473. doi: 10.1021/bi00724a003. [DOI] [PubMed] [Google Scholar]
- Razin A., Riggs A. D. DNA methylation and gene function. Science. 1980 Nov 7;210(4470):604–610. doi: 10.1126/science.6254144. [DOI] [PubMed] [Google Scholar]
- Razin A., Sedat J. Analysis of 5-methylcytosine in DNA. II. Gas chromatography. Anal Biochem. 1977 Feb;77(2):370–377. doi: 10.1016/0003-2697(77)90250-0. [DOI] [PubMed] [Google Scholar]
- Sinclair J., Wells R., Deumling B., Ingle J. The complexity of satellite deoxyribonucleic acid in a higher plant. Biochem J. 1975 Jul;149(1):31–38. doi: 10.1042/bj1490031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Timmis J. N., Ingle J. Variation in satellite DNA from some higher plants. Biochem Genet. 1977 Dec;15(11-12):1159–1173. doi: 10.1007/BF00484506. [DOI] [PubMed] [Google Scholar]
- Varley J. M., Macgregor H. C., Erba H. P. Satellite DNA is transcribed on lampbrush chromosomes. Nature. 1980 Feb 14;283(5748):686–688. doi: 10.1038/283686a0. [DOI] [PubMed] [Google Scholar]
- Varley J. M., Macgregor H. C., Nardi I., Andrews C., Erba H. P. Cytological evidence of transcription of highly repeated DNA sequences during the lampbrush stage in Triturus cristatus carnifex. Chromosoma. 1980;80(3):289–307. doi: 10.1007/BF00292686. [DOI] [PubMed] [Google Scholar]
- Wagner I., Capesius I. Determination of 5-methylcytosine from plant DNA by high-performance liquid chromatography. Biochim Biophys Acta. 1981 Jun 26;654(1):52–56. doi: 10.1016/0005-2787(81)90135-0. [DOI] [PubMed] [Google Scholar]