Abstract
Purpose
To describe visual acuity (VA) and inflammation following cataract surgery in eyes with noninfectious posterior uveitis (NIPU) that were being treated with a fluocinolone acetonide (FA) intravitreal implant compared with those that were not.
Design
Post hoc, subgroup analysis of data from a 3-year, dose-masked, randomized, multicenter trial evaluating the FA implant for the treatment of NIPU.
Participants and controls
The subset of eyes that underwent cataract surgery during the 3-year trial. Eyes were either implanted with a 0.59- or a 2.1-mg FA implant, or, in the case of affected fellow eyes, received standard-of-care local treatment.
Main outcome measures
VA, anterior and posterior chamber inflammation at 1 and 3 months after surgery, and rate of uveitis recurrence and serious postoperative ocular adverse events.
Results
Of 278 patients enrolled in the main trial, 132/142 phakic implanted eyes and 39/186 phakic non-implanted eyes underwent cataract surgery. Mean improvement in VA was significantly greater in implanted than non-implanted eyes at 1 (P = 0.0047) and 3 months (P = 0.0015) postoperatively; significantly fewer anterior chamber cells were seen in implanted than non-implanted eyes at 1 (P = 0.0084) and 3 months (P = 0.0002). Severity of vitreous haze was less in implanted than non-implanted eyes at 3 months postoperatively (P = 0.0005). The postsurgical uveitis recurrence rate was lower in implanted than non-implanted eyes (26.5% vs 44.4%; P = 0.0433). Glaucoma was reported in 19.7% of implanted eyes and no non-implanted eyes (P = 0.0008) postoperatively.
Conclusion
In this post hoc subgroup analysis, eyes with NIPU treated with the FA intravitreal implant demonstrated better vision and less intraocular inflammation following cataract surgery than non-implanted eyes. Recurrent uveitic inflammation did not appear to be triggered by cataract surgery. Glaucoma occurred more frequently in implanted eyes.
Keywords: cataract surgery, posterior chamber inflammation, glaucoma, intraocular pressure, visual acuity, steroid implant
Introduction
Noninfectious posterior uveitis (NIPU) is a sight-threatening but often manageable disease. Current treatment options include corticosteroids delivered topically, systemically, or via sub-Tenon’s or intravitreal depot injection, and immunosuppressive agents delivered systemically.1 These therapies may be effective but are associated with significant adverse events, including those that often accompany topical or systemic corticosteroid or immunosuppressive treatment, and the potential complications of peri-ocular and intraocular injections. Given the need for chronic therapy in patients with NIPU, treatment-associated complications often have cumulative effects, resulting in steroid-induced osteoporosis and intraocular pressure (IOP) elevation.1
An intravitreal implant containing fluocinolone acetonide (FA; Bausch and Lomb Incorporated, Retisert®, Rochester, NY) was approved by the US Food and Drug Administration in April 2005. This sustained-release implant is designed to provide steady-state levels of FA to the posterior segment of the eye for up to 2.5 years. Its efficacy and safety in eyes with NIPU has been demonstrated in several studies.2–4
Cataract formation is a well-documented complication of corticosteroid therapy and, in addition to steroid-induced glaucoma, was a common adverse event in the phakic eyes enrolled in registry trials of the FA intravitreal implant.2 The purpose of this index study was to describe the outcomes of the subgroup of eyes requiring cataract surgery in a clinical trial investigating the safety and efficacy of the FA intravitreal implant. Outcomes of this post hoc analysis included visual acuity (VA), inflammation (measured by anterior chamber cell number and posterior vitreous haze), uveitis recurrence, and ocular serious adverse event (SAE) occurrence.
Methods
Study
This report consists of an analysis of data collected during a Phase IIb/III clinical trial evaluating the role of the FA intravitreal implant in eyes with NIPU. The trial was a multicenter, prospective, dose-masked, randomized, historically controlled trial in patients with unilateral or bilateral disease. One eye of patients with NIPU was randomized to receive a 0.59- or 2.1-mg FA intravitreal implant and was followed for 3 years. In bilaterally affected patients, the eye with more severe disease received the implant (ie, the eye that suffered more recurrences in the previous year; or if equal, the eye that received more treatment in the previous year; or if equal, the eye having the worse VA or if equal, the eye clinically judged by the treating physician to be more severely affected).2 Indication for cataract surgery on either implanted or non-implanted eyes was at the discretion of the individual study center. The trial was approved by the appropriate institutional review board at each institution, and all participating patients provided written informed consent.
Implant
The FA intravitreal implant and the surgical implantation procedure have been described elsewhere.2,3 Briefly, the FA intravitreal implant is designed to provide sustained delivery of FA to the posterior segment of the eye for up to 2.5 years. The implant is placed in the vitreous cavity through a pars plana scleral incision and secured with an 8-0 prolene anchor suture.
Patients and analysis
The analysis was performed on a subset of enrolled patients who received a FA implant and subsequently underwent cataract surgery in the implanted eye or the non-implanted eye at any time within 3 years of implantation (the study period). Some subjects may have undergone cataract extraction in both eyes. Because most patients had only one eye included in the analysis, and because the effects of cataract surgery are primarily monocular, the correlation between eyes in a single patient was not modeled. To optimize comparability between the implanted and non-implanted eyes, non-implanted eyes without a diagnosis of NIPU at the time of enrollment were excluded from this analysis.
Outcomes
Comparisons of VA measured with standard Early Treatment Diabetic Retinopathy Study (ETDRS) charts and procedures, and ocular inflammation between implanted and non-implanted eyes were made 1 and 3 months after cataract surgery. Anterior chamber inflammation was assessed by the number of anterior chamber cells seen under high magnification using a 1-mm beam, reported on a 0–4 scale ranging from Trace (0–4 cells; grade 0) to 4+ (>50 cells; grade 4).5 Posterior inflammation was assessed by the severity of vitreous haze (grade 0–5) observed during ophthalmoscopy and compared to photographic standards.6 Investigator meetings prior to the initiation of the main trial included standardized training on the grading scales. All ETDRS VA evaluators were certified.
Non-implanted eyes had a higher degree of vitreous haze prior to cataract surgery than implanted eyes. To account for this difference, an adjusted mean change was calculated from a linear model of change from baseline vitreous haze and implant status of the eye. Because the increased severity of vitreous haze in an eye that starts at a grade of 3+ is limited to 1 grade while the increase in an eye that starts at 1+ can be up to 3 grades, this technique accounts for the vitreous haze after cataract surgery in a more statistically meaningful manner. At both 1 and 3 months, analyses were performed on the changes between the grades at the most recent examination prior to the cataract surgery and those obtained after surgery. In addition, uveitis recurrence rates and the occurrence of ocular SAEs were evaluated from the time of cataract surgery until the end of the study and were compared between implanted and non-implanted eyes.
Ocular SAEs were defined as any event that required surgical intervention, endophthalmitis, retinal detachment, repeated IOP >30 mmHg, any event causing a six-line drop in VA, or other events the investigator considered sight threatening or serious. Ocular SAEs that have been observed in clinical trials of the FA implant include cataract formation, increased IOP, glaucoma, hypotony, and procedural complications such as eye pain, endophthalmitis, retinal detachment, and vitreous hemorrhage or loss.2–4
Statistics
All hypothesis tests were two-sided and employed a level of significance of α = 0.05, conducted in statistical analysis software (v 8; SAS Institute Inc, Cary, NC). No adjustment for type I error was made for multiple comparisons. For the comparison of implanted versus non-implanted eyes, with respect to continuously distributed parameters, an analysis of covariance (ANCOVA) was employed, which included the fixed-effect treatment (implanted or non-implanted), with the pre-surgery measurement as a covariate. Within-treatment changes from before to after surgery were evaluated using one-sample t-tests in concert with ANCOVA. Between-treatment comparisons of implanted and non-implanted eyes with respect to parameters measured on a dichotomous scale employed Fisher’s exact test.
Results
Of 142 phakic study eyes (eyes that received an implant) and 186 phakic eyes that did not receive an implant, 132 and 39, respectively, underwent cataract surgery while the patients were enrolled in the uveitis trial. Patient demographics are indicated in Table 1. More patients were male (71.9%) and Caucasian (68.4%); their ages ranged from 7 to 72 years. Because results were similar in eyes receiving the 0.59- and 2.1-mg FA intravitreal implants, all implanted eyes were pooled for analysis.
Table 1.
Patient demographics
| Parameter | Implanted eyes (n = 132) | Non-implanted eyes (n = 39) |
|---|---|---|
| Age (years) | ||
| Mean ± SD | 40.5 ± 13.68 | 46.5 ± 13.43 |
| Median | 41 | 46 |
| Range | 7–72 | 16–71 |
| Gender, n (%) | ||
| Female | 39 (29.5) | 9 (23.1) |
| Male | 93 (70.5) | 30 (76.9) |
| Ethnicity, n (%) | ||
| Caucasian | 90 (68.2) | 27 (69.2) |
| African American | 17 (12.9) | 7 (17.9) |
| Asian | 14 (10.6) | 2 (5.1) |
| H ispanic | 8 (6.1) | 3 (7.7) |
| Other | 3 (2.3) | 0 (0) |
Abbreviation: SD, standard deviation.
Visual acuity
Baseline VA measured at the most recent visit preceding cataract surgery demonstrated that implanted and non-implanted eyes undergoing cataract extraction had similar preoperative VA (mean ± standard deviation [SD] log-MAR, 0.84 ± 0.50 for implanted eyes and 0.80 ± 0.50 for non-implanted eyes). Following cataract extraction, implanted eyes demonstrated greater improvement in vision than non-implanted eyes at both 1 and 3 months postoperatively (Table 2). At 1 month after cataract surgery, VA improved by 0.45 ± 0.43 logMAR (22.5 ± 21.5 ETDRS letters) in implanted eyes compared with 0.27 ± 0.46 logMAR (13.5 ± 23.0 ETDRS letters) in non-implanted eyes (P = 0.0047). At 3 months after cataract surgery, VA improved by 0.49 ± 0.49 logMAR (24.5 ± 24.5 ETDRS letters) in implanted eyes compared with 0.26 ± 0.45 log-MAR (13.0 ± 22.5 ETDRS letters) in non-implanted eyes (P = 0.0015).
Table 2.
Visual acuity outcomes
| Treatment | Mean ± SD visual acuity change (logMAR) from baselinea | |||
|---|---|---|---|---|
|
|
||||
| Nb | 1 month | Nb | 3 months | |
| Implanted eyes | 91 | 0.450 ± 0.429 | 130 | 0.493 ± 0.490 |
| Non-implanted eyes | 28 | 0.270 ± 0.456 | 38 | 0.260 ± 0.447 |
| Pc | 0.0047 | 0.0015 | ||
Notes: Changes from scores obtained at the most recent examination prior to cataract surgery (higher value indicates greater improvement);
eyes without missing data;
comparison between implanted and non-implanted eyes.
Abbreviations: logMAR, logarithm of the minimum angle of resolution; SD, standard deviation.
Post-cataract surgery inflammation
On average, non-implanted eyes had more anterior chamber inflammation than implanted eyes prior to cataract surgery. The mean ± SD anterior chamber cell severity at the most recent visit preceding cataract extraction was 0.11 ± 0.50 for implanted eyes and 0.28 ± 0.69 for non-implanted eyes. Inflammation outcomes after cataract surgery are presented in Table 3. One month after cataract extraction the mean change in anterior chamber cell grades was 0.06 ± 0.59 in implanted eyes and 0.15 ± 1.06 in non-implanted eyes (P = 0.0084). Three months after cataract surgery the mean change in anterior chamber cell grades was 0.00 ± 0.63 in implanted eyes and 0.21 ± 1.23 in non-implanted eyes (P = 0.0002).
Table 3.
Changes in anterior chamber cell and vitreous haze severity among studied patients
| Inflammation score | Mean ± SD change from baselinea | |||
|---|---|---|---|---|
|
|
||||
| Nb | 1 month | Nb | 3 months | |
| Anterior chamber cell gradec | ||||
| Implanted eyes | 108 | 0.056 ± 0.593 | 131 | 0.000 ± 0.632 |
| Non-implanted eyes | 33 | 0.152 ± 1.064 | 38 | 0.211 ± 1.234 |
| Pd | 0.0084 | 0.0002 | ||
| Adjusted vitreous haze gradec | ||||
| Implanted eyes | 99 | −0.256 ± 0.706 | 131 | −0.248 ± 0.714 |
| Non-implanted eyes | 32 | −0.083 ± 0.738 | 38 | 0.250 ± 0.745 |
| Pd | 0.2613 | 0.0005 | ||
Notes: Changes from grade assessed at the most recent examination prior to cataract surgery;
eyes without missing data;
see text for definition;
comparison between implanted and non-implanted eyes.
Abbreviation: SD, standard deviation.
Similarly, non-implanted eyes had, on average, more vitreous haze than implanted eyes prior to cataract surgery (mean ± SD vitreous haze severity, 0.42 ± 0.80 and 1.28 ± 1.32 for implanted and non-implanted eyes, respectively), which led to a substantial difference between the observed mean change and the adjusted mean change results. Implanted eyes demonstrated a mean change in vitreous haze severity of −0.09 ± 0.83 (−0.26 ± 0.71, adjusted) at 1 month and a change of −0.13 ± 0.83 (−0.25 ± 0.71, adjusted) at 3 months after cataract surgery. The change for non-implanted eyes was −0.59 ± 1.27 (−0.08 ± 0.74, adjusted) at 1 month and −0.16 ± 1.08 (0.25 ± 0.75, adjusted) at 3 months after cataract surgery. The difference in the vitreous haze change was significant only at 3 months (P = 0.0005) (Table 3).
Uveitis recurrence
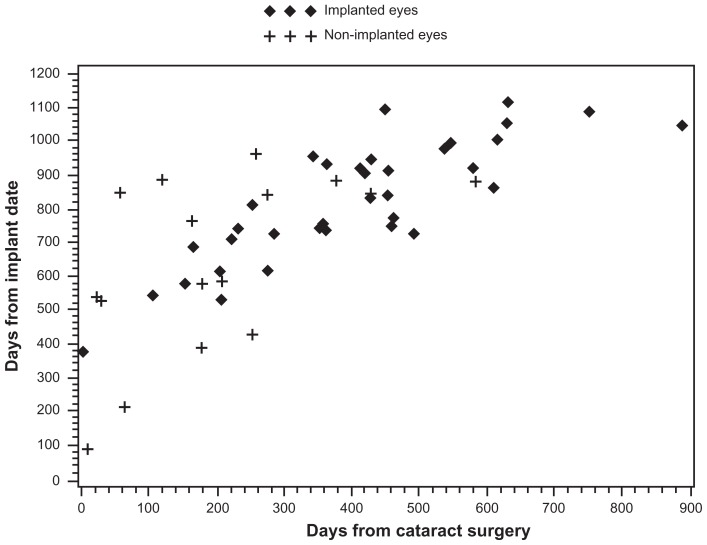
The incidence of uveitis recurrence after cataract surgery, to the end of the study, was compared in implanted and non-implanted eyes. This analysis was not limited to the 3-month postoperative period. The rate of uveitis recurrence after cataract surgery was statistically significantly greater in non-implanted eyes than in implanted eyes (44.4% vs 26.5%; P = 0.0433). Figure 1 illustrates the time course to recurrence of uveitis for implanted and non-implanted eyes as a function of time following both procedures undergone by all study patients (FA implantation and cataract extraction). The figure demonstrates that more non-implanted eyes than implanted eyes experienced a recurrence of uveitis within the first 3 months after cataract surgery. Onset of many of the recurrences reported for implanted eyes occurred well after cataract extraction and intravitreal FA implantation, suggesting that, in at least some of these eyes, drug depletion may have played a role in the recurrence.
Figure 1.
Uveitis recurrences, which were significantly more likely in non-implanted than implanted eyes (16/36 [44.4%] vs 35/132 [26.5%], P = 0.0433), as a function of time since fluocinolone acetonide implant surgery and cataract extraction surgery. Likelihood of recurrence within 3 months of cataract surgery was greater in non-implanted than implanted eyes; a large proportion of the recurrences documented in implanted eyes occurred more than 2 years after implantation.
Serious adverse events
The most common ocular SAE, and the only one reported at statistically significantly different rates following cataract surgery in these eyes, was glaucoma (19.7% of implanted eyes compared with 0% of non-implanted eyes; P = 0.0008). Other ocular SAEs occurring at a rate >5% in either group included IOP increase (15.15% in implanted eyes vs 10.26% in non-implanted eyes, P = 0.6017), hypotony of the eye (7.58% vs 2.56%, respectively, P = 0.4507), and retinal detachment (1.52% vs 5.13%, respectively, P = 0.2238).
Discussion
Cataract formation and progression is common in eyes with uveitis. Cataractogenesis is attributable both to the inflammatory process and to the chronic use of corticosteroids to control the disease.7,8 The present study demonstrated that eyes with NIPU that were being treated with the FA intravitreal implant demonstrated better VA and less intraocular inflammation following cataract surgery than eyes with NIPU that were not being treated with the implant.
In this study, intravitreal FA-implanted eyes demonstrated significantly greater improvements from preoperative visual acuity than non-implanted eyes 3 months after cataract surgery with intraocular lens implantation. Only non-implanted eyes with NIPU were included in this analysis, and in all cases the worse eye was selected to receive the implant. Thus, on average, the eyes with more severe NIPU had the better visual outcomes, with a nearly two-fold greater improvement in vision attributable to ongoing treatment with the FA intravitreal implant.
In our index study, the VA outcomes compared favorably with outcomes following cataract surgery in uveitic eyes as reported by others. Okhravi et al9 reported a median improvement in VA of four Snellen lines in 37 eyes with posterior uveitis undergoing cataract surgery. Similarly, Estafanous et al10 reported a gain in VA of four Snellen lines among 39 eyes with anterior and/or posterior uveitis undergoing phacoemulsification. Ganesh et al11 reported that 77% of eyes with pars planitis undergoing cataract surgery gained ≥2 Snellen lines of VA, with 79% achieving a final acuity of 20/40 or better, and Kawaguchi et al12 reported that 85% of eyes with anterior and/or posterior uveitis experienced improvement in VA following cataract surgery, with 74% achieving acuity of 0.5 logMAR or better.
Cataract surgery in uveitic eyes poses the risk of significant postoperative inflammation, especially if an intraocular lens is placed. The superior VA outcomes following cataract surgery in intravitreal FA-implanted eyes are likely due, in part, to better suppression of postoperative inflammation in implanted eyes compared with non-implanted eyes. At both 1 and 3 months following cataract extraction, changes from preoperative levels of anterior chamber cells were significantly smaller in implanted eyes compared with non-implanted eyes, which still had increased levels of anterior chamber cells 3 months after cataract surgery. In addition, postoperative inflammation in the posterior segment, as measured by the adjusted change in vitreous haze severity, was better suppressed in the implanted eyes compared with the non-implanted eyes at 3 months.
Cataract surgery may precipitate the recurrence of uveitis in eyes that were well controlled preoperatively. In the present study, implanted eyes were significantly less likely to experience recurrence of active uveitis after cataract surgery than were non-implanted eyes. Unlike VA and postoperative inflammation endpoints, which were monitored only through the third postoperative month, recurrence of uveitis was monitored from the time of cataract surgery to the completion of the study. The majority of uveitis recurrences occurred well after the 3-month postoperative period, raising the question of whether these recurrences were related to cataract surgery. A significant proportion of uveitis recurrences were documented more than 1 year after cataract surgery and more than 2 years after intravitreal FA implantation (Figure 1). These observations suggest that cataract surgery was not directly responsible for recurrence in many eyes. Instead, the depletion of FA from the implant (designed to last for 2.5 years) may be manifesting as breakthrough inflammation during the third year after FA implantation. Uveitis recurrence following cataract extraction was reported in 13% of eyes by Kawaguchi et al,12 51% of eyes by Ganesh et al,13 and 41% of eyes by Estafanous et al.10 The rates of uveitis recurrence seen in the present study fall within the range of rates reported by these investigators. Differences between studies are likely attributable, in part, to the notoriously heterogeneous presentation of uveitis, uveitis therapies employed, surgical techniques, lens materials, and postsurgery follow-up as well as the many different types of uveitis enrolled, which vary by anatomic location, chronicity, etiology, and laterality.
The only ocular SAE noted to occur more frequently in implanted eyes than in non-implanted eyes after cataract surgery was glaucoma. Although glaucoma in this cohort was primarily defined by the investigator, the protocol specified that two consecutive IOP measurements ≥30 mmHg in a patient already on two glaucoma medications be reported as a SAE, regardless of the investigator’s opinion. Significant rates of elevated IOP associated with glaucoma in the FA-implanted eyes regardless of cataract status have been reported previously.2,14 The difference between implanted versus non-implanted eyes in the incidence of elevated IOP levels is likely related to the sustained delivery of corticosteroid to the posterior segment of the eye, as well as superior resolution of inflammatory ciliary body insufficiency by the FA implant and subsequent return to normal aqueous humor production.
Conclusion
Eyes with uveitis experience a host of insults arising from both the disease process, including chronicity and recurrence, and the corticosteroids frequently used to control it. The long-term management of chronic uveitis requires aggressive control of inflammation. Complications resulting from aggressive therapy, such as cataract formation, can more safely be addressed once the underlying disease process is controlled. The FA intravitreal implant provides excellent control of uveitis in the majority of eyes with NIPU for up to 2.5 years. Eyes with the FA implant that eventually underwent cataract surgery demonstrated better postoperative VA and less frequent recurrence of uveitis than non-implanted eyes, even though the FA-implanted eyes represented each patient’s worse eye. The only ocular SAE reported at a significantly higher rate for implanted eyes in the post-cataract extraction period was glaucoma, which, although lower than the overall rate due to the selective time frame in this analysis, is consistent with data previously reported for the full study cohort.14 Cataractogenesis is a common and manageable complication of FA intravitreal implantation for NIPU, particularly in light of the potentially severe irreversible complications frequently seen in patients receiving inadequate anti-inflammatory therapy.
Acknowledgments
This research was sponsored by Bausch and Lomb Incorporated, Rochester, NY. The sponsor participated in the design of the study; conducting the study; data collection, management, and analysis; interpretation of the data; and preparation, review, and approval of the manuscript. We would like to thank Richa Attre, PhD, for her assistance in preparing the manuscript.
Fluocinolone Acetonide Uveitis Study Group Principal Investigators
Rajiv Anand, MD, Dallas, TX; Brian Berger, MD, Austin, TX; David Callanan, MD, Arlington, TX; Kakarla V Chalam, MD, Jacksonville, FL; Janet L Davis, MD, Miami, FL; Pravin U Dugel, MD, Phoenix, AZ; JP Dunn Jr, MD, Baltimore, MD; C Stephen Foster, MD, Boston, MA; William Freeman, MD, La Jolla, CA; Debra A Goldstein, MD, Chicago, IL; Allen Ho, MD, Philadelphia, PA; Glenn J Jaffe, MD, Durham, NC; Baruch Kuppermann, MD, PhD, Irvine, CA; Paul Latkany, MD, New York, NY; Careen Y Lowder, MD, PhD, Cleveland, OH; Daniel F Martin, MD, Atlanta, GA; Pauline Merrill, MD, Chicago, IL; Ramana Moorthy, MD, Indianapolis, IN; Lawrence Morse, MD, PhD, Davis, CA; Stuart Noorily, MD, Teaneck, NJ; Peter Pavan, MD, Tampa, FL; Chee Soon Phaik, MD, Singapore; Michael B Raizman, MD, Boston, MA; James T Rosenbaum, MD, Portland, OR; John D Sheppard Jr, MD, Norfolk, VA; Russell van Gelder, MD, PhD, St Louis, MO; Albert Vitale, MD, Salt Lake City, UT.
Data and safety monitoring committee members
Alexander Brucker, MD, Philadelphia, PA (Chair); Theodore Colton, ScD, Boston, MA; Karen Gehrs, MD, Iowa City, IA; Lee Jampol, MD, Chicago, IL; Mark Johnson, MD, Ann Arbor, MI; Robert Levine, MD, New Haven, CT; David Musch, PhD, Ann Arbor, MI.
Footnotes
Disclosures
No conflicting relationship exists for any author except Dale W Usner, who was employed, and Timothy L Comstock, who is employed, by Bausch and Lomb Incorporated, Rochester, NY. John D Sheppard Jr and Quan Dong Nguyen are consultants to Bausch and Lomb Inc, Rochester, NY.
References
- 1.Becker MD, Smith JR, Max R, Fiehn C. Management of sight-threatening uveitis: new therapeutic options. Drugs. 2005;65(4):497–519. doi: 10.2165/00003495-200565040-00005. [DOI] [PubMed] [Google Scholar]
- 2.Callanan DG, Jaffe GJ, Martin DF, Pearson A, Comstock TL. Treatment of posterior uveitis with a fluocinolone acetonide implant. Arch Ophthalmol. 2008;126(9):1191–1201. doi: 10.1001/archopht.126.9.1191. [DOI] [PubMed] [Google Scholar]
- 3.Jaffe GJ, Ben-Nun J, Guo H, Dunn JP, Ashton P. Fluocinolone acetonide sustained drug delivery device to treat severe uveitis. Ophthalmology. 2000;107(11):2024–2033. doi: 10.1016/s0161-6420(00)00466-8. [DOI] [PubMed] [Google Scholar]
- 4.Jaffe GJ, McCallum RM, Branchaud B, Skalak C, Butuner Z, Ashton P. Long-term follow-up results of a pilot trial of a fluocinolone acetonide implant to treat posterior uveitis. Ophthalmology. 2005;112(7):1192–1198. doi: 10.1016/j.ophtha.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 5.Kimura SJ, Thygeson P, Hogan MJ. Signs and symptoms of uveitis. II. Classification of the posterior manifestations of uveitis. Am J Ophthalmol. 1959;47(5 Part 2):171–176. doi: 10.1016/s0002-9394(14)78240-6. [DOI] [PubMed] [Google Scholar]
- 6.Nussenblatt RB, Palestine AG, Chan CC, Roberge F. Standardization of vitreal inflammatory activity in intermediate and posterior uveitis. Ophthalmology. 1985;92(4):467–471. doi: 10.1016/s0161-6420(85)34001-0. [DOI] [PubMed] [Google Scholar]
- 7.Molteno AC, Bosma NJ, Kittelson JM. Otago glaucoma surgery outcome study: long-term results of trabeculectomy – 1976 to 1995. Ophthalmology. 1999;106(9):1742–1750. doi: 10.1016/S0161-6420(99)90351-2. [DOI] [PubMed] [Google Scholar]
- 8.Park UC, Ahn JK, Park KH, Yu HG. Phacotrabeculectomy with mitomycin C in patients with uveitis. Am J Ophthalmol. 2006;142(6):1005–1012. doi: 10.1016/j.ajo.2006.07.018. [DOI] [PubMed] [Google Scholar]
- 9.Okhravi N, Lightman SL, Towler HM. Assessment of visual outcome after cataract surgery in patients with uveitis. Ophthalmology. 1999;106(4):710–722. doi: 10.1016/S0161-6420(99)90155-0. [DOI] [PubMed] [Google Scholar]
- 10.Estafanous MF, Lowder CY, Meisler DM, Chauhan R. Phacoemulsification cataract extraction and posterior chamber lens implantation in patients with uveitis. Am J Ophthalmol. 2001;131(5):620–625. doi: 10.1016/s0002-9394(00)00909-0. [DOI] [PubMed] [Google Scholar]
- 11.Ganesh SK, Babu K, Biswas J. Phacoemulsification with intraocular lens implantation in cases of pars planitis. J Cataract Refract Surg. 2004;30(10):2072–2076. doi: 10.1016/j.jcrs.2004.02.090. [DOI] [PubMed] [Google Scholar]
- 12.Kawaguchi T, Mochizuki M, Miyata K, Miyata N. Phacoemulsification cataract extraction and intraocular lens implantation in patients with uveitis. J Cataract Refract Surg. 2007;33(2):305–309. doi: 10.1016/j.jcrs.2006.10.038. [DOI] [PubMed] [Google Scholar]
- 13.Ganesh SK, Padmaja, Babu K, Biswas J. Cataract surgery in patients with Vogt-Koyanagi-Harada syndrome. J Cataract Refract Surg. 2004;30(1):95–100. doi: 10.1016/S0886-3350(03)00552-2. [DOI] [PubMed] [Google Scholar]
- 14.Goldstein DA, Godfrey DG, Hall A, et al. Intraocular pressure in patients with uveitis treated with fluocinolone acetonide implants. Arch Ophthalmol. 2007;125(11):1478–1485. doi: 10.1001/archopht.125.11.ecs70063. [DOI] [PubMed] [Google Scholar]