Abstract
Ezrin, which cross-links the cytoskeleton and plasma membrane, was involved in a wide variety of cellular processes. Here, to investigate the distribution of ezrin, tissue microarray technology was employed to perform immunohistochemical experiments on human embryos, fetuses at 4 to 22 weeks’ gestation, and adult tissue specimens. Results showed that ezrin was widely expressed in the gastrointestinal tract throughout the human developmental stages studied. At 6 to 8 weeks’ gestation, ezrin was found in epithelial cells, and this staining pattern was particularly pronounced in the brush border of mature absorptive cells lining the villus in later developmental stages and adult tissues. Throughout neural development, ezrin was only expressed in the neural tube at 4 weeks’ gestation. Ezrin was also detected in the cortex and medulla of the adrenal gland at 8 to 12 weeks’ gestation, whereas its immunoreactivity was increased from the zona glomerulosa through the zona reticularis and was essentially undetectable in the adrenal medulla of adult tissues. Significant expression of ezrin was seen throughout development in the kidney, spleen, lymph nodes, and cells of stratified squamous epithelia. However, ezrin was undetectable in lung, liver, heart, and blood vessels. These results demonstrated that the expression pattern of ezrin was highly time specific and tissue specific.
Keywords: ezrin expression, embryo, development, tissue microarray, immunohistochemistry
Ezrin, a membrane-cytoskeleton linker protein, is a member of the ERM (Ezrin-Radixin-Moesin) family and is well documented to participate in a wide variety of cellular processes such as cell adhesion, cell survival, cell motility, extracellular matrix interactions, cell-cell communication, apoptosis, development, carcinogenesis, and metastasis (Bretscher et al. 2002; Saotome et al. 2004; Fehon et al. 2010). Moreover, it has been reported to be involved in the regulation mechanism of signal transduction by binding tyrosine kinase (Gould et al. 1989).
Ezrin was first identified as a downstream target of epidermal growth factor (EGF) signaling and isolated from microvilli (Bretscher 1983; Pakkanen 1988; Hanzel et al. 1989). It contains a globular N-terminal domain similar to the N-terminus of the superfamily of band 4.1 proteins (Correas et al. 1986). Certain reports have described the expression of ezrin. In adult mouse tissues, ezrin was expressed at high levels in small intestine, stomach, lung, pancreas, and kidney; at intermediate levels in spleen, thymus, lymph nodes, and bone marrow; at very low levels in heart, brain, and testis; and undetectable in muscle and liver (Berryman et al. 1993; Shaffer et al. 2010). Expression of ezrin has also been detected in human transformed cells and carcinoma cells (Ohtani et al. 1999; Schindelmann et al. 2002; Kobayashi et al. 2003; Koon et al. 2004; Elliott et al. 2005; Yang et al. 2005; Craven et al. 2006; Madan et al. 2006; Kobel et al. 2006; Zhang Y et al. 2006; Zeng et al. 2006; Deng et al. 2007; Song et al. 2007; Wong et al. 2007; Bal et al. 2007; Torer et al. 2007; Gao et al. 2009; Xie et al. 2011) and in animal models during early fetal development (Gould et al. 1986; Barilá et al. 1995; Gimeno et al. 2004; Richter et al. 2004). However, little is known about the systematic expression of ezrin in human fetal and embryonic tissues.
In this study, to better understand the function of this important cytoskeletal regulator in human development, we demonstrated the expression of ezrin in human embryos, fetuses, and normal adult tissues by employing tissue microarray technology and immunohistochemical staining.
Materials and Methods
Cell Lines and Western Blotting
Esophageal cancer cell lines EC109 and KYSE180 were respectively maintained in 199 medium (Invitrogen, Carlsbad, CA) or DMEM (Invitrogen) containing 10% fetal calf serum. Cells were incubated at 37C in a humidified atmosphere of 5% CO2 in air. Western blotting was performed as described before (Xie et al. 2009). Ezrin/Radixin/Moesin Antibody (#3142; Cell Signaling Technology, Danvers, MA), Ezrin (C19) Antibody (sc6407; Santa Cruz Biotechnology, Santa Cruz, CA), and Ezrin (3C12) Antibody (MS-661-P; Lab Vision, Fremont, CA) were employed in Western blotting analysis.
Samples
Forty human samples were used consisting of 3 embryos, 11 fetuses, and 26 postnatal specimens, as summarized in Table 1. Intact embryos and fetuses were acquired from the Gynecology & Obstetrics Department of Shantou Central Hospital. Samples were collected from 14 healthy pregnant women undergoing elective termination of pregnancy at 4 to 22 weeks’ gestation. Specimens were immediately fixed in 4% buffered formalin solution, and subsequently, visible organs were embedded in paraffin blocks. Normal human tissue sections (from autopsy specimens) were acquired from the Department of Forensic Medicine of Shantou University Medical College. These specimens were collected from 2002 to 2005. The following tissues were collected: cerebellum, cerebrum, lung, trachea, heart, esophagus, stomach, large intestine, small intestine, liver, pancreas, kidney, spleen, thymus, lymph node, pituitary, adrenal gland, and thyroid gland. All research was carried out with the permission of local ethics committees.
Table 1.
Description of Specimens
| Human Stages | Number of Cases |
|---|---|
| Embryo | 3 |
| 4 weeks’ gestation | 1 |
| 5–8 weeks’ gestation | 2 |
| Fetus | 11 |
| 9–12 weeks’ gestation | 5 |
| 13–16 weeks’ gestation | 3 |
| 17–22 weeks’ gestation | 3 |
| Postnatal stages | 26 |
| P1: Neonate (<28 days) | 5 |
| P2: 1–19 years | 6 |
| P3: 20–39 years | 11 |
| P4: >40 years | 4 |
Construction of Tissue Microarrays
Representative regions of each tissue were selected from hematoxylin- and eosin-stained sections and marked on individual paraffin blocks. Samples were chosen from those specimens for which more tissues were available so that availability of tissue for correlative studies would not be compromised. Two tissue cores were obtained from each specimen measuring 1.8 mm in diameter and ranging in length from 1.0 to 3.0 mm depending on the depth of tissue in the donor block (Zhang FR et al. 2008). Each core was precisely arrayed into a new paraffin block. These microarrays were serially sectioned (4 µm), and stained with hematoxylin and eosin to verify tissue sampling and completeness. Unstained sections were baked overnight at 56C in preparation for immunohistochemistry.
Immunohistochemical Staining
Slides were dried in an oven (55–60C) before removing paraffin in several changes of xylene and hydrated through a series of graded alcohols to water, followed by incubation with 3% hydrogen peroxide for 10 min. For antigen retrieval, slides were autoclaved in 0.01 M citrate buffer (pH 6.0) at 120C for 3 min. Sections were then incubated with 10% normal goat serum in PBS for 15 min at room temperature to block nonspecific binding. After rinsing with PBS, slides were incubated overnight at 4C with mouse anti-human ezrin monoclonal antibody (1:400 dilution; Lab Vision). After rinsing with PBS, tissue sections were incubated for 15 min at room temperature with polymer helper solution (Polymer Detection System Kit, Golden Bridge International, Inc.; Mukilteo, WA), then rinsed with PBS. Slides were then incubated for 20 min at room temperature with streptavidin peroxidase-conjugated goat anti-mouse IgG (Polymer Detection System Kit, Golden Bridge International). Subsequently, slides were stained with 0.003% 3,3-diaminobenzide tetrahydrochloride and 0.005% hydrogen peroxide in 0.05 M Tris-HCl (pH 7.2) and counterstained with Mayer’s hematoxylin, dehydrated, and mounted. A metastatic esophageal carcinoma shown previously to have immunoreactivity (Zeng et al. 2006) was used as a positive control in each series of experiments. Negative controls were prepared by substituting PBS for primary antibody.
Ezrin-positive samples were defined as those showing brown signals in the cell membrane and cytosol. Immuno-reactivity was measured semiquantitatively using a scale from (−) to (+++), where (−) indicates no detectable immunostaining, (+) represents fewer than 25% of the cells are reactive, (++) represents 25% to 50% of the cells are reactive, and (+++) represents more than 50% of the cells are reactive. Finally, scales of (−), (+), (++), and (+++) were judged as negative, weakly positive, moderately positive, and strongly positive staining, respectively.
Results
First, we analyzed the specificity of the antibodies used in this study. Three ezrin antibodies were subjected to Western blotting to detect the expression of ezrin in two esophageal cancer cell lines. Results showed that two bands corresponding to ezrin/radixin (80 kDa) and moesin (75 kDa) were present when blotted by using antibody #3142 and antibody sc6407, suggesting that ezrin, radixin, and moesin were all expressed in the two cell lines (Fig. 1A,B). When blotted by using antibody MS-661-P, only one specific band (80 kDa, ezrin) was obtained, indicating that antibody MS-661-P was specific for ezrin detection, and it was selected for immunohistochemical staining (Fig. 1C).
Figure 1.
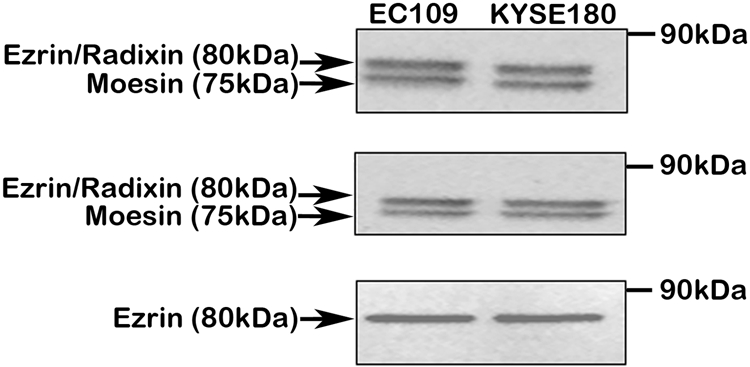
Western blotting analysis of extracts from two esophageal cancer cell lines by using different ezrin antibodies. (A) Blotted by using Ezrin/Radixin/Moesin Antibody (Cell Signaling Technology, #3142). This antibody has cross-reactivity with radixin and moesin and is recommended for detection of ezrin, radixin, and moesin. (B) Blotted by using Ezrin (C19) Antibody (Santa Cruz Biotechnology, sc6407). This antibody also has cross-reactivity with radixin and moesin and is recommended for detection of ezrin, radixin, and moesin. (C) Blotted by using Ezrin (3C12) Antibody (Lab Vision, #MS-661-P). This antibody is recommended for detection of ezrin.
Table 2 summarizes the findings for all tissues studied. Expression of ezrin was heterogeneous among postnatal tissues of different origin, but in a given tissue, no variations were observed in the intensity of ezrin in different postnatal stages. During embryonic and fetal development, however, differential expression of ezrin can be seen.
Table 2.
Distribution of Ezrin in Normal Human Tissues Determined by Immunostaining
| Findings |
||||||||
|---|---|---|---|---|---|---|---|---|
| Embryo |
Fetus |
Postnatal Stages |
||||||
| Organ/System | Tissue | 4–8 Weeks’ Gestation | 8–16 Weeks’ Gestation | 17–22 Weeks’ Gestation | P1: <28 Days | P2: 1–19 Years | P3: 20–39 Years | P4: >40 Years |
| Nervous system | ||||||||
| Nerve cell | ++ | − | − | − | − | − | − | |
| Nerve fiber | − | − | − | − | − | − | − | |
| Cardiovascular system | ||||||||
| Heart | − | − | − | − | − | − | ||
| Artery | − | − | − | − | − | − | ||
| Respiratory system | ||||||||
| Lung | − | − | − | − | − | − | − | |
| Trachea | + | + | − | − | − | − | ||
| Alimentary system | ||||||||
| Gastrointestinal tract | Epithelium mucosae | ++ | − | − | − | − | − | − |
| Glandular epithelium | ++ | ++ | ++ | ++ | ++ | ++ | ||
| Smooth muscle | ++ | ++ | − | − | − | − | ||
| Liver | − | − | − | − | − | − | − | |
| Pancreas | − | − | − | − | − | − | − | |
| Genitourinary system | ||||||||
| Kidney | Glomerulus | ++ | ++ | ++ | ++ | ++ | ++ | |
| Proximal tubule | +++ | +++ | +++ | +++ | +++ | +++ | ||
| Distal tubule | ++ | ++ | ++ | ++ | ++ | ++ | ||
| Collecting duct | ++ | ++ | ++ | ++ | ++ | ++ | ||
| Urothelium | − | − | − | − | ||||
| Prostate gland | − | − | − | − | ||||
| Endocrine system | ||||||||
| Adrenal gland | Zona glomerulosa | +++ | +++ | + | + | + | + | |
| Zona fasciculata | +++ | +++ | + | + | + | + | ||
| Zona reticularis | +++ | +++ | +++ | +++ | +++ | +++ | ||
| Medulla | +++ | +++ | − | − | − | − | ||
| Thyroid gland | − | − | − | − | − | |||
| Pituitary | − | − | − | − | − | |||
| Immune system | ||||||||
| Spleen | ++ | ++ | ++ | ++ | ++ | ++ | ||
| Lymphoid node | + | + | + | + | ||||
| Thymus | + | + | ||||||
| Skin | Basal layer | ++ | ++ | ++ | ++ | ++ | ++ | |
Immunoreactivity was measured semi-quantitatively using a scale from (−) to (+++): (−) no immunostaining, (+) less than 25% of the cells are reactive, (++) 25% to 50% of the cells are reactive, and (+++) more than 50% of the cells are reactive. Scales of (−), (+), (++), and (+++). (+) and (−) denote relative staining intensities as judged visually by microscopy. However, we cannot exclude the possibility that trace amounts of these proteins were undetectable in some cells judged negative.
Ezrin Expression in the Alimentary System
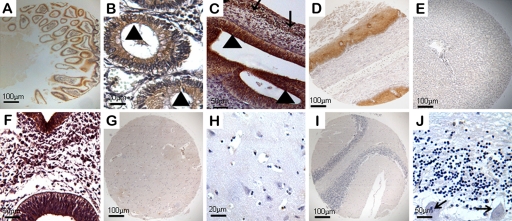
A very specific expression pattern of ezrin was observed in the gastrointestinal tract throughout human developmental stages. Ezrin protein was expressed in glandular epithelium and exhibited a pronounced immunoreactivity in the brush border of mature absorptive cells lining the villus (Figs. 2A and 1B). At 6 to 8 weeks’ gestation, ezrin expression was mainly found in epithelial cells of the gastrointestinal tract (Fig. 2C). Interestingly, this staining pattern was particularly pronounced in embryonic smooth muscle cells (Fig. 2C) and gastric parietal cells. Ezrin protein was also present in cells of esophageal squamous epithelium (Fig. 2D) but undetectable in liver (Fig. 2E).
Figure 2.
Expression of ezrin in the alimentary system and the nervous system of human embryonic, fetal, and normal adult tissues. Ezrin-positive reactivity was defined as those showing brown spotting. (A) Ezrin was positive in adult intestine (40×). (B) Ezrin protein was mainly expressed in the brush border (triangles) of the absorptive cells of the gastrointestinal tract (200×). (C) Immunoreactivity for ezrin was present in epithelial cells (triangles) and smooth muscle cells (arrows) of the gastrointestinal tract at 8 weeks’ gestation (200×). Ezrin was expressed in adult esophagus (D, 40×) but was undetectable in liver tissues (E, 40×). (F) Medullary epithelium and all nerve cells showed significant expression of ezrin at 4 weeks’ gestation (200×). (G–H) Ezrin was undetectable in the nervous system of fetuses and adults. (G) Cerebrum (40×). (H) Nerve fibers and cells of the cerebrum (200×). (I) Cerebellum (40×). (J) Cells of Purkinje cell layer (arrows), granular layer, and molecular layer of the cerebellum (200×).
Ezrin Expression in the Nervous System
In the development of the nervous system, expression of ezrin was detectable in early embryos and undetectable in later developmental stages. At 4 weeks’ gestation, neuroepithelium of the neural tube showed ezrin staining (Fig. 2F). However, in fetuses and adult tissues, no ezrin immunoreactivity was observed in nerve cells and fibers of the cerebrum (Fig. 2G,H) and in all cells of the Purkinje cell layer, granular layer, and molecular layer of the cerebellum (Fig. 2I,J).
Ezrin Expression in the Genitourinary System
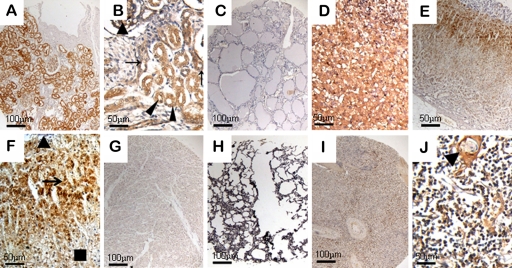
In kidney, epithelial cells of the acinus renis demonstrated strong ezrin staining throughout human development (Fig. 3A). Epithelial cells of the pars convoluta of the proximal tubule showed strong and uniform expression of ezrin, whereas epithelial cells of the distal tubule and collecting duct were moderately positive (Fig. 3B). Ezrin expressions in ladder epithelium and prostate tissues were all undetectable.
Figure 3.
Expression of ezrin in the genitourinary system, endocrine system, and other systems of human embryonic, fetal, and normal adult tissues. (A) Ezrin showed a strong staining in epithelial cells of the acinus renis (40×). (B) Ezrin was strongly positive in the acinus renis (triangles) and epithelial cells of the pars convoluta of the proximal tubule (arrowheads) and moderately positive in epithelial cells of the distal tubule (arrows) at 22 weeks’ gestation (200×). (C) Ezrin was undetectable in adult thyroid (40×). (D) At 12 weeks’ gestation, adrenal cortex and medulla showed strong reactivity (200×). (E) Ezrin was positive in the adult adrenal gland (40×). (F) In normal adult adrenal gland, ezrin immunoreactivity was exhibited in cells of the zona reticularis (arrow) and decreased in the zona glomerulosa (rectangle) and undetectable entirely in the medulla (triangle) (200×). Ezrin was undetectable in adult heart (G, 40×) and lung (H, 40×) but weakly positive in adult spleen (I, 40×). (J) Ezrin protein was present in subcapsular lymphocytes of the thymus and pronounced in Hassall’s corpuscle epithelial cells (triangle) (200×).
Ezrin Expression in the Endocrine System
Examination of endocrine organs presented no ezrin reactivity in follicular cells and C cells of the thyroid gland throughout human development (Fig. 3C). In the anterior pituitary, no staining for ezrin was shown in most cells except for the basophilic cells, which showed moderately positive staining. In the adrenal gland, the cortex and medulla showed a strong ezrin-positive reaction at 8 to 22 weeks’ gestation (Fig. 3D). However, in later stages of development, this protein in particular existed in cells of the inner layers of the cortex, was decreased in the peripheral cortex, and was undetectable in the medulla (Fig. 3E,F).
Ezrin Expression in Other Systems
In the cardiovascular system, ezrin was undetectable in the vascular endothelial cells and myocardial cells (Fig. 3G). In the respiratory system, an intriguing pattern of ezrin expression was seen in the epithelium of the fetal trachea, although no definite reactivity was seen in cells of the lung throughout human developmental stages (Fig. 3H). In lymph tissues, the lymphocytes in spleen stained strongly as well as small B cells and large B cell blasts (Fig. 3I). Ezrin protein was present in some lymphocytes in the red pulp and absent in white cell precursors, red cells, and endothelial cells. In thymus, ezrin was most prevalent in the subcapsular lymphocytes and Hassall’s corpuscle epithelial cells (Fig. 3J). Trophoblastic tissue decidua basalis cells in placenta were ezrin positive, but ezrin was undetectable in mesenchymal fibers.
Discussion
The present study is the first systematic demonstration of the immunoreactive presence of ezrin in human embryonic, fetal, and normal adult tissues. Results showed that expression of ezrin was heterogeneous among postnatal tissues of different origin, but in a given tissue, no variations were observed in the intensity of ezrin in different postnatal stages. In addition, during embryonic and fetal development, ezrin expression was diverse. These results suggest that ezrin expression is highly tissue specific and time specific.
Others have reported that high levels of ezrin transcripts were detected in the lung of adult mouse (Gould et al. 1986). Here we showed that no immunostaining was detectable in cells of the human lung. In the development of the nervous system, however, we found that ezrin expression was found only in the neuroepithelium of the neural tube in early embryos, which is consistent with some previous studies showing ezrin-positive expression in the roof plate of neural tubes in young and middle-gestation mouse embryos (Gimeno et al. 2004), in the fine filopodia of astrocytes of rat brain (Derouiche and Frotscher 2001; Grönholm et al. 2005), and within radial glia in prenatal human cerebrum (Johnson et al. 2002). In addition, ezrin was positive in the glandular epithelium and remarkably expressed in the brush border of mature absorptive cells lining the villus, which were extremely similar to the expression of ezrin in the enterocytes of the rat gastrointestinal tract (Barilá et al. 1995) and in the foregut (pharynx) of chick gastrula stage embryos (Richter et al. 2004). These findings suggest that ezrin is an evolutionarily conserved protein.
The differential expression of ezrin in human embryonic, fetal, and normal adult tissues suggests that it was adapted to tissue-specific and cell-specific throughout human development. Our results showed that in the normal cells of the liver, lung, heart, and blood vessels, ezrin expression is undetectable. However, ezrin expression is abundant in the spleen and kidney, and at the cellular level, ezrin expression is specific to lymphocytes and intestinal epithelium. In addition to a broad range of simple epithelial cell types, mature cells of the outermost stratified layers display a distribution of ezrin in skin and esophagus tissue (Zeng et al. 2006; Park et al. 2010; Xie et al. 2011). We also found that ezrin is expressed in stratified epithelia. A notable characteristic common to these normal, specialized ezrin-positive cells is that ezrin concentrates at the apical actin-rich surface structures such as microvilli, membrane ruffles, and protrusions. These results confirm some previous findings on the distribution of ezrin in differentiating intestinal epithelial cells (Bretscher 1989; Hanzel et al. 1991), suggesting that ezrin might play a physiological role in the assembly or stabilization of actin-containing cell surface structures.
Another point that should be highlighted is that expression of ezrin has been found to be time specific during human development. Ezrin immunoreactivity is positive in early embryo stages but undetectable during both later development and postnatal stages. For example, the nerve cells of the neural tube exhibit ezrin expression at 8 to 12 weeks’ gestation, whereas ezrin is undetectable in derivative tissues at later stages of development, such as cerebral tissue, the cerebellum, the medulla of the adrenal gland, and even adult nervous tissue. In addition, a pronounced expression pattern for ezrin has been detected throughout the gastrointestinal tract at early fetal stages, whereas cells of the muscular layer are essentially undetectable at postnatal stages, suggesting that ezrin might be more important in early development. These results are consistent with previous reports showing that ezrin is involved in certain early development events such as oocyte polarity, mitosis, epithelial morphogenesis, and integrity (Fehon et al. 2010; Larson et al. 2010). The precise mechanism for the upregulation of ezrin protein at early developmental stages needs further study.
Recently, reports have shown that ezrin was upregulated in several tumors, including prostatic intraepithelial neoplasma (Pang et al. 2004), pancreatic adenocarcinoma (Tokunou et al. 2000), breast carcinoma (Sarrió et al. 2006), and hepatocellular carcinoma (Zhang Y et al. 2006), and participated in the regulation of cell growth, invasiveness, adhesion, and metastasis (Xie et al. 2009; Khanna et al. 2004). In the present study, we found no immunoreactivity for ezrin in hepatocytes throughout developmental stages. Thus, the alteration of ezrin expression might play important roles in the progression of these cancers, and the expression of ezrin can be used as a tool for diagnosis. These findings also support that several aspects of ezrin expression have been evolutionary conserved across vertebrates (Ohtani et al. 2002; Pang et al. 2004; Zeng et al. 2006).
Acknowledgments
We are very grateful to Professor X. J. Yu from the Department Forensic Medicine of Shantou University Medical College for assistance in providing autopsy specimens.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the authorship and/or publication of this article.
The author(s) disclosed receipt of the following financial support for the research and/or authorship of this article: This work was supported by grants from the National High Technology Research and Development Program of China (No. 2006AA02A403), the Natural Science Foundation of China–Guangdong Joint Fund (No. U0932001), the National Natural Science Foundation of China (No. 30772485; No. 31000347), Guangdong Scientific Fund Key Items (No. 5104541), and Foundation for Distinguished Young Talents in Higher Education of Guangdong, China (LYM09080).
References
- Bal N, Yildirim S, Nursal TZ, Bolat F, Kayaselcuk F. 2007. Association of ezrin expression in intestinal and diffuse gastric carcinoma with clinicopathological parameters and tumor type. World J Gastroenterol. 13:3726–3729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barilá D, Murgia C, Nobili F, Perozzi G. 1995. Transcriptional regulation of the ezrin gene during rat intestinal development and epithelial differentiation. Biochim Biophys Acta. 1263:133–140 [DOI] [PubMed] [Google Scholar]
- Berryman M, Franck Z, Bretscher A. 1993. Ezrin is concentrated in the apical microvilli of a wide variety of epithelial cells whereas moesin is found primarily in endothelial cells. J Cell Sci. 105:1025–1043 [DOI] [PubMed] [Google Scholar]
- Bretscher A. 1983. Purification of an 80,000-dalton protein that is a component of the isolated microvillus cytoskeleton, and its localization in nonmuscle cells. J Cell Biol. 97:425–432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretscher A. 1989. Rapid phosphorylation and reorganization of ezrin and spectrin accompany morphological changes induced in A-431 cells by epidermal growth factor. J Cell Biol. 108:921–930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretscher A, Edwards K, Fehon RG. 2002. ERM proteins and merlin: integrators at the cell cortex. Nat Rev Mol Cell Biol. 3:586–599 [DOI] [PubMed] [Google Scholar]
- Correas I, Speicher DW, Marchesi VT. 1986. Structure of the spectrin-actin binding site of erythrocyte protein 4.1. J Biol Chem. 261:13362–13366 [PubMed] [Google Scholar]
- Craven RA, Stanley AJ, Hanrahan S, Dods J, Unwin R, Totty N, Harnden P, Eardley I, Selby PJ, Banks RE. 2006. Proteomic analysis of primary cell lines identifies protein changes present in renal cell carcinoma. Proteomics. 6:2853–2864 [DOI] [PubMed] [Google Scholar]
- Deng X, Tannehill-Gregg SH, Nadella MV, He G, Levine A, Cao Y, Rosol TJ. 2007. Parathyroid hormone-related protein and ezrin are up-regulated in human lung cancer bone metastases. Clin Exp Metastasis. 24:107–119 [DOI] [PubMed] [Google Scholar]
- Derouiche A, Frotscher M. 2001. Peripheral astrocyte processes: monitoring by selective immunostaining for the actin-binding ERM proteins. Glia. 36:330–341 [DOI] [PubMed] [Google Scholar]
- Elliott BE, Meens JA, SenGupta SK, Louvard D, Arpin M. 2005. The membrane cytoskeletal crosslinker ezrin is required for metastasis of breast carcinoma cells. Breast Cancer Res. 7:365–373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehon RG, McClatchey AI, Bretscher A. 2010. Organizing the cell cortex: the role of ERM proteins. Nat Rev Mol Cell Biol. 11:276–287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao SY, Li EM, Cui L, Lu XF, Meng LY, Yuan HM, Xie JJ, Du ZP, Pang JX, Xu LY. 2009. Sp1 and AP-1 regulate expression of the human gene VIL2 in esophageal carcinoma cells. J Biol Chem. 284:7995–8004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimeno L, Corradi A, Cobos I, Consalez GG, Martinez S. 2004. Ezrin gene, coding for a membrane-cytoskeleton linker protein, is regionally expressed in the developing mouse neuroepithelium. Gene Expr Patterns. 4:749–754 [DOI] [PubMed] [Google Scholar]
- Gould KL, Bretscher A, Esch FS, Hunter T. 1989. cDNA cloning and sequencing of the protein-tyrosine kinase substrate, ezrin, reveals homology to band 4.1. EMBO J. 8:4133–4142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould KL, Cooper JA, Bretscher A, Hunter T. 1986. The protein-tyrosine kinase substrate, p81, is homologous to a chicken microvillar core protein. J Cell Biol. 102:660–669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grönholm M, Teesalu T, Tyynelä J, Piltti K, Böhling T, Wartiovaara K, Vaheri A, Carpén O. 2005. Characterization of the NF2 protein merlin and the ERM protein ezrin in human, rat, and mouse central nervous system. Mol Cell Neurosci. 28:683–693 [DOI] [PubMed] [Google Scholar]
- Hanzel D, Reggio H, Bretscher A, Forte JG, Mangeat P. 1991. The secretion-stimulated 80K phosphoprotein of parietal cells is ezrin, and has properties of a membrane cytoskeletal linker in the induced apical microvilli. EMBO J. 10:2363–2373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanzel DK, Urushidani T, Usinger WR, Smolka A, Forte JG. 1989. Immunological localization of an 80-kDa phosphoprotein to the apical membrane of gastric parietal cells. Am J Physiol. 256:1082–1089 [DOI] [PubMed] [Google Scholar]
- Johnson MW, Miyata H, Vinters HV. 2002. Ezrin and moesin expression within the developing human cerebrum and tuberous sclerosis-associated cortical tubers. Acta Neuropathol. 104:188–196 [DOI] [PubMed] [Google Scholar]
- Khanna C, Wan X, Bose S, Cassaday R, Olomu O, Mendoza A, Yeung C, Gorlick R, Hewitt SM, Helman LJ. 2004. The membrane-cytoskeleton linker ezrin is necessary for osteosarcoma metastasis. Nat Med. 10:182–186 [DOI] [PubMed] [Google Scholar]
- Kobayashi H, Sagara J, Masumoto J, Kurita H, Kurashina K, Taniguchi S. 2003. Shifts in cellular localization of moesin in normal oral epithelium, oral epithelial dysplasia, verrucous carcinoma and oral squamous cell carcinoma. J Oral Pathol Med. 32:344–349 [DOI] [PubMed] [Google Scholar]
- Kobel M, Langhammer T, Huttelmaier S, Schmitt WD, Kriese K, Dittmer J, Strauss HG, Thomssen C, Hauptmann S. 2006. Ezrin expression is related to poor prognosis in FIGO stage I endometrioid carcinomas. Mod Pathol. 19:581–587 [DOI] [PubMed] [Google Scholar]
- Koon N, Schneider-Stock R, Sarlomo-Rikala M, Lasota J, Smolkin M, Petroni G, Zaika A, Boltze C, Meyer F, Andersson L, et al. 2004. Molecular targets for tumour progression in gastrointestinal stromal tumours. Gut. 53:235–240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson SM, Lee HJ, Hung PH, Matthews LM, Robinson DN, Evans JP. 2010. Cortical mechanics and meiosis II completion in mammalian oocytes are mediated by myosin-II and Ezrin-Radixin-Moesin (ERM) proteins. Mol Biol Cell. 21:3182–3192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madan R, Brandwein-Gensler M, Schlecht NF, Elias K, Gorbovitsky E, Belbin TJ, Mahmood R, Breining D, Qian H, Childs G, et al. 2006. Differential tissue and subcellular expression of ERM proteins in normal and malignant tissues: cytoplasmic ezrin expression has prognostic significance for head and neck squamous cell carcinoma. Head Neck. 28:1018–1027 [DOI] [PubMed] [Google Scholar]
- Ohtani K, Sakamoto H, Rutherford T, Chen Z, Kikuchi A, Yamamoto T, Satoh K, Naftolin F. 2002. Ezrin, a membrane-cytoskeletal linking protein, is highly expressed in atypical endometrial hyperplasia and uterine endometrioid adenocarcinoma. Cancer Lett. 179:79–86 [DOI] [PubMed] [Google Scholar]
- Ohtani K, Sakamoto H, Rutherford T, Chen Z, Satoh K, Naftolin F. 1999. Ezrin, a membrane-cytoskeletal linking protein, is involved in the process of invasion of endometrial cancer cells. Cancer Lett. 147:31–38 [DOI] [PubMed] [Google Scholar]
- Pakkanen R. 1988. Immunofluorescent and immunochemical evidence for the expression of cytovillin in the microvilli of a wide range of cultured human cells. J Cell Biochem. 38:65–75 [DOI] [PubMed] [Google Scholar]
- Pang ST, Fang X, Valdman A, Norstedt G, Pousette A, Egevad L, Ekman P. 2004. Expression of ezrin in prostatic intraepithelial neoplasia. Urology. 63:609–612 [DOI] [PubMed] [Google Scholar]
- Park HR, Min SK, Min K, Jun SY, Seo J, Kim KH, Choi J. 2010. Differential expression of ezrin in epithelial skin tumors: cytoplasmic ezrin immunoreactivity in squamous cell carcinoma. Int J Dermatol. 49:48–52 [DOI] [PubMed] [Google Scholar]
- Richter U, Wittler L, Kessel M. 2004. Restricted expression domains of Ezrin in developing epithelia of the chick. Gene Expr Patterns. 4:199–204 [DOI] [PubMed] [Google Scholar]
- Saotome I, Curto M, McClatchey AI. 2004. Ezrin is essential for epithelial organization and villus morphogenesis in the developing intestine. Dev Cell. 6:855–864 [DOI] [PubMed] [Google Scholar]
- Sarrió D, Rodríguez-Pinilla SM, Dotor A, Calero F, Hardisson D, Palacios J. 2006. Abnormal ezrin localization is associated with clinicopathological features in invasive breast carcinomas. Breast Cancer Res Treat. 98:71–79 [DOI] [PubMed] [Google Scholar]
- Schindelmann S, Windisch J, Grundmann R, Kreienberg R, Zeillinger R, Deissler H. 2002. Expression profiling of mammary carcinoma cell lines: correlation of in vitro invasiveness with expression of CD24. Tumour Biol. 23:139–145 [DOI] [PubMed] [Google Scholar]
- Shaffer MH, Huang Y, Corbo E, Wu GF, Velez M, Choi JK, Saotome I, Cannon JL, McClatchey AI, Sperling AI, et al. 2010. Ezrin is highly expressed in early thymocytes, but dispensable for T cell development in mice. PLoS One. 5:e12404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J, Bai J, Yang W, Gabrielson EW, Chan DW, Zhang Z. 2007. Expression and clinicopathological significance of oestrogen-responsive ezrin-radixin-moesin-binding phosphoprotein 50 in breast cancer. Histopathology. 51:40–53 [DOI] [PubMed] [Google Scholar]
- Tokunou M, Niki T, Saitoh Y, Imamura H, Sakamoto M, Hirohashi S. 2000. Altered expression of the ERM proteins in lung adenocarcinoma. Lab Invest. 80:1643–1650 [DOI] [PubMed] [Google Scholar]
- Torer N, Kayaselcuk F, Nursal TZ, Yildirim S, Tarim A, Nòyan T, Karakayali H. 2007. Adhesion molecules as prognostic markers in pancreatic adenocarcinoma. J Surg Oncol. 96:419–423 [DOI] [PubMed] [Google Scholar]
- Wong SY, Haack H, Kissil JL, Barry M, Bronson RT, Shen SS, Whittaker CA, Crowley D, Hynes RO. 2007. Protein 4.1B suppresses prostate cancer progression and metastasis. Proc Natl Acad Sci U S A. 104:12784–12789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie JJ, Xu LY, Wu ZY, Zhao Q, Xu XE, Wu JY, Huang Q, Li EM. 2011. Prognostic implication of ezrin expression in esophageal squamous cell carcinoma. J Surg Oncol. March 17 [Epub ahead of print]. doi: 10.1002/jso.21909 10.1002/jso.21909 [DOI] [PubMed] [Google Scholar]
- Xie JJ, Xu LY, Xie YM, Zhang HH, Cai WJ, Zhou F, Shen ZY, Li EM. 2009. Roles of ezrin in the growth and invasiveness of esophageal squamous carcinoma cells. Int J Cancer. 124:2549–2558 [DOI] [PubMed] [Google Scholar]
- Yang XY, Ren CP, Wang L, Li H, Jiang CJ, Zhang HB, Zhao M, Yao KT. 2005. Identification of differentially expressed genes in metastatic and non-metastatic nasopharyngeal carcinoma cells by suppression subtractive hybridization. Cell Oncol. 27:215–223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng H, Xu L, Xiao D, Zhang H, Wu X, Zheng R, Li Q, Niu Y, Shen Z, Li E. 2006. Altered expression of ezrin in esophageal squamous cell carcinoma. J Histochem Cytochem. 54:889–896 [DOI] [PubMed] [Google Scholar]
- Zhang FR, Tao LH, Shen ZY, Lv Z, Xu LY, Li EM. 2008. Fascin expression in human embryonic, fetal, and normal adult tissue. J Histochem Cytochem. 56:193–199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Hu MY, Wu WZ, Wang ZJ, Zhou K, Zha XL, Liu KD. 2006. The membrane-cytoskeleton organizer ezrin is necessary for hepatocellular carcinoma cell growth and invasiveness. J Cancer Res Clin Oncol. 132:685–697 [DOI] [PubMed] [Google Scholar]