Abstract
When you are looking for an object, does hearing its characteristic sound make you find it more quickly? Our recent results supported this possibility by demonstrating that when a cat target, for example, was presented among other objects, a simultaneously presented “meow” sound (containing no spatial information) reduced the manual response time for visual localization of the target. To extend these results, we determined how rapidly an object-specific auditory signal can facilitate target detection in visual search. On each trial, participants fixated a specified target object as quickly as possible. The target’s characteristic sound speeded the saccadic search time within 215–220 ms and also guided the initial saccade toward the target, compared to presentation of a distractor’s sound or to no sound. These results suggest that object-based auditory-visual interactions rapidly increase the target object’s salience in visual search.
Introduction
Sounds facilitate visual localization based on spatial coincidence. For example, a sound coming from the location of a visual target facilitates its detection (e.g., Bolognini, Frassinetti, Serino, & Làdavas, 2005; Driver & Spence, 1998; Stein, Meredith, Huneycutt, & McDade, 1989). A sound also facilitates visual localization based on temporal coincidence when a visual target has unique dynamics (compared to distractors) and a sound is synchronized to the target’s dynamics (Van der Burg, Olivers, Bronkhorst, & Theeuwes, 2008).
In addition to these well-established spatial and temporal auditory-visual interactions, neuroimaging results suggest that auditory-visual interactions also occur in an object specific manner in polysensory areas in the temporal cortex (e.g., Bauchamp, Argall, Bodurka, Duyn, & Martin, 2004; Bauchamp, Lee, Argall, & Martin, 2004; von Kriegstein, Kleinschmidt, Sterzer, & Giraud, 2005; Molholm, Ritter, Javitt, & Foxe, 2004). It is therefore possible that feedback from polysensory areas to visual areas could speed visual processing in an object specific manner. Consistent with this possibility, behavioral responses to target objects are faster when the target object (e.g., a cat) is presented together with its characteristic sound (e.g., a “meow” sound) for recognizing the visual target (Molholm et al., 2004) and for localizing the target among distractor objects (Iordanescu, Guzman-Martinez, Grabowecky, & Suzuki, 2008).
However, because these studies used manual responses (via key presses), it was not possible to directly demonstrate that characteristic sounds facilitated perception of the target object. Manual response times include additional processes such as confirming the identity of the target object, mapping the perceptual decision to an arbitrarily defined motor response, and executing the motor response. The present study was designed to circumvent these confounds associated with manual responses to more directly demonstrate that hearing a characteristic sound of an object facilitates its visual localization.
We used saccades as the mode of response in the context of visual search. Because people naturally look at objects of interest, asking participants to quickly fixate targets does not require an arbitrary response mapping. We measured the time it took for participants to saccade to the target object. It has been shown that even when a target location is known, it typically takes 150–350 ms (averaging 200–250 ms) to initiate a saccade (e.g., Darrien, Herd, Starling, Rosenberg, & Morrison, 2001; Yang, Pucci, & Kapoula, 2002). Thus, if we obtained significant speeding of saccades by characteristic sounds for fast saccadic responses (< 250 ms), we could reasonably conclude that characteristic sounds rapidly facilitate the process of target selection during the initial engagement of attention. Furthermore, the result would provide an upper estimate of how rapidly object-based auditory-visual neural interactions (potentially mediated by temporal polysensory areas) influence the retinotopic visual processing required for target localization.
Methods
Participants
Sixteen undergraduate students at Northwestern University gave informed consent to participate for partial course credit. They all had normal or corrected-to-normal visual acuity and normal hearing, and were tested individually in a normally lit room.
Stimuli
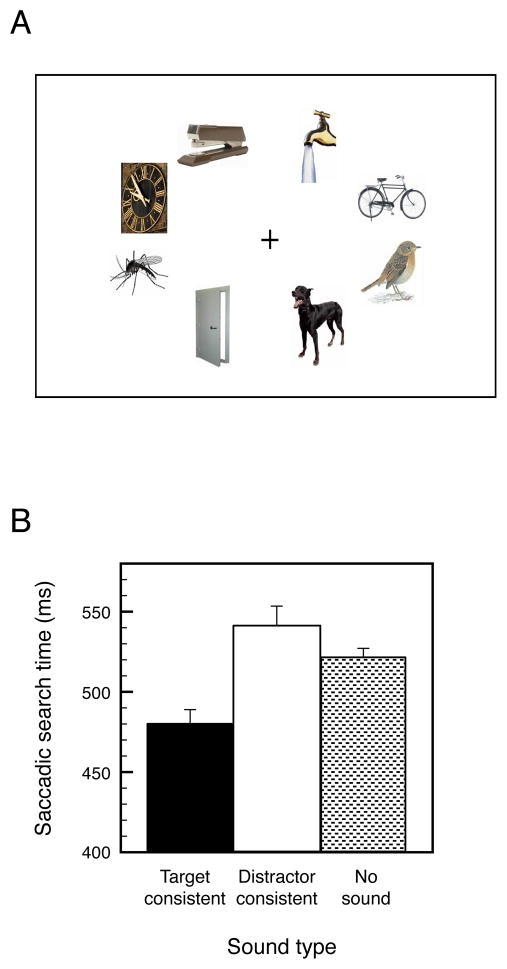
Each search display (see Figure 1A for an example) contained eight colored pictures of common objects (each confined within a 5.14° by 5.11° rectangular region). The centers of the eight pictures were placed along an approximate iso-acuity ellipse (20° horizontal by 15° vertical, the aspect ratio based on Rovamo & Virsu, 1979). One of these pictures was the target and the remaining pictures were the distractors. Search stimuli (some with backgrounds) and their characteristic sounds were selected from a set of 20 objects (bike, bird, car, cat, clock, coins, dog, door, running faucet, keys, kiss, lighter, mosquito, phone, piano, stapler, lightning, toilet, train, and wine glass; see Iordanescu et al., 2008 for the full set of images). We avoided inclusion of objects with similar characteristic sounds (e.g., keys and coins) within the same search display. The durations of characteristic sounds varied due to differences in their natural durations (M = 862 ms with SD = 451 ms, all sounds < 1500 ms). These heterogeneities, however, should not have affected our measurement of auditory-visual interactions because our design was fully counterbalanced (see below). The sounds were clearly audible (~70 dB SPL), presented via two loudspeakers, one on each side of the display monitor; the sounds carried no information about the target’s location.
Figure 1.
(A) An example of a search display; participants fixated the specified target object as quickly as possible. (B). Saccadic search times when a search display was presented simultaneously with a characteristic sound of the target object (Target-consistent), a characteristic sound of a distractor object (Distractor-consistent), or with no sound. The error bars represent ±1 SEM (adjusted to be appropriate for the within-participant design of the experiment).
On each trial, the sound was either consistent with the target object (target consistent), consistent with a distractor object (distractor consistent), or was absent (no sound). In the distractor-consistent-sound condition, the relevant distractor object was always presented in the quadrant diagonally opposite from the target across the fixation marker so that any potential cross-modal enhancement of the distractor did not direct attention near the target. Within a block of 60 trials, each of the 20 sounds was presented once as the target-consistent sound and once as the distractor-consistent sound (with sounds absent in the remaining 20 trials), and each picture was presented once as the target in each of the three sound conditions. This counterbalancing ensured that any facilitative effect of target-consistent sounds would be attributable to the sounds’ associations with the visual targets, rather than to the properties of the pictures or the sounds themselves. Aside from these constraints, the objects were randomly selected and placed on each trial. Each participant was tested in four blocks of 60 trials. Ten practice trials were given prior to the experimental trials.
The stimuli were displayed on a color CRT monitor (1024 × 768 pixels) with a 60 Hz refresh rate, and the experiment was controlled by a Sony VAIO computer using Matlab (Mathworks Inc) and PsychToolbox software (Brainard, 1997; Pelli, 1997). An EyeLink 1000 Tower Mount eye tracker (1000 Hz sampling rate and 0.25° spatial resolution) with a combined chin and forehead rest was used to monitor eye movements and to stabilize the viewing distance at 81 cm. Onsets and offsets of saccades were detected using the EyeLink software which uses saccade-detection criteria based on thresholds for eye position shift (0.1°), velocity (30°/sec) and acceleration (8000°/sec) in conjunction with the general algorithm described in Stampe (1993).
Procedure
Participants looked at a central circle (1° radius) to begin each trial. The name of the current target (e.g., “cat”) was aurally presented at the beginning of each trial. After 2000 ms, the search display appeared synchronously with the onset of one of the two types of sounds, target-consistent, distractor-consistent, or with no sound. Participants were instructed to look at the target as quickly as possible. As soon as the left eye gaze position reached the 4.03° by 4.03° region of the target, the visual display was terminated and the saccadic search time (measured from the onset of the search display) was recorded.
Results
Saccadic search time was significantly faster in the target-consistent-sound condition (M = 480 ms) compared with both the distractor-consistent-sound condition (M = 541 ms), t(15) = 2.967, p < 0.01, d1 = 0.742, and no-sound condition (M = 521 ms), t(15) = 4.113, p < 0.001, d = 1.028; saccadic search time did not differ between the distractor-consistent-sound and no-sound conditions, t(15) = 1.203, n.s., d = 0.301 (Figure 1B). Thus, playing the target objects’ characteristic sounds speeded eye movements to the targets in visual search.
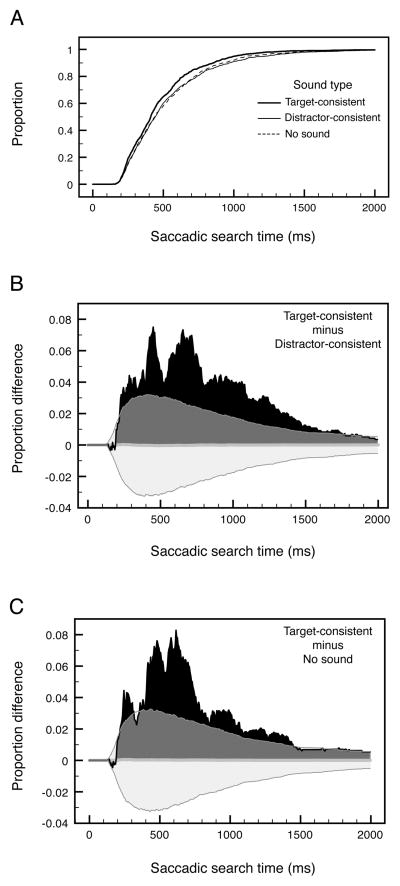
We determined how rapidly characteristic sounds facilitated target selection by computing the proportion of saccadic search times in 5 msec bins and determining the earliest bin for which the target-consistent-sound condition produced a significantly greater cumulative proportion compared to the distractor-consistent-sound and no-sound conditions. For example, if the cumulative proportion for the target-consistent-sound condition significantly exceeded those for the distractor-consistent-sound and no-sound conditions at the 50th cumulative bin, that would indicate that the target-consistent sounds significantly increased the proportion of search times 250 ms and faster. We would then make a conservative inference that the target-consistent sounds facilitated visual search within 250 ms.
As shown in Figure 2A, the cumulative proportion of fast saccadic search times was greater in the target-consistent-sound condition compared with both the distractor-consistent-sound and no-sound conditions, and the distributions do not differ between the distractor-consistent-sound and no-sound conditions; note that the vertical separations in the initial rising portions of the distributions are difficult to discern due to the steep slopes.
Figure 2.
(A) Cumulative distributions of saccadic search times for trials with target-consistent sounds (thick solid curve), distractor-consistent sounds (thin solid curve), and no sounds (thin dashed curve). (B) The difference between the cumulative distribution for trials with target-consistent sounds and the cumulative distribution for trials with distractor-consistent sounds. (C) The difference between the cumulative distribution for trials with target-consistent sounds and the cumulative distribution for trials with no sounds. In (B) and (C), the translucent gray regions indicate the 95% confidence limits (see main text and Appendix for details).
To more clearly illustrate how rapidly the object-specific auditory-visual interactions emerged over time, we plotted the difference between the distribution for the target-consistent-sound condition and those for the distractor-consistent-sound and no-sound conditions. The advantages for target-consistent sounds over distractor-consistent sounds (Figure 2B) and for no sounds (Figure 2C) both rose rapidly after 190 ms. To determine how rapidly the advantages became statistically significant, we computed confidence limits using a bootstrapping method. Under the null hypothesis, saccadic search times from all three conditions would come from the same distribution for each participant. To estimate the extent of condition effects expected from sampling error (under the null hypothesis), we combined data from the three conditions into one saccadic-search-time distribution, and randomly sampled from that distribution to simulate the data for the three conditions for each participant. We then pooled the simulated data from all participants in exactly the same way as we pooled the actual data as shown in Figures 2B and 2C. We repeated this procedure 5,000 times to compute the 2.5th and 97.5th percentile points which are shown as the lower and upper limits of the 95% confidence intervals (the gray regions) in Figures 2B and 2C. The details of this bootstrapping analysis are provided in the Appendix. The advantages of target-consistent sounds over distractor-consistent sounds and no sounds exceeded the 95% confidence limits at the latencies of 220 ms and 215 ms, respectively.
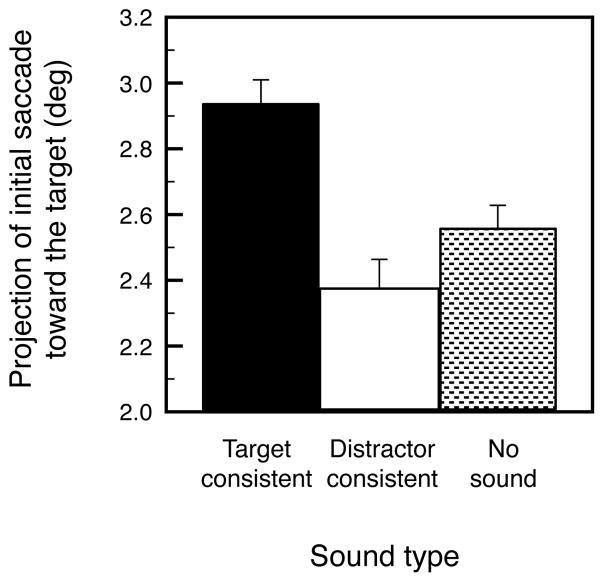
To find converging evidence for the rapid influences of object-based auditory-visual interactions, we also analyzed the impact of characteristic sounds on the trajectory of initial saccades (defined as the first saccade that the participant made following the onset of a search display). We determined whether target-consistent sounds guided initial saccades toward the target compared to distractor-consistent sounds and to no sounds. We quantified the degree to which an initial saccade moved the eyes toward the target by computing the projection of its vector on the axis determined by the fixation point and the target. A larger positive value would indicate that the eyes initially moved closer to the target and a larger negative value would indicate that the eyes initially moved farther away from the target. If a target-consistent sound had an impact on the direction of the initial saccade, the projection value should be significantly greater in the target-consistent-sound condition than in the no-sound condition. Because a distractor-consistent sound was always associated with the distractor placed diagonally opposite from the target, if a distractor-consistent sound had an impact on the direction of the initial saccade, the projection value should be significantly smaller in the distractor-consistent-sound condition compared to no-sound condition.
The average projection values were positive for all conditions indicating that an initial saccade overall moved the eyes toward the target. The projection value was significantly greater in the target-consistent-sound condition compared to both the no-sound, t(15) = 2.912, p < 0.02, d = 0.728, and distractor-consistent-sound, t(15) = 3.740, p < 0.002, d = 0.935, conditions whereas the projection values were not significantly different between the distractor-consistent-sound and no-sound conditions, t(15) = 1.137, n.s., d = 0.284. Thus, target-consistent sounds guided initial saccades toward the targets, whereas distractor-consistent sounds had no significant impact on the trajectory of initial saccades.
Discussion
We investigated how quickly people looked at a target object presented among distractor objects when a sound characteristic of the target object, a sound characteristic of a distractor object, or no sound was concurrently presented with the search display. All of our measures, mean saccadic search times (Figure 1B), cumulative distributions of saccadic search times (Figure 2), and trajectories of initial saccades (Figure 3) provided converging evidence, indicating that playing a characteristic sound of a target object guides and speeds saccades to the target, whereas playing a sound associated with a distractor has little impact.
Figure 3.
Average projections of initial saccade vectors in the target direction when a search display was presented simultaneously with a characteristic sound of the target object (Target-consistent), a characteristic sound of a distractor object (Distractor-consistent), or with no sound. A larger positive value indicates that the initial saccade moved the eyes closer to the target (see main text for details). The error bars represent ±1 SEM (adjusted to be appropriate for the within-participant design of the experiment).
The lack of a measurable effect of distractor-consistent sounds in this study is consistent with previous results (e.g., Molholm et al., 2004; von Kriegstein et al., 2005; Iordanescu et al., 2008), suggesting that object-based auditory-visual enhancements occur in a goal-directed manner. Because neurons in the prefrontal cortex selectively respond to task relevant stimuli (e.g., Duncan, 2001; Miller & Cohen, 2001) and some neurons there respond to both auditory and visual stimuli (e.g., Watanabe, 1992), the locus of the cross-modal effect in our study might be the prefrontal cortex. However, object selectivity in prefrontal cortex might be too weak (e.g., Warden & Miller, 2007) to guide the search mechanisms to specific objects. Moreover, because responses of prefrontal neurons are task dependent (e.g., Asaad et al., 2000; Rainer et al., 1998), it is unclear how their responses would be affected by characteristic sounds in our study where the sounds were task irrelevant in that they were uninformative of target location and consistent with target identity only one third of the time. Alternatively, a target-specific auditory-visual enhancement might arise from a combination of top-down sensitization and cross-modal interaction. For example, a top-down signal, likely from the prefrontal cortex (e.g., Desimone & Duncan, 1995; Duncan, 2001; Miller & Cohen, 2001; Reynolds & Chelazzi, 2004), could sensitize visual representations of the target object, and a target-consistent sound would cross-modally boost activation of this sensitized representation. A distractor-consistent sound would have little effect because the corresponding visual representation would not be sensitized by the top-down signal. The locus of the sensitized representation might be visual object-processing areas or polysensory areas in the temporal lobe (e.g., Amedi et al., 2005; Beauchamp et al., 2004a, 2004b).
For a characteristic sound to influence saccadic latency and trajectory, the complex auditory signal must be processed at the level of encoding sounds of common objects, the auditory and visual processing must interact at the level of object-based processing (potentially in temporal polysensory areas or prefrontal cortex), and then feedback interactions must enhance the retinotopic representation of the target object to facilitate an eye movement to it. These processes would be time consuming if they proceeded serially. An electroencephalographic study examining auditory-visual interactions in visual object recognition showed that a characteristic sound (e.g., a “moo” sound presented with a picture of a cow) enhanced a visual-selection related ERP signal within 210–300 ms (Molholm et al., 2004). The fact that we demonstrated the effect of target-consistent sounds on saccades within 215–220 ms suggests that object-based auditory-visual interactions influence behavior as rapidly as they modulate an ERP correlate. The rapid impact of characteristic sounds on saccades is even more impressive considering the fact that eye movements to even a single predictable target take 150–350 ms (e.g., Darrien et al., 2001; Yang et al., 2002). Our results are thus consistent with the emerging view that sensory processing is fundamentally multimodal, with cross-modal neural interactions influencing all levels of sensory processing including those that were traditionally thought to be unimodal (e.g., Schroeder & Foxe, 2005; Kayser & Logothetis, 2007; Sperdin, Cappe, & Murray, 2010).
Acknowledgments
This research was supported by National Institutes of Health grant R01 EY018197 and National Science Foundation grant BCS0643191.
Appendix
Bootstrapping analysis of cumulative distributions of saccadic search times
In order to determine how rapidly characteristic sounds facilitated saccades to target objects, we compared the cumulative saccadic-search-time distribution for the target-consistent-sound condition with those for the distractor-consistent-sound and no-sound conditions.
Comparing cumulative distributions neither imposes limits on temporal resolution (beyond the measurement error) nor introduces a potential artifact of bin size. A general disadvantage of using cumulative distributions is that beyond the earliest point at which distributions for different conditions diverge, subsequent differences are difficult to interpret because they include earlier differences. Thus, cumulative distributions would not be suited, for example, to determine whether saccadic search times differed among conditions for a specific time interval (say, between 400 ms and 500 ms). However, cumulative distributions are ideal for determining the earliest time point at which saccadic-search-time distributions from different conditions begin to diverge.
To statistically evaluate the distribution differences, we computed confidence intervals. Note that it would be inappropriate to compute confidence intervals in a conventional way based on the inter-participant variability at each time point. Although the overall shape of a probability distribution is free to vary, different time points along each distribution are “yoked.” For example, if values in the lower half of the distribution are frequent, values in the upper half of the distribution must be infrequent.
Consequently, it would be inappropriate to assume that each time point contributes an independent source of variability when comparing probability distributions. We thus evaluated the experimental differences in the distribution shapes between the target-consistent-sound condition and the distractor-consistent-sound and no-sound conditions against the range of random differences expected under the null hypothesis, using a bootstrapping method.
For each participant, we combined all of his or her saccadic search times into a single distribution, assuming the null hypothesis that the sound conditions made no difference. We then randomly sampled from this distribution (with replacement) as many times as the number of saccadic search times in each condition, to simulate the distribution of the participant’s saccadic search times for each of the three sound conditions under the null hypothesis. We converted these simulated saccadic-search-time distributions into cumulative distributions. We then computed the differences between these simulated cumulative distributions, one between the simulated distributions for the target-consistent-sound and distractor-consistent-sound conditions, and the other between the simulated distributions for the target-consistent-sound and no-sound conditions. These two difference distributions were calculated for all participants, and were then averaged across participants to generate a pair of average difference-distribution curves (expected under the null hypothesis) comparable to those shown in Figures 2B and 2C.
To estimate the confidence limits on the variability of these difference distributions under the null hypothesis, we repeated the above procedure 5,000 times, yielding 5,000 simulated average difference distributions of each type (i.e., target-consistent-sound condition minus distractor-consistent-sound condition, or target-consistent-sound condition minus no-sound condition). The 97.5th and 2.5th percentile values of these simulated distributions were used as the upper and lower limits of our 95% confidence intervals.
Footnotes
Each effect size was computed by dividing the mean difference by the standard deviation of the difference scores, consistent with the within-participant design of our experiments.
References
- Amedi A, von Kriegstein K, van Atteveldt MN, Beauchamp MS, Naumer MJ. Functional imaging of human crossmodal identification and object recognition. Experimental Brain Research. 2005;166:559–571. doi: 10.1007/s00221-005-2396-5. [DOI] [PubMed] [Google Scholar]
- Asaad WF, Rainer G, Miller EK. Task-specific neural activity in the primate prefrontal cortex. Journal of Neurophysiology. 2000;84:451–459. doi: 10.1152/jn.2000.84.1.451. [DOI] [PubMed] [Google Scholar]
- Beauchamp MS, Argall BD, Bodurka J, Duyn JH, Martin A. Unraveling multisensory integration: patchy organization within human STS multisensory cortex. Nature Neuroscience. 2004a;7(11):1190–1192. doi: 10.1038/nn1333. [DOI] [PubMed] [Google Scholar]
- Beauchamp MS, Lee KE, Argall BD, Martin A. Integration of auditory and visual information about objects in superior temporal sulcus. Neuron. 2004b;41:809–823. doi: 10.1016/s0896-6273(04)00070-4. [DOI] [PubMed] [Google Scholar]
- Bolognini N, Frassinetti F, Serino A, Làdavas E. “Acoustical vision” of below threshold stimuli: interaction among spatially converging audiovisual inputs. Experimental Brain Research. 2005;160:273–282. doi: 10.1007/s00221-004-2005-z. [DOI] [PubMed] [Google Scholar]
- Brainard DH. The Psychophysics Toolbox. Spatial Vision. 1997;10:433–436. [PubMed] [Google Scholar]
- Darrien JH, Herd K, Starling LJ, Rosenberg JR, Morrison JD. An analysis of the dependence of saccadic latency on target position and target characteristics in human subjects. BMC Neuroscience. 2001;2:13. doi: 10.1186/1471-2202-2-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desimone R, Duncan J. Neural mechanisms of selective visual attention. Annual Review of Neuroscience. 1995;18:193–222. doi: 10.1146/annurev.ne.18.030195.001205. [DOI] [PubMed] [Google Scholar]
- Driver J, Spence C. Attention and the crossmodal construction of space. Trends in Cognitive Sciences. 1998;2(7):254–262. doi: 10.1016/S1364-6613(98)01188-7. [DOI] [PubMed] [Google Scholar]
- Duncan J. An adaptive coding model of neural function in prefrontal cortex. Nature Reviews Neuroscience. 2001;2:820–829. doi: 10.1038/35097575. [DOI] [PubMed] [Google Scholar]
- Sperdin HF, Cappe C, Murray MM. The behavioral relevance of multisensory neural response interactions. Frontiers in Neuroscience. 2010;4(1):9–18. doi: 10.3389/neuro.01.009.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iordanescu L, Guzman-Martinez E, Grabowecky M, Suzuki S. Characteristic sound facilitates visual search. Psychonomic Bulletin & Review. 2008;15:548–554. doi: 10.3758/pbr.15.3.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayser C, Logothetis NK. Do early sensory cortices integrate cross-modal information? Brain Structure and Function. 2007;212:121–132. doi: 10.1007/s00429-007-0154-0. [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annual Review of Neuroscience. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Molholm S, Ritter W, Javitt DC, Foxe JJ. Multisensory visual-auditory object recognition in humans: A high-density electrical mapping study. Cerebral Cortex. 2004;14:452–465. doi: 10.1093/cercor/bhh007. [DOI] [PubMed] [Google Scholar]
- Pelli DG. The VideoToolbox software for visual psychophysics: Transforming numbers into movies. Spatial Vision. 1997;10:437–442. [PubMed] [Google Scholar]
- Rainer G, Asaad WF, Miller EK. Selective representation of relevant information by neurons in the primate prefrontal cortex. Nature. 1998;393:577–579. doi: 10.1038/31235. [DOI] [PubMed] [Google Scholar]
- Reynolds JH, Chelazzi L. Attentional modulation of visual processing. Annual Review of Neuroscience. 2004;27:611–647. doi: 10.1146/annurev.neuro.26.041002.131039. [DOI] [PubMed] [Google Scholar]
- Rovamo J, Virsu V. Visual resolution, contrast sensitivity, and the cortical magnification factor. Experimental Brain Research. 1979;37:475–494. doi: 10.1007/BF00236818. [DOI] [PubMed] [Google Scholar]
- Schroader CE, Foxe J. Multisensory contributions to low-level, ‘unisensory’ processing. Current Opinion in Neurobiology. 2005;15:454–458. doi: 10.1016/j.conb.2005.06.008. [DOI] [PubMed] [Google Scholar]
- Stein BE, Meredith ME, Huneycutt WS, McDade LW. Behavioral indices of multisensory integration: orientation to visual cues is affected by auditory stimuli. Journal of Cognitive Neuroscience. 1989;1:12–24. doi: 10.1162/jocn.1989.1.1.12. [DOI] [PubMed] [Google Scholar]
- Stampe DM. Heuristic filtering and reliable calibration methods for video-based pupil-tracking systems. Behavioral Research Methods, Instruments, & Computers. 1993;25(2):137–142. [Google Scholar]
- Van der Burg E, Olivers CNL, Bronkhorst AW, Theeuwes J. Pip and Pop: nonspatial auditory signals improve spatial visual search. Journal of Experimental Psychology: Human Perception and Performance. 2008;34(5):1053–1065. doi: 10.1037/0096-1523.34.5.1053. [DOI] [PubMed] [Google Scholar]
- von Kriegstein K, Kleinschmidt A, Sterzer P, Giraud A-L. Interaction of face and voice areas during speaker recognition. Journal of Cognitive Neuroscience. 2005;17(3):367–376. doi: 10.1162/0898929053279577. [DOI] [PubMed] [Google Scholar]
- Warden MR, Miller K. The representation of multiple objects in prefrontal neuronal delay activity. Cerebral Cortex. 2007;17:i41–i50. doi: 10.1093/cercor/bhm070. [DOI] [PubMed] [Google Scholar]
- Watanabe M. Frontal units of the monkey coding the associative significance of visual and auditory stimuli. Experimental Brain Research. 1992;89:233–247. doi: 10.1007/BF00228241. [DOI] [PubMed] [Google Scholar]
- Yang Q, Bucci MP, Kapoula Zoï. The latency of saccades, vergence, and combined eye movements in children and in adults. Investigative Ophthalmology & Visual Science. 2002;43(9):2939–2949. [PubMed] [Google Scholar]