Abstract
The BCL-2 family includes both pro- and anti-apoptotic proteins, which regulate programmed cell death during development and in response to various apoptotic stimuli. The BH3-only subgroup of pro-apoptotic BCL-2 family members is critical for the induction of apoptotic signaling, by binding to and neutralizing anti-apoptotic BCL-2 family members. During embryonic development, the anti-apoptotic protein BCL-XL plays a critical role in the survival of neuronal populations by regulating the multi-BH domain protein BAX. In this study, the authors investigated the role of Harakiri (HRK), a relatively recently characterized BH3-only molecule in disrupting the BAX-BCL-XL interaction during nervous system development. Results indicate that HRK deficiency significantly reduces programmed cell death in the nervous system. However, HRK deficiency does not significantly attenuate the widespread apoptosis seen in the Bcl-x−/− embryonic nervous system, indicating that other BH3-only molecules, alone or in combination, may regulate BAX activation in immature neurons.
Keywords: HRK, BCL-XL, BAX, PCD, BH3-only, BCL-2
Apoptosis of excess neural progenitor cells and neurons during embryonic development is essential for normal nervous system morphogenesis and function (Cory et al. 2003). Programmed cell death (PCD) is controlled by the BCL-2 family of anti- and pro-apoptotic regulators (Adams and Cory 2007a). These proteins respond to various death signals such as DNA damage, oxidative stress, or limited trophic support and interact with opposing family members to regulate activation of the apoptosis-inducing proteolytic caspase cascade (Adams and Cory 2007a). The damage signals are transduced by various members of the diverse “BH3-only” protein subfamily, distinguished by their single BCL-2 homology domain (BCL-2 homology domain-3) used to engage their multi-BH domain pro-survival relatives: BCL-2, BCL-XL, BCL-w, MCL-1, and A1. Interaction with BH3-only proteins inhibits the pro-survival function of BCL-2 and BCL-XL and allows activation of the multidomain pro-death family members BAX and BAK, which commit the cell to apoptosis by permeabilizing the outer membrane of the mitochondrion and initiating caspase activation (Adams and Cory 2007b). The critical role of anti-apoptotic proteins in the regulation of PCD was evidenced by targeted gene disruption of Bcl-x. The Bcl-x gene–disrupted mice die approximately on embryonic day 13, and there is extensive apoptotic cell death in postmitotic immature neurons of the developing brain, spinal cord, and dorsal root ganglia (DRG) and in the hematopoetic system (Motoyama et al. 1995). We previously demonstrated that concomitant BAX deficiency protects BCL-XL-deficient embryos from excess neuronal apoptosis, although it does not rescue the embryonic lethality associated with BCL-XL deficiency (Shindler et al. 1997a). Similarly, increased neuron apoptosis in BCL-XL-deficient mice, but not embryonic lethality, can be attenuated by a concomitant deficiency of Apaf-1, caspase-9, or caspase-3 (Zaidi et al. 2001; D’Sa et al. 2003; Cecconi et al. 2004).
The BH3-only members of the BCL-2 protein family are important initiators of PCD and are required for apoptosis induced by diverse cytotoxic stimuli (Huang and Strasser 2000; Bouillet and Strasser 2002). The pro-apoptotic activities of BH3-only proteins are stringently controlled by a variety of transcriptional and posttranslational mechanisms (Puthalakath and Strasser 2002). Harakiri (HRK) is a pro-apoptotic member of the BCL-2 family that physically interacts with the death repressor proteins BCL-2 and BCL-XL but not with death-promoting homologs BAX or BAK (Inohara et al. 1997). Overexpression of HRK in a variety of experimental models induces cell death, which is inhibited by BCL-2 and BCL-XL (Inohara et al. 1997; Sanz et al. 2000). HRK is also a critical regulator of nerve growth factor (NGF) withdrawal-induced apoptosis of sensory neurons and axotomy-induced motoneuron death (Imaizumi et al. 2004). Hrk is expressed at high levels in the developing nervous system, particularly in the DRG, ventral neural tube, and cranial ganglia. In the newborn brain, Hrk is expressed at moderate levels in many areas and at slightly higher levels in the cortical plate, the olfactory tubercle, the piriform cortex, the hippocampus, the ventromedial nuclei of the hypothalamus, the cortical amygdaloid nuclei, the dorsal lateral geniculate nuclei of the thalamus, and the facial motor nuclei of the pons, although in the adult brain, expression of Hrk is restricted mainly to regions of neurogenesis such as the hippocampus (Coultas et al. 2007). Thus, the temporal pattern of HRK expression in the central and peripheral nervous system suggests that it might be playing a role in the regulation of programmed cell death of immature neurons. Apoptosis in this population of cells is known to be regulated by the interaction between BAX and BCL-XL (Motoyama et al. 1995; White et al. 1998). In addition to HRK, the BH3-only protein BIM can also interact with BCL-XL (O’Connor et al. 1998) and may regulate cell death in some cell types (Bouillet et al. 2002). BIM expression is increased in neurons in response to a variety of apoptotic insults (Putcha et al. 2001; Harris and Johnson 2001; Biswas et al. 2005; Puthalakath et al. 2007), and BIM loss partially protects sympathetic neurons from NGF deprivation-induced death in vitro (Putcha et al. 2001; Coultas et al. 2007). However, we have shown previously that BIM deficiency does not attenuate immature neuron death or embryonic lethality in BCL-XL-deficient mice (Akhtar et al. 2008). We thus hypothesized that HRK provides a critical pro-apoptotic stimulus that leads to neuron apoptosis during development and may regulate the extensive neuron apoptosis seen in Bcl-x−/− mice, particularly in DRG neurons where Hrk is highly expressed. To directly test this hypothesis, we examined apoptotic cell death in the nervous system of wild-type and Hrk−/− embryos and generated embryos that were dual-deficient in BCL-XL and HRK. We also examined the effect of a combined deficiency of HRK and BIM in regulating apoptotic death of DRG neurons, as BH3-only molecules may act in concert to initiate PCD. Our results indicate that HRK deficiency attenuates apoptosis in the developing nervous system. However, concomitant HRK deficiency does not prevent the embryonic lethality or neuropathology associated with BCL-XL deficiency. Similarly, a combined deficiency of HRK and BIM attenuates PCD in the nervous system in comparison to wild-type controls, although it does not attenuate BCL-XL deficiency-induced neurodegeneration. Our studies identify HRK as an important regulator of PCD in the developing nervous system, although it is not a critical regulator of BCL-XL-dependent survival pathways.
Materials and Methods
Mice
The generation of mice with gene disruptions in Bcl-x, Bim, and Hrk has been described previously (Motoyama et al. 1995; Bouillet et al. 1999; Coultas et al. 2007). These lines were backcrossed at least six times onto the C57BL/6 background. The Bim and Hrk mutant mice were a kind gift from Dr. Andreas Strasser (Walter and Eliza Hall Institute; Victoria, Australia). To generate mice that were dual-deficient in HRK and BCL-XL, Hrk+/− mice were crossed with Bcl-x+/− mice, and resultant Hrk+/−/Bcl-x+/− mice were interbred. The Hrk+/−/Bcl-x+/− mice were bred to previously generated Bim+/−/Hrk+/− mice to generate mice that were triple deficient in HRK, BIM, and BCL-XL. The morning on which a vaginal plug was seen was designated as embryonic day 0.5 (E0.5). Pregnant mice were anesthetized with pentobarbital and killed by cervical dislocation on gestational day 12.5 or 15.5 to collect the embryos. Viable mice were tailed and weaned at approximately 3 weeks of age. Endogenous and disrupted genes were detected by PCR analysis of tail DNA samples. Mice were cared for in accordance with the guidelines of the National Institutes of Health Guide for the Care and Use of Laboratory Animals. All animal protocols were approved by the Institutional Animal Care and Use Committee of the University of Alabama at Birmingham.
Immunohistochemistry
Embryos were fixed at 4C in Bouin’s fixative overnight. Tissues were dehydrated and paraffin embedded, and 5-µm sections were cut. Sections were deparaffinized and stained with hematoxylin and eosin (H&E) as described previously (Shindler et al. 1997a).
Terminal deoxynucleotidyltransferase-mediated dUPT nick end labeling (TUNEL) staining
TUNEL-positive cells in embryonal sections were detected using ApopTag Peroxidase In Situ Apoptosis Detection Kit (Millipore; Billerica, Massachusetts) as per the manufacturer’s instructions. Briefly, sections were deparaffinized, and endogenous peroxidase activity was blocked with 0.3% hydrogen peroxide in phosphate-buffered saline (PBS) for 10 min. Sections were then permeabilized for 10 min in PBS containing 0.3% Triton X-100. Embryo sections were hybridized with working-strength terminal deoxynucleotidyl transferase (TdT) enzyme diluted in reaction buffer at 37C for 1 hr. The reaction was stopped by incubating the slides in stop/wash buffer for 10 min. After three washes in PBS, the slides were incubated with horseradish peroxidase (HRP)–labeled anti-digoxigenin conjugate for 30 min at room temperature. After several PBS washes, the slides were incubated in 0.05% diaminobenzidine (DAB), a chromogenic peroxidase substrate (Pierce; Rockford, IL), diluted in staining buffer. TUNEL-stained sections were counterstained with hematoxylin.
Cleaved caspase-3 staining
For immunohistochemical detection of cleaved caspase-3, sections were deparaffinized and endogenous peroxidase activity was blocked with 0.3% hydrogen peroxide in PBS for 10 min. To inhibit nonspecific antibody binding, sections were incubated for 30 min at room temperature in PBS blocking buffer (PBS-BB; PBS with 0.1% BSA, 0.3% Triton X-100, and 0.2% nonfat powdered dry milk). Rabbit anti-cleaved caspase-3 antiserum (#9661; Cell Signaling, Danvers, MA) was diluted in PBS-BB and applied to sections overnight at 4C. After washes with PBS, the slides were incubated with SuperPicture HRP polymer conjugate (Invitrogen; Carlsbad, CA). After washes with PBS, biotin-labeled tyramide was deposited using a tyramide signal amplification system (PerkinElmer Life Sciences; Boston, MA) according to the manufacturer’s instructions. Chromogenic detection of antigen-antibody complexes was done using HRP-conjugated streptavidin followed by DAB (Pierce) according to the manufacturer’s instructions. Cleaved caspase-3-immunostained sections were counterstained with hematoxylin. Stained sections were imaged using a Zeiss (Oberkochen, Germany) Axioscop microscope equipped with an Axiocam MRc camera.
Statistics
PCD occurs throughout the developing nervous system but is particularly prominent in the ventral spinal cord and DRG. To quantify the effects of targeted gene disruptions on PCD in the nervous system, TUNEL- or cleaved caspase-3-positive cells were counted in multiple microscopic fields of DRG from each animal. A minimum of three DRG were counted per animal, and at least three animals per group were analyzed in all experiments. The data were represented per mm2 of surface area of the DRG and normalized to wild-type controls. All data points represent the mean ± SEM. The data were analyzed for significance by a one-way or two-way analysis of variance (ANOVA), followed by Bonferroni’s corrections for pairwise comparisons. The data were analyzed using GraphPad (version 4.0; GraphPad Software, La Jolla, CA) software.
Results
Loss of HRK Attenuates PCD in the Developing Nervous System
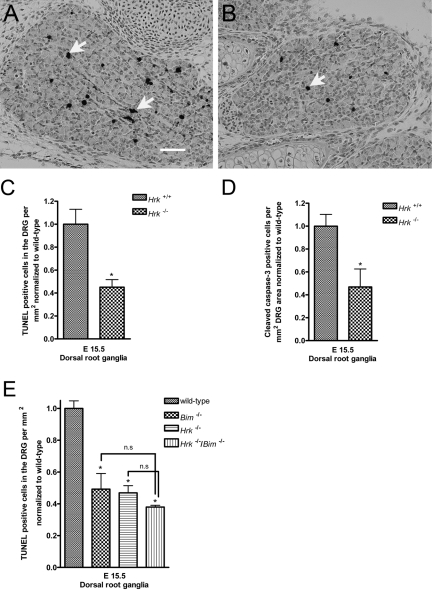
We hypothesized that HRK may be a critical upstream regulator of PCD in the nervous system by altering the balance between pro- and anti-apoptotic BCL-2 family members in response to developmental cues such as trophic factors. To characterize the role of HRK in neuronal PCD, we initially examined the effects of HRK deficiency in the DRG of Hrk−/− mice and compared them to their wild-type littermates at E15.5. HRK-deficient embryos exhibited a significant decrease in PCD in the nervous system in comparison to their wild-type littermates, as evidenced by a decrease in TUNEL-positive staining (Fig. 1C). PCD is typically preceded by the activation of effector caspases, and caspase-3 is the critical effector caspase in the nervous system (Srinivasan et al. 1998), making the detection of cleaved caspase-3 immunoreactivity a reliable marker for detection of apoptotic PCD. We stained sections from wild-type and HRK-deficient mice at E15.5 with an antiserum that recognizes the cleaved (active) fragment of caspase-3 (Fig. 1A,B). In comparison to wild-type controls, there was a significant decrease in the number of cleaved caspase-3 immunoreactive cells in the DRG of HRK-deficient mice at E15.5 (Fig. 1D). These results indicate that HRK significantly contributes to regulation of PCD in the developing nervous system. We also examined the effect of a dual deficiency of BIM and HRK on PCD in the developing nervous system. Deficiency of BIM or HRK significantly reduced the number of TUNEL-positive cells in the DRG of E15.5 mice, although combined deficiency of BIM and HRK showed no additional decrease in apoptosis of DRG neurons (Fig. 1E).
Figure 1.
Harakiri (HRK) loss significantly reduces apoptosis of immature neurons in the developing dorsal root ganglia (DRG) in comparison to wild-type littermates. Hrk−/− and wild-type embryos were collected at E15.5. Sections were stained for cleaved caspase-3 and terminal deoxynucleotidyltransferase-mediated dUPT nick end labeling (TUNEL), and the number of positive cells was counted in the DRG. (A) Wild-type embryos at E15.5 showed a few cleaved caspase-3 immunoreactive cells (indicated by arrows). Scale bar = 20 microns. (B) Loss of Hrk significantly reduced the number of cleaved caspase-3 immunoreactive cells in the immature neurons of the DRG. For quantification of the number of cleaved caspase-3 immunoreactive or TUNEL-positive cells, at least three DRG were counted per animal, and a minimum of three animals were analyzed for the experiments. The number of positive cells was represented in relation to the surface area of the DRG, and the data from Hrk−/− embryos were normalized to wild-type littermate controls. (C) Hrk−/− embryos showed a significant decrease in the number of TUNEL-positive cells in comparison to their wild-type littermate controls at E15.5. (D) Similar to the results observed with TUNEL staining, Hrk−/− embryos showed a significant decrease in the number of cleaved caspase-3 immunoreactive cells in comparison to their wild-type littermate controls at E15.5. (E) Dual deficiency of HRK and BIM was not additive, although HRK or BIM deficiency by itself decreased the number of TUNEL-positive cells in the DRG of E15.5 animals in comparison to wild-type animals. *p<0.05 vs wild-type controls; n.s. = not statistically significant.
HRK Is Not Required for BCL-XL Deficiency-Induced Death in Developing Neurons
BCL-XL functions to support the viability of immature cells during the development of the nervous and hematopoietic systems (Motoyama et al. 1995), and Bcl-x−/− mice die around embryonic day 13. Deficiency of BCL-XL results in embryonic lethality and abnormally increased apoptosis of neuronal cells in the brainstem, DRG, ventral spinal cord, and erythroid progenitors in the fetal liver. It remains unclear whether neurodegeneration, fetal anemia, or still other abnormalities cause embryonic lethality in these mice. To characterize the role of HRK in the regulation of BCL-XL-dependent embryonic lethality, we crossed Hrk+/−/Bcl-x+/− mice to Hrk+/−/Bcl-x+/− mice, and the genotypes of the viable offspring were assessed (Table 1). In 22 litters with 124 viable offspring, no viable Hrk−/−/Bcl-x−/−, Hrk+/+/Bcl-x−/−, or Hrk+/−/Bcl-x−/− mice were identified. To characterize the role of HRK in the regulation of BCL-XL-dependent neuropathology, we crossed Hrk−/−/Bcl-x+/− mice with Hrk+/−/Bcl-x+/− mice, and the embryos were collected at E12.5 to circumvent the BCL-XL deficiency-induced embryonic lethality at E13. Hrk−/−/Bcl-x−/− and Hrk+/−/Bcl-x−/− embryos were observed in the expected Mendelian frequencies (data not shown), indicating that the failure to detect viable postnatal HRK and BCL-XL dual-deficient mice was most likely due to the inability of HRK deficiency to prevent BCL-XL-induced embryonic lethality, which occurs after E12.5.
Table 1.
Genotypes of Viable Mice Generated from Interbreeding of bcl-x+/−/hrk+/− Mice
| Genotypes | Number of Viable Mice | Expected Frequency |
|---|---|---|
| Hrk+/+/Bcl-x+/+ | 10 | 7.75 |
| Hrk+/+/Bcl-x+/− | 27 | 15.5 |
| Hrk+/+/Bcl-x−/− | 0 | 7.75 |
| Hrk+/−/Bcl-x+/+ | 19 | 15.5 |
| Hrk+/−/Bcl-x+/− | 37 | 31.0 |
| Hrk+/−/Bcl-x−/− | 0 | 15.5 |
| Hrk−/−/Bcl-x+/+ | 9 | 7.8 |
| Hrk−/−/Bcl-x+/− | 22 | 15.5 |
| Hrk−/−/Bcl-x−/− | 0 | 7.8 |
Only six of the nine possible genotype combinations resulted from the interbreeding of Bcl-x+/− and Hrk+/− mice. In total, 124 live-born mice were generated in 22 litters. No viable Hrk−/−/Bcl-x−/−, Hrk+/+/ Bcl-x−/−, or Hrk+/−/Bcl-x−/− mice were identified.
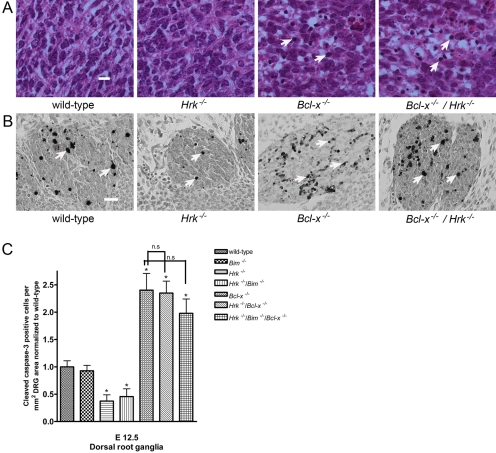
On H&E-stained sections, apoptotic nuclei were defined as nuclei appearing condensed and hyperchromatic, fragmented, and/or exhibiting a marginated chromatin staining pattern. Examination of the ventral spinal cord in wild-type E12.5 animals exhibited a few apoptotic nuclei, indicating that these neurons were undergoing PCD (Fig. 2A). HRK-deficient animals also exhibited occasional apoptotic nuclei on H&E staining (Fig. 2A). As expected, there was a dramatic increase in the number of apoptotic nuclei in the ventral spinal cord of Bcl-x−/− mice, and their frequency was similarly increased in Hrk−/−/Bcl-x−/− mice (Fig. 2A). To define the extent of apoptosis in HRK and BCL-XL dual-deficient animals, we stained sections from E12.5 animals for cleaved caspase-3, and the number immunoreactive cells was counted in the DRG. In comparison to wild-type controls, HRK-deficient animals showed a significant decrease in the number of cleaved caspase-3 immunoreactive cells in the DRG at E12.5, similar to the results observed at E15.5 (Fig. 2C). As reported previously (Akhtar et al. 2008), we did not observe a significant decrease in PCD in the DRG of BIM-deficient embryos in comparison to E12.5 wild-type controls. HRK and BIM dual-deficient animals also exhibited a significant decrease in the number of cleaved caspase-3 immunoreactive cells in the DRG, although there was no additive effect. However, HRK deficiency did not reduce the number of apoptotic cells in the DRG (Fig. 2B) of Bcl-x−/− mice. Quantification of cleaved caspase-3 immunoreactive cells showed no significant difference between BCL-XL-deficient and HRK and BCL-XL dual-deficient animals. Similarly, a combined deficiency of HRK and BIM did not attenuate the widespread apoptosis of immature neurons in the DRG of Bcl-x−/− embryos (Fig. 2C). These findings indicate that neither HRK nor BIM possesses an equivalent pro-apoptotic function as BAX in regulating apoptosis in developing immature neurons.
Figure 2.
Concomitant Hrk deficiency does not reduce apoptosis due to BCL-XL deficiency. Wild-type, Hrk−/−, Bcl-x−/−, and Hrk−/−/Bcl-x −/− E12.5 embryos were sectioned and stained with hematoxylin and eosin (H&E) and for cleaved caspase-3. (A) Occasional apoptotic nuclei were observed in the developing ventral spinal cord of wild-type and Harakiri (HRK)–deficient mice. In contrast, extensive apoptotic nuclei [indicated by arrows] were noted in the ventral spinal cord in BCL-XL-deficient mice and in HRK and BCL-XL dual-deficient mice. Scale bar = 10 microns. (B) Cleaved caspase-3 immunoreactive cells [indicated by arrows] were observed in E12.5 embryos in the developing DRG. BCL-XL deficiency causes a marked increase in the number of cleaved caspase-3 immunoreactive cells in the dorsal root ganglia (DRG). Concomitant HRK deficiency does not attenuate the BCL-XL deficiency-induced apoptosis. Scale bar = 20 microns. (C) Quantification of the number of cleaved caspase-3 immunoreactive cells in the DRG of BCL-XL-deficient, HRK, and BCL-XL dual-deficient mice or HRK, BIM, and BCL-XL triple-deficient mice showed no significant difference in comparison to wild-type controls. *p<0.05 vs wild-type controls; n.s. = not statistically significant.
Discussion
In this report, we assessed the role of the pro-apoptotic molecule HRK in regulating the extensive neuronal apoptotic phenotype and embryonic lethality caused by BCL-XL deficiency. Although viable Hrk−/−/Bcl-x−/− embryos were obtained at E12.5, no viable Hrk−/−/Bcl-x−/− offspring were obtained from the crosses, suggesting that concomitant HRK deficiency did not attenuate BCL-XL deficiency-induced embryonic lethality. Analysis of HRK-deficient embryos revealed reduced PCD in the developing nervous system in comparison to wild-type littermates. These results suggest that HRK plays a significant role in PCD in the immature nervous system in response to developmental cues. Deficiency of BCL-XL results in embryonic lethality and abnormally increased apoptosis of immature neurons in the brainstem, DRG, and ventral spinal cord. Of the various pro-apoptotic BCL-2 family members examined to date, only concomitant BAX deficiency has been found to attenuate the extensive neurodegeneration seen as a result of BCL-XL deficiency (Shindler et al. 1997b; Roth et al. 2000; Zaidi et al. 2001; Klocke et al. 2002; Cecconi et al. 2004), although it is still unable to prevent the embryonic lethality associated with BCL-XL deficiency. In an attempt to identify potential BH3-only regulators of this BAX-BCL-XL interaction, we investigated the role of HRK in regulating BCL-XL deficiency-induced neurodegeneration. HRK is one of the less potent pro-apoptotic members of the BH3-only family on account of its inability to neutralize and inactivate the entire repertoire of anti-apoptotic BCL-2 family members (Willis and Adams 2005). However, the ability of HRK to interact with anti-apoptotic BCL-2 family members such as BCL-2 and BCL-XL (Imaizumi et al. 2004) and its temporal pattern of expression during nervous system development (Coultas et al. 2007) led us to speculate that HRK may critically regulate the BAX-BCL-XL interaction during murine nervous system development. Although it has been reported to protect against NGF withdrawal-induced neuronal cell death in vitro (Coultas et al. 2007), HRK deficiency did not attenuate the massive apoptosis observed in the developing BCL-XL-deficient nervous system. BIM, another pro-apoptotic BH3-only molecule, has also been implicated in NGF withdrawal-induced cell death in certain neuronal populations (Putcha et al. 2001; Harris and Johnson 2001). We have previously identified a role for BIM in regulating BCL-XL deficiency-induced apoptosis of hematopoetic and male germ cells, although BIM deficiency did not attenuate BCL-XL deficiency-induced neurodegeneration (Akhtar et al. 2008). BH3-only proteins have been described to have redundant roles and often work in concert in initiating apoptosis in response to various cytotoxic stimuli (Willis and Adams 2005). Deficiency of HRK or BIM alone reduced neuronal PCD, but dual deficiency did not further attenuate apoptotic death in the embryonic DRG. Concomitant BIM and HRK deficiency also did not rescue the BCL-XL deficiency-induced neurodegeneration or embryonic lethality. Recent reports suggest that activation of the BAX/BAK-dependent apoptotic program depends on the total amount of BH3-only proteins present in the cell (Ren et al. 2010; Harder and Libby 2011). Consistent with this hypothesis, a Bid−/−/Bim−/−/Puma−/− triple knockout mouse recapitulated most of the developmental defects seen in Bax–/–/Bak–/– animals (Ren et al. 2010). BID, BIM, and PUMA are potent activators of BAX and BAK owing to their ability to bind avidly with all the anti-apoptotic BCL-2 family members and their ability to directly activate BAX and BAK. The Bid−/−/Bim−/−/Puma−/− animals exhibited enlarged thymi, splenomegaly lymphadenopathy, and persistent interdigital webs similar to Bax –/–/Bak –/– animals (Lindsten et al. 2000), and triple knockout cells were resistant to a host of cytotoxic stimuli (Ren et al. 2010). In conclusion, these recent reports, together with our findings, suggest that different combinations of BH3-only molecules critically regulate the BAX/BAK-dependent apoptotic program in a cell type– and stimulus-specific manner.
Acknowledgments
The authors thank UAB Neuroscience Core Facilitites (NS 047466 and NS 057098) and Cecelia B. Latham for technical assistance. The authors also thank Dr. Andreas Strasser (Walter and Eliza Hall Institute; Victoria, Australia) for providing the mutant Hrk and Bim mice.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the authorship and/or publication of this article.
The author(s) disclosed receipt of the following financial support for the research and/or authorship of this article: This work was supported by grants from the National Institutes of Health (NS 035107 and NS 041962).
References
- Adams JM, Cory S. 2007a. Bcl-2-regulated apoptosis: mechanism and therapeutic potential. Curr Opin Immunol. 19:488–496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams JM, Cory S. 2007b. The Bcl-2 apoptotic switch in cancer development and therapy. Oncogene. 26:1324–1337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akhtar RS, Klocke BJ, Strasser A, Roth KA. 2008. Loss of BH3-only protein Bim inhibits apoptosis of hemopoietic cells in the fetal liver and male germ cells but not neuronal cells in bcl-x-deficient mice. J Histochem Cytochem. 56:921–927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas SC, Ryu E, Park C, Malagelada C, Greene LA. 2005. Puma and p53 play required roles in death evoked in a cellular model of Parkinson disease. Neurochem Res. 30:839–845 [DOI] [PubMed] [Google Scholar]
- Bouillet P, Metcalf D, Huang DC, Tarlinton DM, Kay TW, Kontgen F, Adams JM, Strasser A. 1999. Proapoptotic Bcl-2 relative Bim required for certain apoptotic responses, leukocyte homeostasis, and to preclude autoimmunity. Science. 286:1735–1738 [DOI] [PubMed] [Google Scholar]
- Bouillet P, Purton JF, Godfrey DI, Zhang LC, Coultas L, Puthalakath H, Pellegrini M, Cory S, Adams JM, Strasser A. 2002. BH3-only Bcl-2 family member Bim is required for apoptosis of autoreactive thymocytes. Nature. 415:922–926 [DOI] [PubMed] [Google Scholar]
- Bouillet P, Strasser A. 2002. BH3-only proteins—evolutionarily conserved proapoptotic Bcl-2 family members essential for initiating programmed cell death. J Cell Sci. 115:1567–1574 [DOI] [PubMed] [Google Scholar]
- Cecconi F, Roth KA, Dolgov O, Munarriz E, Anokhin K, Gruss P, Salminen M. 2004. Apaf1-dependent programmed cell death is required for inner ear morphogenesis and growth. Development. 131:2125–2135 [DOI] [PubMed] [Google Scholar]
- Cory S, Huang DC, Adams JM. 2003. The Bcl-2 family: roles in cell survival and oncogenesis. Oncogene. 22:8590–8607 [DOI] [PubMed] [Google Scholar]
- Coultas L, Terzano S, Thomas T, Voss A, Reid K, Stanley EG, Scott CL, Bouillet P, Bartlett P, Ham J, et al. 2007. Hrk/DP5 contributes to the apoptosis of select neuronal populations but is dispensable for haematopoietic cell apoptosis. J Cell Sci. 120:2044–2052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Sa C, Klocke BJ, Cecconi F, Lindsten T, Thompson CB, Korsmeyer SJ, Flavell RA, Roth KA. 2003. Caspase regulation of genotoxin-induced neural precursor cell death. J Neurosci Res. 74:435–445 [DOI] [PubMed] [Google Scholar]
- Harder JM, Libby RT. 2011. BBC3 (PUMA) regulates developmental apoptosis but not axonal injury induced death in the retina. Mol Neurodegener. 6:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris CA, Johnson EM., Jr 2001. BH3-only Bcl-2 family members are coordinately regulated by the JNK pathway and require Bax to induce apoptosis in neurons. J Biol Chem. 276:37754–37760 [DOI] [PubMed] [Google Scholar]
- Huang DC, Strasser A. 2000. BH3-Only proteins-essential initiators of apoptotic cell death. Cell. 103:839–842 [DOI] [PubMed] [Google Scholar]
- Imaizumi K, Benito A, Kiryu-Seo S, Gonzalez V, Inohara N, Lieberman AP, Kiyama H, Nunez G. 2004. Critical role for DP5/Harakiri, a Bcl-2 homology domain 3-only Bcl-2 family member, in axotomy-induced neuronal cell death. J Neurosci. 24:3721–3725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inohara N, Ding L, Chen S, Nunez G. 1997. harakiri, a novel regulator of cell death, encodes a protein that activates apoptosis and interacts selectively with survival-promoting proteins Bcl-2 and Bcl-X(L). EMBO J. 16:1686–1694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klocke BJ, Latham CB, D’Sa C, Roth KA. 2002. p53 deficiency fails to prevent increased programmed cell death in the Bcl-X(L)-deficient nervous system. Cell Death Differ. 9:1063–1068 [DOI] [PubMed] [Google Scholar]
- Lindsten T, Ross AJ, King A, Zong WX, Rathmell JC, Shiels HA, Ulrich E, Waymire KG, Mahar P, Frauwirth K, et al. 2000. The combined functions of proapoptotic Bcl-2 family members bak and bax are essential for normal development of multiple tissues. Mol Cell. 6:1389–1399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motoyama N, Wang F, Roth KA, Sawa H, Nakayama K, Nakayama K, Negishi I, Senju S, Zhang Q, Fujii S. 1995. Massive cell death of immature hematopoietic cells and neurons in Bcl-x-deficient mice. Science. 267:1506–1510 [DOI] [PubMed] [Google Scholar]
- O’Connor L, Strasser A, O’Reilly LA, Hausmann G, Adams JM, Cory S, Huang DC. 1998. Bim: a novel member of the Bcl-2 family that promotes apoptosis. EMBO J. 17:384–395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putcha GV, Moulder KL, Golden JP, Bouillet P, Adams JA, Strasser A, Johnson EM. 2001. Induction of BIM, a proapoptotic BH3-only BCL-2 family member, is critical for neuronal apoptosis. Neuron 29:615–628 [DOI] [PubMed] [Google Scholar]
- Puthalakath H, O’Reilly LA, Gunn P, Lee L, Kelly PN, Huntington ND, Hughes PD, Michalak EM, McKimm-Breschkin J, Motoyama N, et al. 2007. ER stress triggers apoptosis by activating BH3-only protein Bim. Cell. 129:1337–1349 [DOI] [PubMed] [Google Scholar]
- Puthalakath H, Strasser A. 2002. Keeping killers on a tight leash: transcriptional and post-translational control of the pro-apoptotic activity of BH3-only proteins. Cell Death Differ. 9:505–512 [DOI] [PubMed] [Google Scholar]
- Ren D, Tu HC, Kim H, Wang GX, Bean GR, Takeuchi O, Jeffers JR, Zambetti GP, Hsieh JJ, Cheng EH. 2010. BID, BIM, and PUMA are essential for activation of the BAX- and BAK-dependent cell death program. Science. 330:1390–1393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth KA, Kuan C, Haydar TF, D’Sa-Eipper C, Shindler KS, Zheng TS, Kuida K, Flavell RA, Rakic P. 2000. Epistatic and independent functions of caspase-3 and Bcl-X(L) in developmental programmed cell death. Proc Natl Acad Sci U S A. 97:466–471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanz C, Benito A, Inohara N, Ekhterae D, Nunez G, Fernandez-Luna JL. 2000. Specific and rapid induction of the proapoptotic protein Hrk after growth factor withdrawal in hematopoietic progenitor cells. Blood. 95:2742–2747 [PubMed] [Google Scholar]
- Shindler KS, Latham CB, Roth KA. 1997a. Bax deficiency revents the increased cell death of immature neurons in bcl-x-deficient mice. J Neurosci. 17:3112–3119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shindler KS, Latham CB, Roth KA. 1997b. Bax deficiency revents the increased cell death of immature neurons in bcl-x-deficient mice. J Neurosci. 17:3112–3119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan A, Roth KA, Sayers RO, Shindler KS, Wong AM, Fritz LC, Tomaselli KJ. 1998. In situ immunodetection of activated caspase-3 in apoptotic neurons in the developing nervous system. Cell Death Differ. 5:1004–1016 [DOI] [PubMed] [Google Scholar]
- White FA, Keller-Peck CR, Knudson CM, Korsmeyer SJ, Snider WD. 1998. Widespread elimination of naturally occurring neuronal death in Bax-deficient mice. J Neurosci. 18:1428–1439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis SN, Adams JM. 2005. Life in the balance: how BH3-only proteins induce apoptosis. Curr Opin Cell Biol. 17:617–625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaidi AU, D’Sa-Eipper C, Brenner J, Kuida K, Zheng TS, Flavell RA, Rakic P, Roth KA. 2001. Bcl-X(L)-caspase-9 interactions in the developing nervous system: evidence for multiple death pathways. J Neurosci. 21:169–175 [DOI] [PMC free article] [PubMed] [Google Scholar]