Abstract
Updated classification of urothelial cell cancer differentiates low-grade and high-grade cancers, which determines potential clinical outcome. Substantial interobserver variability necessitates new biomarkers to ensure classification. Claudins’ specific expression pattern characterizes normal tissues, different tumor types, and defined grades of tumor differentiation. The aim of this study was to examine the expression pattern of claudins and proliferation marker Ki-67 in low-grade and high-grade urothelial cell cancers compared with independent control samples of non-tumorous urothelium, as well as to reveal the predictive usefulness of claudins. The expression of claudins-1, -2, -3, -4, -5, -7, and -10 and Ki-67 was studied with quantitative immunohistochemistry and real-time RT-PCR with relative quantification in 103 samples: 86 urothelial cell cancers (27 low grade, 59 high grade) and 17 non-tumorous urothelia. Results were analyzed regarding overall survival and recurrence-free period as well. High-grade tumors overall showed significantly higher claudin-4 and Ki-67 and significantly lower claudin-7 expression when compared with low-grade ones. High-grade tumors revealed significantly shorter overall survival in Kaplan-Meier analysis. Claudin-4, claudin-7, and Ki-67 might be used as potential markers to differentiate low-grade and high-grade urothelial cell cancers, thereby possibly enhancing accuracy of pathological diagnosis and adding further information to clinical outcome.
Keywords: claudin, tight junction, transitional cell carcinoma, urinary bladder cancer
The assessment of pathological grade and stage is still the basic diagnostic method of urothelial cell cancer (UCC) to determine prognosis and treatment (Babjuk et al. 2008; Stenzl et al. 2009). Updated histological classification of UCCs (Damjanov and Mikuz 2007) separates papillary urinary neoplasms of low malignant potential (PUNLMP) into low-grade (LG) and high-grade (HG) carcinoma groups. HG tumors more often become muscle invasive than do LG ones (Heney 1992), and patients have shorter progression-free period and decreased overall survival (Kaufman et al. 2009). Despite the well-defined histologic criteria, there is high interobserver variability in the interpretation of the 2004 WHO classification: almost 30% of UCCs are overgraded. The major variances and difficulties are in the separation of the previous grade 2 group into low- and high-grade groups, whereby pathologists often have a tendency to overgrade and interobserver variability is significant (Chen et al. 2008).
The data on new molecular markers in prognostication and in grading of UCCs are controversial. It is unlikely that a single marker would be able to overcome the predictive strength of grade and stage, but combined independent biomarkers might ensure the accuracy of classification. Consequently, prediction value will be increased as well (Bryan et al. 2010). There is an ongoing effort to unravel new sets of markers enabling safe identification of high-risk patients, such as cytokeratin profiling on squamous cell differentiation in bladder cancer (Gaisa et al. 2010). Claudins (CLDNs) are the major transmembrane components of tight junctions (Lal-Nag and Morin 2009). These dynamic structures have a role in the regulation of paracellular diffusion and maintainance of cellular polarity (Steed et al. 2010). Furthermore, CLDNs are essential for the separation of extracellular compartments, especially in the uroepithelium, where separation of urine is essential to prevent tissue damage (Morita et al. 1999). Moreover, they might influence signaling pathways (Balda and Matter 2009; Lal-Nag and Morin 2009). The expression pattern of 24 different types of CLDNs—both qualitatively and quantitatively—characterizes and usually differentiates normal and tumorous epithelia. Organ-specific changes of CLDN expression pattern cannot be predicted based on the presumption that malignant tumors tend to lose their intercellular connections during tumor progression (Martin and Jiang 2009). Certain CLDNs show decreased expression (Miyamoto et al. 2008; Chao et al. 2009), whereas others are overexpressed (Lodi et al. 2006; Sobel et al. 2006; Lanigan et al. 2009). Data on the CLDN expression profile in different premalignant and malignant alterations suggest that CLDNs might serve as diagnostic and prognostic markers (Paschoud et al. 2007; Lechpammer et al. 2008; Szabo et al. 2009). Furthermore, they might be targets or dockers for future therapy (Saeki et al. 2010).
There are only limited data on CLDN expression in human urothelial carcinoma: CLDN-1, -3, and -4 protein expressions positively correlated with advanced stage in upper urinary tract urothelial carcinoma (Nakanishi et al. 2008). However, CLDN-4 expression was found significantly elevated in well-differentiated UCCs while being decreased in poorly differentiated ones (Boireau et al. 2007). Our recent work established that high claudin-1 protein expression might help to differentiate inverted urothelial papilloma (IUP) from urothelial papillomas (UPs), PUNLMPs, and LG-UCCs. Furthermore, high claudin-4 expression may determine an unfavorable clinical course of LG-UCCs, whereas high claudin-1 expression in PUNLMPs has been associated with markedly better clinical outcome (Szekely et al. 2011).
Our goal was to study the mRNA and protein expressions of CLDNs together with proliferation marker kinase inhibitor 67 (Ki-67) protein expression in non-tumorous uroepithelium as compared with different stages and grades of urothelial carcinoma, with special emphasis on comparison of LG and HG lesions to reveal diagnostic help. Furthermore, our aim was to explore a possible correlation between specific CLDN expression and survival and recurrence-free period.
Material and Methods
Subjects and Samples of Study
A total of 103 human surgically resected, formalin-fixed, paraffin-embedded tissue blocks (86 UCCs; 17 non-tumorous urothelia) were studied with the permission of the Regional Ethical Committee of the Semmelweis University. None of the control cases had any neoplastic urinary bladder lesions earlier. Non-tumorous cases were divided into non-inflammatory (CT, 7 cases) and inflammatory groups (ICT, 10 cases) (Table 1).
Table 1.
Non-Tumorous Samples (n=17)
| Histological Diagnosis |
||
|---|---|---|
| Cause of the Resection | CT (7) | ICT (10) |
| Recurrent infection | 0 | 2 |
| Hematuria | 0 | 6 |
| BPH | 7 | 0 |
| Bladder stone | 0 | 1 |
| Vesicocutaneuos fistule | 0 | 1 |
BPH, benign prostatic hyperplasia; CT, control samples without inflammation; ICT, control samples with inflammation.
The tumor samples were classified according to the latest tumor classification of WHO (2004) (Damjanov and Mikuz 2007). The mean age of the patients was 66.5 years (range, 43-91 years), and the male/female ratio was 2.9:1. Overall survival (OS) intervals were determined as the time period from initial diagnosis to the time of death; recurrence-free survival (RFS) intervals were determined from initial diagnosis to the time of recurrence proved by histology. The mean follow-up period was 45.96 months (range, 2-120 months) (Table 2).
Table 2.
UCC Samples
| Pathological and Clinical Data of Patients | UCCs without T2 (TaT1) | T2 | LG | HG | HG (without T2) |
|---|---|---|---|---|---|
| Total no. (%) of 86 | 64 (74.0) | 22 (26.0) | 27 (31.0) | 59 (69.0) | 37 (43.0) |
| Low grade, No. (%) | 27/64 (42.0) | 0 | 0 | 0 | 0 |
| High grade, No. (%) | 37/64 (58.0) | 22/22 (100.0) | 0 | 0 | 0 |
| Smoker, No. (%) | 29/64 (45.0) | 12/22 (55.0) | 14/27 (52.0) | 27/59 (46.0) | 15/59 (25.0) |
| Sex, men: women (ratio) | 50:14 (3.57:1) | 14:8 (1.75:1) | 20:7 (2.85:1) | 44:15 (2.93:1) | 30:7 (4.3:1) |
| Age, y (range) | 65.38 (46-91) | 67.59 (43-91) | 62.89 (47-81) | 67.34 (43-91) | 67.18 (46-91) |
| Multifocality, No. (%) | 28/64 (44.0) | 6/22 (27.3) | 11/27 (41.0) | 23/59 (39.0) | 17/59 (29.0) |
| Solid growth pattern, No. (%) | 8/64 (12.5) | 9/22 (41.0) | 2/27 (7.4) | 15/59 (25.0) | 4/59 (7.0) |
| Intravesical chemotherapy, No. (%) | 14/64 (22.0) | 2/22 (9.0) | 7/27 (26.0) | 9/59 (15.0) | 7/59 (12.0) |
| Intravesical BCG, No. (%) | 21/64 (33.0) | 0 | 1/27 (4.0) | 20/59 (34.0) | 20/59 (34.0) |
| Systemic chemotherapy, No. (%) | 0 | 5/22 (22.7) | 0 | 5/59 (8.0) | 0 |
| Cystectomy, No. (%) | 2/64 (3) | 7/22 (32.0) | 0 | 9/59 (15.0) | 7/59 (12.0) |
| Tumor recurrence, No. (%) | 20/64 (31.0) | 3/22 (14.0) | 13/27 (48.0) | 10/59 (17.0) | 7/59 (12.0) |
| Progression, No. (%) | 3/64 (5.0) | 0 | 0 | 3/59 (5.0) | 0 |
| Recurrence-free survival, mo (range) | 43.98 (4-110) | 29.75 (4-55) | 38.04 (4-79) | 47.03 (4-110) | 45.39 (4-110) |
| Overall survival, mo (range) | 54.62 (10-120) | 28.27 (4-54) | 57.75 (10-120) | 45.5 (4-110) | 49.15 (4-110) |
| Death, No. | 12 (10a) | 19 (2a) | 2 (2a) | 29 (10a) | 10 (8a) |
BCG, bacillus Calmette-Guérin; LG, low grade; HG, high grade; UCC, urothelial cell cancer. Tumor recurrence: tumor relapse diagnosed after surgery (in case there was a second transurethral resection after the primary operation and the histological diagnosis differing from the reviewed diagnosis was accepted as final). Progression: if a TaT1 tumor progressed into T2. Multifocality: if the tumor was localized to more than one locus. Solid growth pattern: the surface of the tumor was solid (not papillary).
Death not due to UCC.
Early postoperative intravesical chemotherapy (early IVI; within 6 hours after surgery) was administered if there was no suspicion of bladder perforation or postoperative bleeding (five cases: two LG and three HG). If there was any contraindication of early IVI and no contraindication of late IVI and the patient accepted the therapy, late-administrated IVI was performed. Bacillus Calmette-Guérin (BCG) therapy according to guidelines of the European Association of Urology (EAU) was administrated to patients with pT1G3, pTaG3, CIS, or recurrent/therapy-resistant pT1G1-2 according to the following protocol: BCG therapy for 5 weeks (one dose per week) followed by maintenance therapy for at least 1 year (Babjuk et al. 2008).
Radical cystectomy was performed if the patient agreed and there was no contraindication (7/22 T2 cases; Table 2). Five of the 22 T2 patients received systemic chemotherapy, whereas the remaining 10 patients denied any of the available therapeutic options. Contrary to the above, none of the low-grade tumors was operated radically.
Histology and Immunohistochemistry
Tissues were fixed in 10% neutral buffered formalin for 24 hr, followed by paraffin embedding, and then were cut and stained with hematoxylin-eosin to establish the diagnosis.
Paraffin-embedded, 3- to 4-µm-thick sections were used for immunohistochemistry. After deparaffination, slides were washed in PBS (pH 7.4), then microwave oven treated for 3 min with 850 W followed by 170 W for 30 min with antigen retrieval solution (Target Retrieval Solution; DAKO, Glostrup, Denmark). Reactions were carried out in Ventana ES automatic immunostainer (Ventana Medical Systems, Inc.; Tucson, AZ) using avidin-biotin peroxidase technique and diaminobenzidine as chromogen according to the manufacturer’s protocol (iView DAB Detection Kit; Ventana Medical Systems).
Antibodies, dilutions, and positive controls recommended by the manufacturer are listed in Table 3. For negative controls, the specific antibody was omitted and antibody diluent was used alone. The reactions were processed and revealed no signal.
Table 3.
Positive Controls and Antibody Dilutions of CLDNs and Ki-67 for Immunohistochemistry
| Antibody | Dilution | Positive Control | Manufacturer | Antibody Type |
|---|---|---|---|---|
| CLDN-1 | 1:80 | Skin | Zymed | Rabbit polyclonal |
| CLDN-2 | 1:80 | Colon | Zymed | Mouse monoclonal |
| CLDN-3 | 1:80 | Colon | Zymed | Rabbit polyclonal |
| CLDN-4 | 1:100 | Colon | Zymed | Mouse monoclonal |
| CLDN-5 | 1:120 | Endothelium | Zymed | Mouse monoclonal |
| CLDN-7 | 1:100 | Breast | Zymed | Rabbit polyclonal |
| CLDN-10 | 1:60 | Kidney | Zymed | Rabbit polyclonal |
| Ki-67 | 1:100 | Tonsil | DAKO | Mouse monoclonal |
Zymed, South San Francisco, CA; DAKO, Glostrup, Denmark.
Quantitative Real-Time PCR Analysis (Primers and PCR Reaction)
RNA Isolation
Total RNA from formalin-fixed, paraffin-embedded tissues (70 UCCs, 4 CTs, and 4 ICTs) were isolated with the High Pure RNA Paraffin Kit (Roche; Indianapolis, IN) according to the manufacturer’s protocol. Samples were macrodissected to select non-tumorous epithelium or tumor tissue. Proteinase-K digestion time was 16 hr for each sample at 55C.
Reverse Transcription of RNA
In total, 2 µg of total RNA was reverse transcribed in 20 µl mix of High Capacity RNA-to-cDNA Kit (Applied Biosystems [ABI]; Foster City, CA) according to the manufacturer’s protocol.
Real-Time Quantitative RT-PCR
Real-time PCR reactions were carried out in duplicates for CLDNs-1, -2, -3, -4, -5, -7, and -10 and β-actin (reference gene) applying 100 ng of cDNA template in 25 µl total reaction volume of SYBR Green PCR Master Mix (ABI; AB4309155). The primer sequences are listed in Table 4. Real-time PCR conditions were as follows: 2 min at 95C for initial denaturing and then 40 cycles at 95C for 20 sec, 60C for 30 sec, and 72C for 1 min, using the ABI Prism 7000 sequence detection system (Applied Biosystems). Data analysis was performed by E = 2ΔCT (ΔCT = CT-ReferenceGene – CT-TargetGene), using the average value of the β-actin reaction as the reference gene for relative quantification.
Table 4.
Primer Sequences of Claudins and β-Actin
| Gene | Forward | Reverse |
|---|---|---|
| Claudin-1 | gtg cga tat ttc ttc ttg cag gtc | ttc gta cct ggc att gac tgg |
| Claudin-2 | ctc cc tgg cct gca tta tct c | acc tgc tac cgc cac tct gt |
| Claudin-3 | ctg ctc tgc tgc tcg tgt cc | tta gac gta gtc ctt gcg gtc gta g |
| Claudin-4 | ggc tgc ttt gct gca act gtc | gag ccg tgg cac ctt ac acg |
| Claudin-5 | ttc ctg aag tgg tgt cac ctg aac | tgg cag ctc tca atc ttc aca g |
| Claudin-7 | cat cgt gg cag gtc ttg cc | gat ggc agg gcc aaa ctc ata c |
| Claudin-10 | tgg atg ttc cct ata tgc aaa caa | aaa cag agc ggc tcc taa ttc a |
| β-actin | cct ggc acc cag cac aat | ggg ccg gac tcg tca tac |
Morphometry and Statistical Analysis
Immunhistochemical Analysis: Morphometry
Ten non-overlapping photographs were taken from each slide (60× objective, Olympus BX50 microscope; Olympus Corporation, Tokyo, Japan), then evaluated quantitatively with Leica QWin V3 morphometrical software (Leica Microsystems Imaging Solutions Ltd., Cambridge, UK).
Ki-67 Evaluation
Positive cells/total cells were calculated in 10 non-overlapping fields (60× objective, Olympus BX50 microscope) from each slide.
Statistical Analysis
Variables were compared with the Mann-Whitney U test if the sample showed non-Gaussian distribution. Categorical data were compared using χ2 or Fisher’s exact tests. OS and RFS analyses were done using the Kaplan-Meier method. Comparison between survival functions for different strata was assessed with the log-rank statistic. For χ2 or Fisher’s exact test and Kaplan-Meier analysis of Ki-67 and CLDN expressions, UCCs were divided into low (under the median) and high (over the median) expression groups. Differences were considered significant when p<0.05. All statistical analyses were done using Statistica 8.0 (StatSoft, Inc.; Tulsa, OK) software program.
Results
Immunohistochemistry and qRT-PCR Analysis in Non-tumorous Epithelium
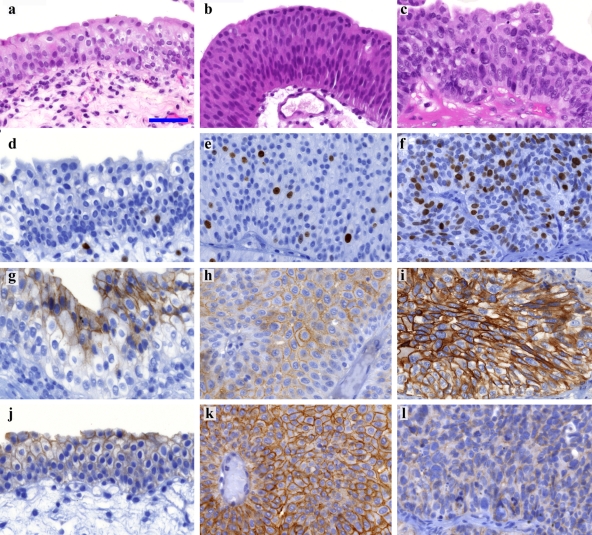
CLDN-1 membrane positivity was mainly found in basal layers of the non-tumorous urothelium. CLDN-4 and -7 membrane positivity was detected in the upper layers, declining toward basal layers of the epithelium, but in some cases, CLDN-7 membrane positivity was found in the whole thickness of the epithelium (Fig. 1g,j). CLDN-3 and -5 were weakly detected in the upper layers in a few cases, mainly at the plasma membrane of umbrella cells. Perimembranous-cytoplasmic granular CLDN-2 expression was detected in the basal and parabasal epithelium. CLDN-10 was not detected. Scattered nuclear Ki-67 positivity was observed (Fig. 1d).
Figure 1.
Claudin-4, -7, and Ki-67 expression in normal urothelium, low- and high-grade urothelial cell cancer (UCC). (a, d, g, j) H&E, Ki-67, CLDN-4, and CLDN-7 in normal urothelium, respectively. (b, e, h, k) H&E, Ki-67, CLDN-4, and CLDN-7 in low-grade UCC, respectively. (c, f, i, l) H&E, Ki-67, CLDN-4, and CLDN-7 in high-grade UCC Ki-67 expression in low-grade UCC, respectively. Photographs were taken with the same magnification; scale bar: 50 µm. High-grade tumors revealed significantly increased (f) Ki-67 (p<0.0001) and (i) CLDN-4 (p=0.037) and significantly decreased (l) CLDN-7 (p<0.0001) protein expression levels in comparison to low-grade tumors, respectively (e, h, k). Only linear adjustments of brightness/contrast and color balance were applied for whole images to create uniform-looking pictures for the composite image.
Inflamed control samples (ICT) revealed significantly higher CLDN-2 (p=0.007) and CLDN-4 (p=0.039) protein levels and CLDN-7 (p=0.047) mRNA expression in comparison to non-inflamed control samples (Table 5). In ICTs CLDN-2 positivity appeared in the umbrella cells as well.
Table 5.
Comparison of Claudin Protein, mRNA, and Ki-67 Protein Expression in Control Samples and UCCs
| CT vs ICT | CT vs UCC | ICT vs UCC | LG vs HGa | LG vs HGb | |
|---|---|---|---|---|---|
| CLDN-1 IHC | ↑(0.386) | ↓(0.014) | ↓(0.001) | ↓(0.078) | ↓(0.035) |
| CLDN-2 IHC | ↑(0.007) | ↑(0.489) | ↓(0.001) | ↑(0.913) | ↑(0.337) |
| CLDN-4 IHC | ↑(0.039) | ↑(0.125) | ↓(0.082) | ↑(0.037) | ↑(0.011) |
| CLDN-7 IHC | ↑(0.791) | ↑(0.115) | ↓(0.062) | ↓(<0.001) | ↓(0.001) |
| CLDN-1 mRNA | ↓(0.624) | ↑(0.101) | ↑(0.117) | ↓(0.025) | ↓(0.017) |
| CLDN-2 mRNA | ↑(0.624) | ↑(0.037) | ↑(0.727) | ↑(0.002) | ↑(0.009) |
| CLDN-4 mRNA | ↓(0.624) | ↑(0.092) | ↑(0.034) | ↑(<0.001) | ↑(<0.001) |
| CLDN-7 mRNA | ↑(0.047) | ↑(0.001) | ↑(0.043) | ↓(0.720) | ↓(0.819) |
| Ki-67 | ↓(0.245) | ↑(0.076) | ↑(0.196) | ↑(<0.001) | ↑(<0.001) |
IHC, immunohistochemistry; CT, non-inflamed control samples; ICT, inflamed control samples; LG, low grade; HG, high grade; UCC, urothelial cell cancer. Significant changes are marked in bold; p-values are in parentheses. The Mann-Whitney U test was used to calculate statistics between two independent groups (TaT1 vs T2; LG vs HG). The non-underlined group is compared to the underlined one (reference). ↑ groups not underlined show higher expression compared with the reference group. ↓ groups not underlined show decreased expression compared with the reference group.
Including T2.
Without T2.
Immunohistochemistry and Real-Time qRT-PCR Analysis in UCCs
CLDN-1, -2, -4, and -7 proteins were detectable in most cases. CLDNs-3 and -5 were found only in few, mostly well-differentiated UCCs. CLDN-10 was not detected. The vertical distribution of CLND-1, -2, and -7 proteins within UCCs was similar to the control epithelium, whereas CLDN-4 positivity was mainly detected in the entire width of tumors.
UCCs versus Non-inflamed Control Samples (CT)
Tumorous samples showed decreased CLDN-1 (p=0.01) and increased CLDN-2, -4, and -7 and Ki-67 protein expressions, whereas CLDN-1, -2, -4, and -7 mRNA levels were found increased in comparison to CT (Table 5).
Low-Grade versus High-Grade UCCs
LG and HG comparisons resulted in similar significant changes, both including T2 UCCs into the HG group (*) or excluding T2 UCCs from the comparison (**) (Table 5).
HG tumors revealed significantly higher protein expression of CLDN-4 (*p=0.037; **p=0.011; Fig. 1h,i) and Ki-67 (*p<0.001; **p<0.001; Fig. 1e,f) in parallel with significantly lower protein expression of CLDN-1 (**p<0.035) and CLDN-7 (*p<0.001; **p=0.001; Fig. 1k,l) in comparison to LG UCCs. Decreased CLDN-1 (*p=0.025; **p=0.017) and elevated CLDN-2 (*p=0.002; **p=0.009) and CLDN-4 (*p<0.001; **p<0.001) mRNA levels were detected in HG in comparison to LG tumors (Table 5). CLDN-4 protein expression characterized HG cases in the entire width of the tumors contrary to LG cases showing vertical distribution similar to the normal pattern (χ2, *p<0.001; **p<0.001).
Patient Follow-ups
None of the LG patients (0/27) whereas 19 of the HG bladder cancer patients (19/59) died of UCC (p<0.01; Fisher’s exact test). Seventeen of these HG patients had T2 stage disease, whereas 2 patients had T1 disease.
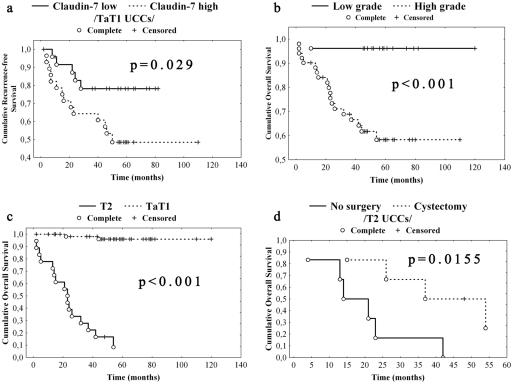
Recurrence appeared in 13 (48%) LG and 10 (17%) HG cases. There was no significant difference in the mean RFS time between LG and HG UCCs (38 months and 45 months, respectively). There was no significant association between RFS and Ki-67 expressions. However, non-muscle invasive UCCs expressing CLDN-7 over the median revealed shorter RFS in comparison to UCCs expressing CLDN-7 under the median (Kaplan-Meier analysis, p=0.029; Fig. 2a).
Figure 2.
Survival analysis of urothelial cell cancers (UCCs) regarding grade, stage, and claudin-7 expression. (a) Low expression (under the median) of claudin-7 was associated with significantly longer recurrence-free survival (RFS) in comparison to high (over the median) expression. (b) High-grade (HG) tumors revealed significantly shorter overall survival (OS) when compared to low-grade (LG) ones. (c) T2 tumors were associated with significantly shorter OS in comparison to Ta/T1 tumors. (d) T2 patients treated with cystectomy showed significantly longer OS compared with those T2 patients not treated with radical surgical resection. Complete: death/recurrence occured. Censored: follow-up was terminated.
Three patients from the HG TaT1 group (two died of UCC) whereas none from the LG TaT1 group progressed into the muscle-invasive stage.
HG tumors (including T2 UCCs) were associated with significantly shorter OS when compared to LG ones (Kaplan-Meier analysis, p<0.001; Fig. 2b). In concordance with international data, T2 tumors (all of them were HG) of our study were associated with shorter OS in comparison to Ta/T1 tumors (Kaplan-Meier analysis, p<0.001; Fig. 2c). Similarly, T2 patients treated with cystectomy had longer OS compared with those T2 patients not treated with radical surgical resection (14 vs 48 months; Kaplan-Meier analysis, p=0.0155; Fig. 2d).
Multivariate analysis, including LG-HG classification, was not done because none of the patients died in the LG group, whereas 19 died from the HG group (17 of 19 were T2).
Discussion
A high rate of interobserver variability underscores the importance of finding new markers, which can help in differentiating LG from HG urothelial carcinomas. Fifty percent of newly diagnosed UCC cases will recur, and 10% to 20% will progress to a higher grade and stage, which highlights the ultimate need for new markers of recurrence prediction and the necessity of more or less agressive therapy. Altered expression of CLDNs proved to have value in differential diagnostics and also in the prognostic/predictive assessment of many human tumors.
Our data showed significantly elevated CLDN-4 and decreased CLDN-7 protein expressions in HG UCCs in comparison to LG ones. mRNA expression of CLDN-4 proved to be significantly higher in high-grade UCCs in comparison to low-grade ones as well. Although the lower CLDN-7 mRNA expression in high-grade UCCs was detected, the difference did not prove to be significant. Even though we did use macrodissection of tumor tissue for RNA isolation, the possible and probable explanation for the discrepancy in the significance between mRNA and IHC expressional data is not only hidden in the posttranscriptional modification but rather related to the tissue homogenization required for RNA isolation. It means that the ratio between normal and tumor tissue might change from one sample to the other.
These features were independent from the presence of muscle invasion because LG and HG comparisons resulted in similar significant changes, both including or excluding T2 UCCs into the HG group. Furthermore, high protein expression of CLDN-7 but not Ki-67 expression was associated with shorter recurrence-free survival in TaT1 UCCs. Interestingly, claudin-7 high and low expressor groups did not reveal any statistical difference regarding postoperative treatment such as BCG or intravesical chemotherapy (χ2 test). Therefore, claudin-7 expression was the only variable related to altered recurrence-free survival in non-muscle invasive bladder cancer. Peculiarly, RFS proved to be longer in the high-grade group compared with the low-grade group. Recent studies also observed similar trends. The recurrence rate of low-grade bladder cancer was more than 50% (Miyamoto et al. 2010) but only 35% in T1 high-grade bladder cancer after intravesical BCG treatment in a long-term follow-up study (Kakiashvili et al. 2011). On the contrary, May et al. (2010) detected a shorter RFS and higher recurrence rate of HG papillary tumors in comparison to LG ones. In our set of data, the low-grade tumors revealed recurrence in 48%, whereas high-grade tumors had a 17% recurrence. The higher recurrence rate might have influence on RFS. Furthermore, the longer RFS in the high-grade group is probably related to the applied BCG therapy (20 treatments in the high-grade cases and only 1 in the low-grade tumor cases), and it is well known that BCG treatment delays recurrence (Jacobs et al. 2010; Kakiashvili et al. 2011).
Boireau et al. (2007) found overexpressed CLDN-4 in well-differentiated superficial UCCs (Ta) when compared with neighboring non-tumorous epithelium, whereas 60% of poorly differentiated T1/T2 tumors revealed decreased CLDN-4 expression compared with the surrounding epithelium. This set of data did not concern CLDN expression of different UCC subgroups (grade) and used only the surrounding non-tumorous epithelium as control. The field cancerization theory in urothelium thereby questions the comparative normal value of nearby situated non-tumorous epithelium because this might already contain genetic changes possibly playing role in urothelial carcinogenesis (Jones et al. 2005).
Dysregulated CLDN-7 expression—higher or lower as compared to overlying normal epithelium—of squamous cell carcinoma of the tongue tended to be associated with lower survival (Bello et al. 2008). Lost or decreased expression of CLDN-7 is frequently found in the invasive squamous cell carcinoma (SCC) of the esophagus, and knockdown expression of CLDN-7 in SCC cell lines led to enhanced invasion (Lioni et al. 2007). On the contrary, strong CLDN-7 expression is associated with poor prognosis in ovarian carcinomas (Kleinberg et al. 2008). CLDN-4 may be useful as a potential marker and therapeutic target for prostate cancer metastases as well as cholangiocellular and pancreatic carcinomas (Nichols et al. 2004; Lodi et al. 2006; Szasz et al. 2010). CLDN-1 acts as a metastasis suppressor in lung carcinomas (Chao et al. 2009).
We found CLDN-1, -3, -4, -5, and -7 expressions similar to the upper urinary tract tight-junction protein expression profile (Nakanishi et al. 2008) as well as that of the normal bladder epithelium (Varley et al. 2006). Control cases featuring inflammatory changes in the urothelium showed altered CLDN expression; CLDN-2 and -4 protein expressions were significantly elevated in these samples in comparison to control samples, revealing no inflammatory changes, which raises their possible association with urinary inflammation. Our working group found that high claudin-4 expression in the case of low-grade papillary urothelial cancer but low claudin-1 expression in the case of PUNLMPs might determine poor clinical outcome, whereas these expressional changes are associated with shorter recurrence-free survival. On the contrary, low claudin-1 expression of PUNLMPs and high claudin-4 expression of LG-UCCs are associated with a markedly better clinical outcome. The claudin-1 expression analysis might help to differentiate IUP from UPs, PUNLMPs, and LG-UCCs because IUP reveals higher claudin-1 protein expression in comparison to the other entities (Szekely et al. 2011).
Many markers, such as Ki-67 (Santos et al. 2003) and p53 (Esrig et al. 1994), have been intensively investigated in recent years. However, none of the molecular markers showed superior diagnostic or predictive value in comparison to histology. Nevertheless, in agreement with our study, Ki-67 may serve as an adjacent marker in the separation of LG and HG UCCs (Chen et al. 2008). Ki-67 expression was found as an independent prognostic factor for time of relapse in patients treated with radical cystectomy, and expression of this marker may perfectuate the prognostication of non-invasive bladder cancers, according to our findings (Margulis et al. 2006). Santos et al. (2003) suggested that a high Ki-67 index together with grade, stage, and multifocality should be included as independent prognostic marker into the multivariate prognostic analysis for low-grade urothelial superficial papillary carcinomas. These authors detected a significant association between high Ki-67 expression and shorter clinical OS and RFS (Santos et al. 2003). In our study, Ki-67 was not independent from grade and stage. On the other hand, LG-HG classification was found to be an important factor in the prediction of clinical outcome because none of the LG patients but 19 from the HG cases (2 of them were TaT1) died of UCC.
Overall, pathological diagnosis and grading is the gold standard to estimate the outcome and planning of appropriate therapy. There is no absolute decisive single marker to differentiate LG and HG lesions. New markers are needed to ensure the classification (May et al. 2010). This is the first report of CLDN expression separating LG versus HG UCCs and providing further information on the clinical outcome of UCCs.
Conclusion
CLDN-4, CLDN-7, and Ki-67 might be used as potential markers to differentiate LG and HG UCCs. Although CLDN-4 and Ki-67 show significantly higher expression, that of CLDN-7 is significantly lower in HG UCCs in contrast to LG ones. On the whole, CLDNs might enhance the accuracy of pathological diagnosis and add further information to clinical outcome.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the authorship and/or publication of this article.
This work was supported by the grant of Hungarian Scientific Research Found (OTKA)# 75468, the grant of Jedlik Anyos National R&D Found (NKFT) # 07-A1/2007 and the grant of Department of Health Scientific Council (ETT) # 252/2009, TAMOP-4.2.1/B-09/1/KMR-2010-0001
Supplementary material for this article is available on the Journal of Histochemistry & Cytochemistry Web site at http://jhc.sagepub.com/supplemental.
References
- Babjuk M, Oosterlinck W, Sylvester R, Kaasinen E, Bohle A, Palou-Redorta J. 2008. EAU guidelines on non-muscle- invasive urothelial carcinoma of the bladder. Eur Urol. 54: 303–314 [DOI] [PubMed] [Google Scholar]
- Balda MS, Matter K. 2009. Tight junctions and the regulation of gene expression. Biochim Biophys Acta. 1788:761–767 [DOI] [PubMed] [Google Scholar]
- Bello IO, Vilen ST, Niinimaa A, Kantola S, Soini Y, Salo T. 2008. Expression of claudins 1, 4, 5, and 7 and occludin, and relationship with prognosis in squamous cell carcinoma of the tongue. Hum Pathol. 39:1212–1220 [DOI] [PubMed] [Google Scholar]
- Boireau S, Buchert M, Samuel MS, Pannequin J, Ryan JL, Choquet A, Chapuis H, Rebillard X, Avances C, Ernst M, et al. 2007. DNA-methylation-dependent alterations of claudin-4 expression in human bladder carcinoma. Carcinogenesis. 28:246–258 [DOI] [PubMed] [Google Scholar]
- Bryan RT, Zeegers MP, James ND, Wallace DM, Cheng KK. 2010. Biomarkers in bladder cancer. BJU Int. 105:608–613 [DOI] [PubMed] [Google Scholar]
- Chao YC, Pan SH, Yang SC, Yu SL, Che TF, Lin CW, Tsai MS, Chang GC, Wu CH, Wu YY, et al. 2009. Claudin-1 is a metastasis suppressor and correlates with clinical outcome in lung adenocarcinoma. Am J Respir Crit Care Med. 179:123–133 [DOI] [PubMed] [Google Scholar]
- Chen YB, Tu JJ, Kao J, Zhou XK, Chen YT. 2008. Survivin as a useful adjunct marker for the grading of papillary urothelial carcinoma. Arch Pathol Lab Med. 132:224–231 [DOI] [PubMed] [Google Scholar]
- Damjanov I, Mikuz G. 2007. Tumors of the Kidney and the Male Urogenital System. In: Damjanov I, Fan F, eds. Cancer Grading Manual. New York: Springer, 55-63 [Google Scholar]
- Esrig D, Elmajian D, Groshen S, Freeman JA, Stein JP, Chen SC, Nichols PW, Skinner DG, Jones PA, Cote RJ. 1994. Accumulation of nuclear p53 and tumor progression in bladder cancer. N Engl J Med. 331:1259–1264 [DOI] [PubMed] [Google Scholar]
- Gaisa N, Braunschweig T, Reimer N, Bornemann J, Eltze E, Siegert S, Toma M, Villa L, Hartmann A, Knuechel R. 2010. Different immunohistochemical and ultrastructural phenotypes of squamous differentiation in bladder cancer. Virchows Arch. 458:301–312 [DOI] [PubMed] [Google Scholar]
- Heney NM. 1992. Natural history of superficial bladder cancer: prognostic features and long-term disease course. Urol Clin North Am. 19:429–433 [PubMed] [Google Scholar]
- Jacobs BL, Lee CT, Montie JE. 2010. Bladder cancer in 2010: how far have we come? CA Cancer J Clin. 60:244–272 [DOI] [PubMed] [Google Scholar]
- Jones TD, Wang M, Eble JN, MacLennan GT, Lopez-Beltran A, Zhang S, Cocco A, Cheng L. 2005. Molecular evidence supporting field effect in urothelial carcinogenesis. Clin Cancer Res. 11:6512–6519 [DOI] [PubMed] [Google Scholar]
- Kakiashvili DM, van Rhijn BW, Trottier G, Jewett MA, Fleshner NE, Finelli A, Azuero J, Bangma CH, Vajpeyi R, Alkhateeb S, et al. 2011. Long-term follow-up of T1 high-grade bladder cancer after intravesical bacille Calmette-Guerin treatment. BJU Int. 107:540–546 [DOI] [PubMed] [Google Scholar]
- Kaufman DS, Shipley WU, Feldman AS. 2009. Bladder cancer. Lancet. 374:239–249 [DOI] [PubMed] [Google Scholar]
- Kleinberg L, Holth A, Trope CG, Reich R, Davidson B. 2008. Claudin upregulation in ovarian carcinoma effusions is associated with poor survival. Hum Pathol. 39:747–757 [DOI] [PubMed] [Google Scholar]
- Lal-Nag M, Morin PJ. 2009. The claudins. Genome Biol. 10:235.231–235.237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanigan F, McKiernan E, Brennan DJ, Hegarty S, Millikan RC, McBryan J, Jirstrom K, Landberg G, Martin F, Duffy MJ, et al. 2009. Increased claudin-4 expression is associated with poor prognosis and high tumour grade in breast cancer. Int J Cancer. 124:2088–2097 [DOI] [PubMed] [Google Scholar]
- Lechpammer M, Resnick MB, Sabo E, Yakirevich E, Greaves WO, Sciandra KT, Tavares R, Noble LC, DeLellis RA, Wang LJ. 2008. The diagnostic and prognostic utility of claudin expression in renal cell neoplasms. Mod Pathol. 21:1320–1329 [DOI] [PubMed] [Google Scholar]
- Lioni M, Brafford P, Andl C, Rustgi A, El Deiry W, Herlyn M, Smalley KS. 2007. Dysregulation of claudin-7 leads to loss of E-cadherin expression and the increased invasion of esophageal squamous cell carcinoma cells. Am J Pathol. 170:709–721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodi C, Szabo E, Holczbauer A, Batmunkh E, Szijarto A, Kupcsulik P, Kovalszky I, Paku S, Illyes G, Kiss A, et al. 2006. Claudin-4 differentiates biliary tract cancers from hepatocellular carcinomas. Mod Pathol. 19:460–469 [DOI] [PubMed] [Google Scholar]
- Margulis V, Shariat SF, Ashfaq R, Sagalowsky AI, Lotan Y. 2006. Ki-67 is an independent predictor of bladder cancer outcome in patients treated with radical cystectomy for organ-confined disease. Clin Cancer Res. 12:7369–7373 [DOI] [PubMed] [Google Scholar]
- Martin TA, Jiang WG. 2009. Loss of tight junction barrier function and its role in cancer metastasis. Biochim Biophys Acta. 1788:872–891 [DOI] [PubMed] [Google Scholar]
- May M, Brookman-Amissah S, Roigas J, Hartmann A, Storkel S, Kristiansen G, Gilfrich C, Borchardt R, Hoschke B, Kaufmann O, et al. 2010. Prognostic accuracy of individual uropathologists in noninvasive urinary bladder carcinoma: a multicentre study comparing the 1973 and 2004 World Health Organisation classifications. Eur Urol. 57:850–858 [DOI] [PubMed] [Google Scholar]
- Miyamoto H, Brimo F, Schultz L, Ye H, Miller JS, Fajardo DA, Lee TK, Epstein JI, Netto GJ. 2010. Low-grade papillary urothelial carcinoma of the urinary bladder: a clinicopathologic analysis of a post–World Health Organization/International Society of Urological Pathology classification cohort from a single academic center. Arch Pathol Lab Med. 134:1160–1163 [DOI] [PubMed] [Google Scholar]
- Miyamoto K, Kusumi T, Sato F, Kawasaki H, Shibata S, Ohashi M, Hakamada K, Sasaki M, Kijima H. 2008. Decreased expression of claudin-1 is correlated with recurrence status in esophageal squamous cell carcinoma. Biomed Res. 29:71–76 [DOI] [PubMed] [Google Scholar]
- Morita K, Furuse M, Fujimoto K, Tsukita S. 1999. Claudin multigene family encoding four-transmembrane domain protein components of tight junction strands. Proc Natl Acad Sci U S A. 96:511–516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakanishi K, Ogata S, Hiroi S, Tominaga S, Aida S, Kawai T. 2008. Expression of occludin and claudins 1, 3, 4, and 7 in urothelial carcinoma of the upper urinary tract. Am J Clin Pathol. 130:43–49 [DOI] [PubMed] [Google Scholar]
- Nichols LS, Ashfaq R, Iacobuzio-Donahue CA. 2004. Claudin 4 protein expression in primary and metastatic pancreatic cancer: support for use as a therapeutic target. Am J Clin Pathol. 121:226–230 [DOI] [PubMed] [Google Scholar]
- Paschoud S, Bongiovanni M, Pache J-C, Citi S. 2007. Claudin-1 and claudin-5 expression patterns differentiate lung squamous cell carcinomas from adenocarcinomas. Mod Pathol. 20:947–954 [DOI] [PubMed] [Google Scholar]
- Saeki R, Kondoh M, Kakutani H, Matsuhisa K, Takahashi A, Suzuki H, Kakamu Y, Watari A, Yagi K. 2010. A claudin-targeting molecule as an inhibitor of tumor metastasis. J Pharmacol Exp Ther. 334:576–582 [DOI] [PubMed] [Google Scholar]
- Santos L, Amaro T, Costa C, Pereira S, Bento MJ, Lopes P, Oliveira J, Criado B, Lopes C. 2003. Ki-67 index enhances the prognostic accuracy of the urothelial superficial bladder carcinoma risk group classification. Int J Cancer. 105:267–272 [DOI] [PubMed] [Google Scholar]
- Sobel G, Nemeth J, Kiss A, Lotz G, Szabo I, Udvarhelyi N, Schaff Z, Paska C. 2006. Claudin 1 differentiates endometrioid and serous papillary endometrial adenocarcinoma. Gynecol Oncol. 103:591–598 [DOI] [PubMed] [Google Scholar]
- Steed E, Balda MS, Matter K. 2010. Dynamics and functions of tight junctions. Trends Cell Biol. 20:142–149 [DOI] [PubMed] [Google Scholar]
- Stenzl A, Cowan NC, De Santis M, Jakse G, Kuczyk MA, Merseburger AS, Ribal MJ, Sherif A, Witjes JA. 2009. The Updated EAU Guidelines on Muscle-Invasive and Metastatic Bladder Cancer. Eur Urol. 55:815–825 [DOI] [PubMed] [Google Scholar]
- Szabo I, Kiss A, Schaff Z, Sobel G. 2009. Claudins as diagnostic and prognostic markers in gynecological cancer. Histol Histopathol. 24:1607–1615 [DOI] [PubMed] [Google Scholar]
- Szasz AM, Nyirady P, Majoros A, Szendroi A, Szucs M, Szekely E, Tokes AM, Romics I, Kulka J. 2010. Beta-catenin expression and claudin expression pattern as prognostic factors of prostatic cancer progression. BJU Int. 105:716–722 [DOI] [PubMed] [Google Scholar]
- Szekely E, Torzsok P, Riesz P, Korompay A, Fintha A, Szekely T, Lotz G, Nyirady P, Romics I, Timar J, Schaff Z, Kiss A. 2011. Expression of claudins and their prognostic significance in noninvasive urothelial neoplasms of the human urinary bladder. J Histochem Cytochem. 59:932-941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varley CL, Garthwaite MA, Cross W, Hinley J, Trejdosiewicz LK, Southgate J. 2006. PPARgamma-regulated tight junction development during human urothelial cytodifferentiation. J Cell Physiol. 208:407–417 [DOI] [PMC free article] [PubMed] [Google Scholar]