Abstract
Galectin-3 is a β-galactoside-binding protein involved in immunomodulation, cell interactions, cancer progression, and pathogenesis of infectious organisms. We report the identification and characterization of galectin-3 in human semen. In the male reproductive tract, the ~30 kDa galectin-3 protein was identified in testis, epididymis, vas deferens, prostate, seminal vesicle, and sperm protein extracts. In seminal plasma, galectin-3 was identified in the soluble fraction and in prostasomes, cholesterol-rich, membranous vesicles that are secreted by the prostate and incorporated into seminal plasma during ejaculation. Two-dimensional immunoblot analysis of purified prostasomes identified five galectin-3 isoelectric variants with a pI range of 7.0 to 9.2. Affinity purification and tandem mass spectrometry of β-galactoside-binding proteins from prostasomes confirmed the presence of galectin-3 in prostasomes and identified a truncated galectin-3 variant. The intact galectin-3 molecule contains a carbohydrate recognition domain and a non-lectin domain that interacts with protein and lipid moieties. The identification of a monovalent galectin-3 fragment with conserved carbohydrate-binding activity indicates the functional relevance of this truncation and suggests a regulatory mechanism for galectin-3 in prostasomes. Surface biotinylation studies suggested that galectin-3 and the truncated galectin-3 variant are localized to the prostasome surface. Prostasomes are proposed to function in immunosuppression and regulation of sperm function in the female reproductive tract, are implicated in facilitating sexually-transmitted infections, and are indicated in prostate cancer progression. Given the overlap in functional significance, the identification of galectin-3 in prostasomes lays the groundwork for future studies of prostasomes in reproduction, disease transmission, and cancer progression.
Keywords: galectin-3, prostate, prostasomes, seminal plasma, lectin
Introduction
Galectin-3 is a member of the galectin protein family, conserved animal lectins that bind to β-galactoside moieties on glycoproteins and glycolipids [1]. To date, fifteen mammalian galectins, designated galectin-1 to galectin-15, have been described in a wide range of tissues. Galectins exhibit multiple, and at times contrasting, functions dependent upon the individual galectin family member, cellular localization, concentration, and/or posttranslational modification. Galectin-3 is one of the most extensively characterized galectins and its multiple intra- and extracellular functions include regulation of cell proliferation and apoptosis, immunomodulation, cell-cell adhesion, cell-extracellular matrix adhesion, tumor progression, pathogen-host interactions, mRNA splicing, and cytoskeletal organization [1–3]. Galectin-3 is expressed as a cytosolic protein that lacks a signal sequence for transport into the endoplasmic reticulum but is secreted via a non-classical pathway.
The ~30 kDa human galectin-3 protein is composed of a C-terminal 135 amino acid carbohydrate recognition domain (CRD) linked to a N-terminal 12 amino acid non-lectin domain via a 103 amino acid collagen-like linker sequence [1]. The CRD has a strong preference for the polylactosamine structure (Galβ1-4/3GlcNAc)n and for terminal GalNAc [4]. The non-lectin domain can interact with protein or lipid moieties, such as the lipid component of bacterial lipopolysaccharide (LPS) [3]. Thus, the galectin-3 monomer can function to crosslink two unrelated galectin-3 binding ligands. Galectin-3 can also form multimers via self-association of the non-lectin domain, leaving the CRDs accessible for binding with multiple glycoconjugate ligands [1]. Extra- and intracellular galectin-3 functions are dependent on the multi-valency of galectin-3 multimers. The collagen-like linker sequence contains multiple protease cleavage sites and proteolytic cleavage has been shown to regulate galectin-3 function [1, 5, 6].
In the male reproductive tract, multiple studies have associated galectin-3 with tumor progression in prostate cancer [7]. In the testis, galectin-3 was identified in Sertoli cells (non-germ line cells that support spermatogenesis) and spermatogenic cells [8]. In the current report, we investigated galectin-3 in human semen, specifically the association of galectin-3 with prostasomes. Prostasomes are cholesterol-rich, membranous vesicles that are secreted by the prostate, incorporated into seminal plasma during ejaculation, and function in immunosuppression and regulation of sperm function [9].
Materials and Methods
Antibodies and Protein Extracts
Mouse monoclonal antibodies against galectin-3 (clone 9C4) and CD26 were purchased from Fitzgerald Industries International (Concord, MA) and Lab Vision (Fremont, CA), respectively. Horse radish peroxidase (HRP)-conjugated goat anti-mouse secondary antibodies and HRP-conjugated streptavidin were from Jackson ImmunoResearch Laboratories (West Grove, PA). Protein extracts of human testis, epididymis, vas deferens, seminal vesicle, and prostate were purchased from Biochain Institute, Inc. (Hayward, CA). These protein extracts were prepared by the company from liquid nitrogen fresh frozen tissues using a proprietary mixture of HEPES (pH 7.9), MgCl2, KCl, EDTA, sucrose, glycerol, sodium deoxycholate, NP-40, sodium orthovanadate and protease inhibitors.
Preparation of Sperm Protein Extract and Clarified Seminal Plasma
Semen samples from healthy human males were obtained from the Assisted Reproductive Technology Center at the University of Arkansas for Medical Sciences (UAMS) following a protocol approved by the UAMS Institutional Review Board. Semen samples were centrifuged at 1000 × g for 20 minutes. Seminal plasma was decanted and clarified at 10,000 × g, 4 °C for 30 minutes. Pelleted sperm were washed twice in Ham’s F-10 medium (Sigma Chemical Company, Saint Louis, Missouri) and extracted in 1% sodium dodecyl sulfate (SDS) containing 1X Complete® protease inhibitor cocktail (Roche Diagnostics, Indianapolis, Indiana) for 30 minutes at 4 °C. Extracted sperm protein and seminal plasma were centrifuged at 10,000 × g, 4 °C for 30 minutes to remove insoluble debris. Protein concentrations were determined using a bicinchoninic acid (BCA) assay (Pierce Chemical Company, Rockford, Illinois).
Differential Ultracentrifugation
Clarified seminal plasma was centrifuged at 100,000 × g, 4 °C for two hours to prepare a soluble fraction (supernatant) and a membrane-enriched fraction (pellet). The membrane-enriched pellet was treated with 0.5% Triton X-100 at 4 °C for 15 minutes in a volume equal to the original seminal plasma sample and centrifuged at 100,000 × g, 4 °C for two hours. The resulting supernatant and pellet contained the detergent-soluble fraction and the detergent-resistant fraction, respectively. The detergent resistant pellet was resuspended in a volume equal to the original seminal plasma sample.
Sucrose Density Gradient Analysis
The membrane fraction from 3 ml clarified seminal plasma was collected by centrifugation at 100,000 × g, 4 °C for 1 hour and resuspended in 250 μl 45% sucrose/Tris-buffered saline (TBS; 10 mM Tris, pH 7.4, 150 mM NaCl). To prepare a discontinuous sucrose density gradient, 200 μl resuspended membrane fraction was overlayed with 600 μl 30% sucrose/TBS and 400 μl 5% sucrose/TBS in a polycarbonate 11 × 34 mm tube. Following centrifugation in a swinging bucket rotor at 100,000 × g, 4 °C for 18 hours, nine equal fractions were collected, the first corresponding to the top of the tube and the ninth corresponding to the bottom of the tube.
Prostasome Isolation and Surface Biotinylation
Prostasomes were separated from amorphous material in the seminal plasma membrane fraction by one of two methods. First, the membrane fraction was resuspended in phosphate buffered saline (PBS, pH 7.2: 1.9 mM sodium phosphate monobasic, 8.1 mM sodium phosphate dibasic, 150 mM NaCl) at the original volume of the seminal plasma sample, subjected to column chromatography on a 35 ml Sephacryl S300 column at 4 °C and prostasomes were collected in the column void volume [10]. The column void volume was determined with blue dextran. Alternatively, the membrane pellet from clarified seminal plasma was washed by ultracentrifugation three times in PBS for one hour each to enrich for prostasomes [11]. In some experiments, surface proteins on purified prostasomes were labeled with membrane-impermeable sulfo-NHS-LC-biotin (Pierce Chemical Company) [12] and excess biotin was removed by dialysis against PBS.
β-Galactoside-Binding Protein Purification and Sequence Analysis
The membrane fraction containing prostasomes was resuspended in column buffer (10 mM Tris, 130 mM NaCl, 4 mM β-mercaptoethanol [BME], 1% octyl-β-glucoside [Pierce Chemical Company], 0.1% SDS, 20 mM methyl-β-cyclodextran [Sigma], 1X protease inhibitors [Roche Diagnostics]) at the original volume of the seminal plasma sample. One ml lactose-agarose beads (Sigma) were packed and equilibrated in column buffer. The membrane fraction was applied and the column was washed with column buffer. Bound material was eluted with 250 mM lactose (Sigma) in TBS. Fractions containing putative β-galactoside-binding proteins were identified by immunoblot analysis and silver staining and pooled. Proteins in the identified fractions were separated by SDS-PAGE and stained with Coomassie blue.
Spots were excised from Coomassie blue stained protein bands into a 96-well plate with the ProPic imaging and spot-picking robot from Genomic Solutions, and trypsin digestion was performed with the ProGest in-gel enzymatic digestion robot (Genomic Solutions) using sequencing grade modified trypsin (Promega, Madison, WI). The resulting peptide mixture was loaded using an autosampler onto a trapping column (Symmetry300 C18 5 μm NanoEase, Waters Corp., Beverly, MA) using a CapLC XE (Waters) system, a switching valve, and a flow rate of 20 μl/min. Peptides were separated by nanoflow capillary HPLC using a CapLC XE pump (Waters) operating at 12 μL/min; flow rate was controlled with a splitter in front of the switching valve. Peptides were eluted at 300 nL/min onto a self-packed PicoFrit (New Objective) 75 μm × 10 cm column (Jupiter 4 μ Proteo 90A, Phenomenex, Torrance, CA). The eluant was analyzed in-line by ESI-MS/MS using a Micromass Q-Tof Micro (Waters) tandem mass spectrometer operating in the positive ion mode. Data acquisition was performed in a data dependent fashion. Data was processed using ProteinLynx 2.0 (Waters), and the resulting peak list was used to search Mascot protein database (www.matrixscience.com) and assigned a Mascot score. Mascot scores for proteins are a summation of the scores for the individual peptides. Higher scores translate to greater probability for a sequence being correct.
Biotinylation and Collagenase Digestion of Recombinant Galectin-3
The human galectin-3 cDNA sequence was amplified by the polymerase chain reaction (PCR) from the pOTB7-galectin-3 plasmid construct (ATCC MGC-2058; American Type Culture Collection, Manassas, VA) using specific primers (IDT, Coralville, IA). The forward primer was 5′-GCCGTCCATATGGCAGACAATTTTTCG 3′ and the reverse primer was 5′-AGGATCCAGATTATATCATGGTATATG-3′. Underlines indicate NdeI and BamHI restriction sites, respectively. Amplified cDNA was digested with NdeI and BamHI (New England Biolabs, Ipswich, MA), and cloned into the pET-11a expression vector. The prepared construct was transformed into competent E. coli XL1 blue cells and the galectin-3 cDNA sequence was confirmed by automated DNA sequencing performed in the DNA Sequencing Core Facility, Department of Microbiology and Immunology, University of Arkansas for Medical Sciences. E. coli BL21 (DE3) competent cells transformed with the galectin-3 expression construct were grown in LB/ampicillin media (Midwest Scientific, St. Louis MO) to A600 nm of 0.5, induced with 1mM isopropyl-1-thio-β-D-galactopyranoside (IPTG; Midwest Scientific) for 4 hours at 37 °C and harvested by centrifugation at 10,000 × g for 30 minutes at 4 °C. The cell pellet was lysed by sonication in lysis buffer (75 mM Tris, pH 7.8, PBS, 0.1% Triton X-100, 1 mM dithiothreitol, 0.2 mg/ml lysozyme [Sigma, St. Louis, Missouri], and 1X protease inhibitors [Roche Diagnostics, Indianapolis, IN]) and centrifuged at 3000 × g for 15 minutes 4 °C. Recombinant galectin-3 was purified from bacterial lysate by lactose affinity column chromatography and biotinylated with twenty-fold molar excess of EZLink ® Sulfo-NHS-LC-Biotin (Pierce) in PBS/4 mM BME for 30 minutes at room temperature. Excess biotin was removed by dialysis against PBS with 4mM BME. Biotinylated galectin-3 was incubated at 37 °C for two hours with Clostridium histolyticum collagenase Type VII (Sigma) [13].
Electrophoresis and Immunoblot Analysis
For one-dimensional immunoblots, protein samples were separated by SDS-PAGE and electroblotted onto nitrocellulose [14]. Blots were developed for 1 minute in Ponceau S, photographically digitized, rinsed in PBS/0.05% Tween-20 to remove staining, and blocked in 3% BSA/PBS/0.05% Tween-20. Blots were incubated with primary antibodies overnight at 4 °C. Dilution factors for all immunoblots were anti-galectin-3 at 1:500 and anti-CD26 at 1:1000. Blots were washed three times with PBS/0.05% Tween-20, incubated in HRP-conjugated goat anti-mouse secondary antibodies (1:5000), washed twice with PBS/0.05% Tween-20, washed once in PBS, and developed with tetramethylbenzidine (TMB) reaction substrate (Kirkegaard & Perry, Gaithersburg, MD) or with enhanced chemiluminescence (GE Healthcare, Piscataway, NJ). In some experiments, SDS-PAGE gels were subjected to silver staining and biotinylated proteins were detected on electroblots with HRP-conjugated streptavidin (1:50,000). Negative controls included blots incubated with secondary antibodies or streptavidin alone.
For two-dimensional analysis, proteins were separated in the first dimension by isoelectric focusing on IEF strips with a pI range of 3.0 to 10.0 using a Bio-Rad Protean IEF Cell (Bio-Rad, Hercules CA) following the manufacturer’s instructions. Proteins were separated through a second dimension on 16% acrylamide SDS-PAGE and evaluated by immunoblotting and silver staining. The pI of electrophoresed proteins was estimated using a pI marker set (Bio-Rad).
Results
Immunoreactivity of the 9C4 mAb with intact galectin-3 but not the galectin-3 CRD
Biotinylated galectin-3 was proteolytically cleaved with C. histolyticum collagenase Type VII which cuts galectin-3 between tyrosine-107 and glycine-108 in the collagen-like linker sequence. This cleavage liberates the CRD from the N-terminal domain with anticipated molecular masses of ~17 kDa and ~10 kDa, respectively [13]. Electroblot analysis identified streptavidin-reactive protein bands of ~30 kDa in the untreated sample and ~30 and ~17 kDa in the collagenase-treated galectin-3 sample (Fig. 1). The absence of Arg and Lys residues in the galectin-3 linker and non-lectin domains results in the exclusive biotinlytation of the galectin-3 CRD; thus, only the intact (~30 kDa) and cleaved galectin-3 CRD (~17 kDa) bands can be identified with streptavidin. Anti-galectin-3 9C4 immunoreactivity with the intact (~30 kDa) galectin-3 bands was identified in the untreated and collagenase-treated samples with an additional ~10 kDa band in the collagenase-treated sample. Thus, the anti-galectin-3 9C4 mAb reacted with an epitope present in intact galectin-3 and in the N-terminal 107 amino acid residues of galectin-3 that contain the non-lectin domain and a portion of the collagen-linker sequence; the 9C4 mAb was not immunoreactive with the cleaved galectin-3 CRD.
Figure 1.
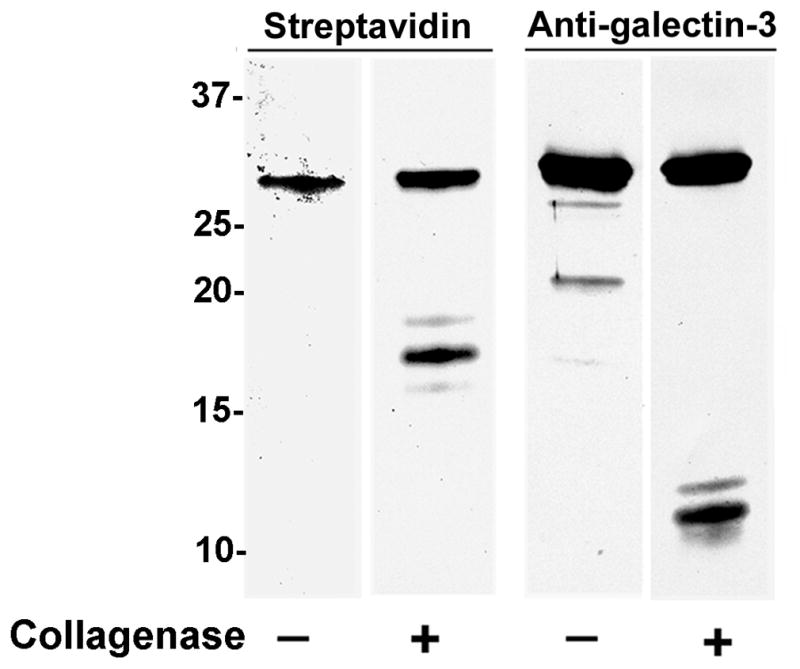
Immunoreactivity of the 9C4 mAb with intact galectin-3 but not with the galectin-3 CRD. Biotinylated, recombinant galectin-3 was incubated with C. histolyticum collagenase and subjected to electroblot analysis. Streptavidin identified biotinylated galectin-3 (~30 kDa) in the untreated and treated samples and the biotinylated, cleaved galectin-3 CRD (~17 kDa) in the treated sample. The 9C4 mAb reacted with intact galectin-3 (~30 kDa) in the untreated and treated samples and with a protein band of the molecular mass (~10 kDa) anticipated for the 107 amino acid, N-terminal galectin-3 protein fragment generated by collagenase Type VII cleavage. 9C4 immunoreactivity with the CRD was not apparent. Molecular weight markers are indicated in kDa.
Galectin-3 immunoreactivity in male reproductive tract samples
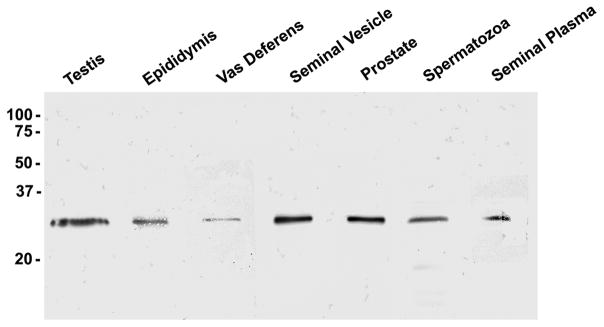
To examine the presence of galectin-3 in the male reproductive tract, clarified human seminal plasma and protein extracts of human testis, epididymis, vas deferens, seminal vesicle, prostate, and sperm were separated by SDS-PAGE under reducing conditions and evaluated by anti-galectin-3 immunoblot analysis (Fig. 2). Galectin-3 immunoreactivity was identified as an ~30 kDa band in all extracts and in clarified seminal plasma.
Figure 2.
Galectin-3 immunoblot analysis of male reproductive tract protein samples. Ten μg human testis, epididymis, vas deferens, seminal vesicle, and prostate extracts and 20 μg sperm extract and clarified seminal plasma were separated by SDS-PAGE and evaluated for galectin-3 reactivity by immunoblot analysis with the anti-galectin-3 mAb. Galectin-3 (~30 kDa) immunoreactivity was identified in all extracts and seminal plasma. Molecular weight markers are indicated in kDa.
Ultracentrifugation analyses of the seminal plasma membrane fraction
Galectin-3 association with the soluble and membrane fractions of seminal plasma was investigated by differential ultracentrifugation. Following SDS-PAGE and anti-galectin-3 immunoblot analysis, galectin-3 was identified in the soluble and membrane fractions of seminal plasma (Fig. 3). Galectin-3 was detected in both the Triton X-100-soluble and Triton X-100-insoluble membrane fractions. Detergent-insolubility of the membrane fraction is characteristic of cholesterol-rich membrane [15].
Figure 3.
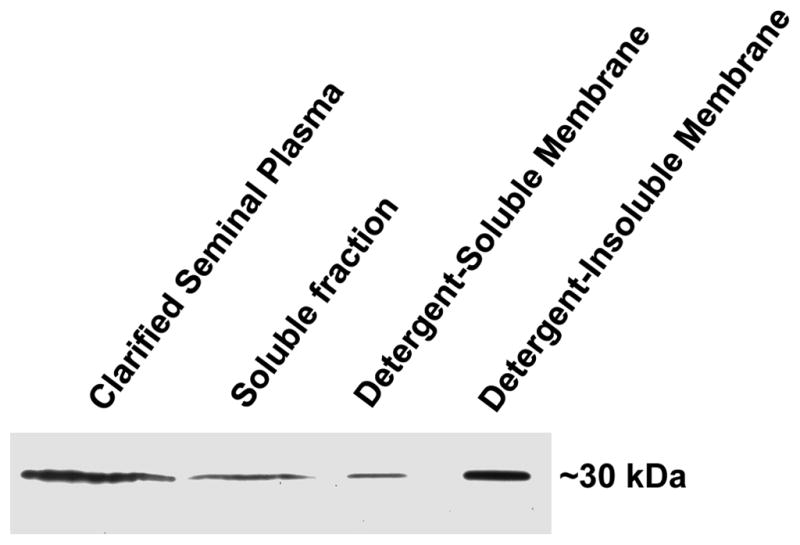
Differential ultracentrifugation analysis of galectin-3 in seminal plasma. Clarified seminal plasma was ultracentrifuged to separate the soluble fraction (supernatant) from the membrane fraction (pellet). The membrane fraction was treated with 0.5% Triton X-100 and ultracentrifuged to separate the detergent-soluble (supernatant) and detergent-insoluble (pellet) membrane fractions. Twenty μg (13.1 μl) clarified seminal plasma and 13.1 μl soluble fraction, detergent-soluble membrane fraction, and detergent-insoluble membrane fraction were evaluated by immunoblot analysis with the anti-galectin-3 mAb to compare the relative amount of galectin-3 in each fraction.
The human seminal plasma membrane fraction was subjected to discontinuous sucrose density gradient ultracentrifugation. Immunoblot analysis of collected fractions demonstrated that galectin-3 ascended into regions of lower sucrose concentration to the interface between the 5% and 30% sucrose phases (Fig. 4). This migration is characteristic of buoyant, low density, cholesterol-rich membrane [15]. CD26 (dipeptidyl peptidase IV) is an integral membrane glycoprotein in prostasomes [9] and was included as a positive control. CD26 co-migrated with galectin-3 into regions of lower sucrose density.
Figure 4.
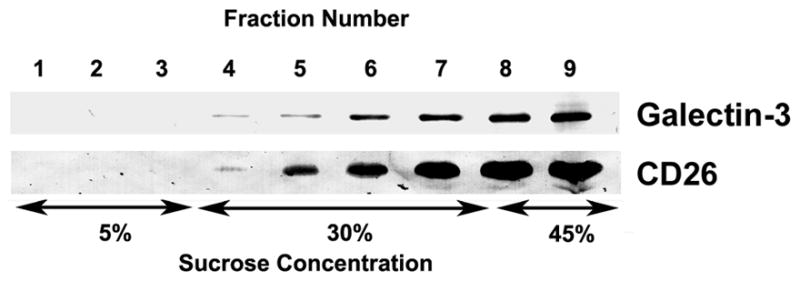
Sucrose density gradient analysis of galectin-3 in the membrane fraction of seminal plasma. The membrane fraction from seminal plasma was subjected to density gradient ultracentrifugation on a 5%/30%/45% discontinuous sucrose gradient. Nine equal fractions were collected, the first corresponding to the top of the tube and the ninth corresponding to the bottom of the tube, and evaluated by immunoblot analysis for galectin-3 and CD26.
Galectin-3 immunoreactivity in purified prostasomes
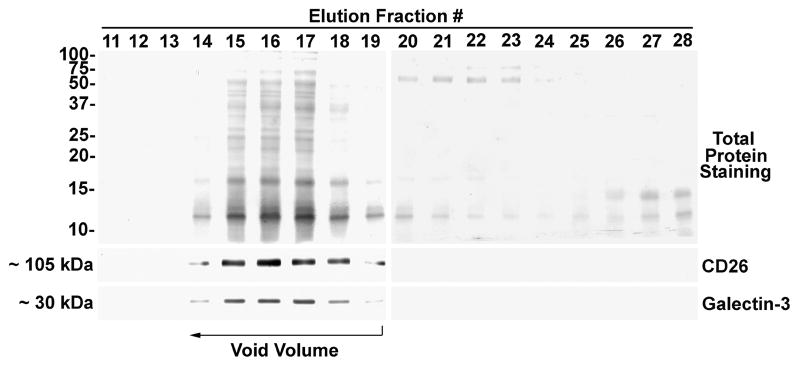
Prostasomes were purified from seminal plasma using a standard method for prostasome isolation [10]. The prostasome-enriched membrane fraction of seminal plasma was subjected to size exclusion chromatography on Sephacryl S300 to separate prostasomes from non-prostasomal material. Prostasomes are larger in mass than the exclusion limit of the column and were collected in the column void volume (fractions 14–19) as demonstrated by total protein staining and immunoblot analysis for the positive control CD26 (Fig. 5). Anti-galectin-3 immunoblot analysis demonstrated galectin-3 immunoreactivity in fractions 14–19, indicating the association of galectin-3 with prostasomes. Neither galectin-3 nor CD26 were detected in later column fractions.
Figure 5.
Prostasome purification by size exclusion chromatography and galectin-3 immunoblot analysis. The membrane fraction from seminal plasma was subjected to size exclusion column chromatography on Sephacryl S300 and prostasomes were collected in the void volume (fractions 14–19). Aliquots of column fractions were separated by SDS-PAGE. Electroblots were stained for total protein with Ponceau S and for galectin-3 and CD26 immunoreactivity. Molecular weight markers are indicated in kDa.
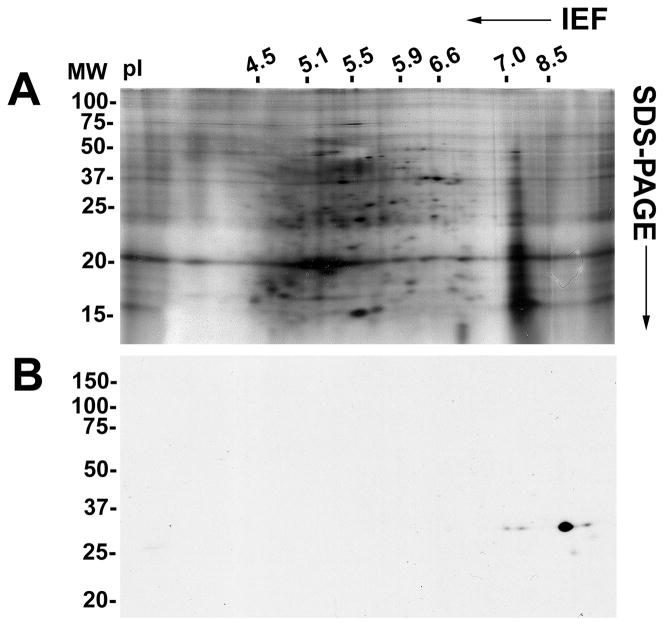
Two-dimensional immunoblot analysis of purified prostasomes with the anti-galectin-3 monoclonal antibody identified five immunoreactive spots with a molecular mass of ~30 kDa (Fig. 6). A major spot was identified at pI 9.0 and three minor spots at pI 7.0, 7.9, and 9.5. An additional minor spot was identified at ~27 kDa and with a pI of 9.2.
Figure 6.
Two-dimensional immunoblot analysis of purified prostasomes. Forty μg purified prostasome protein was separated by pI in a first dimension and by molecular mass in a second dimension. Total protein was identified by silver staining (A). Galectin-3 immunoreactivity was detected by immunoblot analysis (B). pI markers are indicated at the top of the electrophoretic gel and molecular weight (in kDa) markers are indicated on the right.
Purification of β-galactoside-binding proteins and tandem mass spectrometry
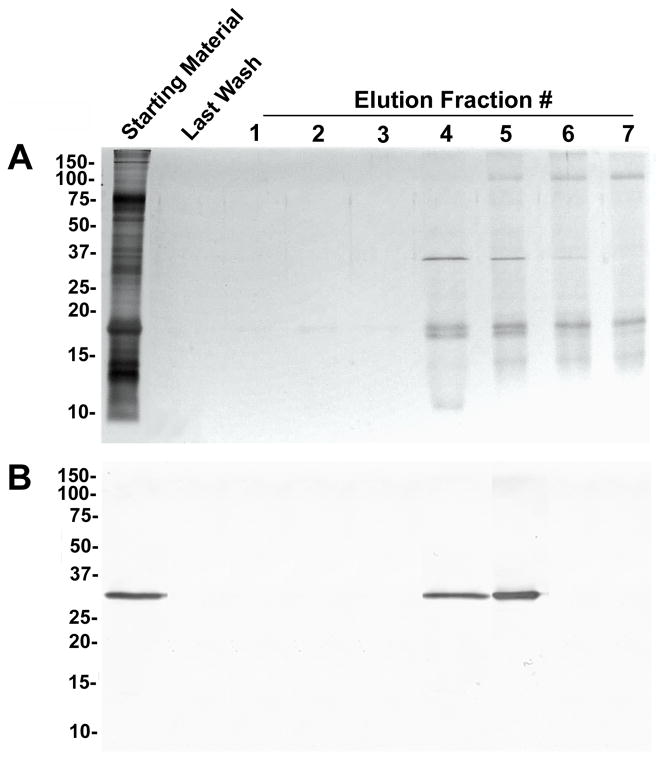
β-galactoside-binding proteins were isolated from prostasomes by lactose-affinity column chromatography. Fractions eluted with lactose were subjected to SDS-PAGE, silver-staining, and anti-galectin-3 immunoblot analysis (Fig. 7). Galectin-3 was identified as an ~30 kDa immunoreactive band in the starting material and in eluted fractions 4 and 5. Silver staining identified specific protein bands of ~15, ~30, and ~90 kDa in the purified fractions. In fractions 4 and 5, the ~15 kDa band appeared as a doublet.
Figure 7.
Purification of β-galactoside-binding proteins from the prostasome-enriched membrane fraction. The seminal plasma membrane fraction was extracted to release proteins from the cholesterol-rich prostasome membrane. Extracted proteins were subjected to affinity column chromatography on immobilized lactose. Aliquots of starting material, the final column wash, and eluted fractions containing β-galactoside-binding proteins were separated by SDS-PAGE. Total protein was identified by silver staining (A), and galectin-3 was identified by immunoblot analysis (B). Molecular weight markers are indicated in kDa.
Tandem mass spectrometry of the lactosyl affinity-purified ~30 kDa protein identified ten separate peptides that matched with the reported amino acid sequence of human galectin-3 with a significant Mascot score of 565 and represented 40% of the galectin-3 sequence (Fig. 8A). Analysis of the ~15 kDa band yielded nine peptide matches with galectin-3 with a significant Mascot score of 427 and represented 31% of the full-length galectin-3 sequence and 59% of the galectin-3 CRD sequence (Fig. 8B). No sequence matches were obtained for any other members of the galectin family, including the prototype galectins that have a molecular mass of ~15 kDa. Analysis of the ~90 kDa band yielded 21 peptide matches with lactoferrin with a Mascot score of 1093 and represented 35% of the lactoferrin sequence (Data not shown).
Figure 8.

Sequence coverage of galectin-3 peptides identified by tandem mass spectrometry. The ~30 and ~15 kDa β-galactoside-binding proteins from the seminal plasma membrane fraction were digested with trypsin and analyzed by tandem mass spectrometry. (A) Analysis of the ~30 kDa protein yielded ten peptides (Bold Underline) that matched with the reported amino acid sequence of human galectin-3 (Mascot score = 565) and represented 40% of the galectin-3 sequence. (B) Analysis of the ~15 kDa protein yielded nine peptides (Bold Underline) that matched with the reported amino acid sequence of human galectin-3 (Mascot score = 427) and represented 31% of the galectin-3 sequence.
Purification of β-galactoside-binding proteins from surface-biotinylated prostasomes
Purified prostasomes were surface-biotinylated with membrane-impermeable sulfo-NHS-LC-biotin and β-galactoside-binding proteins were purified by lactosyl-affinity chromatography. Streptavidin reacted with multiple biotinylated bands on an electroblot of the non-bound material collected in the flow through from the affinity column (Fig. 9). Two predominate biotinylated β-galactoside-binding proteins were identified in the purified fraction at ~15 kDa and ~30 kDa, and a minor band was detected at ~23 kDa. Immunoblot analysis of the same material indicated that the biotinylated ~30 kDa band co-migrated with the ~30 kDa anti-galectin-3 immunoreactive band.
Figure 9.
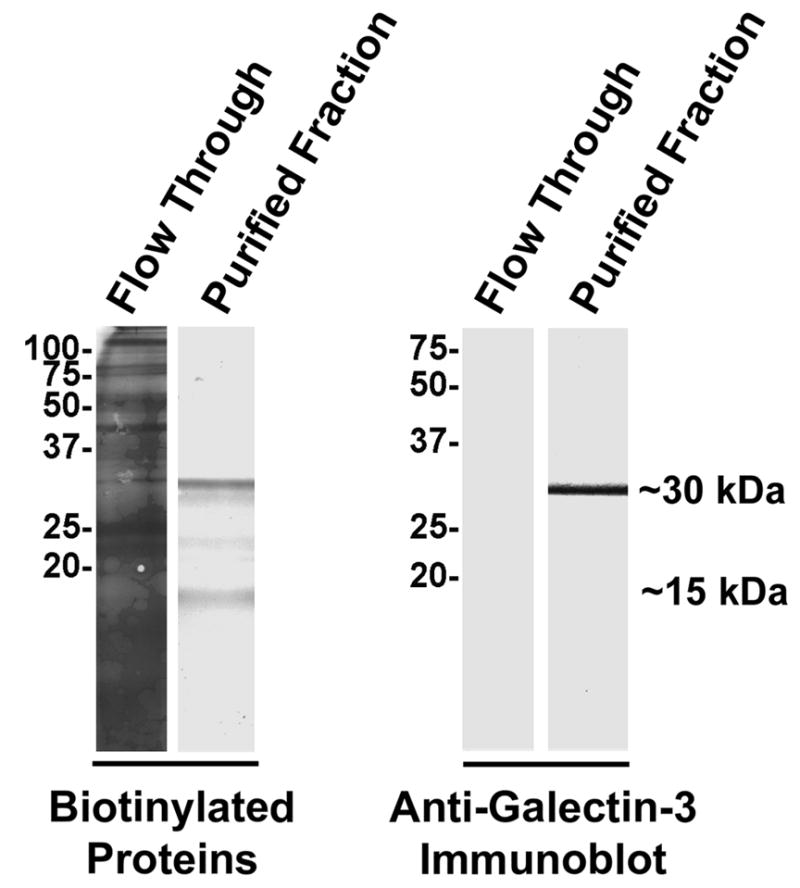
Purification of β-galactoside-binding proteins from purified, surface-biotinylated prostasomes. The surface of isolated prostasomes was biotinylated with membrane-impermeable sulfo-NHS-LC-biotin. β-galactoside-binding proteins were affinity purified on immobilized lactose. Non-bound material in the column flow through and purified β-galactoside-binding proteins were subjected to electroblot analysis to identify biotinylated proteins with HRP-conjugated streptavidin and to identify galectin-3 immunoreactivity. Molecular weight markers are indicated in kDa.
Discussion
Galectin-3 expression was previously identified in the male reproductive tract in human testis [8] and prostate [7]. Our immunochemical results now demonstrate galectin-3 in the human testis, epididymis, vas deferens, seminal vesicle, prostate, sperm, and in the soluble and membrane fractions of seminal plasma. Identification in the seminal plasma membrane fraction suggested that galectin-3 is associated with prostasomes. Prostasomes are multi-lamellar membranous vesicles (50–400 nm) that are cholesterol-rich and are secreted by the prostate into seminal plasma during ejaculation. Differential ultracentrifugation of seminal plasma indicated that galectin-3 is associated with a detergent-insoluble membrane fraction, and sucrose density gradient ultracentrifugation demonstrated that galectin-3 ascended into regions of lower sucrose concentration. These results are consistent with an association with buoyant, cholesterol-rich membranous vesicles such as prostasomes.
Prostasomes are a heterogeneous population of membranous vesicles [16] that are secreted by the prostatic epithelium and exhibit exosome-like structural and functional properties. Exosomes are membranous vesicles that are secreted by an exocytotic pathway by a variety of cell types and tissues including dendritic cells, lymphocytes, the intestinal epithelium, and the parotid gland [17–20]. Exosome-like vesicles, termed epididymosomes, are secreted by the epididymis and contribute to sperm maturation [21]. The majority of characterized exosomes, including prostasomes, exhibit immunomodulatory properties and are proposed to mediate intercellular communication [20]. Significantly, previous proteomic analyses identified galectin-3 associated with exosomes from dendritic cells [18] and saliva [19]. However, the role of galectin-3 in these exosomes remains unclear.
The association of galectin-3 with prostasomes was demonstrated by the identification of galectin-3 in prostasome fractions purified by size exclusion from the membrane fraction of seminal plasma. On two-dimensional immunoblots of purified prostasomes, galectin-3 was detected as one major immunoreactive spot with four additional minor spots. The predominate galectin-3 spot and three minor ~30 kDa galectin-3 spots were identified over a pI range consistent with previously reported galectin-3 isoelectric points [22, 23]. Identification of these four ~30 kDa isoelectric isoforms is potentially due to variations in phosphorylation and/or acetylation of the galectin-3 polypeptide [22, 24]. Wang and colleagues demonstrated that differences in phosphorylation resulted in discrete isoelectric variants of mouse galectin-3 [22], and Huflejt et al. [24] identified phosphorylation of the human galectin-3 N-terminal region. Extensive characterization of the post-translational modifications of prostasome galectin-3 was beyond the scope of the current report and will be the subject of a future study. A minor, lower molecular weight isoform has also been previously observed on two-dimensional blots of galectin-3 and may be the result of an RNA splicing variant [23] or due to proteolytic processing.
The presence of galectin-3 in prostasomes was confirmed by the identification of a lactosyl affinity-purified, ~30 kDa protein band as galectin-3 by tandem mass spectrometry. Identification of peptide sequences in only the C-terminal half of galectin-3 was likely due to the absence of trypsin cleavage sites in the N-terminal region of the molecule. Interestingly, galectin-3 was not identified by the proteomic analysis of prostasomes reported by Utleg et al. [25] and Poliakov et al. [16]. However, our identification of galectin-3 may have resulted from differences in the methods employed to isolate prostasomes from seminal plasma. Similarly, galectin-3 identification in dendritic cell exosomes varied with the exosome isolation methods utilized [17, 18].
Sequence analysis also identified a lactosyl affinity-purified, ~15 kDa band as galectin-3. No sequence matches were obtained for any other members of the galectin family, including the prototype galectins that have a molecular mass of ~15 kDa [1]. The ability of this protein to bind to immobilized lactose demonstrated that it contained a functional CRD. However, the lack of immunoreactivity with the anti-galectin-3 mAb, which is specific to the N-terminal region of galectin-3, indicated that the non-lectin binding domain was absent. The expected molecular weight of the CRD region is ~15 kDa [6]. Therefore, these collective results indicate that the ~15 kDa protein band represents a truncated galectin-3 variant that lacks the non-lectin domain and retains carbohydrate-binding activity. The ability of the galectin-3 CRD fragment to bind carbohydrate moieties following proteolytic cleavage has also been reported by other investigators [1, 26, 27].
Co-purification of lactoferrin with β-galactoside-binding proteins implicates lactoferrin as a potential galectin-3 binding protein. Lactoferrin is a member of the transferrin family of iron-binding proteins and is found in body fluids, including milk and seminal plasma, neutrophil granules, and prostasomes [25, 28]. Although galectin-3 binding has not been described for lactoferrin, this protein contains N-acetyl lactosamine glycans that may include motifs for galectin-3 binding [28], and galectin-3 has been shown to bind to a minor fraction of transferrin in serum [29]. However, the well documented interaction of lactoferrin with advanced glycation end products (AGEs) [30] indicates that lactoferrin exhibits carbohydrate binding activity and might bind directly to an immobilized carbohydrate such as lactose. Therefore, the potential for galectin-3 binding to lactoferrin will require direct confirmation.
Biotinylation of the prostasome surface combined with the purification of β-galactoside-binding lectins was used to examine the localization of galectin-3 on the prostasome surface. Sulfo-NHS-LC-biotin is not membrane permeable; therefore, only molecules on the prostasome surface would be biotinylated. Two predominate biotinylated, β-galactoside-binding protein bands of ~30 kDa and ~15 kDa were identified. The biotinylated ~30 kDa band comigrated with the immunoreactive ~30 kDa galectin-3 band. Significantly, ~30 kDa and ~15 kDa β-galactoside-binding proteins from prostasomes were identified by tandem mass spectrometry as galectin-3 and a truncated galectin-3 CRD product, respectively. The minor ~23 kDa band identified in the biotinylated β-galactoside-binding fraction was not detected by total protein staining of non-biotinylated β-galactoside-binding proteins. The relationship between this putative β-galactoside-binding protein and galectin-3 is unknown and requires further investigation. Absence of the ~90 kDa lactoferrin band in the biotinylated, β-galactoside-binding fraction may have resulted from lack of prostasome surface localization, low efficiency of lactoferrin biotinylation, and/or inhibition of galectin-3-lactoferrin interaction following biotinylation. Definitive confirmation of galectin-3 localization on the prostasome surface and investigation of galectin-3 inside prostasomes will require electron microscopy. Nevertheless, these results suggest that galectin-3 and a galectin-3 CRD fragment are present on the surface of human prostasomes, and this is the first indication that galectin-3 is localized to the surface of an exosome-like vesicle from any cell or tissue source.
Galectin-3 cleavage resulting in the loss of galectin-3 multi-valency has been demonstrated with multiple proteases including collagenases, matrix metalloproteases (MMP)-2, -9, and -13, neutrophil elastase, and leishmanolysin [3, 5, 6, 27]. Proteolytic regulation of galectin-3 function in this manner was indicated in the inactivation of neutrophil NADPH oxidase activity, in the pathogenesis of Leishmania major, and in chondrocyte release from the cartilage matrix [3, 5, 31]. Following cleavage, the galectin-3 CRD fragment retains its carbohydrate binding activity, as evidenced by its ability to bind lactose and other carbohydrate moieties with similar substrate specificity as that observed with intact galectin-3 [26]. However, a physiological function for the galectin-3 CRD fragment remains speculative [1, 27]. MMPs-2 and -9 are present in human seminal plasma [32] and may contribute to galectin-3 truncation in prostasomes. However, whether the truncated galectin-3 variant in prostasomes is the product of proteolytic cleavage or differential mRNA splicing is not known. Nevertheless, the identification of this functional galectin-3 CRD fragment provides a potential regulatory mechanism for galectin-3 function in prostasomes.
Prostasomes are a major component of semen with the total prostasome protein content in semen being twice that of spermatozoa [33]; thus, while millions of sperm are present in an ejaculate, prostasomes likely number in the billions. Two main functional roles for prostasomes have been proposed: enhancement of sperm function and immunosuppressive activity in the female reproductive tract [9]. Prostasomes were shown to fuse with sperm in vitro, to increase sperm motility by delivery of intra-prostasomal calcium stores, and to prevent the premature maturation of sperm. Furthermore, prostasomes are proposed to protect sperm from the female immune system by inhibiting the oxidative burst in neutrophils, preventing phagocytosis in leukocytes, and acting as a reservoir for the complement inhibitor CD59. These general immunosuppressive effects may also facilitate the pathogenesis of sexually-transmitted pathogens [34]. Significantly, prostate cancer cell lines continue to secrete prostasomes, and prostasomes have been proposed to play a role in angiogenesis, metastasis, and immunosuppression during prostate cancer [9]. The identification of galectin-3 on the surface of prostasomes, together with its cell adhesive and immunomodulatory properties, implicates galectin-3 in prostasome regulation of sperm function and immunosuppression in the female reproductive tract. Furthermore, the roles of galectin-3 in apoptosis, tumor cell adhesion, angiogenesis and metastasis in multiple malignancies, including prostate cancer, suggests that galectin-3 may participate in prostasome promotion of prostate cancer progression [1]. Future study of galectin-3 in prostasomes will provide new insights into prostasome function in the molecular mechanisms of intercellular communication in prostate cancer and in intercellular signals communicated from the male genital tract to the female genital tract during normal reproductive function.
Acknowledgments
This work was funded by NIH HD50540 and a pilot study grant from the College of Medicine, University of Arkansas for Medical Sciences (UAMS) to A.B.D. The authors wish to thank the Proteomics Core Facility at UAMS. The final publication is available at www.springerlink.com: http://www.springerlink.com/content/t977167073683416/
Abbreviations
- BME
β-mercaptoethanol
- BSA
bovine serum albumin
- CRD
carbohydrate recognition domain
- Gal
galactose
- GalNAc
N-acetyl galactosamine
- GlcNAc
N-acetyl glucosamine
- HRP
horse radish peroxidase
- kDa
kilodaltons
- PAGE
polyacrylamide gel electrophoresis
- PBS
phosphate-buffered saline
- pI
isoelectric point
- SDS
sodium dodecyl sulfate
- sulfo-NHS-LC-biotin
sulfosuccinimidyl-6-(biotinamido) hexanoate
- TBS
Tris-buffered saline
References
- 1.Dumic J, Dabelic S, Flogel M. Galectin-3: an open-ended story. Biochim Biophys Acta. 2006;1760:616–35. doi: 10.1016/j.bbagen.2005.12.020. [DOI] [PubMed] [Google Scholar]
- 2.Rabinovich GA, Toscano MA. Turning ‘sweet’ on immunity: galectin-glycan interactions in immune tolerance and inflammation. Nat Rev Immunol. 2009;9:338–52. doi: 10.1038/nri2536. [DOI] [PubMed] [Google Scholar]
- 3.Vasta GR. Roles of galectins in infection. Nat Rev Microbiol. 2009;7:424–38. doi: 10.1038/nrmicro2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bachhawat-Sikder K, Thomas CJ, Surolia A. Thermodynamic analysis of the binding of galactose and poly-N-acetyllactosamine derivatives to human galectin-3. FEBS Lett. 2001;500:75–9. doi: 10.1016/s0014-5793(01)02586-8. [DOI] [PubMed] [Google Scholar]
- 5.Guevremont M, Martel-Pelletier J, Boileau C, Liu FT, Richard M, Fernandes JC, Pelletier JP, Reboul P. Galectin-3 surface expression on human adult chondrocytes: a potential substrate for collagenase-3. Ann Rheum Dis. 2004;63:636–43. doi: 10.1136/ard.2003.007229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nieminen J, St-Pierre C, Sato S. Galectin-3 interacts with naive and primed neutrophils, inducing innate immune responses. J Leukoc Biol. 2005;78:1127–35. doi: 10.1189/jlb.1204702. [DOI] [PubMed] [Google Scholar]
- 7.Ellerhorst J, Troncoso P, Xu XC, Lee J, Lotan R. Galectin-1 and galectin-3 expression in human prostate tissue and prostate cancer. Urol Res. 1999;27:362–7. doi: 10.1007/s002400050164. [DOI] [PubMed] [Google Scholar]
- 8.Wollina U, Schreiber G, Gornig M, Feldrappe S, Burchert M, Gabius HJ. Sertoli cell expression of galectin-1 and -3 and accessible binding sites in normal human testis and Sertoli cell only-syndrome. Histol Histopathol. 1999;14:779–84. doi: 10.14670/HH-14.779. [DOI] [PubMed] [Google Scholar]
- 9.Burden HP, Holmes CH, Persad R, Whittington K. Prostasomes--their effects on human male reproduction and fertility. Hum Reprod Update. 2006;12:283–92. doi: 10.1093/humupd/dmi052. [DOI] [PubMed] [Google Scholar]
- 10.Arienti G, Carlini E, Palmerini CA. Fusion of human sperm to prostasomes at acidic pH. J Membr Biol. 1997;155:89–94. doi: 10.1007/s002329900160. [DOI] [PubMed] [Google Scholar]
- 11.Andersson E, Sorensen OE, Frohm B, Borregaard N, Egesten A, Malm J. Isolation of human cationic antimicrobial protein-18 from seminal plasma and its association with prostasomes. Hum Reprod. 2002;17:2529–34. doi: 10.1093/humrep/17.10.2529. [DOI] [PubMed] [Google Scholar]
- 12.Shetty J, Diekman AB, Jayes FC, Sherman NE, Naaby-Hansen S, Flickinger CJ, Herr JC. Differential extraction and enrichment of human sperm surface proteins in a proteome: identification of immunocontraceptive candidates. Electrophoresis. 2001;22:3053–66. doi: 10.1002/1522-2683(200108)22:14<3053::AID-ELPS3053>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 13.Massa SM, Cooper DN, Leffler H, Barondes SH. L-29, an endogenous lectin, binds to glycoconjugate ligands with positive cooperativity. Biochemistry. 1993;32:260–7. doi: 10.1021/bi00052a033. [DOI] [PubMed] [Google Scholar]
- 14.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979;76:4350–4. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Taylor CM, Coetzee T, Pfeiffer SE. Detergent-insoluble glycosphingolipid/cholesterol microdomains of the myelin membrane. J Neurochem. 2002;81:993–1004. doi: 10.1046/j.1471-4159.2002.00884.x. [DOI] [PubMed] [Google Scholar]
- 16.Poliakov A, Spilman M, Dokland T, Amling CL, Mobley JA. Structural heterogeneity and protein composition of exosome-like vesicles (prostasomes) in human semen. Prostate. 2009;69:159–67. doi: 10.1002/pros.20860. [DOI] [PubMed] [Google Scholar]
- 17.Thery C, Regnault A, Garin J, Wolfers J, Zitvogel L, Ricciardi-Castagnoli P, Raposo G, Amigorena S. Molecular characterization of dendritic cell-derived exosomes. Selective accumulation of the heat shock protein hsc73. J Cell Biol. 1999;147:599–610. doi: 10.1083/jcb.147.3.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thery C, Boussac M, Veron P, Ricciardi-Castagnoli P, Raposo G, Garin J, Amigorena S. Proteomic analysis of dendritic cell-derived exosomes: a secreted subcellular compartment distinct from apoptotic vesicles. J Immunol. 2001;166:7309–18. doi: 10.4049/jimmunol.166.12.7309. [DOI] [PubMed] [Google Scholar]
- 19.Gonzalez-Begne M, Lu B, Han X, Hagen FK, Hand AR, Melvin JE, Yates JR. Proteomic analysis of human parotid gland exosomes by multidimensional protein identification technology (MudPIT) J Proteome Res. 2009;8:1304–14. doi: 10.1021/pr800658c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Simpson RJ, Jensen SS, Lim JW. Proteomic profiling of exosomes: current perspectives. Proteomics. 2008;8:4083–99. doi: 10.1002/pmic.200800109. [DOI] [PubMed] [Google Scholar]
- 21.Sullivan R, Saez F, Girouard J, Frenette G. Role of exosomes in sperm maturation during the transit along the male reproductive tract. Blood Cells Mol Dis. 2005;35:1–10. doi: 10.1016/j.bcmd.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 22.Cowles EA, Agrwal N, Anderson RL, Wang JL. Carbohydrate-binding protein 35. Isoelectric points of the polypeptide and a phosphorylated derivative. J Biol Chem. 1990;265:17706–12. [PubMed] [Google Scholar]
- 23.Subhasitanont P, Srisomsap C, Punyarit P, Svasti J. Proteomic studies of galectin-3 expression in human thyroid diseases by immunodection. Cancer Genomics and Proteomics. 2006;3:389–394. [PubMed] [Google Scholar]
- 24.Huflejt ME, Turck CW, Lindstedt R, Barondes SH, Leffler H. L-29, a soluble lactose-binding lectin, is phosphorylated on serine 6 and serine 12 in vivo and by casein kinase I. J Biol Chem. 1993;268:26712–8. [PubMed] [Google Scholar]
- 25.Utleg AG, Yi EC, Xie T, Shannon P, White JT, Goodlett DR, Hood L, Lin B. Proteomic analysis of human prostasomes. Prostate. 2003;56:150–61. doi: 10.1002/pros.10255. [DOI] [PubMed] [Google Scholar]
- 26.Ahmad N, Gabius HJ, Sabesan S, Oscarson S, Brewer CF. Thermodynamic binding studies of bivalent oligosaccharides to galectin-1, galectin-3, and the carbohydrate recognition domain of galectin-3. Glycobiology. 2004;14:817–25. doi: 10.1093/glycob/cwh095. [DOI] [PubMed] [Google Scholar]
- 27.Ochieng J, Green B, Evans S, James O, Warfield P. Modulation of the biological functions of galectin-3 by matrix metalloproteinases. Biochim Biophys Acta. 1998;1379:97–106. doi: 10.1016/s0304-4165(97)00086-x. [DOI] [PubMed] [Google Scholar]
- 28.van Berkel PH, Geerts ME, van Veen HA, Kooiman PM, Pieper FR, de Boer HA, Nuijens JH. Glycosylated and unglycosylated human lactoferrins both bind iron and show identical affinities towards human lysozyme and bacterial lipopolysaccharide, but differ in their susceptibilities towards tryptic proteolysis. Biochem J. 1995;312 (Pt 1):107–14. doi: 10.1042/bj3120107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cederfur C, Salomonsson E, Nilsson J, Halim A, Oberg CT, Larson G, Nilsson UJ, Leffler H. Different affinity of galectins for human serum glycoproteins: galectin-3 binds many protease inhibitors and acute phase proteins. Glycobiology. 2008;18:384–94. doi: 10.1093/glycob/cwn015. [DOI] [PubMed] [Google Scholar]
- 30.Li YM. Glycation ligand binding motif in lactoferrin. Implications in diabetic infection. Adv Exp Med Biol. 1998;443:57–63. doi: 10.1007/978-1-4757-9068-9_7. [DOI] [PubMed] [Google Scholar]
- 31.Karlsson A, Follin P, Leffler H, Dahlgren C. Galectin-3 activates the NADPH-oxidase in exudated but not peripheral blood neutrophils. Blood. 1998;91:3430–8. [PubMed] [Google Scholar]
- 32.Shimokawa Ki K, Katayama M, Matsuda Y, Takahashi H, Hara I, Sato H, Kaneko S. Matrix metalloproteinase (MMP)-2 and MMP-9 activities in human seminal plasma. Mol Hum Reprod. 2002;8:32–6. doi: 10.1093/molehr/8.1.32. [DOI] [PubMed] [Google Scholar]
- 33.Arienti G, Carlini E, Saccardi C, Palmerini CA. Role of human prostasomes in the activation of spermatozoa. J Cell Mol Med. 2004;8:77–84. doi: 10.1111/j.1582-4934.2004.tb00261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kelly RW, Critchley HO. Immunomodulation by human seminal plasma: a benefit for spermatozoon and pathogen? Hum Reprod. 1997;12:2200–7. doi: 10.1093/oxfordjournals.humrep.a019559. [DOI] [PubMed] [Google Scholar]