Abstract
Dentin sialophosphoprotein (DSPP) and its cleaved products, dentin phosphoprotein (DPP) and dentin sialoprotein (DSP), play important roles in biomineralization. Believed to be tooth specific, the authors’ group revealed its expression in bone, and more recently, they and other groups also showed its expression in a few types of soft tissues. In this study, the authors systematically examined the expression of DSPP in a variety of non-mineralized tissues using reverse-transcription polymerase chain reaction (RT-PCR), real-time PCR, Western immunoblotting, and immunohistochemistry analyses in wild-type mice as well as β-galactosidase assays in the Dspp lacZ knock-in mice. These approaches showed the presence of DSPP in the salivary glands, cartilage, liver, kidney, and brain and its absence in the heart and spleen. Real-time PCR showed that the expression levels of DSPP mRNA in salivary glands, cartilage, liver, and kidney were higher than in the bone. Interestingly, DSPP was observed in the pericytes of blood vessels in the dental pulp, which are believed to be able to differentiate into odontoblasts. On the basis of these observations, the authors conclude that DSPP and/or its cleaved products may fulfill important functions in certain non-mineralized tissues in addition to its role in biomineralization.
Keywords: gene expression, dentin sialophosphoprotein, biomineralization, non-mineralized tissues, DSPP knockout mice
Dentin sialophosphoprotein (DSPP) is a non-collagenous protein (NCP) belonging to a family of proteins known as SIBLING (small integrin-binding ligand, N-linked glycoprotein) (Fisher et al. 2001). The SIBLING family members share a number of similarities in their genomic organization, posttranslational modifications, and tissue localization. Other members of this family include osteopontin (OPN), bone sialoprotein (BSP), dentin matrix protein 1 (DMP1), and matrix extracellular phosphoglycoprotein (MEPE) (Fisher and Fedarko 2003).
DSPP was initially discovered by cDNA cloning using a mouse odontoblast cDNA library (MacDougall et al. 1997). The proteolytically cleaved fragments of DSPP, however, were discovered much earlier and were believed to be separate entities until the discovery of a single gene (Dspp) encoding both dentin sialoprotein (DSP) and dentin phosphoprotein (DPP) (MacDougall et al. 1997). DPP, which was discovered in 1967, is the most abundant of the NCPs in dentin (Veis and Perry 1967). It is an unusually polyanionic protein, containing a large number of aspartic acids (Asp) and phosphoserines (Pse) in the repeating sequences of (Asp-Pse)n and (Asp-Pse-Pse)n (George et al. 1996). On the other hand, DSP, a glycoprotein with little or no phosphate, was discovered in 1981 (Butler et al. 1981). The importance of DSPP in biomineralization has been illustrated in human and mouse genetic studies that revealed the association of Dspp mutations or ablations with mineralization defects in the dentin and bone (Sreenath et al. 2003; Verdelis et al. 2008; Xiao et al. 2001; Zhang X et al. 2001).
DSPP was originally thought to be dentin specific. Later on, studies by our research group demonstrated the expression of DSPP in bone and cementum (Qin et al. 2002; Qin, Brunn, Cadena, et al. 2003; Baba et al. 2004). More recently, DSPP was found in some non-mineralized tissues by immunohistochemical staining (Alvares et al. 2006; Ogbureke and Fisher 2004, 2007). To date, no study has been performed using multidimensional methodology to systematically evaluate the expression of DSPP in non-mineralized tissues. Our major objective in this study was to analyze the expression and distribution of DSPP in non-mineralized tissues: salivary glands, cartilage, liver, kidney, brain, heart, and spleen. The methodology used in this investigation included reverse-transcription polymerase chain reaction (RT-PCR), real-time PCR, Western immunoblotting, immunohistochemical staining in wild-type mice, and β-galactosidase expression assays in the Dspp null mice. This multidimensional approach not only indicated the presence of DSPP in salivary glands, cartilage, liver, kidney, and brain but also demonstrated that DSPP is expressed in the salivary glands, cartilage, liver, and kidney at a level higher than that in bone. The results of this study show that DSPP and/or its cleaved products may play an important role in some non-mineralized tissues such as salivary glands and cartilages, which sets the stage for future studies exploring the newly discovered role of DSPP in the soft tissues.
Materials and Methods
Tissue Acquisition/Sample Preparation
The non-mineralized tissues from 1-month-old wild-type (WT) male C57BL/6J mice (The Jackson Laboratory; Bar Harbor, ME) were used for the mRNA analyses using RT-PCR and real-time PCR. To obtain RNA extracts from the teeth, long bone, salivary gland, articular cartilage, liver, kidney, brain, heart, and spleen, the 1-month-old mice were sacrificed using CO2, and the tissues were dissected out and then frozen. One-month- and 3-month-old Dspp knockout (Dspp-KO) mice (strain name: B6; 129-Dspptm1Kul/Mmnc; MMRRC, UNC, Chapel Hill, NC) were used for the β-galactosidase expression assay. To perform protein chemistry and immunohistochemistry analyses, tissue samples were obtained from 3-month-old C57BL/6J mice. Dspp knockout mice of the same age were used as negative controls in protein chemistry and immunoblotting experiments. The mice were sacrificed using CO2 to obtain samples for protein extraction and β-galactosidase expression assay or first anesthetized and then perfused for immunohistochemical analysis. The animal protocol used in this study was approved by the Animal Welfare Committee of Texas A&M Health Science Center Baylor College of Dentistry (Dallas, TX).
Isolation of RNA, RT-PCR, and Real-time PCR
Total RNA was extracted from the tissues of 1-month-old mice (C57BL/6J) with the RNeasy mini kit (Qiagen; Germantown, MD) according to the manufacturer’s protocol. The RNA (1 µg/per sample) was reverse transcribed into first-strand cDNA using the QuantiTect Rev Transcription Kit (Qiagen; Germantown, MD). The PCR amplification of the DSPP cDNA was then performed using a forward primer, 5′-TTGTTGAAAACTCTGTGGCTGTGC-3′, and a reverse primer, 5′-GGCATCGTTGAAAATGCGGAGGAA-3′. The sequence of the forward primer came from exon 3, whereas that of reverse primer was from exon 4 of the mouse Dspp. Exons 3 and 4 encode amino acid residues in the DSP region of the Dspp gene. The highly repetitive DPP region of the Dspp gene was not amplified in this investigation. The housekeeping gene, glyceraldehyde-3-phosphate dehydrogenase (GAPDH), was used as the internal control. The PCR conditions were as follows: initial denaturation at 95C for 10 min, followed by 30 cycles of 94C for 30 sec, 60C for 30 sec, and 72C for 30 sec. The size of the amplified DSPP product was 510 base pairs (bp) long and was visualized on 1% agarose gel (Sigma; St. Louis, MO) stained with ethidium bromide and observed under ultraviolet light. The RT-PCR product (510 bp) from the salivary glands was then sequenced (Northwoods DNA; Solway, MN).
Real-time PCR was carried out to estimate the relative levels of DSPP expression in the various non-mineralized tissues compared to those in the bone and teeth. For quantitative comparison, the same amount of cDNA (1 µg/µl) was used as the template. The primers used for real-time PCR were as follows: forward, 5′AACTCTGTGGCTGTGCCTCT-3′ (in exon 3) and reverse, 5′TATTGACTCGGAGCCATTCC-3′ (in exon 4). The real-time PCR reactions were performed using the Brilliant SYBR Green QPCR Master Mix (Applied Biosystems; Foster City, CA) and the CFX-96 Real-Time PCR Detection System (Bio-Rad; Hercules, CA). GAPDH was again used as the control. The PCR conditions were as follows: initial denaturation at 95C for 10 min, followed by 45 cycles of 95C for 30 sec, 60C for 1 min, and 72C for 30 sec. The PCR product accumulation was monitored by the increase in fluorescence intensity caused by the binding of SYBR Green to double-stranded DNA. The mean values from the triplicate analyses were then compared.
β-Galactosidase Expression Assay
For the β-galactosidase expression assay, 1-month- and 3-month-old Dspp knockout mice (strain name: B6; 129-Dspptm1Kul/Mmnc; MMRRC, UNC, Chapel Hill, NC) were used. In these Dspp knockout mice, exons 2 to 5 of the Dspp gene, spanning the entire coding region, were replaced by the lacZ gene, followed by a neomycin resistance gene. A previous study showed that the expression pattern of the lacZ gene driven by the Dspp promoter reflects that of the endogenous Dspp (Sreenath et al. 2003). The β-galactosidase activity was assessed in the tissues from 1-month-old and 3-month-old mice as previously described (Feng et al. 2002; Zhang J et al. 2002). Briefly, the mice were fixed with ice-cold 4% paraformaldehyde for 1 hr, then stained overnight in freshly made X-Gal solution (1 mg/ml) at 37C, followed by re-fixation, decalcification (for bone and teeth), wax embedding, sectioning, and counterstaining. The 1-month-old Dspp knockout mice were used for whole-tissue staining, whereas the 3-month-old Dspp knockout mice were stained and then processed for paraffin embedding. Serial 5-µm sections were prepared and counterstained with hematoxylin. Wild-type mice of the same age (The Jackson Laboratory) were used as negative controls in this experiment.
SDS-PAGE and Western Immunoblotting
For the extraction of DSPP protein, ten 3-month-old WT mice were used. Teeth, bone, salivary gland, articular cartilage, liver, kidney, brain, heart, and spleen tissues were carefully removed under a dissecting microscope. To extract DSPP from the non-mineralized tissues, the samples were placed in a 4-M guanidium-HCl (Gdm-HCl) solution of pH 7.2 (Acros Organics; Fairlawn, NJ) containing proteinase inhibitors (0.78 mg ml−1 of benzamidine-HCl, 0.18 mg ml−1 of sodium iodoacetate, 1.8 µg ml−1 of soybean trypsin inhibitor, 0.17 mg ml−1 of phenylmethylsufonyl fluoride, and 5 µg ml−1 of pepstatin) for 48 hr. For the protein extraction from teeth and bone, Gdm-HCl/0.5 M EDTA was used following the protocol previously described (Qin et al. 2001). The Gdm-HCl extracts were subjected to Q-Sepharose (Amersham Biosciences; Uppsala, Sweden) ion-exchange chromatography with a gradient ranging from 0.1 to 0.8 M NaCl in 6 M urea solution (pH 7.2) (Qin et al. 2001). This method separated the NCPs into 100 to 120 fractions (0.5 ml each). The samples were treated with 3% β-mercaptoethanol (β-ME) to break the disulfide bonds of the dimeric form of DSP. For SDS-PAGE, 60 µl of sample from each chromatographic fraction was loaded onto 10% SDS-PAGE gels. First, Stains-all staining was used to profile and locate NCPs in all the chromatographic fractions. Western immunoblotting was then carried out on all of the fractions that showed proteins by Stains-all staining, to confirm and/or identify DSP in those specific fractions. Protein extracts from Dspp knockout mouse tissues were used as negative controls, and 0.3 µg of DSP isolated from incisor dentin of rat was used as the positive control in the Western immunoblotting analyses. To detect DSP, we used three types of antibodies: (1) an anti-DSP monoclonal antibody (anti-DSP-2G7.3) (Qin, Brunn, Baba, et al. 2003), (2) a polyclonal anti-DSP antibody (Butler et al. 1992), and (3) a monoclonal antibody (anti-DSP-3A11) raised in mouse using the technique previously described (Qin, Brunn, Baba, et al. 2003). For the generation of the monoclonal antibody anti-DSP-3A11, rat dentin DSP was injected into the footpad of BALB/c mice. Lymphocytes from the local lymph nodes were fused with P3 myeloma cells. The fusion cells showing a positive reaction to DSP were screened and cloned. Among several positive clones, 3A11 showed a strong reaction to DSP. Clone 3A11 was expanded in the abdominal cavity of the BALB/c mice, and the antibody in ascites fluid was purified with ImmunoPure Immobilized (A/G) IgG Purification Kit (Pierce; Rockford, IL) following the manufacturer’s instructions.
All three antibodies were used at a dilution of 1:2000. The blots were washed three times in PBS containing 0.3% Tween 20, followed by incubation in the alkaline phosphate-conjugated anti-mouse IgG (Sigma Aldrich; St. Louis, MO) at a dilution of 1:5000. Finally, the blots were incubated with the chemiluminescent substrate CDP-Star (Ambion; Austin, TX) for 5 min and exposed to X-ray films.
Immunohistochemistry Analyses
Immunohistochemistry (IHC) was done to analyze the difference in the distribution of DSPP in the various non-mineralized tissues. Dspp knockout mice of same age were used as negative controls. For the IHC analyses, 3-month-old mice were perfused from the ascending aorta with 4% paraformaldehyde in 0.1 M phosphate buffer. The samples were fixed in 4% paraformaldehyde for 48 hr. The mineralized tissues (bone and teeth) were then decalcified in 8% EDTA (pH 7.4) at 4C. The fixed non-mineralized samples, as well as the decalcified specimens (bone and teeth), were processed for paraffin embedding, and serial 5-µm sections were prepared. The anti-DSP monoclonal antibody, referred to as “anti-DSP-2C12.3” (Baba et al. 2004), was used at a dilution of 1:800. Mouse IgG at the same concentration as that of the anti-DSP-2C12.3 was used to replace the primary antibody as the negative control in these experiments. All the IHC experiments were carried out using the M.O.M. kit and DAB kit (Vector Laboratories; Burlingame, CA) according to the manufacturer’s instructions.
Results
Evaluation of DSPP mRNA in Non-mineralized Tissues
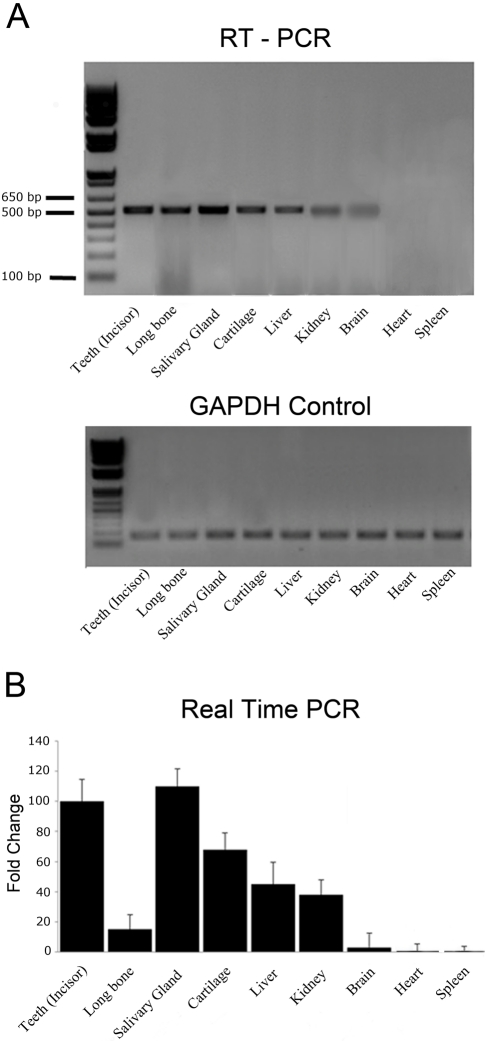
The RT-PCR analyses showed the presence of DSPP mRNA in the salivary glands, articular cartilage, liver, kidney, and brain tissues (Fig. 1A). DNA sequencing analyses confirmed that the 510-bp product amplified from the salivary gland was from the DSPP mRNA sequence. The heart and spleen did not indicate the presence of DSPP mRNA.
Figure 1.
(A) Dentin sialophosphoprotein (DSPP) mRNA in different tissues. The expression of DSPP and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) control was examined by reverse transcriptase-polymerase chain reaction (RT-PCR) analysis using RNA extracted from the tissues of 1-month-old mice. Lane 1, teeth (incisor); lane 2, bone; lane 3, salivary glands; lane 4, articular cartilage from mandibular condyle; lane 5, liver; lane 6, kidney; lane 7, brain; lane 8, heart; lane 9, spleen. Note that positive results were observed in the salivary glands, cartilage, liver, kidney, and brain but not in the heart and spleen. GAPDH control shows similar positive result for all samples. (B) Relative expression level of DSPP mRNA in non-mineralized tissues. The mRNA level of teeth was set at 100% for real-time PCR calculations. The level of DSPP mRNA in other tissues was then calculated relative to the level found in the teeth. Note that the expression levels of DSPP in salivary glands, articular cartilage, liver, and kidney are higher than that of bone.
These RT-PCR results were also confirmed by real-time PCR (Fig. 1B). To calculate the relative expression levels, the mRNA level of the teeth was considered the standard, and its value was set at 100% in the real-time PCR analyses. The expression level of the DSPP in other soft tissues was then calculated relative to the level observed in the teeth. In the real-time PCR analyses, the salivary glands, cartilage, liver, and kidney exhibited higher levels of DSPP mRNA than in the bone (Fig. 1B). We observed that the size of salivary glands of Dspp knockout mice appeared smaller than that of the wild-type mice. However, we did not find significant difference histologically between the salivary glands of the Dspp knockout and wild-type mice. The expressions of DSPP mRNA in the salivary glands, cartilage, liver, and kidney were further confirmed by the β-galactosidase staining, Western immunoblotting, and immunohistochemical staining (see below).
β-Galactosidase Expression Assay
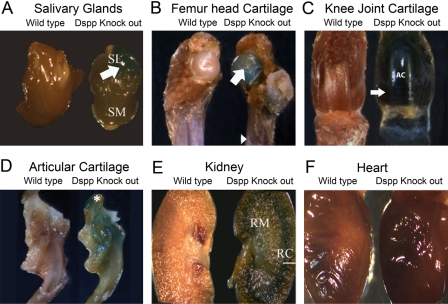
We used 1-month-old and 3-month-old Dspp knockout mice to assess the expression of DSPP in non-mineralized tissues. We dissected and used the whole organs/tissues of the 1-month-old Dspp knockout mice for X-gal staining. Strong lacZ signals (blue) were observed in the salivary glands (in particular, the sublingual glands) (Fig. 2A); other locations of strong lacZ signals were found in the femoral head cartilage (Fig. 2B), the articular cartilage and distal femoral condyle in the knee joint (Fig. 2C), the articular cartilage of the mandibular condyle (Fig. 2D), and the junction between the renal cortex and renal medulla in the kidney (Fig. 2E), as well as in the teeth (Fig. 2D). Consistent with the PCR results, the diaphysis region of the long bones demonstrated a relatively lower level of β-galactosidase activity. The WT mice used as controls did not exhibit any β-galactosidase staining, nor did the heart (Fig. 2F) and spleen show positive staining (not shown).
Figure 2.
Dentin sialophosphoprotein (DSPP)–lacZ expression pattern in 1-month-old homozygous Dspp knockout mice. These mice were selected for tracking the expression pattern in various soft tissues. The wild-type C57BL/6J mice of same age were used as a control. The expression of the lacZ gene was observed in (A) submandibular salivary gland (denoted as SM) and more strongly in the sublingual (denoted as SL and pointed by white arrow) salivary gland of the Dspp null mice (right); (B) femoral head cartilage of the Dspp null mice (right) was pointed by a white arrow, whereas the diaphysis region of the long bone was indicted by an arrowhead; (C) articular cartilage (AC) and distal femoral condyle (white arrow) in the knee and growth plate region of the long bone (right); (D) articular cartilage of the mandibular condyle (denoted by white asterisk) of Dspp knockout mice; and (E) the kidneys, in which the junction of the renal cortex (marked by a white line as RC) and renal medulla (shown as RM) demonstrated strong lacZ signals. (F) Expression of lacZ gene was not observed in the heart.
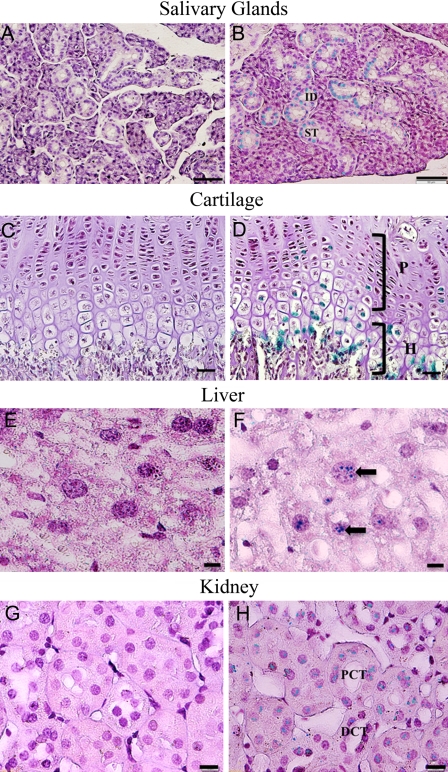
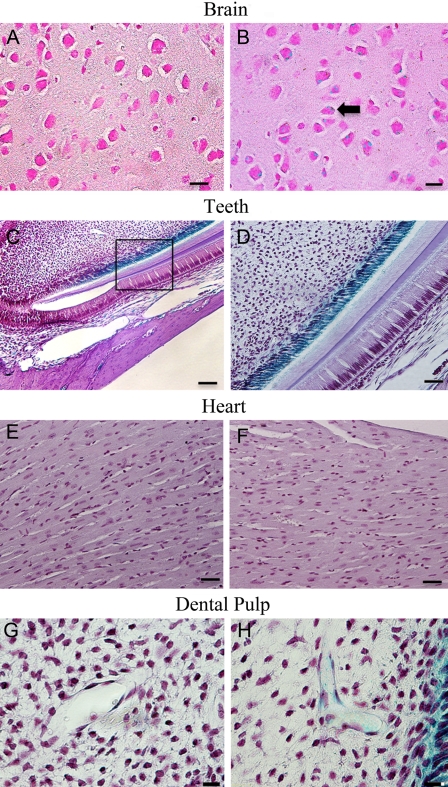
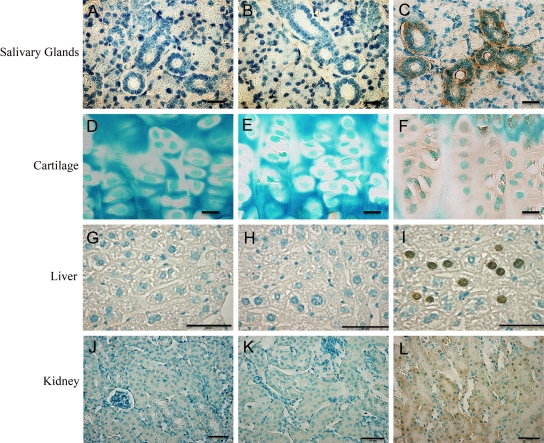
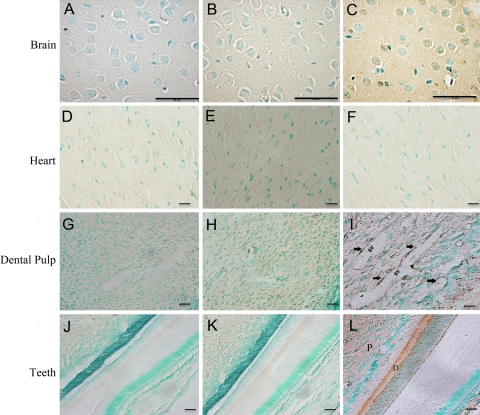
The specimens from the 3-month-old Dspp knockout mice were stained with X-gal and then processed for paraffin embedding. Serial 5-µm sections were prepared and counterstained with hematoxylin. In these 3-month-old Dspp knockout mice, the following expression patterns were observed: In the submandibular salivary glands, positive staining was seen in the interlobular ducts and striated ducts (Fig. 3A,B); in the growth plate cartilage, positive signals were observed in the proliferative and hypertrophic zones (Fig. 3C,D); in the liver, a lacZ expression was noted in the hepatocytes (Fig. 3E,F); in the kidney, positive staining was seen in the distal and proximal convoluted tubule (Fig. 3G,H); in the brain, the neuron bodies of the ganglionic cell layer in the cerebral cortex showed positive staining (Fig. 4A,B); and in the teeth, a positive signal was found in the odontoblast layer (Fig. 4C,D). Consistent with the results of the whole-organ/tissue X-gal staining, the cells in the heart did not yield any positive signals (Fig. 4E,F). Interestingly, the β-galactosidase activity was also observed around the blood vessels in the dental pulp, especially in the pericytes located in the peripheral region of these blood vessels (Fig. 4G,H).
Figure 3.
Dentin sialophosphoprotein (DSPP)–lacZ expression pattern in 3-month-old homozygous Dspp knockout mice. The following expression patterns were observed: Salivary glands: (A) salivary gland samples from C57BL/6J wild-type mice were used as negative control; (B) the interlobular duct (ID) as well as the striated duct (SD) of the submandibular salivary gland of the Dspp knockout mice showed positive lacZ staining; bar = 50 µm. Cartilage: (C) growth plate cartilage control from C57BL/6J wild-type mice; (D) growth plate cartilage of Dspp knockout mice expressing positive stain for lacZ in the proliferative (P) and hypertrophic zones (H) of the cartilage; bar = 25 µm. Liver: (E) liver tissues from C57BL/6J mice were used as control and showed no reaction to lacZ staining; (F) in Dspp knockout mouse liver, hepatocytes (black arrow) exhibited lacZ expression; bar = 10 µm. Kidney: (G) samples from C57BL/6J mice were used as negative control; (H) Dspp knockout mice exhibited positive signals in the distal (DCT) and proximal convoluted tubule (PCT) of the kidney; bar = 10 µm.
Figure 4.
Dentin sialophosphoprotein (DSPP)–lacZ expression pattern in 3-month-old homozygous Dspp knockout mice. The following expression patterns were observed: Brain: (A) cerebral cortex samples from the wild-type C57BL/6J mice used as control; (B), Dspp knockout mice showed bodies of neurons of ganglionic cell layer (black arrow) positively stained for DSPP-lacZ expression; bar = 25 µm. Teeth: (C) Dspp knockout mouse incisor exhibiting positive staining for DSPP-lacZ expression in the odontoblast layer of incisors; (D) higher magnification of the boxed area in C. The blue staining in the cytoplasm of odontoblast layer was possibly caused by the diffusion of β-galactosidase staining from the nuclei into the cytoplasm. Bar: C = 100 µm; D = 50 µm. Heart: no difference was observed between the wild-type C57BL/6J (E) and Dspp knockout (F) mice; bar = 25 µm. Dental pulp: (G) control sample from the wild-type mice; (H) LacZ signaling was noticed in the blood vessels in the dental pulp, particularly in the pericytes surrounding the blood vessels; bar = 10 µm.
SDS-PAGE and Western Immunoblotting
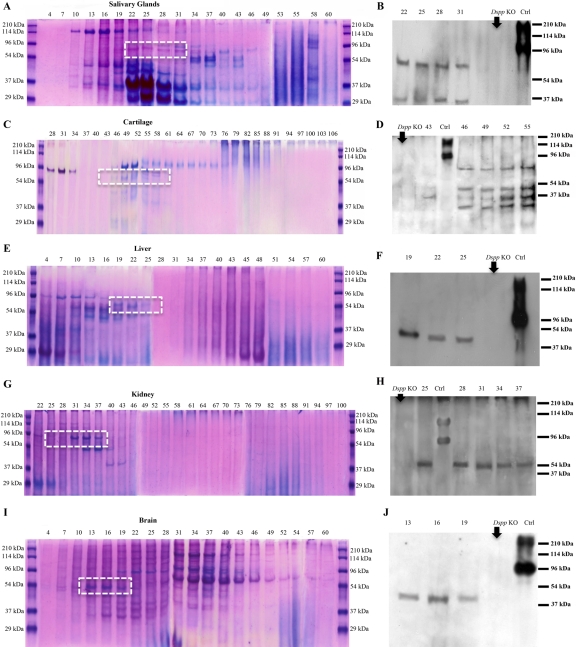
The Gdm-HCl extracts of each of the non-mineralized tissues were separated into 120 fractions by Q-Sepharose ion-exchange chromatography. Each of the chromatographic fractions was assayed by SDS-PAGE and Stains-all staining (Fig. 5A,C,E,G,I) and subsequently by Western immunoblotting (Fig. 5B,D,F,H,J). Stains-all staining of fractions from these non-mineralized tissues did not clearly demonstrate any protein corresponding to the migration rates of DSPP or DSP (Fig. 5A,C,E,G,I). The fractions that might contain DSPP/DSP were probed further with the anti-DSP antibodies. Western immunoblotting analyses using the anti-DSP-2G7.3 revealed the presence of DSP in several of the chromatographic fractions from the non-mineralized tissue extracts, which migrated between the 54- and 96-kDa molecular mass markers (Fig. 5B,D,F,H,J). Because the migration rate of the protein in the extracts from the salivary gland, cartilage, liver, kidney, and brain, recognized by the anti-DSP-2G7.3 monoclonal antibody, was faster than that from rat dentin (~100 kDa), we further tested the identity of these polypeptides using two more different anti-DSP antibodies. For the later experiments, representative fractions containing the protein recognized by the anti-DSP-2G7.3 monoclonal antibody were analyzed by Western immunoblotting using the polyclonal anti-DSP antibody and a second type of monoclonal anti-DSP antibody (anti-DSPP-3A11). The protein recognized by the anti-DSP-2G7.3 monoclonal antibody was also immunoreactive to the two different types of anti-DSP antibodies (Fig. 6A,B). Thus, our data indicated that these polypeptides did represent DSP, although their migration rates were different from those of DSP isolated from rat dentin. The corresponding chromatographic fractions from the extracts of the heart and spleen did not show any protein polypeptide that could be recognized by these anti-DSP antibodies (not shown). Because anti-DPP antibodies are not available, information about the presence or absence of DPP in these non-mineralized tissues could not be obtained. However, DSP and DPP are from a single mRNA, and the presence of DSPP mRNA was proven by PCR (see above). Thus, the presence of DSP would indicate the expression of DSPP.
Figure 5.
Stains-all staining and Western immunoblotting in salivary glands (A, B), articular cartilage (C, D), liver (E, F), kidney (G, H), and brain (I, J). Numbers at the top of each image represent fraction numbers of the Q-Sepharose chromatography. (A, C, E, G, I) Stains-all staining for every third fraction in the chromatographic region of interest was performed to illustrate the existence of acidic proteins. The identity of dentin sialoprotein (DSP) in the corresponding fractions (dotted white boxed region) was confirmed by Western immunoblotting (B, D, F, H, J) using the anti-DSP-2G7.3 antibody. DSP was detected in fractions numbered on the top of the figures. The differences in the fractions in which DSP eluted may be attributed to the posttranslational modifications of DSP in these different tissues. The tissue extracts from Dspp knockout (Dspp KO) mice were used as negative controls (B, D, F, H, J). Ctrl: 0.3 µg of DSP isolated from incisor dentin of rat. Note that DSP extracted from these tissues migrated faster than that isolated from rat dentin (i.e., the positive Ctrl).
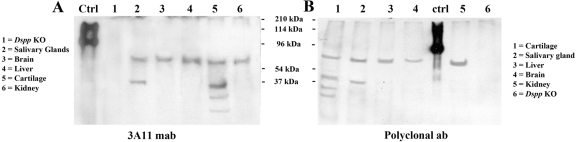
Figure 6.
One representative fraction from each tissue extract showing the highest level of dentin sialoprotein (DSP) in Western immunoblotting with the anti-DSP-2G7.3 monoclonal antibody was further probed by two additional anti-DSP antibodies: the anti-DSP-3A11 (A) and the polyclonal anti-DSP antibody (B). The polypeptides recognized by the anti-DSP-2G7.3 monoclonal antibody were also immunoreactive to the anti-DSP-3A11 and the polyclonal anti-DSP antibody, indicating that they represented DSP, although the migration rate of DSP from these soft tissues differed from that of rat dentin.
Immunohistochemistry Analyses
Immunohistochemical staining of the non-mineralized tissues showed a positive reaction in the salivary gland, cartilage, liver, kidney, and brain tissues in the 3-month-old wild-type mice (Figs. 7 and 8). The tissues from Dspp knockout mice of the same age were used as negative controls. In the submandibular salivary gland, DSPP immunoreactivity was identified in the interlobular duct and striated duct (Fig. 7A-C). In the growth plate cartilage, immunostaining was observed in the proliferative and hypertrophic zones of the cartilage (Fig. 7D-F). In the liver, DSPP immunoreactivity was detected in the hepatocytes (Fig. 7G-I), and in the kidney, immunostaining for DSPP was widely distributed throughout the distal and proximal convoluted tubule (Fig. 7J-L). The neuron bodies of the ganglionic cell layer showed a positive signal for DSPP in the cerebral cortex (Fig. 8A-C). The heart tissue displayed no anti-DSP immunoreactivity (Fig. 8D-F). As seen in the β-galactosidase staining, DSPP immunoreactivity was found in the pericyte cells in the peripheral blood vessels in the dental pulp (Fig. 8G-I). Teeth samples also showed strong anti-DSP immunoreactivity (Fig. 8J-L).
Figure 7.
Immunohistochemistry using the anti-DSP-2C12.3 monoclonal antibody (C, F, I, L). Mouse IgG of the same concentration as the anti-DSP-2C12.3 was used as the negative control (B, E, H, K). The tissues from Dspp knockout mice were also used as negative controls (A, D, G, J). DSP immunoreactivity was identified in the interlobular duct as well as striated duct of the submandibular salivary gland (C). In the cartilage, immunostaining was identified in the proliferative and hypertrophic zones (F). Dentin sialoprotein (DSP) immunoreactivity was also detected in the liver sac containing hepatocytes (I). In the kidney, immunostaining for DSP was widely distributed throughout the distal as well as proximal convoluted tubule (L). Bar: A-C = 50 µm; D-F = 10 µm; G-L = 50 µm.
Figure 8.
Immunohistochemistry using the anti-DSP-2C12.3 monoclonal antibody (C, F, I, L). Mouse IgG of the same concentration as the anti-DSP-2C12.3 was used as the negative control (B, E, H, K). The tissues from Dspp knockout mice were also used as negative controls (A, D, G, J). Dentin sialoprotein (DSP) immunoreactivity was identified in the cerebral cortex, and bodies of neurons of the ganglionic cell layer showed a positive signal for DSP expression (C). Heart showed no difference between the Dspp knockout mouse sample (D), mouse IgG control (E), and the anti-DSP antibody specimens (F). The blood vessels (BV) in the dental pulp showed positive immunostaining in the areas containing pericytes (I, arrows). Teeth sample, showing pulp (P) and dentin (D) region, from wild-type mice was used as the positive control (L). Bar: A-L = 50 µm.
Discussion
In the present study, we systematically examined the expression of the DSPP in the mouse salivary glands, cartilage, liver, kidney, brain, heart, and spleen using real-time PCR, β-galactosidase expression assays in Dspp knockout mice, Western immunoblotting, and immunohistochemistry. These multidimensional techniques showed unequivocal evidence of DSPP expression in the salivary glands, cartilage, liver, kidney, and brain and its absence in the heart and spleen.
The RT-PCR and real-time PCR analyses revealed that the expression level of DSPP in the salivary glands, cartilage, liver, and kidney is higher than that in bone. This difference in the DSPP expression levels between these non-mineralized tissues and bone was further confirmed by other approaches, such as β-galactosidase expression assays, in the Dspp knockout mice. A previous study has shown phenotypic changes in the long bone in the Dspp knockout mice (Verdelis et al. 2008). The significantly higher level of DSPP expression in the salivary glands, cartilage, liver, and kidney suggests that DSPP might play important roles in these soft tissues. Clearly, there is a need to examine if these soft tissues, particularly the salivary glands, are affected in the Dspp knockout mice. The results from the present study also warrant further evaluation of the roles of DSPP in the development or homeostasis maintenance of such soft tissues.
DSP was observed in the extracts from the non-mineralized tissues in the Western immunoblotting analyses using three different antibodies. Although these experiments suggest that the protein recognized by the anti-DSP antibodies represents DSP, the migration rate of this protein extracted from the non-mineralized tissues was faster than that isolated from rat incisor dentin. However, the protein polypeptide appeared similar in size to rat dentin DSP sequentially treated with sialidase. O-glycosidase N-glycosidase F (Qin, Brunn, Baba, et al., 2003) indicated that the DSP in the non-mineralized tissues may be devoid of any carbohydrate moieties. A recent study in our lab also showed that DSP has a proteoglycan form referred to as “DSP-PG” (Zhu et al. 2010). In this investigation, we failed to detect DSP-PG in the protein extracts, which could further indicate that DSP in non-mineralized tissues is not glycosylated. This difference in the posttranslational modifications of DSP might also suggest that the role of DSPP/DSP in non-mineralized tissues differs from that in hard tissues.
Another interesting finding in the present study according to the β-galactosidase assay was that the cells (especially pericytes) around the blood vessels in the dental pulp were positive to lac Z staining, indicating DSPP expression in those cells. Previous studies have suggested that, in addition to participating in the maintenance of blood vessel homeostasis, the pericytes may also represent multipotential mesenchymal stem cells (Doherty et al. 1998; Schor et al. 1995). Researchers have demonstrated that pericytes differentiate into osteoblasts (Brighton et al. 1992; Diaz-Flores et al. 1992; Schor et al. 1995; Doherty et al. 1998), chondrocytes, adipocytes, smooth muscle cells, macrophages, and fibroblasts (Doherty and Canfield 1999; Farrington-Rock et al. 2004).
Several research groups have hypothesized that the pericytes present within the dental pulp tissue may also be able to differentiate into the odontoblast-like cells involved in reparative dentin formation (Yamamura 1985; Fitzgerald et al. 1990; Shi and Gronthos 2003). Recently, some studies showed that dental pulp tissue contains postnatal stem cells co-expressing STRO-1 (a cell surface antigen used to identify osteogenic precursors in bone marrow stromal cells) and CD146 (a pericyte marker), suggesting indirectly that odontoblast-like progenitors derive from a perivascular niche within the dental pulp (Miura et al. 2003; Shi and Gronthos 2003).
Interestingly, DMP1, which is another member of the SIBLING family of proteins, was recently shown to act as a morphogen on the undifferentiated mesenchymal cells present in the dentin-pulp complex, inducing de novo dentinogenesis (Almushayt et al. 2006). Several studies have already indicated the presence of DSPP/DSP in dental pulp stem cells (DPSC) (Yu et al. 2005). DSPP, DSP, and DPP have all been implicated as specific markers for odontoblast differentiation (Braut et al. 2003; Liu et al. 2005; Nakashima et al. 2002). The presence of DSPP in the region characterized by β-galactosidase assay and immunohistochemistry provides further evidence of this particular role of DSPP (and/or DSP/DPP). Therefore, these interesting data from our present study show DSPP to be present in the pericytes of the dental pulp, marking its probable role as an odontoblast progenitor cell.
DSPP belongs to the SIBLING family of proteins, which have similar characteristics (Fisher and Fedarko 2003; Fisher et al. 2001). The expression of dentin matrix protein 1 (DMP1), which is also a member of the SIBLING group, has already been established in non-mineralized tissues (Terasawa et al. 2004). Studies in our lab have also proved the expression of SIBLING proteins in the rat articular cartilage (Sun et al. 2010). In addition, several other SIBLING family members have been associated with non-mineralized tissues and, in some cases, even cancer (Bellahcene et al. 2008; Ogbureke and Fisher 2004, 2005, 2007; Ogbureke et al. 2007). The results obtained in previous reports by other laboratories have led us to hypothesize that DSPP, as with other members of the SIBLING family, may have functions apart from regulating biomineralization. To test the above hypothesis, we designed this investigation using multipronged approaches to systematically evaluate the expression of DSPP in multiple soft tissues. In conclusion, this study indicates that DSPP may serve multiple purposes in addition to its role in biomineralization. Further studies are needed to elucidate the molecular mechanisms by which DSPP functions in non-mineralized tissues.
Acknowledgments
The authors are grateful to Ms. Jeanne Santa Cruz for her assistance with the editing of this article.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the authorship and/or publication of this article.
The author(s) disclosed receipt of the following financial support for the research and/or authorship of this article: This work was supported by grant DE005092 (to CQ) from the National Institutes of Health.
References
- Almushayt A, Narayanan K, Zaki AE, George A. 2006. Dentin matrix protein 1 induces cytodifferentiation of dental pulp stem cells into odontoblasts. Gene Ther. 13:611–620 [DOI] [PubMed] [Google Scholar]
- Alvares K, Kanwar YS, Veis A. 2006. Expression and potential role of dentin phosphophoryn (DPP) in mouse embryonic tissues involved in epithelial-mesenchymal interactions and branching morphogenesis. Dev Dyn. 235:2980–2990 [DOI] [PubMed] [Google Scholar]
- Baba O, Qin C, Brunn JC, Jones JE, Wygant JN, McIntyre BW, Butler WT. 2004. Detection of dentin sialoprotein in rat periodontium. Eur J Oral Sci. 112:163–170 [DOI] [PubMed] [Google Scholar]
- Bellahcene A, Castronovo V, Ogbureke KU, Fisher LW, Fedarko NS. 2008. Small integrin-binding ligand N-linked glycoproteins (SIBLINGs): multifunctional proteins in cancer. Nat Rev Cancer. 8:212–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braut A, Kollar EJ, Mina M. 2003. Analysis of the odontogenic and osteogenic potentials of dental pulp in vivo using a Col1a1-2.3-GFP transgene. Int J Dev Biol. 47:281–292 [PubMed] [Google Scholar]
- Brighton CT, Lorich DG, Kupcha R, Reilly TM, Jones AR, Woodbury RA., II 1992. The pericyte as a possible osteoblast progenitor cell. Clin Orthop Relat Res. 275:287–299 [PubMed] [Google Scholar]
- Butler WT, Bhown M, Brunn JC, D’Souza RN, Farach-Carson MC, Happonen RP, Schrohenloher RE, Seyer JM, Somerman MJ, Foster RA, et al. 1992. Isolation, characterization and immunolocalization of a 53-kDal dentin sialoprotein (DSP). Matrix. 12:343–351 [DOI] [PubMed] [Google Scholar]
- Butler WT, Bhown M, Dimuzio MT, Linde A. 1981. Noncollagenous proteins of dentin: isolation and partial characterization of rat dentin proteins and proteoglycans using a three-step preparative method. Coll Relat Res. 1:187–199 [DOI] [PubMed] [Google Scholar]
- Diaz-Flores L, Gutierrez R, Lopez-Alonso A, Gonzalez R, Varela H. 1992. Pericytes as a supplementary source of osteoblasts in periosteal osteogenesis. Clin Orthop Relat Res. 275:280–286 [PubMed] [Google Scholar]
- Doherty MJ, Ashton BA, Walsh S, Beresford JN, Grant ME, Canfield AE. 1998. Vascular pericytes express osteogenic potential in vitro and in vivo. J Bone Miner Res. 13:828–838 [DOI] [PubMed] [Google Scholar]
- Doherty MJ, Canfield AE. 1999. Gene expression during vascular pericyte differentiation. Crit Rev Eukaryot Gene Expr. 9:1–17 [PubMed] [Google Scholar]
- Farrington-Rock C, Crofts NJ, Doherty MJ, Ashton BA, Griffin-Jones C, Canfield AE. 2004. Chondrogenic and adipogenic potential of microvascular pericytes. Circulation. 110:2226–2232 [DOI] [PubMed] [Google Scholar]
- Feng JQ, Zhang J, Tan X, Lu Y, Guo D, Harris SE. 2002. Identification of Cis-DNA Regions controlling Bmp4 expression during tooth morphogenesis in vivo. J Dent Res. 81:6–10 [DOI] [PubMed] [Google Scholar]
- Fisher LW, Fedarko NS. 2003. Six genes expressed in bones and teeth encode the current members of the SIBLING family of proteins. Connect Tissue Res. 44(Suppl 1):33–40 [PubMed] [Google Scholar]
- Fisher LW, Torchia DA, Fohr B, Young MF, Fedarko NS. 2001. Flexible structures of SIBLING proteins, bone sialoprotein, and osteopontin. Biochem Biophys Res Commun. 280:460–465 [DOI] [PubMed] [Google Scholar]
- Fitzgerald M, Chiego DJ, Jr, Heys DR. 1990. Autoradiographic analysis of odontoblast replacement following pulp exposure in primate teeth. Arch Oral Biol. 35:707–715 [DOI] [PubMed] [Google Scholar]
- George A, Bannon L, Sabsay B, Dillon JW, Malone J, Veis A, Jenkins NA, Gilbert DJ, Copeland NG, et al. 1996. The carboxyl-terminal domain of phosphophoryn contains unique extended triplet amino acid repeat sequences forming ordered carboxyl-phosphate interaction ridges that may be essential in the biomineralization process. J Biol Chem. 271:32869–32873 [DOI] [PubMed] [Google Scholar]
- Liu H, Li W, Shi S, Habelitz S, Gao C, Denbesten P. 2005. MEPE is downregulated as dental pulp stem cells differentiate. Arch Oral Biol. 50:923–928 [DOI] [PubMed] [Google Scholar]
- MacDougall M, Simmons D, Luan X, Nydegger J, Feng J, Gu TT. 1997. Dentin phosphoprotein and dentin sialoprotein are cleavage products expressed from a single transcript coded by a gene on human chromosome 4: dentin phosphoprotein DNA sequence determination. J Biol Chem. 272:835–842 [DOI] [PubMed] [Google Scholar]
- Miura M, Gronthos S, Zhao M, Lu B, Fisher LW, Robey PG, Shi S. 2003. SHED: stem cells from human exfoliated deciduous teeth. Proc Natl Acad Sci U S A. 100:5807–5812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashima M, Mizunuma K, Murakami T, Akamine A. 2002. Induction of dental pulp stem cell differentiation into odontoblasts by electroporation-mediated gene delivery of growth/differentiation factor 11 (Gdf11). Gene Ther. 9:814–818 [DOI] [PubMed] [Google Scholar]
- Ogbureke KU, Fisher LW. 2004. Expression of SIBLINGs and their partner MMPs in salivary glands. J Dent Res. 83:664–670 [DOI] [PubMed] [Google Scholar]
- Ogbureke KU, Fisher LW. 2005. Renal expression of SIBLING proteins and their partner matrix metalloproteinases (MMPs). Kidney Int. 68:155–166 [DOI] [PubMed] [Google Scholar]
- Ogbureke KU, Fisher LW. 2007. SIBLING expression patterns in duct epithelia reflect the degree of metabolic activity. J Histochem Cytochem. 55:403–409 [DOI] [PubMed] [Google Scholar]
- Ogbureke KU, Nikitakis NG, Warburton G, Ord RA, Sauk JJ, Waller JL, Fisher LW. 2007. Upregulation of SIBLING proteins and correlation with cognate MMP expression in oral cancer. Oral Oncol. 43:920–932 [DOI] [PubMed] [Google Scholar]
- Qin C, Brunn JC, Baba O, Wygant JN, McIntyre BW, Butler WT. 2003. Dentin sialoprotein isoforms: detection and characterization of a high molecular weight dentin sialoprotein. Eur J Oral Sci. 111:235–242 [DOI] [PubMed] [Google Scholar]
- Qin C, Brunn JC, Cadena E, Ridall A, Butler WT. 2003. Dentin sialoprotein in bone and dentin sialophosphoprotein gene expressed by osteoblasts. Connect Tissue Res. 44(Suppl 1):179–183 [PubMed] [Google Scholar]
- Qin C, Brunn JC, Cadena E, Ridall A, Tsujigiwa H, Nagatsuka H, Nagai N, Butler WT, et al. 2002. The expression of dentin sialophosphoprotein gene in bone. J Dent Res. 81:392–394 [DOI] [PubMed] [Google Scholar]
- Qin C, Brunn JC, Jones J, George A, Ramachandran A, Gorski JP, Butler WT. 2001. A comparative study of sialic acid-rich proteins in rat bone and dentin. Eur J Oral Sci. 109:133–141 [DOI] [PubMed] [Google Scholar]
- Schor AM, Canfield AE, Sutton AB, Arciniegas E, Allen TD. 1995. Pericyte differentiation. Clin Orthop Relat Res. 313:81–91 [PubMed] [Google Scholar]
- Shi S, Gronthos S. 2003. Perivascular niche of postnatal mesenchymal stem cells in human bone marrow and dental pulp. J Bone Miner Res. 18:696–704 [DOI] [PubMed] [Google Scholar]
- Sreenath T, Thyagarajan T, Hall B, Longenecker G, D’Souza R, Hong S, Wright JT, MacDougall M, Sauk J, Kulkarni AB, et al. 2003. Dentin sialophosphoprotein knockout mouse teeth display widened predentin zone and develop defective dentin mineralization similar to human dentinogenesis imperfecta type III. J Biol Chem. 278:24874–24880 [DOI] [PubMed] [Google Scholar]
- Sun Y, Ma S, Zhou J, Yamoah AK, Feng JQ, Hinton RJ, Qin C. 2010. Distribution of small integrin-binding ligand, N-linked glycoproteins (SIBLING) in the articular cartilage of the rat femoral head. J Histochem Cytochem. 58:1033–1043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terasawa M, Shimokawa R, Terashima T, Ohya K, Takagi Y, Shimokawa H. 2004. Expression of dentin matrix protein 1 (DMP1) in non-mineralized tissues. J Bone Miner Metab. 22:430–438 [DOI] [PubMed] [Google Scholar]
- Veis A, Perry A. 1967. The phosphoprotein of the dentin matrix. Biochemistry. 6:2409–2416 [DOI] [PubMed] [Google Scholar]
- Verdelis K, Ling Y, Sreenath T, Haruyama N, MacDougall M, van der Meulen MC, Lukashova L, Spevak L, Kulkarni AB, Boskey AL, et al. 2008. DSPP effects on in vivo bone mineralization. Bone. 43:983–990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao S, Yu C, Chou X, Yuan W, Wang Y, Bu L, Fu G, Qian M, Yang J, Shi Y, Hu L, Han B, Wang Z, Huang W, Liu J, Chen Z, Zhao G, Kong X, et al. 2001. Dentinogenesis imperfecta 1 with or without progressive hearing loss is associated with distinct mutations in DSPP. Nat Genet. 27:201–204 [DOI] [PubMed] [Google Scholar]
- Yamamura T. 1985. Differentiation of pulpal cells and inductive influences of various matrices with reference to pulpal wound healing. J Dent Res. 64:530–540 [DOI] [PubMed] [Google Scholar]
- Yu J, Deng Z, Shi J, Zhai H, Xie X, Zhuang X, Li Y, Jin Y. 2005. Differentiation of dental pulp stem cells into regular-shaped dentin-pulp complex induced by tooth germ cell conditioned medium. Tissue Eng. 40:408–411 [DOI] [PubMed] [Google Scholar]
- Zhang J, Tan X, Contag CH, Lu Y, Guo D, Harris SE. 2002. Dissection of promoter control modules that direct Bmp4 expression in the epithelium-derived components of hair follicles. Biochem Biophys Res Commun. 293:1412–1419 [DOI] [PubMed] [Google Scholar]
- Zhang X, Zhao J, Li C, Gao S, Qiu C, Liu P, Wu G, Qiang B, Lo WH, Shen Y, et al. 2001. DSPP mutation in dentinogenesis imperfecta Shields type II. Nat Genet. 27:151–152 [DOI] [PubMed] [Google Scholar]
- Zhu Q, Sun Y, Prasad M, Wang X, Yamoah AK, Li Y, Feng J, et al. 2010. Glycosaminoglycan chain of dentin sialoprotein proteoglycan. J Dent Res. 89:808–812 [DOI] [PMC free article] [PubMed] [Google Scholar]