Abstract
It is well documented that the gut injury plays a critical role in the development of systemic inflammation and distant organ injury in conditions associated with splanchnic ischemia. Consequently understanding the mechanisms leading to gut injury is important. In this context, recent work suggests a protective role for the intestinal mucus layer and an injury-inducing role for luminal pancreatic proteases. Thus, we explored the role of the mucus layer in gut barrier function by observing how the removal of the mucus layer affects ischemia/reperfusion-mediated gut injury in rats as well as the potential role of luminal pancreatic proteases in the pathogenesis of gut injury. Ischemia was induced by the ligation of blood vessels to segments of the ileum for 45 min, followed by up to three hours of reperfusion. The ileal segments were divided into 5 groups. These included a non-ischemic control, ischemic segments exposed to saline, the mucolytic N-acetylcholine (NAC), pancreatic proteases or NAC plus pancreatic proteases. Changes in gut barrier function were assessed by the permeation of fluorescein isothiocyanate dextran (MW 4000 Da; FD4) in ileal everted sacs. Gut injury was measured morphologically and by the luminal content of protein, DNA and hemoglobin. The mucus layer was assessed functionally by measuring its hydrophobicity and morphologically. Gut barrier function was promptly and effectively re-established during reperfusion, which was accompanied by the restoration of the mucus layer. In contrast, treatment of the gut with the mucolytic NAC for 10 min during ischemia resulted in a failure of mucus restitution and further increases in gut permeability and injury. The presence of digestive proteases by themselves did not exacerbate gut injury but in combination with NAC, they caused an even greater increase in gut injury and permeability. These results suggest that the mucus layer not only serves as a barrier between the luminal contents and gut surface epithelia, but also plays a critical role in the maintenance and restitution of gut barrier function.
Keywords: Mucus layer, Ischemia/reperfusion, intestinal permeability, gut barrier function, digestive proteases
INTRODUCTION
Many studies have shown that the gut plays a critical role in the systemic consequences of trauma/hemorrhagic shock (1), as well as in other disease processes such as burn injury, sepsis, acute pancreatitis, obstructive jaundice, and chronic liver disease (1, 2, 3, 4). Because the gut contains large amounts of bacteria, much attention has focused on the increased infiltration of endotoxin and the translocation of bacteria from the lumen of the gut to the systemic circulation as a potential cause or contributor to the development of the multiple organ dysfunction syndrome (MODS) (1). More recently, it has been found that, under several circumstances associated with gut injury, increased infiltration of pancreatic digestive proteases into the gut wall may be involved in the pathogenesis of gut-induced MODS (5, 6). This association is not surprising, considering the destructive power these digestive enzymes possess and the large amount of these enzymes released into the gut lumen each day. This notion of a role for pancreatic proteases in gut injury and gut-associated MODS is supported by studies documenting that trauma-hemorrhagic shock (T/HS)-induced intestinal and lung injury can be alleviated by pancreatic duct ligation (7) as well by the intraluminal, but not the intravenous infusion, of a serine pancreatic protease inhibitor (8, 9).
In investigating the possible role of pancreatic proteases in gut injury and gut-associated MODS in conditions associated with splanchnic hypoperfusion, it is important to recognize that the physical gut barrier consists of two distinct elements. First is the well described mucosal layer of epithelial cells lining the villous, with their tight junctions that serve to limit gut permeability (10). Yet, there is another component that contributes to gut barrier function, which is the mucus layer overlying the enterocytes (11). This layer of hydrophobic mucus prevents direct contact of the epithelial cells with the luminal contents and thereby limits the penetration of harmful agents, like digestive proteases as well as bacteria, across the mucosal barrier (12). That loss of the mucus layer is likely important in loss of gut barrier function is supported by a series of recent studies. This work showed that increased gut permeability after T/HS was associated with disruption of the mucus layer (13) and that increasing periods of gut ischemia resulted in dose-dependent reductions in mucus layer function including mucosal hydrophobicity, which correlated significantly with increased in intestinal permeability (14). Lastly, treatment of the gut with the mucolytic N-acetyl cysteine (NAC) caused a dose-dependent decrease in mucosal hydrophobicity and an increase in intestinal permeability (14, 15), further suggesting that reductions in the mucus layer itself may affect gut barrier function. Thus, the goal of the current study was to test the hypothesis that loss of the mucus layer contributes to gut injury and that the magnitude of gut injury associated with loss of the mucus layer is exacerbated by the presence of pancreatic proteases within the lumen of the gut.
MATERIALS AND METHODS
Animals
Specific pathogen-free male Sprague-Dawley rats (Taconic Farms, Germantown, NY) weighing 300 to 350 g were housed under barrier-sustained conditions and kept at 25°C with 12-hour light/dark cycles. The rats had free access to water and chow (Teklad 22/5 Rodent Diet W-8640, Harlan Teklad, Madison, Wis). All rats were maintained and all experiments were conducted in accordance with the recommendations of the Guide for the Care and Use of Laboratory Animals and the experiments were approved by the New Jersey Medical School Animal Care and Use Committee.
Experimental Design
To test the general hypothesis that the magnitude of an intestinal ischemia-reperfusion injury is augmented by loss of the mucus layer and further increased by the presence of pancreatic proteases in the gut lumen, we utilized a rat model where the distal one-third of the small bowel of each animal was divided to create 5 distinct ileal segments, which were exposed to different luminal conditions in the presence of an intestinal ischemia-reperfusion insult. To limit the amount of luminal material, the rats were fasted prior to study. An advantage of this model is that it is an in vivo ischemic insult, where the animal remains alive and all interventions are carried out in the intestine of a living rat. Only at the time of sacrifice is the intestine removed for study.
In the first set of experiments, we serially determined the time course of gut injury and recovery after a 45 minute period of segmental arterial occlusion by measuring intestinal permeability, luminal DNA and protein levels (reflects enterocyte shedding into the lumen) as well as mucosal hydrophobicity. These measurements were made at the end of the 45 minute ischemic period and at 1, 2 or 3 hours after reperfusion.
In the second set of experiments, we tested if removing the mucus layer increased the injury observed. To accomplish this, we treated the gut with the mucolytic N-acetylcysteine (NAC) and measured intestinal permeability at the end of the 45 min ischemic period as well as after 3 hours of reperfusion. Thus, in this experiment, the ileum was divided into five segments as follows: 1) control (no ischemia); 2) Ischemia 45 min; 3) Ischemia 45 min + NAC; 4) Ischemia 45 min with Reperfusion 3 hr and 5) Ischemia 45 min, Reperfusion 3 hr + NAC. Just after the start of ischemia, the NAC-treated segments were infused with 10% NAC at 0.1 ml/cm for 10 min following which the NAC was flushed out with 50 ml of saline. Our previous study showed that such a treatment can result in effective removal of the mucus (14).
In the third set of experiments, we tested the effects of physiologic concentrations of pancreatic proteases on gut permeability by adding pancreatic proteases to NAC-treated and non-treated intestinal segments subjected to ischemia-reperfusion. Controls for these mucus and/or protease treated intestinal segments were untreated intestinal segments subjected to ischemia plus reperfusion. The pancreatic protease solution consisted of a mixture of trypsin and chymotrypsin at a concentration of 1 mg/ml for each. The protease mixture was injected into the lumen at 0.1 ml/cm just after the start of ischemia. This concentration of proteases recreates their physiological levels in the gut lumen (16, 17). Thus, in this experiment, the ileum was divided into five segments as follows: 1) Naïve (taken just before starting ischemia); 2) Ischemia 45 min, Reperfusion 3h + Saline; 3) Ischemia 45 min, Reperfusion 3h + Proteases; 4) Ischemia 45 min, Reperfusion 3h + NAC and 5) Ischemia 45 min, Reperfusion 3h + NAC + proteases (the mixture of digestive proteases as described above was injected just after flushing out NAC). To avoid bias, the segments exposed to the various conditions were varied among the different animals.
Experimental model and Time-course of ischemia/reperfusion-mediated gut injury
The experimental approach utilized is illustrated in Figure 1. After the rats were anesthetized with an intraperitoneal injection of sodium pentobarbital (50 mg/kg), a laparotomy was performed and the distal segment of the ileum, about 65 cm long, was isolated and ligated at both ends with 4-0 silk (Figure 1a). Using saline, the luminal contents were gently flushed out of the isolated ileal segment through cuts made near the ligated ends of the segment as well as one made two-fifths of the distance from the distal end of the segment (Figure 1b). The rationale for flushing out the luminal contents was two-fold. First, to reduce any confounding effects caused by differences in the amount as well as composition (digestive proteases, bile salts, etc) of luminal contents between the segments and secondly to facilitate the performance of assays on the luminal contents. After gently milking out the flushing solution, the 65 cm segment was divided into five smaller isolated segments of about 12 cm in length, with care to avoid injuring the blood vessels to these segments. Then these individually ligated segments were injected with 0.1 ml/cm of saline to ensure a comparable degree of intestinal distension (Figure 1c).
Figure 1.
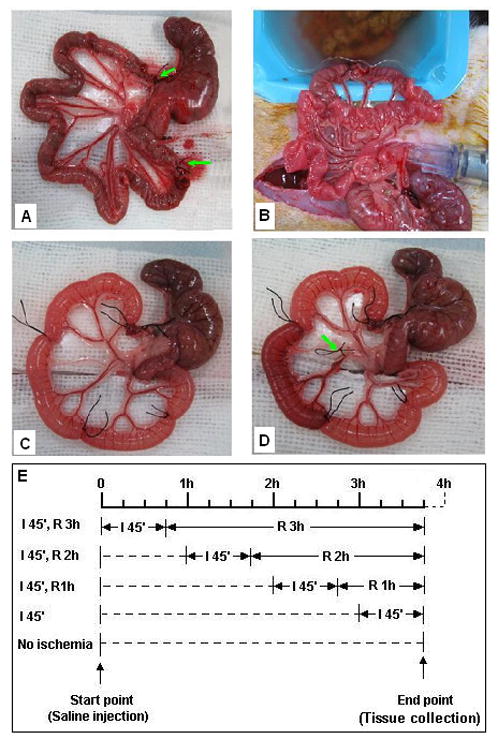
Experimental approach utilized. A segment of distal ileum (about 65 cm) is isolated and ligated at both ends (arrows) (A), following which the luminal contents are gently flushed out with saline through cuts made at the ends of the ligated segment (B); the initial ileal segment is divided into five smaller segments (about 12 cm for each) each of which is distended with 0.5 ml of fluid (C); segmental ischemia is produced by occlusion of the blood vessels to that segment (arrow) (D). Successful occlusion and release of the blood supply was demonstrated by the change of color of that segment during (becomes pale) and after the release of the blood vessel (restoration of blood flow). The time schedule for ischemia/reperfusion of the individual segments is staggered such that all of the segments can be harvested at the same time (E).
Ischemia of each specific intestinal segment was achieved by occlusion of the blood vessels that supplied that segment with a small piece of rubber tubing to avoid vascular injury. A slip knot was used to facilitate vascular release and reperfusion (Figure 1d). The successful occlusion of the blood supply was ascertained by the change of color of the corresponding segment during the ligation as well as its release.
By initiating ischemia at different times, the reperfusion period was controlled (Figure 1e). This design has been used in studies by others (18) to make it possible to compare adjacent segments with different treatments in the same animal. This approach reduces the variability caused by differences among individual animals and also greatly reduced the numbers of animals needed for the experiments. At the end of the experiment period, each segment was harvested and its exact length was measured following which the segment was cut open at both ends and flushed with 5 ml saline. The flushing solution was saved and put in the −80 °C freezer for further analysis. Each intestinal segment was then divided into following pieces: one (6 cm) for the permeability assay, one (3 cm) for hydrophobicity, and the remaining piece for evaluation of the mucus layer and morphology.
Measurement of intestinal mucosal permeability
Intestinal permeability was measured using the everted gut sac method and the fluorescent tracer, fluorescein isothiocyanate dextran (MW 4000 Da; FD4) as described previously (14). Briefly, the intestinal segment was everted using a thin metal rod. One end of the segment was secured with 4-0 silk to the grooved tip of a 1-mL plastic syringe containing 0.5 ml modified Krebs-Henseleit bicarbonate buffer (KHBB, pH 7.4). After tying off the end, the everted gut sac was distended gently with the 0.5 ml of KHBB and the second end was ligated following which the gut sac was placed in a 100-mL beaker containing 80 mL of KHBB plus FD4 (20 μg/mL). The solution in the beaker was maintained at 37°C temperature in a water bath, and a gas mixture containing 95% O2 and 5% CO2 was bubbled continuously. A 1.0-mL sample was taken from the beaker before placing the everted gut sac to determine the initial external (mucosal surface) FD4 concentration. After a 30 minute incubation, the concentration of FD4 within the gut sac was measured using a Perkin-Elmer LS-50 fluorescence spectrophotometer (Palo Alto, CA) at an excitation wavelength of 492 (slit width, 510 nm) and an emission wavelength of 515 nm (slit width, 510 nm). Permeability was expressed as the mucosal-to-serosal clearance of FD4 calculated using the following equations:
where M is the mass (in ng) of FD4 in the gut sac at the end of the 30-min incubation period, [FD4]serosal is the FD4 concentration in the serosal fluid aspirated from the sac at the end of the 30 minute incubation period, F is the flux of FD4 (in ng/min) across the mucosa, [FD4]mucosal is the FD4 concentration measured in the beaker at the beginning of the 30 minute incubation period, A = Π LD which is the calculated area (in cm2) of the mucosal surface, and C is the clearance of FD4 (in nl · min−1 · cm−2) across the mucosa.
Measurement of DNA, protein, glycoprotein and Hemoglobin in the lumen
To determine the luminal content of DNA, protein, glycoprotein and hemoglobin, the previously frozen solution that had been flushed through each intestinal segment was thawed and assayed for these substances as described below. Since the length of small intestine from which each of the luminal flushing solutions had been obtained had been measured, to optimize comparison between groups data was expressed as mg of material per cm of bowel length.
The amount of DNA in the lumen was measured by the diphenylamine (DPA) method (19). In brief, 25 μl of the collected homogenized flushing solutions were added to the wells of a microplate, followed by the addition of 25 μl of a mixture of 40% perchloric acid and 0.32 % acetaldehyde at a ratio of 5:1, and 100 μl of 4% diphenylamine (in glacial acetic acid). After mixing and sealing the cover with parafilm, the microplate was incubated at room temperature overnight. The next day, the microplate was read with a microplate reader at 595 and 750 nm, with the reading at 750 nm as the background. Harp sperm DNA was used as the standard.
The amount of protein in the lumen was measured using the protein assay reagent from Bio-Rad according to the manufacturer’s protocol. In brief, after thawing, the flushing solution was homogenized and an aliquot of the solution was diluted 10 times with distilled water. Then 5 μl of the diluted specimens as well as a protein standard (bovine serum albumin) were added to the wells of a microplate, followed by the addition of 195 μl of 1X reagent. Five minutes later, the microplate was read with a microplate reader at 595 nm.
The amount of glycoprotein in the lumen was measured as described previously by Mantle et al. (20). Hemoglobin was measured using QuantiChrom Hemoglobin Assay Kit from BioAssays System (Hayward, CA) according to the manufacture’s protocol.
Measurement of hydrophobicity
Hydrophobicity was measured by a goniometer (Rame-Hart, Mountain Lakes, NJ), which contains an adjustable sample stage, a syringe, a light source, and a microscope linked to a computer as described previously (14). In brief, the segment of the ileum was put on a piece of paper (about 5 cm long by 4 cm wide), where it was cut open, spread out and rinsed gently with saline. Then the moist supporting paper containing the intestinal segment was mounted on a slide by wrapping both edges of the paper around the slide. This maneuver ensured that the intestinal segment would remain flat during drying of the tissue. The tissue was allowed to dry until the mucosal surface showed a matted appearance. Then, the paper along with the tissue was taken off from the slide and a strip of the tissue with the underlying paper was cut and mounted onto a stand of Styrofoam with a narrow edge to ensure a good view of the droplet once it was placed on the tissue. After placing the styrofoam stand on the stage of the goniometer, 5 μl of saline was gently applied from a syringe onto the surface of the tissue and adjustments were made to create a good exposure of the droplet in the view on the computer screen. The contact angle of the droplet was measured and recorded by the machine. Four to five measurements were taken for each segment and their average was used in the analysis. The more hydrophobic the surface, the greater the contact angle between the water droplet and the mucosal surface. Thus, a greater contact angle means a higher degree of hydrophobicity.
Morphologic analysis of gut injury and mucus layer
The morphology of the mucus layer was evaluated using either frozen or non-frozen tissue sections in a blinded fashion. Frozen sections were made from a 3 cm segment of ileum that was ligated on both sides, filled with saline and then snap frozen in liquid nitrogen. Tissue sections were then produced by cutting the tissue on a cryostat at a thickness of 16 μm. Then the tissue sections were stained with 2.5 % alcian blue in 3% acetic acid for 2.5 h. After washing with tap water, the extent of mucus (glycoprotein) staining were observed under a microscope.
In the second technique, mucus staining was combined with hematoxylin and eosin (H&E) staining. In this technique, segments of ileum were opened along the mesenteric border, laid flat with the mucosal surface up, and pinned to a cardboard base. To stain the mucus layer, 3% Alcian Blue (Sigma) solution in distilled water was sprayed on the mucosal surface, immediately following which the tissue section was immersed in Carnoy’s solution (60% ethanol, 30% acetic acid and 10% chloroform) for 2 hours at 4°C. The samples were then placed in 100% ethanol, cleared in xylene and embedded in paraffin (21). The tissue sections were cut longitudinally in 4 μm sections for histological analysis. The Carnoy-stained cut sections of tissue were then stained with H&E to evaluate ileal villous injury and mucus coverage simultaneously.
Five random fields with 150 to 250 villi from each animal were analyzed in a blinded fashion using light microscopy at 100× magnification. The overall incidence of villous damage was expressed as a percentage of injured villi to total villi counted. Injury was graded using the method described by Chiu et al. (22). Briefly, Grade 0 corresponds to normal mucosal villi, Grade 1 is development of subepithelial space, Grade 2 is an extension of this subepithelial space with moderate lifting of epithelial layer from the lamina propria, Grade 3 is massive epithelial lifting, Grade 4 has denuded villi and Grade 5 involves digestion and disintegration of lamina propria, hemorrhage and ulceration. The percentage of mucus coverage of the ileal surface was measured in microns (μm) with an objective micrometer in a blinded fashion as described by Rupani et al. (13).
Statistics
Data were analyzed using SAS software (SAS Institute, Carey, NC). Quantitative parameters such as changes of mucosal permeability and hydrophobicity, and luminal DNA, protein, hemoglobin, and glycoprotein, of the different treatments were analyzed by repeated-measures ANOVA, in which the different segments of gut were used as the repeated variable. Differences between means were determined by post hoc analysis. A difference of p < 0.05 was considered statistically significant. Data are expressed as means ± SEM.
RESULTS
In this model of segmental gut ischemia and reperfusion, the greatest increase in permeability was observed at the end of the 45 minute ischemic period and over the ensuing 3 hour reperfusion period, gut permeability progressively returned to baseline levels (Figure 2). Consistent with gut injury and sloughing of the injured enterocytes into the gut lumen, the concentration of luminal DNA and protein was increased by 1 hour after the initiation of reperfusion and remained elevated throughout the experimental period (Figure 3).
Figure 2.
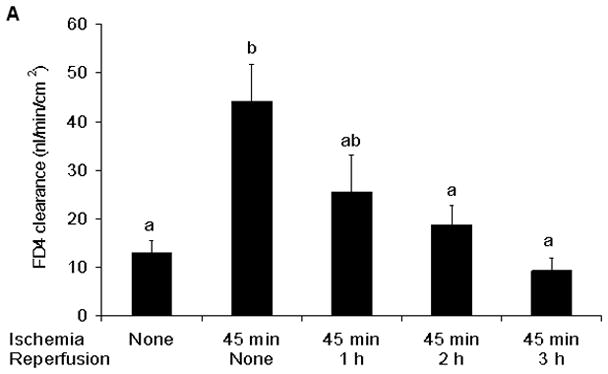
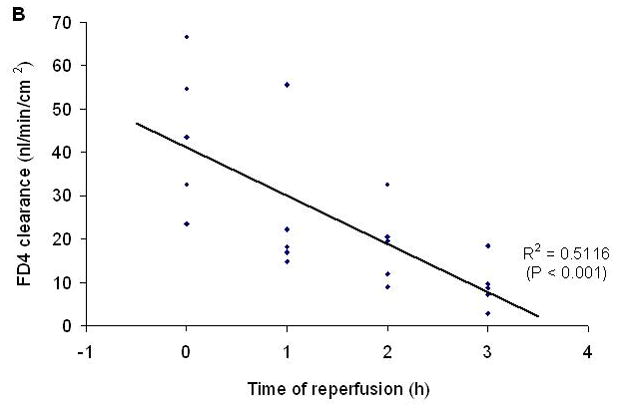
A) Intestinal mucosal permeability was increased at the end of the ischemic period and had returned to baseline during the reperfusion periods. B) The return of gut barrier function correlated with the length of time that passed after the reestablishment of gut blood flow. Data are expressed as mean ± SEM (n=5); groups not sharing a letter are significantly different from each other (p< 0.05).
Figure 3.
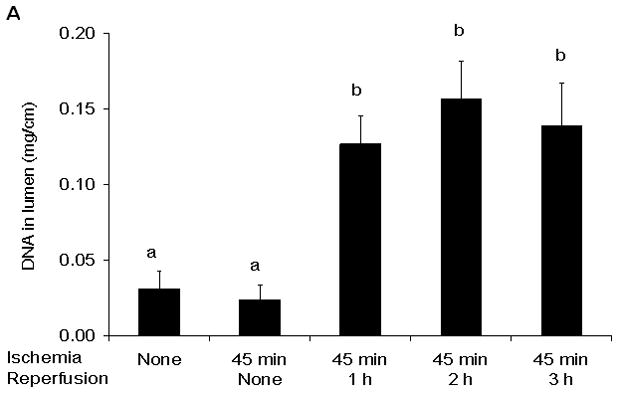
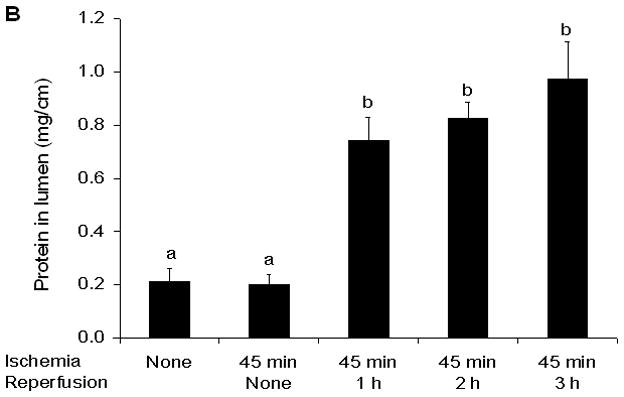
Luminal DNA (A) and Protein (B) concentrations were increased at the 1 hour reperfusion period and remained elevated thereafter. Data are expressed as mean ± SEM (n=5) with groups not sharing a letter being significantly different from each other (p< 0.05).
Although morphologic evidence of gut injury was present at all time points, it appears that the mucus layer was rapidly being reconstituted during the reperfusion period as reflected by alcian blue staining of the mucus layer (Figure 4a). Functional measurements of gut hydrophobicity made at these time points showed that the intestinal mucosal hydrophobicity had significantly decreased by the end of the 45 min ischemic period (Figure 4b). However, mucosal hydrophobicity had increased back to normal levels during the reperfusion period. This pattern of rapid return to normal hydrophobicity levels with reperfusion were similar to the patterns observed with intestinal permeability measurements and alcian blue mucus staining.
Figure 4.
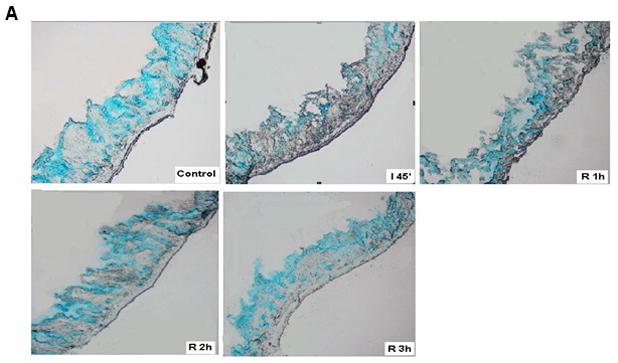
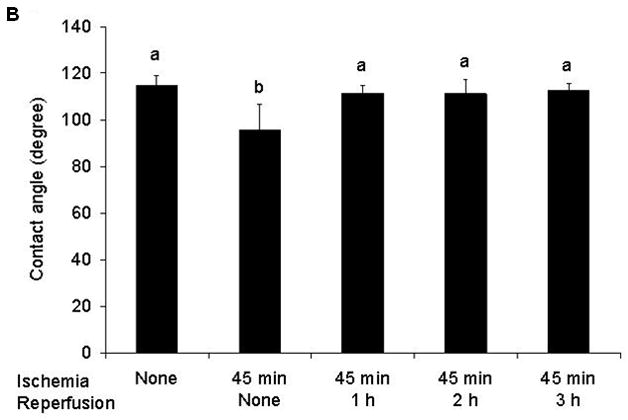
A) Alcian blue staining of the mucus layer of frozen sections of the gut collected at the different points of ischemia/reperfusion (40×). The control gut segment (no ischemia) shows abundant amounts of mucus on the surface or within the mucosa in goblet cells. However, after 45 min of ischemia, there is a dramatic decrease in the intensity of the stain. During reperfusion, the intensity of the mucus stain gradually increased back to normal. B) Intestinal mucosal hydrophobicity obtained at different time points after the ischemic or ischemia/reperfusion periods show that the decrease in hydrophobicity observed after the ischemic period returned to normal by the end of the 1st hour of reperfusion and thus correlated with Alcian blue mucus staining. Data are expressed as mean ± SEM (n=5); groups not sharing a letter are significant different from each other (p< 0.05).
Next, we tested the hypothesis that a brief treatment with NAC to disrupt the mucus layer would augment and prolong ischemia-reperfusion-mediated increases in gut permeability by measuring the effect of NAC on gut permeability at the end of the ischemic period, when permeability is increased as well as at the 3 hour reperfusion period, when gut permeability had returned to normal. Treatment with NAC caused about a two-fold increase in gut permeability at the end of the ischemic period indicating that loss of the mucus layer augmented the magnitude of ischemia-induced gut permeability (Figure 5). Furthermore, in contrast to the non-NAC exposed intestinal segments subjected to the ischemia-reperfusion insult where gut barrier function had returned to baseline levels at 3 hrs after reperfusion, increased gut permeability persisted in the NAC-treated intestinal segments. In fact, not only did NAC prolong the length of time gut permeability was increased after the ischemia-reperfusion insult, but the magnitude of gut permeability at this time point was approximately 10-fold higher in the NAC-treated than the non-NAC-treated intestinal segments (Figure 5).
Figure 5.
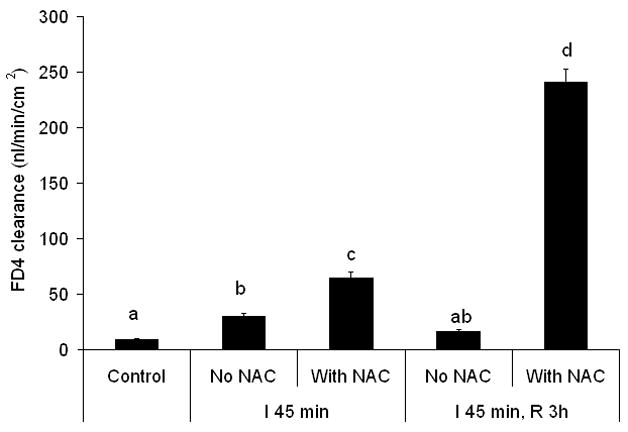
Treatment with 10% N-acetyl cysteine (NAC) increased and prolonged the magnitude of the increased gut permeability observed after ischemia or ischemia-reperfusion. Data are expressed as mean ± SEM (n=5); groups not sharing a letter are significantly different from each other (p< 0.05).
To further evaluate the effect of the loss of the mucus layer, we tested the hypothesis that NAC-induced loss of the mucus layer would result in luminal pancreatic proteases augmenting gut permeability after the ischemia-reperfusion insult. To test this hypothesis we measured gut permeability at 3 hours after reperfusion in intestinal segments exposed to NAC or pancreatic proteases alone as well as in gut segments exposed to NAC plus pancreatic proteases. As shown in the earlier experiments, gut permeability had returned to baseline levels in the non-NAC-treated intestinal segments by 3 hours after the end of the ischemic period, while gut permeability was increased in the NAC-treated group (Figure 6). In the absence of NAC, the addition of pancreatic proteases into the lumen of the gut segments exposed to ischemia-reperfusion did not increase gut permeability. However, the combination of NAC plus pancreatic proteases resulted in a greater increase in gut permeability than that observed with NAC alone (Figure 6). This observation suggests that luminal pancreatic proteases can potentiate gut injury in the absence of a mucus layer. Furthermore, as can be seen by looking at the gross appearance of the variously-treated gut segments, the ischemia-reperfused segment treated with NAC plus proteases appeared necrotic (Figure 7a), while the other segments remained viable. Consistent with the gross appearance of the intestinal segments, the luminal levels of hemoglobin, DNA, protein and glycoprotein (mucus) were increased to a greater extent in the NAC plus protease-treated ileal segments than the other ileal segments (Table 1). Histologic evaluation of the extent of morphologic injury showed that all of the manipulated gut segments showed some degree of injury with the highest incidence occurring in the gut segments exposed to NAC or NAC plus proteases (Figure 7b). However, the greatest magnitude of injury was observed in the NAC plus protease intestinal segments followed by the NAC only segments (Figure 7c). Furthermore, although some gut injury occurred in the naïve, saline and protease only-treated segments, the magnitude of villous injury was similar between these groups and significantly less than the NAC-treated and the NAC plus protease-treated intestinal segments (Figure 7c). As expected, the percentage of the mucus layer lost was highest in the NAC and the NAC plus proteases groups, less loss observed in the protease and saline only groups as compared to the naïve intestinal segments (Figure 7d).
Figure 6.
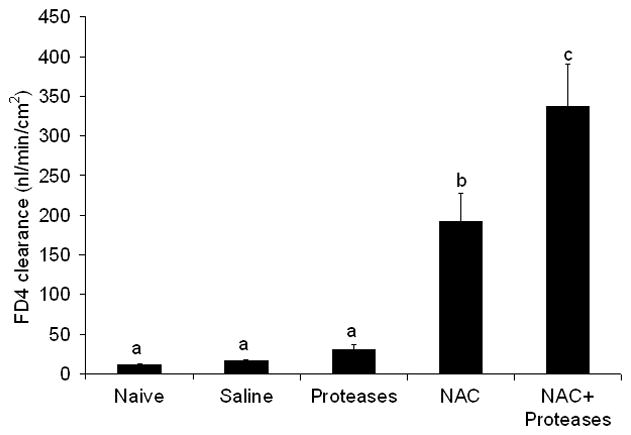
Gut permeability was increased by the addition of pancreatic proteases to NAC-treated but not non-NAC-treated gut segments subjected to ischemia and 3 hours of reperfusion. Data are expressed as mean ± SEM (n=5); groups not sharing a letter are significantly different from each other (p< 0.05).
Figure 7.
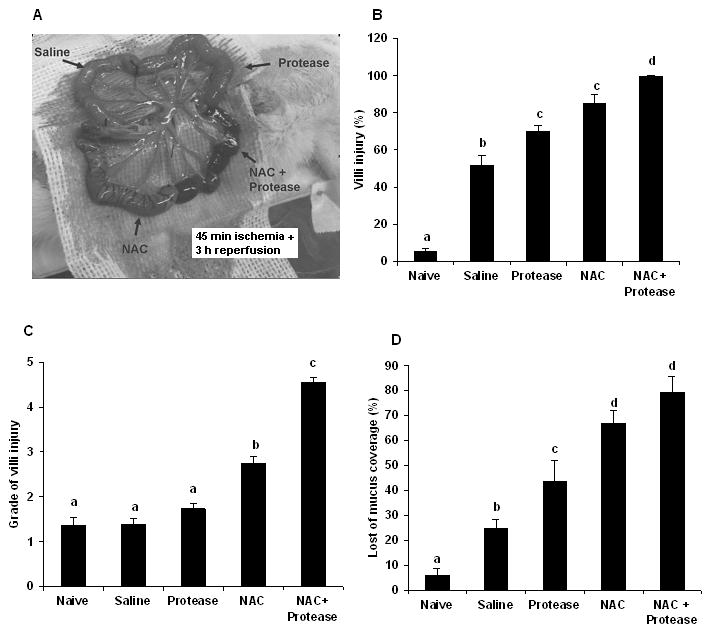
A) The appearance of the NAC plus protease segments was grossly necrotic at the end of the 3 hr reperfusion period. B) By histology, morphologic evidence of villous injury was present to some extent in all of the segments with the greatest incidence of injury in the NAC plus protease group. C) Likewise, the magnitude of villous injury was greatest in the NAC plus protease segments. D) Loss of the mucus layer was present to some extent in all of the segments with the greatest losses being observed in the NAC and NAC plus proteases groups. Data are expressed as mean ± SEM (n=5); groups not sharing a letter are significantly different from each other (p< 0.05).
Table 1.
Effect of N-Acetyl Cysteine (NAC) and digestive proteases on ischemia/reperfusion-mediated gut injury
| Groups | Luminal Hemoglobin (mg/cm) | Luminal DNA (mg/cm) | Luminal protein (mg/cm) | Luminal glycoprotein (mg/cm) | Hydrophobicity |
|---|---|---|---|---|---|
| Naïve | 2.3 ± 0.4 | 0.002 ± 0.001 | 0.02 ± 0.01 | 0.10 ± 0.03 | 107.7 ± 2.9 |
| Saline | 22.8 ± 6.3 | 0.11 ± 0.02 # | 0.47 ± 0.07 # | 2.19 ± 0.58 # | 114.9 ± 4.2 |
| NAC | 19.2 ± 5.8 | 0.03 ± 0.01 | 0.36 ± 0.04 | 1.78 ± 0.14 # | 85.5 ± 3.4 $ |
| Proteases | 23.1 ± 4.9 | 0.12 ± 0.03 # | 0.30 ± 0.06 | 2.37 ± 0.64 # | 101.8 ± 8.3 |
| NAC + Proteases | 225.2± 39.2 * | 0.40 ± 0.05 * | 1.88 ± 0.20 * | 6.77 ± 0.46 * | 88.5 ± 5.7 $ |
P < 0.01 vs all other groups
P < 0.05 vs naïve subgroup
P < 0.05 vs naïve, proteases, saline groups
DISCUSSION
The results of the current study support the general hypothesis that loss of the mucus layer contributes to ischemia/reperfusion-induced gut injury and that the magnitude of gut injury associated with loss of the mucus layer is exacerbated by the presence of pancreatic proteases within the lumen of the gut. This conclusion is based on the following key sets of observations. First, the time of maximal gut permeability, loss of the mucus layer and decreased mucosal hydrophobicity all occurred at the same time (i.e. end of the period of ischemia). Secondly, the restitution of gut barrier function over the 3 hr post-reperfusion period was associated with comparable improvements in reconstitution of the mucus layer and increased mucosal hydrophobicity. Additionally, a brief exposure to the mucolytic NAC at the end of the ischemic period, not only significantly increased the magnitude of gut permeability, but also limited the ability of the ischemic gut to recover during the reperfusion period. Lastly, the presence of intraluminal pancreatic proteases potentiated ischemia/reperfusion-induced gut injury only in the NAC-treated gut segments thereby high-lighting the importance of the mucus layer in limiting pancreatic protease-mediated gut injury. This ability of NAC placed in the lumen of the gut to potentiate gut injury is especially compelling in that NAC has anti-oxidant as well as mucolytic properties and its systemic administration has been shown to limit ischemia-reperfusion-mediated injury of the gut as well as other tissues (23, 24). Although the fact that NAC has anti-oxidant properties is a potential limitation of this work, nonetheless, the results of the current study are consistent with the concept of the mucus layer being important in limiting ischemia/reperfusion-induced gut injury as well as the notion that intraluminal digestive proteins have the potential to be injury-inducing factors, especially when the mucus layer is lost.
This idea that the small intestinal mucus layer is an important protective barrier against proteolytic and other potentially injurious factors within the lumen of the gut is consistent with other relatively recent observations (5, 13, 14). This intestinal-protective effect of mucus is not surprising in light of the well recognized role of the gastric mucus layer as an important protective defense against gastric acid-induced injury in the stomach (25, 26). The exact mechanism(s) by which a gut ischemia-reperfusion injury, such as superior mesenteric arterial occlusion (SMAO), leads to loss of the mucus layer is not known. However, it has been shown that mucins, which are the major component of the mucus layer, lose their viscoelastic and hydrophobic properties under oxidant stress (27). Furthermore, these modified mucins are more susceptible to cleavage and further destruction by pancreatic proteases (28, 29) thereby setting up a potential situation where a weakened mucus layer loses its resistance to enzymatic degradation by digestive enzymes contained within the gut lumen. In this scenario of gut ischemia-reperfusion induced oxidative stress, not only could the intraluminal digestive enzymes destroy the mucus layer but they would now be able to come into direct contact with the enterocytes lining the intestinal villi and thereby injure these cells resulting in further loss of gut barrier function. This potential sequence of events, where luminal pancreatic enzymes contribute to loss of the mucus layer and contribute to gut injury is supported by earlier work showing that pancreatic duct ligation greatly reduces trauma hemorrhagic shock-induced disruption of the mucus layer, enterocyte and villous injury as well as loss of gut barrier function (7). In fact, the concept that the pancreas plays an important pathologic role in the development of organ failure observed after hemorrhagic or traumatic shock was initially proposed approximately 30 years ago (30, 31). More recently, work from the laboratory of Kistler and Schmid-Schonbein (9, 32, 33) have re-focused attention on the role of pancreatic proteases acting on the ischemic gut as a critical event in the pathogenesis of gut-induced distant organ injury and hemodynamic instability.
Taken together, the current results support our working hypothesis that gut injury after an intestinal-ischemia-reperfusion insult is influenced not just by events occurring within the systemic compartment but also by factors within the gut luminal compartment. Furthermore, it is the interaction between the systemic and gut luminal compartments that results in gut injury and thereby sets the stage for gut-induced distant organ dysfunction. While this notion is supported by studies showing that pancreatic duct ligation (7) as well as the intraluminal neutralization of pancreatic proteases with antiproteases protects against gut injury and gut-induced distant organ injury in models of splanchnic ischemia or hemorrhagic shock (8, 9), further work is needed to better define the relationships between various components of the luminal compartment as well the interactions between the systemic and luminal compartments.
In summary, this study indicates that the mucus layer not only serves as a barrier that prevents direct contact between the villous epithelium and potentially harmful luminal contents, it also appears to play an important role in the maintenance and re-establishment of gut barrier function. Consequently, mucus surrogates or drugs capable of fortifying the mucus layer might be a promising approach for the prevention or treatment of gut injury and gut-induced MODS.
Acknowledgments
Supported by NIH grants GM 59841 and T32 069330
References
- 1.Deitch EA, Xu D, Kaise VL. Role of the gut in the development of injury- and shock induced SIRS and MODS: the gut-lymph hypothesis, a review. Front Biosci. 2006;11:520–8. doi: 10.2741/1816. [DOI] [PubMed] [Google Scholar]
- 2.Magnotti LJ, Deitch EA. Burns, bacterial translocation, gut barrier function, and failure. J Burn Care Rehabil. 2005;26:383–91. doi: 10.1097/01.bcr.0000176878.79267.e8. [DOI] [PubMed] [Google Scholar]
- 3.Andersson R, Wang XD. Gut barrier dysfunction in experimental acute pancreatitis. Ann Acad Med Singapore. 1999;28:141–6. [PubMed] [Google Scholar]
- 4.Almeida J, Galhenage S, Yu J, Kurtovic J, Riordan SM. Gut flora and bacterial translocation in chronic liver disease. World J Gastroenterol. 2006;12:1493–502. doi: 10.3748/wjg.v12.i10.1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sharpe S, Doucet D, Qin X, Deitch EA. Role of intestinal mucus and pancreatic proteases in the pathogenesis of trauma-hemorrhagic shock-induced gut barrier failure and multiple organ dysfunction syndrome. Journal of Organ Dysfunction. 2008;4:168–76. [Google Scholar]
- 6.Acosta JA, Hoyt DB, Schmid-Schonbein GW, Hugli TE, Anjaria DJ, Frankel DA, Coimbra R. Intraluminal pancreatic serine protease activity, mucosal permeability, and shock: a review. Shock. 2006;26:3–9. doi: 10.1097/01.shk.0000209557.31457.ae. [DOI] [PubMed] [Google Scholar]
- 7.Caputo FJ, Rupani B, Watkins AC, Barlos D, Vega D, Senthil M, Deitch EA. Pancreatic duct ligation abrogates the trauma hemorrhage-induced gut barrier failure and the subsequent production of biologically active intestinal lymph. Shock. 2007;28:441–6. doi: 10.1097/shk.0b013e31804858f2. [DOI] [PubMed] [Google Scholar]
- 8.Deitch EA, Shi HP, Lu Q, Feketeova E, Xu DZ. Serine proteases are involved in the pathogenesis of trauma-hemorrhagic shock-induced gut and lung injury. Shock. 2003;19:452–6. doi: 10.1097/01.shk.0000048899.46342.f6. [DOI] [PubMed] [Google Scholar]
- 9.Mitsuoka H, Kistler EB, Schmid-Schonbein GW. Protease inhibition in the intestinal lumen: attenuation of systemic inflammation and early indicators of multiple organ failure in shock. Shock. 2002;17:205–9. doi: 10.1097/00024382-200203000-00008. [DOI] [PubMed] [Google Scholar]
- 10.Fink MP. Intestinal epithelial hyperpermeability: update on the pathogenesis of gut mucosal barrier dysfunction in critical illness. Curr Opin Crit Care. 2003;9:143–51. doi: 10.1097/00075198-200304000-00011. [DOI] [PubMed] [Google Scholar]
- 11.Allen A, Bell A, Mantle M, Pearson JP. The structure and physiology of gastrointestinal mucus. Adv Exp Med Biol. 1982;144:115–33. doi: 10.1007/978-1-4615-9254-9_15. [DOI] [PubMed] [Google Scholar]
- 12.Lichtenberger LM. The hydrophobic barrier properties of gastrointestinal mucus. Annu Rev Physiol. 1995;57:565–83. doi: 10.1146/annurev.ph.57.030195.003025. [DOI] [PubMed] [Google Scholar]
- 13.Rupani B, Caputo FJ, Watkins AC, Vega D, Magnotti LJ, Lu Q, Xu da Z, Deitch EA. Relationship between disruption of the unstirred mucus layer and intestinal restitution in loss of gut barrier function after trauma hemorrhagic shock. Surgery. 2007;141:481–9. doi: 10.1016/j.surg.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 14.Qin X, Caputo FJ, Xu DZ, Deitch EA. Hydrophobicity of mucosal surface and its relationship to gut barrier function. Shock. 2008;29:372–6. doi: 10.1097/shk.0b013e3181453f4e. [DOI] [PubMed] [Google Scholar]
- 15.Sharpe SM, Qin X, Lu Q, Feketeova E, Palange DC, Dong W, Sheth SU, Lee MA, Reino D, Xu DZ, Deitch EA. Loss of the intestinal mucus layer in the normal rat causes gut Injury, but not toxic mesenteric lymph nor lung injury. Shock. 2010 doi: 10.1097/SHK.0b013e3181dc3ff5. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rosario HS, Waldo SW, Becker SA, Schmid-Schonbein GW. Pancreatic trypsin increases matrix metalloproteinase-9 accumulation and activation during acute intestinal ischemia-reperfusion in the rat. Am J Pathol. 2004;164:1707–16. doi: 10.1016/S0002-9440(10)63729-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Norin KE, Midtvedt T, Gustafsson BE. Influence of intestinal microflora on the tryptic activity during lactation in rats. Lab Anim. 1986;20:234–7. doi: 10.1258/002367786780865656. [DOI] [PubMed] [Google Scholar]
- 18.Musemeche CA, Kosloske AM, Bartow SA, Umland ET. Comparative effects of ischemia, bacteria, and substrate on the pathogenesis of intestinal necrosis. J Pediatr Surg. 1986;21:536–8. doi: 10.1016/s0022-3468(86)80228-7. [DOI] [PubMed] [Google Scholar]
- 19.Giles K, Myers A. An improved diphenylamine method for the estimation of deoxyribonucleic acid. Nature. 1965;206:93. [Google Scholar]
- 20.Mantle M, Allen A. A colorimetric assay for glycoproteins based on the periodic acid/Schiff stain [proceedings] Biochem Soc Trans. 1978;6:607–9. doi: 10.1042/bst0060607. [DOI] [PubMed] [Google Scholar]
- 21.Matsuo K, Ota H, Akamatsu T, Sugiyama A, Katsuyama T. Histochemistry of the surface mucous gel layer of the human colon. Gut. 1997;40:782–9. doi: 10.1136/gut.40.6.782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chiu CJ, McArdle AH, Brown R, Scott HJ, Gurd FN. Intestinal mucosal lesion in low-flow states. I. A morphological, hemodynamic, and metabolic reappraisal. Arch Surg. 1970;101:478–83. doi: 10.1001/archsurg.1970.01340280030009. [DOI] [PubMed] [Google Scholar]
- 23.Freire Cerqueira N, Hussni CA, Bonetti Yoshida W, Lopes Sequeira J, Padovani CR. Effects of pentoxifylline and n-acetylcysteine on injuries caused by ischemia and reperfusion of splanchnic organs in rats. Int Angiol. 2008;27:512–21. [PubMed] [Google Scholar]
- 24.Turut H, Ciralik H, Kilinc M, Ozbag D, Imrek SS. Effects of early administration of dexamethasone, N-acetylcysteine and aprotinin on inflammatory and oxidant-antioxidant status after lung contusion in rats. Injury. 2009;40:521–7. doi: 10.1016/j.injury.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 25.Hills BA, Butler BD, Lichtenberger LM. Gastric mucosal barrier: hydrophobic lining to the lumen of the stomach. Am J Physiol. 1983;244:G561–8. doi: 10.1152/ajpgi.1983.244.5.G561. [DOI] [PubMed] [Google Scholar]
- 26.Goddard PJ, Kao YC, Lichtenberger LM. Luminal surface hydrophobicity of canine gastric mucosa is dependent on a surface mucous gel. Gastroenterology. 1990;98:361–70. doi: 10.1016/0016-5085(90)90826-m. [DOI] [PubMed] [Google Scholar]
- 27.Grisham MB, Von Ritter C, Smith BF, Lamont JT, Granger DN. Interaction between oxygen radicals and gastric mucin. Am J Physiol. 1987;253:G93–6. doi: 10.1152/ajpgi.1987.253.1.G93. [DOI] [PubMed] [Google Scholar]
- 28.Forstner JF. Intestinal mucins in health and disease. Digestion. 1978;17:234–63. doi: 10.1159/000198115. [DOI] [PubMed] [Google Scholar]
- 29.Kemper AC, Specian RD. Rat small intestinal mucins: a quantitative analysis. Anat Rec. 1991;229:219–26. doi: 10.1002/ar.1092290209. [DOI] [PubMed] [Google Scholar]
- 30.Bounous G, Brown RA, Mulder DS, Hampson LG, Gurd FN. Abolition of ‘tryptic enteritis’ in the shocked dog. Creation of an experimental model for study of human shock and its sequelae. Arch Surg. 1965;91:371–5. doi: 10.1001/archsurg.1965.01320150001001. [DOI] [PubMed] [Google Scholar]
- 31.Lefer AM, Glenn TM. Role of the pancreas in the pathogenesis of circulatory shock. Adv Exp Med Biol. 1971;23:311–35. doi: 10.1007/978-1-4615-9014-9_31. [DOI] [PubMed] [Google Scholar]
- 32.Kistler EB, Hugli TE, Schmid-Schonbein GW. The pancreas as a source of cardiovascular cell activating factors. Microcirculation. 2000;7:183–92. [PubMed] [Google Scholar]
- 33.Mitsuoka H, Kistler EB, Schmid-Schonbein GW. Generation of in vivo activating factors in the ischemic intestine by pancreatic enzymes. Proc Natl Acad Sci U S A. 2000;97:1772–7. doi: 10.1073/pnas.97.4.1772. [DOI] [PMC free article] [PubMed] [Google Scholar]


