Abstract
Intestinal mucosal barrier is the first line of defense against bacteria and their products originating from the intestinal lumen. We have shown a role for IL-18 in impaired gut barrier function following acute alcohol (EtOH) intoxication combined with burn injury. To further delineate the mechanism, this study examined whether IL-18 alters intestine tight junction proteins or induces mucosal apoptosis under these conditions. To accomplish this, rats were gavaged with EtOH (3.2 g/Kg) prior to ~12.5% total body surface area burn or sham injury. One day after injury, EtOH combined with burn injury resulted in a significant decrease in total occludin protein and its phosphorylation in small intestine compared to either EtOH or burn injury alone. There was no change in claudin-1 protein content but its phosphorylation on tyrosine was decreased following EtOH and burn injury. This was accompanied with an increase in mucosal apoptosis (p<0.05). The treatment of rats with anti-IL-18 antibody at the time of burn injury prevented intestine apoptosis and normalized tight junction proteins following EtOH and burn injury. Altogether, these findings suggest that IL-18 modulates tight junction proteins and cause apoptosis leading to impaired intestinal mucosal integrity following EtOH intoxication combined with burn injury.
1. Introduction
The intestine is the second largest immunological organ in the body. It has a large surface area with multiple functions. One of the primary functions of intestine is to absorb nutrition. Another major task for the gut is to maintain a local barrier which prevents the translocation of bacteria and endotoxin contained within the intestinal lumen to the extra-intestinal sites. The intestinal barrier is mainly formed by a layer of epithelial cells joined together by tight junction (TJ). TJ is a complex of membrane-bound proteins (e.g., occludin and claudins) and their adaptor and scaffolding proteins (e. g., junctional adhesion molecule, ZO-1, ZO-2 and ZO-3 [1; 2]. These proteins form a structure at the boundary of two adjacent cells working as a barrier within the epithelial cell space [2]. The TJ proteins are the rate-limiting step in the paracellular pathway and form a selectively permeable barrier to the solutes, fluid and other nutrition elements as well as the bacterial movement across the intestinal mucosa [3]. Therefore, an intact intestinal epithelial barrier plays a critical role in maintaining the normal physiological function and protecting from gut-derived pathogens. In addition to the physical epithelial barrier, the secretion of immunoglobulin A (IgA) is another important defense factor on mucosal surfaces [4; 5]. IgA is secreted by mucosal plasma cells residing under epithelial cells. Following release, IgA is coated on intestinal epithelium to prevent adherence of bacteria to mucosal surface. IgA can also neutralize toxin, regulate the microbial environment of intestine, and prevent local inflammation [4; 5].
Several lines of evidence indicate that intestine barrier is impaired following major trauma; burn injury as well as alcohol/ethanol (EtOH) exposure [6-9]. Nearly, one million burn injuries are reported every year within the United States and almost half of these injuries are reported to occur under the influence of EtOH [10-14]. Studies have also indicated that the intoxicated patients require frequent intubations, experience delayed wound healing and longer hospital stay. The intoxicated patients were found to be more susceptible to infection and had significantly higher mortality rate compared to burn patients who were not intoxicated at the time of injury. In addition, intoxicated patients died of smaller burns [11-15]. Similar findings were obtained in experimental models of EtOH and burn injury [7; 14; 16; 17]. We have shown that a single dose of EtOH or a minor burn injury alone was not able to produce severe adverse effects in the intestine; however, when the EtOH and minor burn injury were combined, they caused intestinal tissue damage, leakiness, and a significant increase in bacterial translocation [18-21]. This was accompanied with an increase in intestinal IL-18 levels [18-21]. IL-18, a proinflammatory cytokine, belongs to IL-1 cytokine superfamily. It is synthesized as a precursor protein (pro-IL-18) which in the presence of IL-1β-converting enzyme (ICE, or caspase-1) matures into 18-kDa active protein [22-26]. It is produced by macrophages, dendritic cells, neutrophils, and epithelial cells. IL-18, like IL-12 was discovered initially to be a factor that drives T cell towards Th1 cells as an IFN-γ-inducing factor [22-26]. However, later studies have indicated that IL-18 induces tissue damage in inflammatory bowel disease, arthritis and sepsis [22; 23; 25-28]. In our previous studies we have shown that IL-18 plays a role in increased gut leakiness following EtOH and burn injury. We also showed that IL-18 plays a key role in increased neutrophil recruitment to the intestine and the lung following EtOH intoxication and burn injury [18-21]. However, the mechanism by which IL-18 causes gut leakiness following EtOH and burn injury remains largely unknown. This study examined whether IL-18 alters intestine tight junction proteins or induces mucosal apoptosis as changes in any of these parameters may cause gut leakiness following EtOH intoxication and burn injury.
2. Materials and Methods
2.1. Animals and reagents
Male Sprague-Dawley rats (250-275g) were obtained from Charles River Laboratories (Wilmington, MA). Anti-occludin antibody and anti-claudin-1 antibody were obtained from Invitrogen (Carlsbad, CA). Anti-cleaved caspase-3 antibody, anti-phospho tyrosine antibody (pTyr100) and anti-phospho threonine antibody (p-Thr-polyclonal) were obtained from Cell Signaling (Danvers, MA). Anti-rat IL-18 antibody was obtained from R&D Systems (Minneapolis, MN). Cell death detection ELISA kit was obtained from Roche Applied Science (Indianapolis, IN).
2.2. Rat model of acute EtOH intoxication and burn injury
As described previously [18; 21], rats were randomly divided into four groups: sham vehicle, sham EtOH, burn vehicle and burn EtOH. In EtOH treated groups, blood EtOH levels in the range of 90-100 mg/dL were achieved by gavage feeding of 5 ml of 20% EtOH (~3.2 g/kg body weight) in water. In vehicle group, animals were gavaged with 5 ml of water. Four hours after gavage, all animals were anesthetized and transferred into a template fabricated to expose ~12.5% of the total body surface area. Animals were then immersed in a boiling water bath (95-97°C) for 10-12 sec. Sham-injured rats were subjected to identical anesthesia and immersed in lukewarm water (37°C) for 10-12 sec. The animals were dried immediately and resuscitated intraperitoneally with 10 ml of physiological saline. After recovery from anesthesia, the animals were returned to their cages and allowed food and water ad libitum. In some experiments, rats were treated intraperitoneally with anti-IL-18 antibody (100 μg/kg BW) or same amount of isotype IgG (Santa Cruz Biotechnology, Santa Cruz, CA) immediately after burn injury. One day after injury, rats were sacrificed.
All the experiments were carried out in adherence to the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals. These studies were initiated at the University of Alabama at Birmingham (UAB) and were approved by UAB and Loyola University Chicago Medical Center Institutional Animal Care and Use Committees.
2.3. Preparation of mucosal homogenates
Leaving approximately the first 15 cm proximal segment of intestine, a 20 cm long small intestine piece was horizontally opened and cleaned. Mucosa was removed by scraping the intestine with a glass slide, suspended in 1 ml lysis buffer containing 50 mM HEPES, 150 mM NaCl, 1 mM EDTA, 100 mM NaF, 1 mM MgCl2, 10 mM Na4P2O7, 200 μM Na3VO4, 0.5% Triton X-100, 10% glycerol and protease inhibitor cocktail (Sigma Chemical Co. St. Louis, MO), and sonicated twice for 10 sec on ice [18; 20]. Homogenates were cleared by centrifuging at 10,000 rpm for 15 min at 4°C, and supernatants were collected and stored at −70oC until use. Protein levels in the homogenates were measured by BioRad assay kit (Hercules, CA).
2.4. Immunofluorescence localization of intestinal occludin and claudin-1 protein expression
About 1.0 cm long segment of last part of ileum was fixed in 10% formalin and sent to Histology Laboratory at Loyola University Medical Center where they were embedded in paraffin, and cut into ~5 μm thick sections. After dewaxing and rehydrating, the antigenic site retrieval of the sections was accomplished by boiling slides for 20 min in 0.01 M citric acid buffer (pH 6.0) [29]. Nonspecific binding sites were blocked with 5% goat serum for 2 hrs. The sections were incubated with rabbit anti-occludin or rabbit anti-claudin-1 antibodies for 2 hrs at room temperature. The sections were washed in PBS and incubated with goat anti-rabbit IgG conjugated with Alexa Fluor® 488 or Texas Red® (Invitrogen) for 1 hr at room temperature. For nuclear staining, the sections were incubated with Hoechst (Invitrogen) for 2 min at room temperature. The sections were washed again, covered with cover slides by using gel mounting media (Fluoro-gel, Electron Microscopy sciences, Hatfield, PA) and stored in the dark at 4oC. The distribution of occludin and claudin-1 were examined by using a Zeiss Axiovert 200M fluorescence microscope (Carl Zeiss MicroImaging Inc. Thornwood, NY). For non specific staining controls, the sections were stained directly with secondary antibody (e.g. fluorescent labeled anti goat anti-rabbit IgG). We did not find any fluorescence signal in sections stained directly with secondary antibody.
2.5. Phosphorylation of mucosal occludin and claudin-1
To accomplish this, first occludin and claudin-1 proteins were immunoprecipitated from the mucosal homogenates using their respective antibodies as described previously in our study [30]. Briefly, equal amount of protein from mucosal homogenates was incubated with anti-occludin or anti-claudin-1 antibody for 1 hr at 4oC and then incubated with Protein G-Sepharose beads for another 2 hrs at 4oC. The beads were washed five times with wash buffer containing 50 mM HEPES, 150 mM NaCl, 1 mM EDTA, 100 mM NaF, 1 mM MgCl2, 10 mM Na4P2O7, 200 μM Na3VO4, 0.5% Triton X-100 and centrifuged at 10,000 rpm for 3 min at 4°C. Bound proteins were analyzed by SDS-PAGE and transferred to Immobilon P membranes. Membranes were probed with anti-phospho-tyrosine antibody and were re-blotted with anti-phospho-threonine antibody after stripping [21; 30; 31]. Then membranes were stripped again and re-blotted with antibodies to occludin or claudin-1 to confirm the respective protein levels. Representative blots shown in the result section come from the same membrane which may have more samples in various groups.
2.6. Measurement of mucosal apoptosis and cleaved caspase-3 activity
The mucosal apoptosis was assessed by measuring cytoplasmic histone-associated DNA fragments (mono-and oligonucleosomes) using ELISA kit. The apoptosis in the intestinal mucosa was further confirmed by measuring caspase-3 activity by Western Blot analysis using anti-cleaved caspase-3 antibody as described in our previous study [21]. Representative blots shown in the result section come from the same membrane which may have more samples in various groups.
2.7. Measurement of IL-18-mediated epithelial barrier disruption
In order to establish the effect of IL-18 on epithelial barrier, the present study determined the effect of IL-18 on paracellular permeability as described previously [32]. Briefly, 105 YAMC (murine colonic epithelial cell line obtained from Dr. Xiao-Di Tan, Northwestern University Children’s Memorial Hospital) were seeded in transwells (0.4 μm pore size) and cultured in RPMI 1640 containing 50μg/ml gentamicin, 100 units/ml penicillin, 100 μg/ml streptomycin, 1% ITS+Premix (BD Biosciences, Bedford, MA), 5% fetal bovine serum (FBS) and 5 units/ml mouse IFN-γ treated with recombinant mouse IL-18 (10, 50 or 100 ng/ml) in RPMI 1640 medium containing all the above ingredients except FBS and IFN-γ. After 16 hrs of incubation, the RPMI 1640 medium was replaced with HBSS. FITC-dextran (30μg/ml; MW 4 kD, Sigma Chemicals) was added to the apical chamber. Samples were collected from the apical and basal chambers at 30 min, 1 hr, 2 hrs and 4 hrs after addition of FITC-dextran. The concentration of FITC-dextran in these samples was determined using Synergy 2 Multi-Mode Microplate Reader (BioTek, Winooski, VT) at an excitation wavelength of 490 nm and emissions at 530 nm. Paracellular permeability of dextran was expressed as ratio of apical to basolateral flux of the tracer FITC-dextran.
2.8. Statistical analysis
The data, wherever applicable, are presented as means ± SEM and were analyzed using ANOVA. The significance between the groups was determined by Tukey’s test (GraphPad InStat). A p<0.05 between two groups was considered statistically significant.
3. Results
3.1. Localization of occludin and claudin-1 proteins in the small intestine
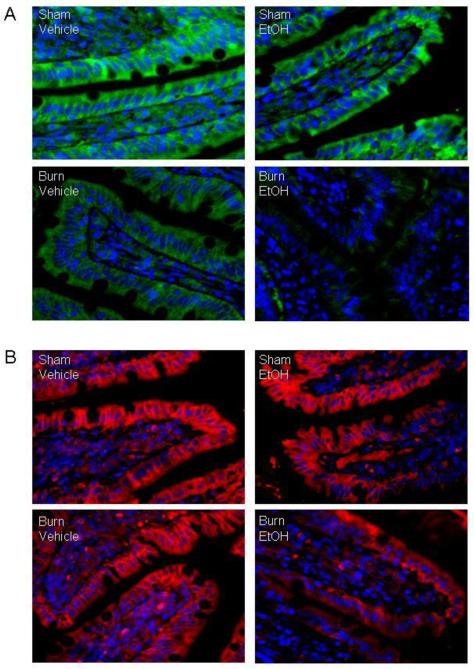
Fluorescent immunostaining revealed that occludin (Fig. 1A) and claudin-1 (Fig. 1B) predominantly localized at the boundary between the apical and the basolateral plasma membrane. As shown in Fig. 1A, there was a tendency of a decrease in density of occludin expression following EtOH or burn injury alone as compared to sham vehicle group. However, a substantial decrease in occludin protein expression was observed in small intestine harvested from animal receiving EtOH intoxication combined with burn injury compared to sham animals regardless of EtOH intoxication or burn injury alone. In contrast, there was no demonstrable change in the density of claudin-1 protein expression either in EtOH or burn injury alone animals compared with sham animal without EtOH intoxication. There was a tendency of a decrease in claudin-1 expression in small intestine harvested from EtOH and burn injured animals (Fig. 1B) compared to shams and burn alone group.
Fig. 1. Representative micrographs showing immunofluorescence localization of occludin and claudin-1 protein in small intestine one day after EtOH and burn injury.
Representative photomicrograph showing small intestine sections stained with anti-occludin antibody and Alexa Fluor® 488 conjugated goat anti-rabbit IgG (Panel A) or with anti-claudin-1 antibody and Texas Red® conjugated goat anti-rabbit IgG (Panel B). Nucleus was stained with Hoechst (blue). Localization of occludin (green) and claudin-1(red) were observed by fluorescence microcopy (x1000). Similar results were obtained in 3-4 animals in each group.
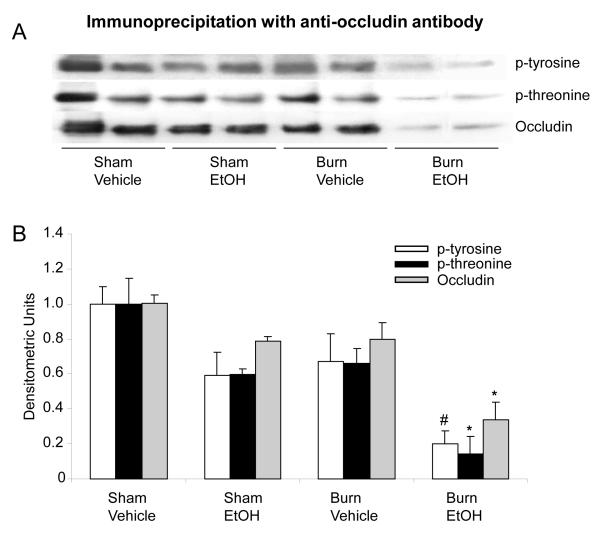
To further quantitate and confirm the findings obtained from immunofluorescence, the levels of mucosal occludin and claudin-1 protein expression were determined by Western Blot. A several fold decrease in occludin protein expression was observed in the mucosal homogenates from rats receiving a combined insult of EtOH intoxication and burn injury compared to animals receiving either sham injury regardless of EtOH intoxication or burn injury alone (data not shown). We next examined whether EtOH intoxication combined with burn injury influences the phosphorylation of occludin. The results from these experiment as shown in Fig. 2 indicate that the phosphorylation of occludin on both tyrosine and threonine residues was decreased by more than 2-fold in the mucosal homogenates from rats which had undergone a combined insult of EtOH intoxication and burn injury compared to rats receiving either insult alone. No significant change in occludin phosphorylation was observed in mucosal homogenates prepared from rats receiving either EtOH intoxication or burn injury alone compared with sham rats gavaged with vehicle.
Fig. 2. Representative blots showing occludin protein expression and phosphorylation in small intestine mucosa one day after EtOH and burn injury.
Mucosal homogenates were immunoprecipitated with anti-occludin antibody and immunoblotted with phospho-antibodies (A). Band densities were quantitated by image analysis, normalized to the average of the band densities of sham animals and are shown as mean ± SEM from 3-4 animals in each group in Panel B.*, p<0.05 compared with other respective groups; #, p<0.05 compared with respective sham vehicle.
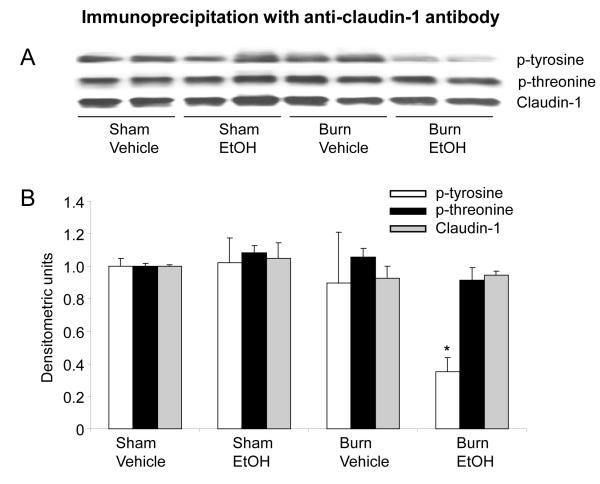
The expression of claudin-1, on the other hand, was not found to be different following a combined insult of EtOH intoxication and burn injury compared to either EtOH intoxication or burn injury alone (Fig. 3). However, a more than 2-fold decrease in claudin-1 tyrosine phosphorylation was observed following a combined insult of EtOH intoxication and burn injury compared to shams (Fig. 3B). Although there was a tendency of a decrease in claudin-1 tyrosine phosphorylation compared to sham EtOH and burn vehicle group, this was not found to be significantly different (p>0.05). In contrast, no difference in the threonine phosphorylation was observed following a combined insult of EtOH and burn injury compared to either shams or burn injury alone.
Fig. 3. Representative blots showing claudin-1 protein expression and phosphorylation in small intestine mucosa one day after EtOH and burn injury.
Mucosal homogenates were immunoprecipitated with anti-claudin-1 antibody and immunoblotted with phospho-antibodies (A). Band densities were quantitated by image analysis, normalized to the average of the band densities of sham animals and are shown as mean ± SEM from 3-4 animals in each group in Panel B.*, p<0.05 compared with respective sham vehicle group.
3.2. Mucosal apoptosis and caspase-3 activity
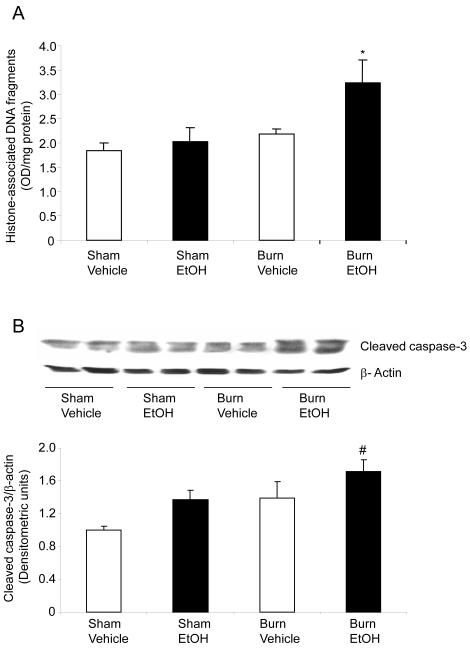
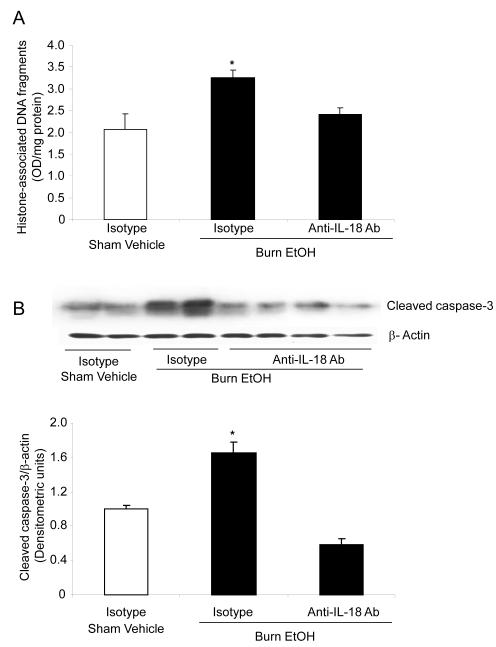
Mucosal apoptosis was determined by measuring cytoplasmic histone-associated DNA fragments (Fig. 4A). As compared to sham vehicle, no difference in mucosal apoptosis was observed following EtOH intoxication or burn injury alone. However, EtOH intoxication combined with burn injury resulted in nearly a 2-fold increase in mucosal apoptosis compared to rats receiving either sham injury regardless of EtOH exposure or ~1.5-fold increase compared to burn injury alone (Fig. 4A). Similarly, caspase-3 activity, another marker of apoptosis, was also not found to be significantly different in the mucosal homogenates prepared from rats receiving either EtOH exposure or burn injury alone compared to sham vehicle (Fig. 4B). However, a significant increase in mucosal cleaved caspase-3 expression was observed in rats receiving a combined insult of EtOH intoxication and burn injury compared to rats receiving sham injury (Fig. 4B).
Fig. 4. EtOH and burn injury increases intestinal mucosal apoptosis one day after EtOH and burn injury.
Apoptosis was assessed by measuring cytoplasmic histone-associated DNA fragments in the mucosal homogenates using ELISA and the values are normalized to the protein contents (A). The levels of cleaved caspase-3 were measured in mucosal homogenates by Western Blot (B). Band densities were quantitated by image analysis, normalized to the average of the band densities of sham animals and are shown as mean ± SEM from 5 animals in each group in Panel B.*, p<0.05 compared with other groups; #, p<0.05 compared with sham vehicle group.
3.3. Effect of Anti-IL-18 antibody treatment on mucosal apoptosis
Recent findings from our laboratory have suggested a role of IL-18 in the intestinal leakiness following EtOH intoxication and burn injury [18-21]. Similar to the findings reported in our previous studies [18-21], we found a significant increase in mucosal IL-18 levels following EtOH intoxication and burn injury compared to either insult alone. The administration of anti-IL-18 antibody normalized mucosal IL-18 levels following EtOH and burn injury (data not shown). We determined whether restoration of IL-18 levels prevent mucosal apoptosis. Treatment of animals with anti-IL-18 antibody (100μg/kg body weight) immediately after burn injury significantly prevented the increase in mucosal apoptosis (Fig. 5A) and caspase-3 activity (Fig. 5B) following a combined insult of EtOH intoxication and burn injury.
Fig. 5. IL-18 antibody normalizes mucosa apoptosis and caspase-3 activity following EtOH and burn injury.
Animals were treated intraperitoneally with anti-IL-18 antibody (100 μg/kg) immediately after burn injury. One day after injury, small intestine mucosa was collected and homogenized. For apoptosis, the homogenates were analyzed for cytoplasmic histone-associated DNA fragments using ELISA (A). The levels of cleaved caspase-3 were measured in mucosal homogenates by Western Blot (B). Band densities were quantitated by image analysis, normalized to the average of the band densities of sham animals and are shown as mean ± SEM in Panel B.*, p<0.05 compared with other groups.
3.4. Effect of anti-IL-18 antibody treatment on mucosal occludin and claudin-1 expression and phosphorylation
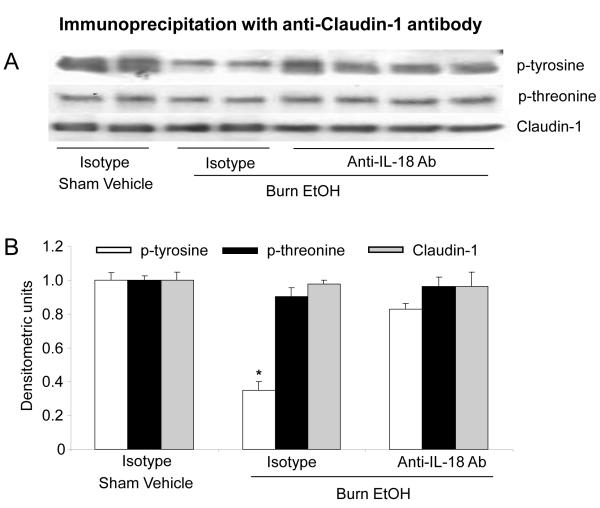
The results shown in Fig. 6 indicated that treatment of animals with anti-IL-18 antibody prevented the decrease in occludin protein expression and its phosphorylation on both tyrosine and threonine residues following EtOH intoxication and burn injury. Furthermore, anti-IL-18 antibody treatment also prevented the decrease in claudin-1 tyrosine phosphorylation following EtOH intoxication and burn injury (Fig. 7).
Fig. 6. Effect of IL-18 antibody on expression of occludin protein and phosphorylation after EtOH and burn injury.
Animals were treated intraperitoneally with neutralizing anti-IL-18 antibody (100 μg/kg BW) immediately after burn injury. One day after injury, small intestine mucosa was collected and homogenized. Mucosal homogenates were immunoprecipitated with anti-occludin antibody and immunoblotted with phospho-antibodies (A). Band densities were quantitated by image analysis, normalized to the average of the band densities of sham animals and are shown as mean ± SEM in Panel B.*, p<0.05 compared with other respective groups.
Fig. 7. Effect of IL-18 anti-body on expression of claudin-1 protein and phosphorylation after EtOH and burn injury.
Animals were treated intraperitoneally with neutralizing anti-IL-18 antibody (100 μg/kg BW) immediately after burn injury. One day after injury, small intestine mucosa was collected and homogenized. Mucosal homogenates were immunoprecipitated with anti-claudin-1 antibody and immunoblotted with phospho-antibodies (A). Band densities were quantitated by image analysis, normalized to the average of the band densities of sham animals and are shown as mean ± SEM in Panel B.*, p<0.05 compared with respective other groups.
3.5. IL-18 increases paracellular permeability in epithelial cell
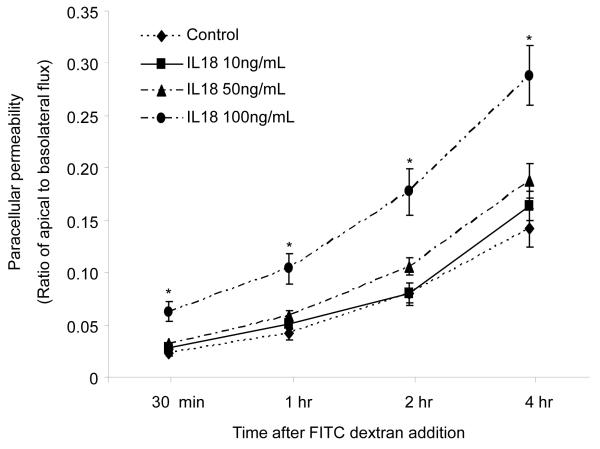
To confirm whether IL-18 directly increases paracellular permeability, we treated YAMCs with different doses of rIL-18. Paracellular permeability was determined by measuring the flux of tracer (4kD FITC-dextran) from the apical to lower chambers of the transwells. Results presented in Fig. 8 clearly show that IL-18 dose-dependently increased the paracellular flux of FITC dextran. At lower concentrations (10 and 50 ng/ml) IL-18-mediated increase in paracelluar permeability was not found to be significantly different compared to control group. However, at higher concentration (100 ng/mL), IL-18 caused a significant increased in paracellular permeability at any time points compared with other groups.
Fig. 8. IL-18 increases epithelial cells paracellular permeability.
YAM cells were treated with 10, 50 and 100ng/ml of recombinant IL-18 for ~16 hours. FITC-dextran 30μg/ml was added to the apical chamber. Samples were collected from the apical and basal chambers at various time points and the concentration of tracer (FITC dextran) was determined. Paracellular permeability to dextran was expressed as ratio of apical to basolateral flux. Value is means ± SEM from two different experiments and each group contains three transwells in each experiment. *p<0.05 vs. other groups.
4. Discussion
In this study, we observed that a combined insult of EtOH intoxication and burn injury inreases intestinal mucosal apoptosis and alter tight junction proteins, occludin and claudin-1. Administration of anti-IL-18 antibody normalized these parameters (apoptosis and tight junction proteins) following EtOH intoxication and burn injury. Furthermore, our finding that IL-18 increases paracellular permeability in colonic epithelial cells suggest that IL-18 plays a role in altered intestine barrier function following EtOH and burn injury.
Similar roles of IL-18 were demonstrated in experiment models of inflammatory bowel disease and other inflammatory and infectious diseases [22; 23; 25-28]. Pizarro et al. have reported that IL-18 mRNA transcripts and expression of mature IL-18 protein significantly increase in freshly isolated intestinal epithelial cells (IEC) from patients with Crohn’s disease (CD) compared to non inflamed control patients [28]. Halpern et al. have used IL-18 knockout mice to develop experimental necrotizing enterocolitis (NEC) and found lower incidence and severity of NEC in IL-18 knockout compared to WT mice [27]. However, the mechanism by which IL-18 influences intestine barrier remains largely unknown.
We observed that IL-18 decreases the phosphorylation of both occludin and claudin-1 following EtOH and burn injury. Occludin and claudin-1 are integral membrane proteins localized at the points of membrane-membrane interaction of the tight junction [33]. Previous finding indicates that the overexpression of full-length occludin in cultured Madin-Darby Canine Kidney (MDCK) cells resulted in an increase in their trans-epithelial resistance (TER). The introduction of COOH-terminally truncated occludin into MDCK cells resulted in a several fold increased paracellular leakage of small molecular weight tracers [34]. In addition, the phosphorylation of occludin is a key step in tight junction assembly. It is highly phosphorylated at serine/threonine and tyrosine residues in the intact epithelium [35]. It has been shown that the highly phosphorylated occludin is selectively concentrated at complex region of tight junction and less phosphorylated occludin is targeted to basolateral membrane from cytoplasm [36]. The MDCK cells treated with 12-O-tetradecanoylphorbol-13-acetate (TPA) an activator of protein kinase C, caused dephosphorylation of occludin and disrupt the formation of tight junction [37].
Furthermore, phosphoaminoacid analysis showed that occludin is phosphorylated on serine and threonine residues [36; 37]. Additional findings suggest that the tyrosine phosphorylation of occludin is also involved in the assembly and disassembly of tight junction [38; 39]. Consistent with these findings, we have observed that acute EtOH intoxication combined with burn injury significantly decreased the phosphorylation on both tyrosine and threonine residues of occludin compared to sham animals. The treatment of animals with anti-IL-18 antibody normalizes the both phosphorylation and the protein levels. Since the phosphorylation of occludin is a key to tight junction assembly, a decrease in it as observed in this study may perturb the tight junction assembly resulting in impaired gut barrier function.
In contrast, others have shown that tyrosine phosphorylation of tight junction proteins may increase permeability in epithelial and endothelial cells [40; 41]. Rao et al. have observed that Caco-2 cell treated with a mixture of xanthine oxidase and xanthine exhibited a decrease in TER and increase in permeability to mannitol [42]. Furthermore, Hydrogen peroxide administration also induced tyrosine phosphorylation of occludin. The tyrosine kinase inhibitor, genistein prevented the oxidative-induced decrease in TER [42]. Additionally their findings suggest that Tyr-379 and Tyr-383 in chicken occludin, and Tyr-398 and Tyr-402 in human occludin are exclusive sites of phosphorylation by c-Src. The deletion or mutation of these tyrosine in occludin abolished the c-Src-mediated phosphorylation and regulation of ZO-1 [42; 43]. However, most of these studies have been performed in vitro using cell lines and it remains unclear whether a similar change occurs under pathological conditions. In addition, phosphorylation can be activated by different kinases on distinct residues on the same tight junction proteins [44]. This could account for the difference between the findings reported here and previous studies; however a definitive cause for these differences remains to be established.
Studies have indicated that similar to occludin, claudins are also integral component of tight junction. Nearly 24 members of claudin family are reported in eukaryotes; their expression is suggested to be tissue and cell specific which can be affected in various inflammatory conditions [45]. A recent study suggested an increase in claudin-1 and claudin-2 expression in active inflammatory bowl disease [46]. We observed that IL-18 also reduces the phosphorylation of claudin-1 following a combined insult of EtOH intoxication and burn injury. Several kinases including mitogen activated protein kinase and protein kinase C are involved in claudin-1 phosphorylation [45]. In contrast, protein phosphatase 2A induced dephosphorylation of claudin-1 and increased in paracellular permeability [47]. Similarly, Ko et al. also observed human corneal epithelial cells (HCE) treated with neurotransmitter substance P increased the expression of ZO-1, but not claudin-1 which was accompanied by an increase in TER in HCE cell monolayer [48]. Taken together, these findings suggest that tight junction proteins are critical for the proper functioning of tight junctions. However, more studies are needed to confirm whether EtOH modulates other claudin members in the intestinal tissue following burn injury. Any alteration in their distribution or their state of phosphorylation as observed in our study may adversely affect this barrier leading to gut leakiness as observed following EtOH intoxication and burn injury [21].
An increase in apoptosis might be another reason for intestinal epithelial barrier dysfunction in EtOH and burn injury. The increase in epithelial apoptosis has been observed in many pathological conditions, such as sepsis, active Crohn’s disease, ischemia/reperfusion and burn injury [49-53]. A balance between epithelial cell proliferation and apoptosis is critical to normal functioning of epithelial barrier Several lines of evidence suggest that the epithelial cells and cells of lamina propria can synthesize and produce IL-18 [24; 28]. Studies have also shown that IL-18 is synthesized as a non-functional precursor, pro-IL-18 which in the presence of IL-1 converting enzyme (ICE) also referred to as caspase-1, matures into a functional biologically active form of IL-18 [24]. The activity of IL-18 is further regulated after its release from the cell by a naturally occurring protein referred to as IL-18 binding protein [24]. In addition to IL-18, several other inflammatory mediators (i.e., IFN-γ, TNF-α, IL-1, IL-6 and IL-10 etc.) are also implicated in altered tight junction function and enhanced apoptosis of endothelial and epithelial cells [54-59]. There is evidence suggesting an increase in the levels of many of these cytokines following EtOH and burn injury [60] but whether they play a role in altered epithelial barrier under those conditions remains to be established.
The exact mechanism by which IL-18 modulates the expression and phosphorylation of claudin-1 and occludin, remains unknown. Our previous findings suggest that IL-18 causes an increase in neutrophil infiltration into the intestine tissue following EtOH and burn injury [20; 21]. Neutrophils produce ROS and proteases and thus neutrophils-derived factors may contribute to altered tight junction proteins and apoptosis in the intestine following EtOH and burn injury. IL-18 receptors are constitutively expressed on the intestinal epithelia cell [24]. Since the cytoplasmic regions of the receptors for IL-1 and IL-18 are homologous to each other and to members of the Toll family, IL-18 shares signaling pathway with IL-1 and Toll receptors [24] including the recruitment of myeloid differentiation factor (MyD)88 and activation of IL-1 receptor-associated kinase (IRAK). Thus IL-18 may utilize MyD88 and/or IRAK pathways in mediating its effect on intestine mucosal barrier following EtOH and burn injury. Furthermore, IL-18 may also modulate MyD88/IRAK downstream signaling cascade including mitogen activated protein kinase (MAPK) and protein kinase C. Alternatively, it may up-regulate the phosphatases and perturb the balance between protein phosphatase and kinases leading to more intestinal apoptosis and altered tight junction assembly. Thus multiple mechanisms may exist by which IL-18 may modulate tight junction proteins and cause apoptosis in the gut. In summary, the results presented here suggest that an increase in IL-18 disrupt the distribution and activation of the tight junction proteins occludin and claudin-1 in the intestine following EtOH intoxication combined with burn injury. Furthermore, it increases cell death in the intestinal mucosa. Altogether these findings suggest that IL-18 plays a critical role in impaired intestinal mucosal integrity following EtOH intoxication combined with burn injury.
Highlights.
An increase in IL-18 following alcohol and burn injury:
decreases the expression and phosphorylation of occludin in intestine
decreases claudin-1 phosphorylation but does not affect its expression in intestine
increases the levels of cleaved caspase-3 in intestine
increases intestinal cell death
Acknowledgements
This study was supported from NIH through R01AA015731 and R21AA015979.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure
The authors have no financial conflict of interests.
Reference List
- 1.Will C, Fromm M, Muller D. Claudin tight junction proteins: novel aspects in paracellular transport. Perit.Dial.Int. 2008;28:577–584. [PubMed] [Google Scholar]
- 2.Forster C. Tight junctions and the modulation of barrier function in disease. Histochem.Cell Biol. 2008;130:55–70. doi: 10.1007/s00418-008-0424-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clayburgh DR, Shen L, Turner JR. A porous defense: the leaky epithelial barrier in intestinal disease. Lab Invest. 2004;84:282–291. doi: 10.1038/labinvest.3700050. [DOI] [PubMed] [Google Scholar]
- 4.Kunisawa J, Kiyono H. A marvel of mucosal T cells and secretory antibodies for the creation of first lines of defense. Cell Mol.Life Sci. 2005;62:1308–1321. doi: 10.1007/s00018-005-5035-1. [DOI] [PubMed] [Google Scholar]
- 5.Mestecky J, Russell MW, Elson CO. Intestinal IgA: novel views on its function in the defence of the largest mucosal surface. Gut. 1999;44:2–5. doi: 10.1136/gut.44.1.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baker JW, Deitch EA, Li M, Berg RD, Specian RD. Hemorrhagic shock induces bacterial translocation from the gut. J.Trauma. 1988;28:896–906. doi: 10.1097/00005373-198807000-00002. [DOI] [PubMed] [Google Scholar]
- 7.Choudhry MA, Fazal N, Goto M, Gamelli RL, Sayeed MM. Gut-associated lymphoid T cell suppression enhances bacterial translocation in alcohol and burn injury. Am.J.Physiol Gastrointest.Liver Physiol. 2002;282:G937–G947. doi: 10.1152/ajpgi.00235.2001. [DOI] [PubMed] [Google Scholar]
- 8.Faries PL, Simon RJ, Martella AT, Lee MJ, Machiedo GW. Intestinal permeability correlates with severity of injury in trauma patients. J.Trauma. 1998;44:1031–1035. doi: 10.1097/00005373-199806000-00016. [DOI] [PubMed] [Google Scholar]
- 9.Herndon DN, Zeigler ST. Bacterial translocation after thermal injury. Crit Care Med. 1993;21:S50–S54. doi: 10.1097/00003246-199302001-00010. [DOI] [PubMed] [Google Scholar]
- 10.American Burn Association . Anonymous. 2000. Burn incidence and treatment in the US: 2000 fact sheet. [Google Scholar]
- 11.Grobmyer SR, Maniscalco SP, Purdue GF, Hunt JL. Alcohol, drug intoxication, or both at the time of burn injury as a predictor of complications and mortality in hospitalized patients with burns. J.Burn Care Rehabil. 1996;17:532–539. doi: 10.1097/00004630-199611000-00010. [DOI] [PubMed] [Google Scholar]
- 12.Haum A, Perbix W, Hack HJ, Stark GB, Spilker G, Doehn M. Alcohol and drug abuse in burn injuries. Burns. 1995;21:194–199. doi: 10.1016/0305-4179(95)80008-c. [DOI] [PubMed] [Google Scholar]
- 13.Jones JD, Barber B, Engrav L, Heimbach D. Alcohol use and burn injury. J.Burn Care Rehabil. 1991;12:148–152. doi: 10.1097/00004630-199103000-00012. [DOI] [PubMed] [Google Scholar]
- 14.Choudhry MA, Rana SN, Kavanaugh MJ, Kovacs EJ, Gamelli RL, Sayeed MM. Impaired intestinal immunity and barrier function: a cause for enhanced bacterial translocation in alcohol intoxication and burn injury. Alcohol. 2004;33:199–208. doi: 10.1016/j.alcohol.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 15.Marshall SW, Runyan CW, Bangdiwala SI, Linzer MA, Sacks JJ, Butts JD. Fatal residential fires: who dies and who survives? JAMA. 1998;279:1633–1637. doi: 10.1001/jama.279.20.1633. [DOI] [PubMed] [Google Scholar]
- 16.Faunce DE, Garner JL, Llanas JN, Patel PJ, Gregory MS, Duffner LA, Gamelli RL, Kovacs EJ. Effect of acute ethanol exposure on the dermal inflammatory response after burn injury. Alcohol Clin.Exp.Res. 2003;27:1199–1206. doi: 10.1097/01.ALC.0000075833.92139.35. [DOI] [PubMed] [Google Scholar]
- 17.Napolitano LM, Koruda MJ, Zimmerman K, McCowan K, Chang J, Meyer AA. Chronic ethanol intake and burn injury: evidence for synergistic alteration in gut and immune integrity. J.Trauma. 1995;38:198–207. doi: 10.1097/00005373-199502000-00008. [DOI] [PubMed] [Google Scholar]
- 18.Li X, Rana SN, Schwacha MG, Chaudry IH, Choudhry MA. A novel role for IL-18 in corticosterone-mediated intestinal damage in a two-hit rodent model of alcohol intoxication and injury. J Leukoc Biol. 2006;80:367–375. doi: 10.1189/jlb.1205745. [DOI] [PubMed] [Google Scholar]
- 19.Rana SN, Li X, Chaudry IH, Bland KI, Choudhry MA. Inhibition of IL-18 reduces myeloperoxidase activity and prevents edema in intestine following alcohol and burn injury. J Leukoc Biol. 2005;77:719–728. doi: 10.1189/jlb.0704396. [DOI] [PubMed] [Google Scholar]
- 20.Li X, Schwacha MG, Chaudry IH, Choudhry MA. Acute Alcohol intoxication potentiates neutrophil-mediated intestine tissue damage following burn injury. Shock. 2008;29:377–383. doi: 10.1097/shk.0b013e31815abe80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li X, Schwacha MG, Chaudry IH, Choudhry MA. Heme oxygenase-1 protects against neutrophil-mediated intestinal damage by down-regulation of neutrophil p47phox and p67phox activity and O2-production in a two-hit model of alcohol intoxication and burn injury. J Immunol. 2008;180:6933–6940. doi: 10.4049/jimmunol.180.10.6933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jordan JA, Guo RF, Yun EC, Sarma V, Warner RL, Crouch LD, Senaldi G, Ulich TR, Ward PA. Role of IL-18 in acute lung inflammation. J.Immunol. 2001;167:7060–7068. doi: 10.4049/jimmunol.167.12.7060. [DOI] [PubMed] [Google Scholar]
- 23.Kashiwamura S, Ueda H, Okamura H. Roles of interleukin-18 in tissue destruction and compensatory reactions. J.Immunother. 2002;25(Suppl 1):S4–11. doi: 10.1097/00002371-200203001-00002. [DOI] [PubMed] [Google Scholar]
- 24.Nakanishi K, Yoshimoto T, Tsutsui H, Okamura H. Interleukin-18 regulates both Th1 and Th2 responses. Annu.Rev.Immunol. 2001;19:423–474. doi: 10.1146/annurev.immunol.19.1.423. [DOI] [PubMed] [Google Scholar]
- 25.Netea MG, Fantuzzi G, Kullberg BJ, Stuyt RJ, Pulido EJ, McIntyre RC, Jr., Joosten LA, Van der Meer JW, Dinarello CA. Neutralization of IL-18 reduces neutrophil tissue accumulation and protects mice against lethal Escherichia coli and Salmonella typhimurium endotoxemia. J.Immunol. 2000;164:2644–2649. doi: 10.4049/jimmunol.164.5.2644. [DOI] [PubMed] [Google Scholar]
- 26.Sivakumar PV, Westrich GM, Kanaly S, Garka K, Born TL, Derry JM, Viney JL. Interleukin 18 is a primary mediator of the inflammation associated with dextran sulphate sodium induced colitis: blocking interleukin 18 attenuates intestinal damage. Gut. 2002;50:812–820. doi: 10.1136/gut.50.6.812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Halpern MD, Khailova L, Molla-Hosseini D, Arganbright K, Reynolds C, Yajima M, Hoshiba J, Dvorak B. Decreased development of necrotizing enterocolitis in IL-18-deficient mice, Am.J.Physiol Gastrointest. Liver Physiol. 2008;294:G20–G26. doi: 10.1152/ajpgi.00168.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pizarro TT, Michie MH, Bentz M, Woraratanadharm J, Smith MF, Jr., Foley E, Moskaluk CA, Bickston SJ, Cominelli F. IL-18, a novel immunoregulatory cytokine, is up-regulated in Crohn’s disease: expression and localization in intestinal mucosal cells. J.Immunol. 1999;162:6829–6835. [PubMed] [Google Scholar]
- 29.Akhtar S, Li X, Chaudry IH, Choudhry MA. Neutrophil chemokines and their role in IL-18-mediated increase in neutrophil O2-production and intestinal edema following alcohol intoxication and burn injury. Am.J.Physiol Gastrointest.Liver Physiol. 2009;297:G340–G347. doi: 10.1152/ajpgi.00044.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Choudhry MA, Uddin S, Sayeed MM. Prostaglandin E2 modulation of p59fyn tyrosine kinase in T lymphocytes during sepsis. J.Immunol. 1998;160:929–935. [PubMed] [Google Scholar]
- 31.Li X, Chaudry IH, Choudhry MA. ERK and not p38 pathway is required for IL-12 restoration of T cell IL-2 and IFN-gamma in a rodent model of alcohol intoxication and burn injury. J.Immunol. 2009;183:3955–3962. doi: 10.4049/jimmunol.0804103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yu LC, Flynn AN, Turner JR, Buret AG. SGLT-1-mediated glucose uptake protects intestinal epithelial cells against LPS-induced apoptosis and barrier defects: a novel cellular rescue mechanism? FASEB J. 2005;19:1822–1835. doi: 10.1096/fj.05-4226com. [DOI] [PubMed] [Google Scholar]
- 33.Furuse M, Hirase T, Itoh M, Nagafuchi A, Yonemura S, Tsukita S, Tsukita S. Occludin: a novel integral membrane protein localizing at tight junctions. J.Cell Biol. 1993;123:1777–1788. doi: 10.1083/jcb.123.6.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Balda MS, Whitney JA, Flores C, Gonzalez S, Cereijido M, Matter K. Functional dissociation of paracellular permeability and transepithelial electrical resistance and disruption of the apical-basolateral intramembrane diffusion barrier by expression of a mutant tight junction membrane protein. J.Cell Biol. 1996;134:1031–1049. doi: 10.1083/jcb.134.4.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Feldman GJ, Mullin JM, Ryan MP. Occludin: structure, function and regulation. Adv.Drug Deliv.Rev. 2005;57:883–917. doi: 10.1016/j.addr.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 36.Sakakibara A, Furuse M, Saitou M, ndo-Akatsuka Y, Tsukita S. Possible involvement of phosphorylation of occludin in tight junction formation. J.Cell Biol. 1997;137:1393–1401. doi: 10.1083/jcb.137.6.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Farshori P, Kachar B. Redistribution and phosphorylation of occludin during opening and resealing of tight junctions in cultured epithelial cells. J.Membr.Biol. 1999;170:147–156. doi: 10.1007/s002329900544. [DOI] [PubMed] [Google Scholar]
- 38.Chen YH, Lu Q, Goodenough DA, Jeansonne B. Nonreceptor tyrosine kinase c-Yes interacts with occludin during tight junction formation in canine kidney epithelial cells. Mol.Biol.Cell. 2002;13:1227–1237. doi: 10.1091/mbc.01-08-0423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tsukamoto T, Nigam SK. Role of tyrosine phosphorylation in the reassembly of occludin and other tight junction proteins. Am.J.Physiol. 1999;276:F737–F750. doi: 10.1152/ajprenal.1999.276.5.F737. [DOI] [PubMed] [Google Scholar]
- 40.Gloor SM, Weber A, Adachi N, Frei K. Interleukin-1 modulates protein tyrosine phosphatase activity and permeability of brain endothelial cells. Biochem.Biophys.Res.Commun. 1997;239:804–809. doi: 10.1006/bbrc.1997.7557. [DOI] [PubMed] [Google Scholar]
- 41.Staddon JM, Herrenknecht K, Smales C, Rubin LL. Evidence that tyrosine phosphorylation may increase tight junction permeability. J.Cell Sci. 1995;108(Pt 2):609–619. doi: 10.1242/jcs.108.2.609. [DOI] [PubMed] [Google Scholar]
- 42.Rao RK, Basuroy S, Rao VU, Karnaky KJ, Jr, Gupta A. Tyrosine phosphorylation and dissociation of occludin-ZO-1 and E-cadherin-beta-catenin complexes from the cytoskeleton by oxidative stress. Biochem.J. 2002;368:471–481. doi: 10.1042/BJ20011804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Elias BC, Suzuki T, Seth A, Giorgianni F, Kale G, Shen L, Turner JR, Naren A, Desiderio DM, Rao R. Phosphorylation of Tyr-398 and Tyr-402 in occludin prevents its interaction with ZO-1 and destabilizes its assembly at the tight junctions. J.Biol.Chem. 2009;284:1559–1569. doi: 10.1074/jbc.M804783200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gonzalez-Mariscal L, Tapia R, Chamorro D. Crosstalk of tight junction components with signaling pathways. Biochim.Biophys.Acta. 2008;1778:729–756. doi: 10.1016/j.bbamem.2007.08.018. [DOI] [PubMed] [Google Scholar]
- 45.Fujibe M, Chiba H, Kojima T, Soma T, Wada T, Yamashita T, Sawada N. Thr203 of claudin-1, a putative phosphorylation site for MAP kinase, is required to promote the barrier function of tight junctions. Exp.Cell Res. 2004;295:36–47. doi: 10.1016/j.yexcr.2003.12.014. [DOI] [PubMed] [Google Scholar]
- 46.Weber CR, Nalle SC, Tretiakova M, Rubin DT, Turner JR. Claudin-1 and claudin-2 expression is elevated in inflammatory bowel disease and may contribute to early neoplastic transformation. Lab Invest. 2008;88:1110–1120. doi: 10.1038/labinvest.2008.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nunbhakdi-Craig V, Machleidt T, Ogris E, Bellotto D, White CL, III, Sontag E. Protein phosphatase 2A associates with and regulates atypical PKC and the epithelial tight junction complex. J.Cell Biol. 2002;158:967–978. doi: 10.1083/jcb.200206114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ko JA, Yanai R, Nishida T. Up-regulation of ZO-1 expression and barrier function in cultured human corneal epithelial cells by substance P. FEBS Lett. 2009;583:2148–2153. doi: 10.1016/j.febslet.2009.05.010. [DOI] [PubMed] [Google Scholar]
- 49.Coopersmith CM, Stromberg PE, Dunne WM, Davis CG, Amiot DM, Buchman TG, Karl IE, Hotchkiss RS. Inhibition of intestinal epithelial apoptosis and survival in a murine model of pneumonia-induced sepsis. JAMA. 2002;287:1716–1721. doi: 10.1001/jama.287.13.1716. [DOI] [PubMed] [Google Scholar]
- 50.Hagiwara C, Tanaka M, Kudo H. Increase in colorectal epithelial apoptotic cells in patients with ulcerative colitis ultimately requiring surgery. J.Gastroenterol.Hepatol. 2002;17:758–764. doi: 10.1046/j.1440-1746.2002.02791.x. [DOI] [PubMed] [Google Scholar]
- 51.Noda T, Iwakiri R, Fujimoto K, Matsuo S, Aw TY. Programmed cell death induced by ischemia-reperfusion in rat intestinal mucosa. Am.J.Physiol. 1998;274:G270–G276. doi: 10.1152/ajpgi.1998.274.2.G270. [DOI] [PubMed] [Google Scholar]
- 52.Wolf SE, Ikeda H, Matin S, DebRoy MA, Rajaraman S, Herndon DN, Thompson JC. Cutaneous burn increases apoptosis in the gut epithelium of mice. J.Am.Coll.Surg. 1999;188:10–16. doi: 10.1016/s1072-7515(98)00260-9. [DOI] [PubMed] [Google Scholar]
- 53.Zeissig S, Bojarski C, Buergel N, Mankertz J, Zeitz M, Fromm M, Schulzke JD. Downregulation of epithelial apoptosis and barrier repair in active Crohn’s disease by tumour necrosis factor alpha antibody treatment. Gut. 2004;53:1295–1302. doi: 10.1136/gut.2003.036632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Al-Sadi RM, Ma TY. IL-1beta causes an increase in intestinal epithelial tight junction permeability. J.Immunol. 2007;178:4641–4649. doi: 10.4049/jimmunol.178.7.4641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Capaldo CT, Nusrat A. Cytokine regulation of tight junctions. Biochim.Biophys.Acta. 2009;1788:864–871. doi: 10.1016/j.bbamem.2008.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Colgan SP, Resnick MB, Parkos CA, lp-Archer C, McGuirk D, Bacarra AE, Weller PF, Madara JL. IL-4 directly modulates function of a model human intestinal epithelium. J.Immunol. 1994;153:2122–2129. [PubMed] [Google Scholar]
- 57.Mazzon E, Puzzolo D, Caputi AP, Cuzzocrea S. Role of IL-10 in hepatocyte tight junction alteration in mouse model of experimental colitis. Mol.Med. 2002;8:353–366. [PMC free article] [PubMed] [Google Scholar]
- 58.Yang R, Han X, Uchiyama T, Watkins SK, Yaguchi A, Delude RL, Fink MP. IL-6 is essential for development of gut barrier dysfunction after hemorrhagic shock and resuscitation in mice. Am.J.Physiol Gastrointest.Liver Physiol. 2003;285:G621–G629. doi: 10.1152/ajpgi.00177.2003. [DOI] [PubMed] [Google Scholar]
- 59.Youakim A, Ahdieh M. Interferon-gamma decreases barrier function in T84 cells by reducing ZO-1 levels and disrupting apical actin. Am.J.Physiol. 1999;276:G1279–G1288. doi: 10.1152/ajpgi.1999.276.5.G1279. [DOI] [PubMed] [Google Scholar]
- 60.Li X, Akhtar S, Kovacs EJ, Gamelli RL, Choudhry MA. Inflammatory response in multiple organs in a mouse model of acute alcohol intoxication and burn injury Journal of Burn Care & Research. 2011;32:489–497. doi: 10.1097/BCR.0b013e3182223c9e. [DOI] [PMC free article] [PubMed] [Google Scholar]