Abstract
Although local interactions involving orientation and spatial frequency are well understood, less is known about spatial interactions involving higher level pattern features. We examined interactive coding of aspect ratio, a prevalent two-dimensional feature. We measured perception of two simultaneously flashed ellipses by randomly post-cueing one of them and having observers indicate its aspect ratio. Aspect ratios interacted in two ways. One manifested as an aspect-ratio-repulsion effect. For example, when a slightly tall ellipse and a taller ellipse were simultaneously flashed, the less tall ellipse appeared flatter and the taller ellipse appeared even taller. This repulsive interaction was long range, occurring even when the ellipses were presented in different visual hemifields. The other interaction manifested as a global assimilation effect. An ellipse appeared taller when it was a part of a global vertical organization than when it was a part of a global horizontal organization. The repulsion and assimilation effects temporally dissociated as the former slightly strengthened, and the latter disappeared when the ellipse-to-mask stimulus onset asynchrony was increased from 40 to 140 ms. These results are consistent with the idea that shape perception emerges from rapid lateral and hierarchical neural interactions.
Keywords: aspect ratio, repulsion, assimilation, lateral interaction, hierarchical interaction, shape perception
Introduction
When two stimuli are simultaneously presented, their neural representations can interact within the same level of processing through lateral connections and across different levels of processing through hierarchical connections (e.g., Felleman & Van Essen, 1991; Gilbert, 1992; Lamme, Supèr, & Spekreijse, 1998). The primary goal of the current study was to characterize how lateral and hierarchical interactions influenced the perception of aspect ratio when two ellipses were simultaneously presented. We characterized lateral interactions in the coding of aspect ratio by determining how a nearby ellipse influenced the perceived aspect ratio of a target ellipse and characterized hierarchical interactions by determining how the global spatial organization of a pair of ellipses influenced the perceived aspect ratio of one ellipse within the pair.
Aspect ratio is a simple but important two-dimensional feature defined by closed contours. It is relevant to perception of figure versus ground (e.g., Elder & Zucker, 1992; Koffka, 1935), objects (e.g., faces, drinking glasses; Biederman & Kalocsai, 1997; Rhodes, 1988; Young & Yamane, 1992), as well as 3D surface orientations (e.g., Biederman, 2001; Biederman & Kalocsai, 1997; Knill, 1998a, 1998b). Demonstrations of sequential repulsive aftereffects (e.g., looking at a tall ellipse makes a subsequently presented circle appear flat) have suggested that aspect ratio is coded as a unique pattern feature (e.g., Regan & Hamstra, 1992; Suzuki, 2003; Suzuki & Cavanagh, 1998). Aspect ratio exemplifies intermediate-level processing as distinct from low-level processing because (1) it is a two-dimensional rather than a one-dimensional feature, (2) its coding does not emerge until intermediate-level visual areas such as V4 (e.g., Desimone & Schein, 1987; Dumoulin & Hess, 2007), and (3) aftereffects for aspect ratio require awareness of the adaptor as do aftereffects for high-level features (e.g., facial identity), whereas aftereffects for low-level features such as orientation, spatial frequency (e.g., De Valois, Albrecht, & Thorell, 1982; Hubel & Wiesel, 1968), and low/intermediate-level features such as curvature (e.g., Hegdé & Van Essen, 2000, 2007; Pasupathy & Connor, 1999, 2001, 2002) do not require awareness of the adaptor (e.g., Blake & Fox, 1974; He & MacLeod, 2001; Moradi, Koch, & Shimojo, 2005; Sweeny, Grabowekcy, & Suzuki, 2010).
Our primary goal was to determine if simultaneous coding of multiple aspect ratios involves neural interactions that are similar to those involved in low-level coding of orientation and spatial frequency and low/intermediate-level coding of curvature, in which lateral inhibitory interactions create perceptual repulsion effects. In orientation coding, neighboring contours with similar orientations tend to repel each other. For example, when a small vertical grating is presented within a larger slightly leftward-tilted grating, the vertical grating appears to be slightly tilted in the opposite (rightward) direction. This repulsive interaction, known as the tilt illusion (e.g., Gibson, 1937; Magnussen & Kurtenbach, 1980), is thought to be mediated by orientation-specific inhibitory interactions among low-level orientation-tuned neurons in V1 and/or V2 (e.g., Blakemore, Carpenter, & Georgeson, 1970; Carpenter & Blakemore, 1973; see Schwartz, Hsu, & Dayan, 2007, for a recent review). Similar repulsive interactions have also been demonstrated for the perception of spatial frequency (Klein, Stromeyer, & Ganz, 1974) and curvature (Gibson, 1933). Thus, repulsive interactions are common in low-level and low/intermediate-level pattern vision. Spatial interactions in the perception of aspect ratio have not been investigated, however. A demonstration of repulsive interactions for the perception of simultaneously presented ellipses (e.g., when a slightly tall and a taller ellipse are simultaneously presented, the slightly tall ellipse may appear flatter and the taller ellipse may appear even taller) would suggest that lateral inhibitory mechanisms similar to those involved in low-level coding of orientation and spatial frequency, and low/intermediate-level coding of curvature, are also operative in intermediate-level coding of aspect ratio.
Hierarchical mechanisms may also be operative in the coding of aspect ratio. Neurons in intermediate-level visual areas such as V4 (e.g., Desimone & Schein, 1987) as well as those in high-level visual areas such as inferotemporal cortex (e.g., Kayaert, Biederman, & Vogels, 2003) show tuning for aspect ratio. Through feedback connections (e.g., Lamme & Roelfsema, 2000; Lamme et al., 1998), the activity of high-level aspect-ratio-tuned neurons with large receptive fields that encode global aspect ratios might influence the activity of intermediate-level aspect-ratio-tuned neurons with smaller receptive fields that encode local aspect ratios. We thus hypothesized that a global aspect ratio detected in high-level processing might bias local coding of aspect ratio in intermediate-level processing. Specifically, two ellipses presented side by side generate a horizontally stretched global organization, whereas two ellipses presented one above the other generate a vertically stretched global organization. Note that, for stimuli of the scale used in our experiments, perceptual processing of global patterns initially takes precedence over processing of local patterns (e.g., Bar, 2003; Navon, 1977; Paquet & Merikle, 1988). At the neuronal level, initial firing of neurons in macaque inferotemporal cortex encodes global categories prior to finer details (e.g., Sugase, Yamane, Ueno, & Kawano, 1999; Tamura & Tanaka, 2001). Furthermore, based on a broad range of behavioral evidence including visual search and perceptual learning, Hochstein and Ahissar (2002) postulate in their reverse hierarchy theory that perceptual dominance of visual information rapidly flows backward from global gist to local details. We therefore hypothesized that the influences of horizontally and vertically stretched global organizations on the perceived aspect ratios of individual ellipses would be limited to the initial moments of perception. In other words, presenting two ellipses side by side might make each ellipse appear flatter than it actually is, and presenting two ellipses one above the other might make each ellipse appear taller than it actually is, but only when ellipses are briefly presented.
To summarize, we determined whether two simultaneously (and briefly) presented ellipses produced an aspect-ratio-repulsion effect similar to those demonstrated for low-level coding of orientation, spatial frequency, and low/intermediate-level coding of curvature. Such an effect would suggest that lateral inhibitory interactions are also operative in intermediate-level coding of aspect ratio. We also determined whether the global organization of an ellipse pair was assimilated into the perception of local aspect ratio. Such a global assimilation effect would suggest that hierarchical interactions are operative in the coding of aspect ratio. We further determined whether this global-to-local interaction only affected initial perception as predicted by the hypothesized rapid shifting of perceptual information from the global to the local level.
Experiment 1: Simultaneous aspect-ratio-repulsion and global assimilation effects
Methods
Observers
Eight undergraduate students at Northwestern University gave informed consent to participate for course credit. All had normal or corrected-to-normal visual acuity and were tested individually in a dimly lit room.
Stimuli and procedure
All stimuli were dark gray (26.0 cd/m2), embedded in a white square (2.52° × 2.52°, 94.0 cd/m2), and presented against a black (1.1 cd/m2) background. We embedded each ellipse in a white square for two reasons. First, we wanted to ensure that the aspect ratio of each ellipse would be processed in reference to the same neutral aspect ratio of the immediately surrounding square. Second, we wanted to ensure that any perceptual distortions would be due to interactions between higher level shape representations and not to interactions between the proximal contours of neighboring ellipses. The strongest local contour interactions for each ellipse would be with the vertical and horizontal contours of the surrounding square.
We created ellipses (drawn with 0.057° thick lines) with 11 aspect ratios ranging from flat to tall. The aspect ratios were symmetrically distributed (in log scale) around the neutral aspect ratio (i.e., circle), −0.22 (1.23° × 2.05°), −0.18 (1.23° × 1.89°), −0.14 (1.23° × 1.73°), −0.10 (1.23° × 1.55°), −0.05 (1.23° × 1.39°), 0.00 (circle; 1.23° × 1.23), 0.05 (1.39° × 1.23° 0.10 (1.55° × 1.23°), 0.14 (1.73° × 1.23° 0.18 (1.89° × 1.23°), and 0.22 (2.05° × 1.23°). The areas of the ellipses were not exactly matched, but this should not have influenced our results in a systematic way because perceptual coding of aspect ratio has been shown to occur independently of size (e.g., Regan & Hamstra, 1992). Each ellipse was treated with a Gaussian blur of 2.0-pixel radius to reduce aliasing. Seven ellipses (log aspect ratios −0.18, −0.14, −0.10, 0.00, 0.10, 0.14, and 0.18) were used as the stimuli. Ten ellipses excluding the circle were used in a magnitude-matching screen.
Ellipse pairs were presented in two spatial organizations: horizontally paired across the vertical meridian (i.e., across the left and right visual hemifields) and presented in either the upper or lower visual field—the between-hemifield condition, or vertically paired and presented solely within either the left or right visual hemifield—the within-hemifield condition (Figure 1A). We used these organizations to induce global horizontal and global vertical organizations, respectively, and also to allow comparisons between longer range cortical interactions across hemifields and shorter range cortical interactions within a hemifield. The inter-ellipse distances were identical (3.740°) for both the between-hemifield and within-hemifield conditions, and all ellipses were presented along an approximate iso-acuity orbit (10.8° horizontal diameter and 8.81° vertical diameter; e.g., Rovamo & Virsu, 1979) centered at the fixation cross.
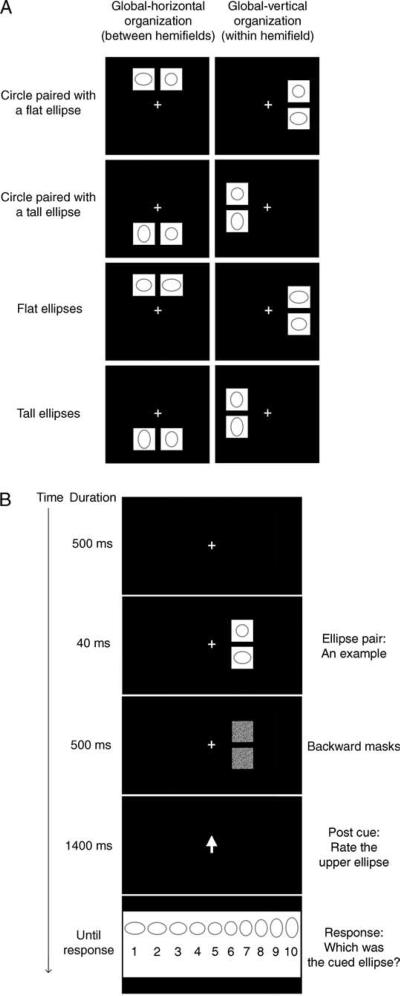
Figure 1.
(A) Examples of ellipse pairs presented in the between-hemifield condition (forming a global horizontal organization) or the within-hemifield condition (forming a global vertical organization). Ellipse pairs were presented side by side (in separate visual hemifields either above or below fixation) in the between-hemifield condition or one above the other (either within the left or right visual hemifield) in the within-hemifield condition. (B) A typical trial sequence. Two ellipses were simultaneously flashed on each trial, and the post-ellipse cue (a central arrow) indicated which ellipse the observer should rate for its aspect ratio.
Note that each of the paired ellipses always fell in a separate quadrant of the visual field. Receptive fields of neurons in intermediate-level ventral visual areas such as V4 and TEO are primarily confined within a single visual quadrant (e.g., Boussaoud, Desimone, & Ungerleider, 1991; Gattass, Sousa, & Gross, 1988; Kastner et al., 2001). Thus, each ellipse most likely fell in separate receptive fields of aspect-ratio-tuned neurons. We reasoned that an arrangement in which ellipses fell in separate receptive fields would be most likely to produce strong lateral inhibitory interactions. This is because when multiple stimuli fall within the same receptive field of a ventral visual neuron, its response tends to be the average of its responses to the individual stimuli separately (e.g., Chelazzi, Duncan, Miller, & Desimone, 1998; Kastner et al., 2001; Miller, Gochin, & Gross, 1993; Reynolds, Chelazzi, & Desimone, 1999; Rolls & Tovée, 1995; Sato, 1989; Zoccolan, Cox, & Di Carlo, 2005). Such neural averaging may result in perceptual averaging (Sweeny, Paller, Grabowecky, & Suzuki, 2009), which would counteract perceptual repulsion.
To measure perceptual bias and also to measure the global assimilation effect independently of the aspect-ratio-repulsion effect, each of the seven ellipses was paired with itself on 16 trials. To measure the aspect-ratio-repulsion effect involving a circle, the circle was paired with either a flat ellipse (log aspect ratio = −0.14) or a tall ellipse (log aspect ratio = 0.14); each pair was presented on 32 trials, and the circle and elongated ellipse were post-cued (see below) on an equal number of trials. To measure the aspect-ratio-repulsion effect between elongated ellipses, either two flat ellipses (log aspect ratios of −0.10 and −0.18) or two tall ellipses (log aspect ratios of 0.10 and 0.18) were paired; each pair was presented on 32 trials, and the ellipses with the greater and lesser elongations were post-cued on an equal number of trials. Paired ellipses were presented in the between-hemifield and within-hemifield organizations with an equal probability, and each post-cued ellipse was presented equally often at each of the eight locations. All pairings were randomly intermixed across 240 trials.
Procedure
Each trial began with the presentation of a fixation cross for 500 ms (Figure 1B). The experimenter strongly encouraged observers to fixate the central cross, emphasizing that looking at any other location on the screen would make the task more difficult because the to-be-rated ellipse could appear in any of the eight locations. Next, an ellipse pair appeared for 40 ms. This brief stimulus presentation did not allow time for deliberate saccades to a specific stimulus. A Gaussian-noise square (2.52° × 2.52° with pixel luminance values ranging from 1.1 to 88.2 cd/m2) replaced each stimulus and remained on the screen for 500 ms. Upon the offset of the masking squares, the fixation cross was replaced by an arrow pointing to the location in which one of the ellipses was presented. We asked observers to report the perceived aspect ratio of one of the two ellipses rather than asking them to report the aspect ratios of both ellipses in order to prevent potential uses of an attention/response strategy such as always reporting the more elongated ellipse first. The post-stimulus arrow cue was either pointing leftward or rightward on between-hemifield trials or upward or downward on within-hemifield trials. This post-stimulus cue lasted 1400 ms. Upon its offset, a magnitude-matching screen appeared prompting observers to rate the perceived aspect ratio of the post-cued ellipse by indicating the number below the ellipse that most closely matched the perceived aspect ratio (Figure 1B). To avoid potential response bias due to spatial placement of response buttons, observers spoke the number and the experimenter recorded the responses while sitting out of sight.
We cued the to-be-rated ellipse after the offset of the masking squares so that observers did not know which ellipse they would rate until after the display disappeared. This design promoted a strategy of allocating attention equally between the two ellipses. Although it might seem reasonable to present the arrow cue during the ellipse presentation, doing so might bias attention to the target ellipse. This might make visual neurons respond primarily to the attended target ellipse (e.g., Chelazzi et al., 1998; Reynolds et al., 1999), and thus potentially reduce or eliminate spatial interactions. It is also likely that making observers split attention between the central arrow cue and the ellipses would degrade perceptual encoding of the briefly presented ellipses. Post-cueing has the advantage of encouraging observers to fully attend to both ellipses. It, however, has a potential drawback because observers have to keep the ellipses in memory at least during the 500-ms masking period until the post-cue is presented. Memory degradation that might occur during this period might affect the results. We will argue in the Discussion section that the specific pattern of results we obtained is unlikely to be attributable to memory degradation. For now, we point out that the memory retention period (from the onset of the masking squares to the onset of the arrow cue, and then to the onset of the response screen) was the same for all three experiments. Thus, any differences in the results across the experiments could not be attributable to memory degradation.
In order to encourage observers to make subtle aspect-ratio discriminations, the rating scale did not include an exact “circle” option. If observers perceived exact circles, their responses over trials should average to the midpoint of the scale. The experimenter instructed observers to respond quickly and to rely on their “gut feeling” if they were unsure. Six practice trials preceded the experiment. All stimuli were presented on a 19″ CRT monitor using Presentation software (www.neurobs.com) at a viewing distance of 100 cm.
Results and discussion
Lateral aspect-ratio interaction
To simplify our data presentation, we computed an aspect-ratio-repulsion index where we subtracted the aspect-ratio rating of each ellipse when paired with an ellipse of the same aspect ratio from its rating when paired with an ellipse of a different aspect ratio. In this way, we subtracted out any general bias in perceiving either a tall or flat aspect ratio. The sign of the aspect-ratio-repulsion index was assigned such that a positive sign was given to a distortion in the repulsive direction and a negative sign was given to a distortion in the averaging direction. For example, if a tall ellipse appeared less tall when paired with a taller ellipse than when paired with an identical ellipse (i.e., a repulsive effect), a positive sign was given, whereas if a tall ellipse appeared taller when paired with a taller ellipse than when paired with an identical ellipse (i.e., an averaging effect), a negative sign was given.
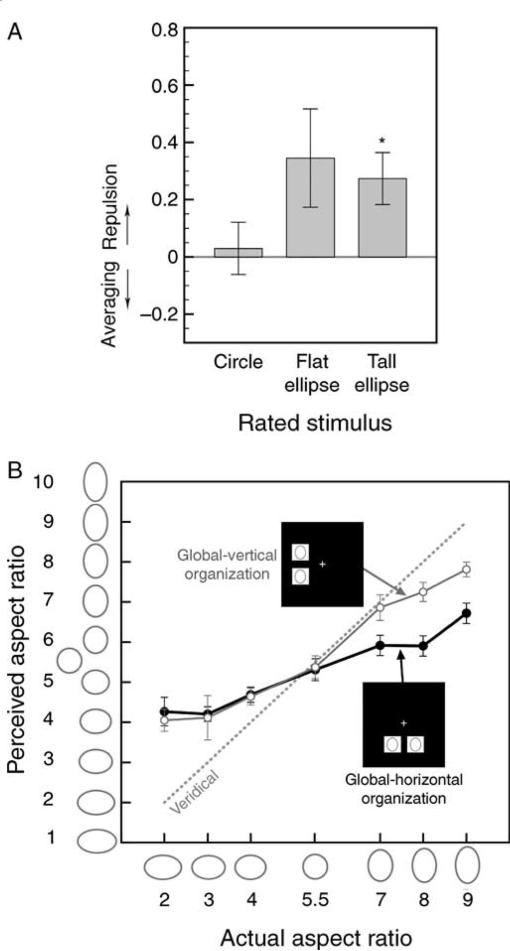
We found an aspect-ratio-repulsion effect. When the aspect-ratio-repulsion index was averaged across all ellipse pairs, the value was significantly greater than zero (M = 0.274, SEM = 0.089), t(7) = 3.061, p < 0.02, d = 1.082. When the data were analyzed separately for the perception of tall and flat ellipses (Figure 2A), the index was significantly positive for tall ellipses, t(7) = 2.982, p < 0.03, d = 1.054, and marginally significant for flat ellipses, t(7) = 2.002, p < 0.09, d = 0.708 (the index was not significantly different between the tall and flat ellipses, t[7] = 0.391, n.s.). We note that the aspect-ratio-repulsion index was significant for the perception of both tall and flat ellipses in Experiments 2 and 3.
Figure 2.
Experiment 1 (ellipses aligned, ellipse-to-mask SOA = 40 ms). (A) The aspect-ratio-repulsion index (see main text) for the perception of circles, flat ellipses, and tall ellipses (with data averaged across the between- and within-hemifield conditions); positive values indicate repulsive effects and negative values indicate averaging effects. * Represents p < 0.05. (B) Ratings of ellipses with different aspect ratios (including circle) when paired with ellipses with identical aspect ratios and presented in a vertical global organization (open symbols) or a horizontal global organization (filled symbols). The x-axis indicates the rated ellipses and the y-axis indicates the observer's ratings of those ellipses. The dashed gray line represents the expected aspect-ratio ratings if perception was veridical. Ellipses in pairs that formed global vertical organizations were perceived to be taller than ellipses in pairs that formed global horizontal organizations, but global organization only affected the perception of tall ellipses. For both (A) and (B), the error bars represent ±1 SEM (adjusted for repeated-measures comparisons in (B)).
Interestingly, perception of the circle was unaffected by the paired ellipses (Figure 2A), t(7) = 0.325, n.s. (also the case in Experiments 2 and 3). We suspect that this is the result of a perceptual scaling effect. Because observers reported aspect ratios of strongly elongated ellipses in many of the trials, their sensitivity for detecting subtle elongations induced on a circle could have been reduced. Consistent with this idea, a pilot experiment showed that when observers always reported the perceived aspect ratio of a circle paired with a tall or flat ellipse, significant aspect-ratio-repulsion effects were obtained for circles.
The overall aspect-ratio-repulsion index did not significantly differ between the within-hemifield (M = 0.335, SEM = 0.149) and between-hemifield (M = 0.212, SEM = 0.119) conditions, t(7) = 0.609, n.s. In other words, the magnitude of aspect-ratio interactions was equivalent whether the underlying neural interactions occurred within a cerebral hemisphere or across hemispheres. The equivalent magnitudes of the within- and between-hemifield effects were replicated in Experiments 2 and 3.
Finally, the aspect-ratio-repulsion index was significantly greater in the outward direction (i.e., a flat ellipse appearing flatter when paired with a less flat ellipse, and a tall ellipse appearing taller when paired with a less tall ellipse; M = 0.478, SEM = 0.137) than in the inward direction (i.e., a flat ellipse appearing less flat when paired with a flatter ellipse, and a tall ellipse appearing less tall when paired with a taller ellipse; M = 0.065, SEM = 0.048), t(7) = 2.796, p < 0.03, d = 0.988. However, this outward–inward asymmetry was not obtained in Experiment 2 or 3.
Hierarchical aspect-ratio interaction
Global organizations (vertical in the within-hemifield condition and horizontal in the between-hemifield condition) may have affected perception of the cued ellipse. To see how global organizations influenced the perception of ellipses, we plotted the average ratings of seven ellipses paired with themselves (thus unaffected by repulsive interactions), separately for the vertical and horizontal organizations. Two effects are evident in Figure 2B. First, aspect ratios were generally underestimated, as evidenced by the fact that the slope of the perceived-vs.-actual-aspect-ratio function was significantly less than 1 (M = 0.471, SEM = 0.064; all standard errors reported for hierarchical aspect-ratio interactions have been adjusted for within-observer comparisons), t(7) = 7.401, p < 0.01, d = 2.617. When we conducted a control experiment in which we presented one ellipse at a time, the slope was closer to 1 and comparable to the slope obtained for paired ellipses with a longer ellipse-to-mask SOA (see Figure 4B in Experiment 3). Furthermore, when a single ellipse with a small aspect ratio (elongation of 13% or less) was briefly presented, its perceived aspect ratio was systematically exaggerated (Suzuki & Cavanagh, 1998). Thus, the general underestimation of aspect ratio obtained in this experiment is likely to be caused by a combination of factors, including paired presentations of ellipses (potentially causing a crowding effect) and the inclusion of substantially elongated (up to 66% elongation) ellipses (potentially causing adaptation to large elongations).
Figure 4.
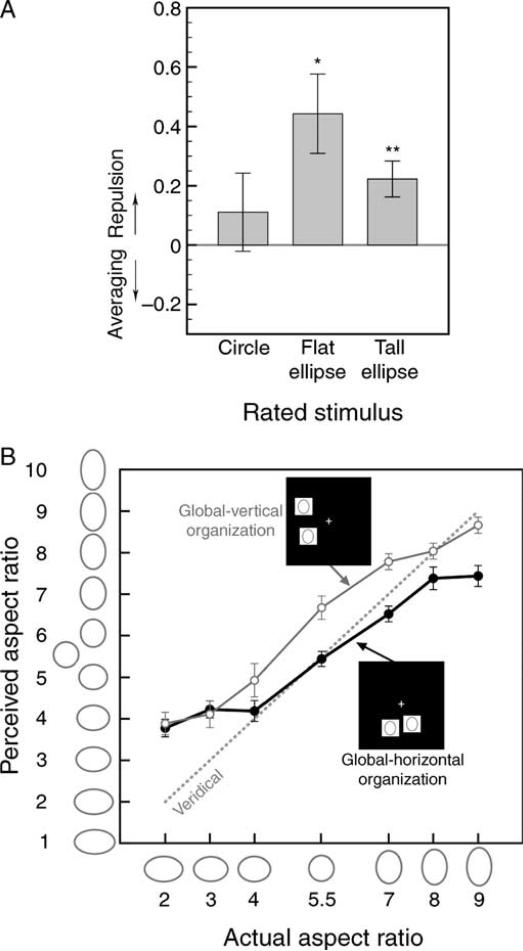
Experiment 3 (ellipses aligned, ellipse-to-mask SOA = 140 ms). (A) The aspect-ratio-repulsion index for the perception of circles, flat ellipses, and tall ellipses (with data averaged across the between- and within-hemifield conditions); positive values indicate repulsive effects and negative values indicate averaging effects. * Represents p < 0.05, and ** represents p < 0.01. (B) Ratings of ellipses with different aspect ratios (including circle) when paired with ellipses with identical aspect ratios and presented in a global vertical organization (open symbols) or a global horizontal organization (filled symbols). The x-axis indicates the rated ellipses and the y-axis indicates the observer's ratings of those ellipses. The dashed gray line represents the expected aspect-ratio ratings if perception was veridical. The longer ellipse-to-mask SOA (140 ms) eliminated the effect of global organization on the perceived aspect ratio of individual ellipses. For both (A) and (B), the error bars represent ±1 SEM (adjusted for repeated-measures comparisons in (B)).
The second effect apparent from Figure 2B is that the vertical organization made the individual ellipses appear taller compared to the horizontal organization, but the organization only affected the perception of tall ellipses. This effect was confirmed by a three-way ANOVA with global organization (vertical or horizontal), aspect ratio of the rated ellipse (seven values), and aspect ratio of the paired ellipse (same or different) as the independent variables and aspect-ratio rating as the dependent variable. We found a significant main effect of global organization, F(1, 7) = 9.248, p < 0.05, , and a significant interaction between global organization and aspect ratio of the rated ellipse, F(6, 42) = 4.720, p < 0.001, . This pattern of results did not depend on whether the same ellipses or ellipses with different aspect ratios were paired because aspect ratio of the paired ellipse (same or different) interacted neither with the effect of global organization (F[1, 7] = 1.039, n.s.) nor with the effect of organization-by-aspect-ratio interaction (F[6, 42] = 0.436, n.s.). Thus, although Figure 2B shows the “pure” global organization effects (without aspect-ratio-repulsion effects) for pairs of identical ellipses, the same global organization effects occurred when paired ellipses had different aspect ratios.
We note that ellipses were presented at slightly different retinal positions in the horizontal and vertical organizations. Although the visibility of ellipses was matched across these positions, which were distributed along an approximate iso-acuity orbit (Rovamo & Virsu, 1979), ellipses in a horizontal pair were slightly closer to the vertical meridian than those in a vertical pair, and ellipses in a vertical pair were slightly closer to the horizontal meridian than those in a horizontal pair. It is thus possible that ellipses flashed near the vertical meridian tend to appear flatter and those presented near the horizontal meridian tend to appear taller. We evaluated this possibility by presenting ellipses one at a time with six new observers. There were no significant effects of position; notably, for vertical ellipses (for which we obtained robust global organization effects), only two observers on average rated ellipses presented near the horizontal meridian to be taller than those presented near the vertical meridian, while the remaining four observers produced the opposite pattern. It is thus unlikely that the global organization effect reflects a position effect. This conclusion is further supported by the result of the next experiment where we obtained robust global organization effects when ellipse positions were randomly jittered.
Overall, we have demonstrated a simultaneous aspect-ratio-repulsion effect for the perception of elongated ellipses. Simultaneously presented aspect ratios repulsively interact as do simultaneously presented orientations, spatial frequencies, and curvatures, presumably due to lateral inhibitory interactions between populations of aspect-ratio-tuned neurons. The overall aspect-ratio-repulsion effect was not diminished when the ellipses were presented in separate visual hemifields, indicating an involvement of long-range cortical connections (even across cerebral hemispheres) in coding aspect ratios. We have also demonstrated a hierarchical aspect-ratio interaction where global organization is assimilated into perception of tall ellipses—a global assimilation effect; a tall ellipse appears taller when it is a part of a vertical organization than when it is a part of a horizontal organization.
Experiment 2: Simultaneous aspect-ratio-repulsion and global assimilation effects with randomly jittered ellipse locations
This experiment was identical to Experiment 1 except that the x and y coordinates of each ellipse were randomly jittered so that the ellipses were always misaligned in an unpredictable manner. This random jittering addressed the question of whether the simultaneous aspect-ratio-repulsion effect and/or the global assimilation effect depended on the alignment of the paired ellipses along the cardinal axes.
Methods
Observers
Eight undergraduate students at Northwestern University gave informed consent to participate for course credit. All had normal or corrected-to-normal visual acuity and were tested individually in a dimly lit room.
Stimuli and procedure
The stimulus design and procedure were identical to those in Experiment 1, except that the location of each ellipse was randomly jittered on each trial. The amounts of horizontal and vertical jitters for each ellipse were independently and randomly sampled from a uniform distribution between ±0.57°.
Results and discussion
Overall, we replicated the aspect-ratio-repulsion and global assimilation effects from Experiment 1.
Lateral aspect-ratio interaction
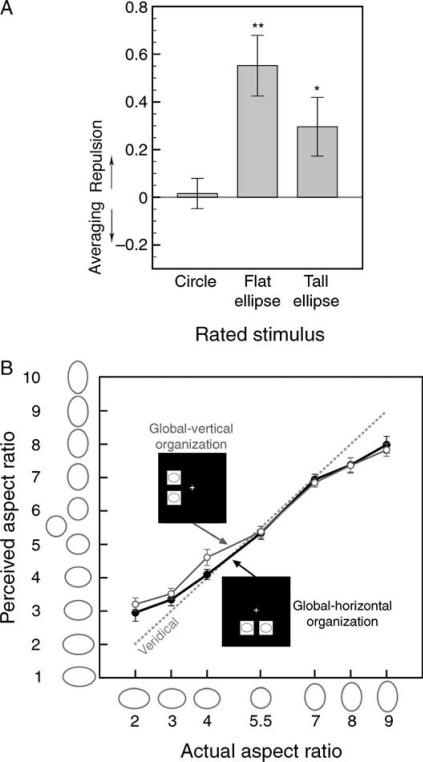
The overall aspect-ratio-repulsion index was significantly positive (i.e., in the repulsive direction; M = 0.303, SEM = 0.093), t(7) = 3.270, p < 0.02, d = 1.156, and was significantly positive for both tall ellipses, t(7) = 3.667, p < 0.01, d = 1.297, and flat ellipses, t(7) = 3.314, p < 0.02, d = 1.171, but was not significantly different from zero for circles, t(7) = 0.838, n.s. (Figure 3A). There was a trend that the aspect-ratio-repulsion index was greater for flat ellipses than for tall ellipses, t(7) = 2.026, p < 0.09, d = 0.716. Thus, as in Experiment 1, the aspect-ratio-repulsion effect influenced the perception of both tall and flat ellipses but did not affect the perception of circles. As in Experiment 1, the aspect-ratio-repulsion index did not significantly differ between the within-hemifield (M = 0.442, SEM = 0.155) and between-hemifield (M = 0.163, SEM = 0.141) conditions, t(7) = 1.192, n.s. (note that the indices were numerically larger for the within-hemifield condition than for the between-hemifield condition in Experiments 1 and 2, but this was not the case in Experiment 3). Unlike Experiment 1, however, the aspect-ratio-repulsion index did not differ between the outward (M = 0.209, SEM = 0.116) and inward (M = 0.419, SEM = 0.091) directions, t(7) = 1.438, n.s.
Figure 3.
Experiment 2 (ellipse positions jittered, ellipse-to-mask SOA= 40 ms). (A) The aspect-ratio-repulsion index for the perception of circles, flat ellipses, and tall ellipses (with data averaged across the between- and within-hemifield conditions); positive values indicate repulsive effects and negative values indicate averaging effects. * Represents p < 0.05, and ** represents p < 0.01. (B) Ratings of ellipses with different aspect ratios (including circle) when paired with ellipses with identical aspect ratios and presented in a global vertical organization (open symbols) or a global horizontal organization (filled symbols). The x-axis indicates the rated ellipses and the y-axis indicates the observer's ratings of those ellipses. The dashed gray line represents the expected aspect-ratio ratings if perception was veridical. Ellipses in pairs that formed global vertical organizations were perceived to be taller than ellipses in pairs that formed global horizontal organizations, but global organization primarily affected the perception of tall ellipses. For both (A) and (B), the error bars represent ±1 SEM (adjusted for repeated-measures comparisons in (B)).
Hierarchical aspect-ratio interaction
As in Experiment 1, aspect ratios were generally underestimated in perception as the slope of the perceived-vs.-actual-aspect-ratio function was significantly less than 1 (M = 0.662, SEM = 0.042), t(7) = 15.829, p < 0.01, d = 5.596. We also replicated the global assimilation effect on the perception of tall ellipses. As in Experiment 1, a tall ellipse appeared taller when it was a part of a vertical organization than when it was a part of a horizontal organization, but the perception of a flat ellipse was relatively unaffected (Figure 3B). This pattern of results was again confirmed by a three-way ANOVA with a significant main effect of global organization, F(1, 7) = 12.085, p < 0.01, ηp2 = 0.633, a significant interaction between global organization and aspect ratio of the rated ellipse, F(6, 42) = 2.615, p < 0.05, ηp2 = 0.272, with no significant interactions between either of these effects and aspect ratio of the paired ellipse (suggesting that the global assimilation effect is orthogonal to the aspect-ratio-repulsion effect), F(1, 7) = 4.852, n.s., and F(6, 42) = 0.862, n.s., respectively.
We replicated both the aspect-ratio-repulsion and global assimilation effects when the alignment of ellipses was randomly jittered on each trial. It is clear from comparing Figure 2A with Figure 3A and Figure 2B with Figure 3B that neither the aspect-ratio-repulsion effect nor the global assimilation effect was diminished by jittered ellipse alignment. Neither effect depends on the exact alignment of ellipses.
In the next experiment, we began to investigate the underlying mechanisms of the aspect-ratio-repulsion and global assimilation effects. In particular, global precedence effects, reverse hierarchy theory, and the progression of information processing from global categories to finer details in high-level visual neurons suggest that, although the feed-forward flow of neural activation proceeds from low-level to high-level visual areas, perception of visual information flows backward from global organization to local details (see the Introduction section for citations). The global assimilation effect might thus be transient, occurring only when perception is initially dominated by global processing. If true, the global assimilation effect should diminish or be eliminated when the ellipse-to-mask SOA is increased to allow longer stimulus processing time so that perception is dominated by local processing. In contrast, longer processing time might strengthen the aspect-ratio-repulsion effect due to increased temporal summation of lateral inhibitory interactions between the aspect-ratio-tuned neural populations responding to the two ellipses.
Experiment 3: How does longer processing time affect the simultaneous aspect-ratio-repulsion and global assimilation effects?
Methods
Observers
Eight undergraduate students at Northwestern University gave informed consent to participate for course credit. All had normal or corrected-to-normal visual acuity and were tested individually in a dimly lit room.
Stimuli and procedure
The stimulus design and procedure were identical to those in Experiment 1 with two exceptions: the ellipses were presented for an additional 10 ms (50 ms total), and there was a 90-ms blank screen between the offset of the ellipses and the onset of the noise masks, resulting in a 140-ms ellipse-to-mask SOA instead of a 40-ms SOA. The 140-ms value of ellipse-to-mask SOA was chosen for the following reason. We assume that when a backward mask is applied, neural processing lasts for about the duration of the stimulus-to-mask SOA (verified for neurons in macaque inferotemporal cortex; Rolls, Tovée, & Panzeri, 1999). We also assume that local aspect ratios are processed in an intermediate-level visual area such as V4 and global organizations are processed in a high-level visual area such as inferotemporal cortex (see the Introduction section). A 40-ms ellipse-to-mask SOA, as used in Experiments 1 and 2, would terminate the bottom-up activation of aspect-ratio-tuned neurons within about 90 ms (50-ms response latency [Zipser, Lamme, & Schiller, 1996] plus 40-ms ellipse-to-mask SOA). If the latency of feedback from inferotemporal cortex to V4 were similar to that to V1, the feedback would arrive within about 80–100 ms (e.g., Lee, Mumford, Romero, & Lamme, 1998; Rockland & Van Hoesen, 1994; Zipser et al., 1996). These timing estimates suggest that the bottom-up ellipse signals to the intermediate-level aspect-ratio-tuned neurons would end (by ~90 ms) by the time the feedback signals from high-level global processing arrive (80–100 ms). Feedback signals carrying the global organization of ellipses may thus strongly influence aspect-ratio perception in this case with little in the way of concurrent bottom-up ellipse signals to counter their effects. In contrast, a 140-ms ellipse-to-mask SOA, used in this experiment, would prolong the bottom-up processing of ellipse signals by 100 ms, allowing the intermediate-level aspect-ratio-tuned neurons to continue processing the bottom-up ellipse signals while receiving top-down feedback. This may counter the strong initial top-down influences. Note that neural responses to brief (tens of milliseconds) stimuli continue for hundreds of milliseconds when no backward masking is applied (e.g., Hikosaka, 1999; Müller, Metha, Krauskoph, & Lennie, 1999; Rolls et al., 1999; Sato, Kawamura, & Iwai, 1980). Thus, the combination of brief (50 ms) ellipse duration with 140-ms ellipse-to-mask SOA allowed us to increase the stimulus processing duration to counter the initially strong top-down influences, while at the same time allowing us to present the ellipses briefly enough to prevent strategic attention shifts and saccades during ellipse presentation.
Results and discussion
Lateral aspect-ratio interaction
We replicated the aspect-ratio-repulsion effect from Experiments 1 and 2. The aspect-ratio-repulsion index was overall significantly positive (i.e., in the repulsive direction; M = 0.352, SEM = 0.104), t(7) = 3.377, p < 0.02, d = 1.194. The index was significantly positive for the perception of both tall ellipses, t(7) = 2.396, p < 0.05, d = 0.847, and flat ellipses, t(7) = 4.35, p < 0.01, d = 1.539, but not significantly different from zero for the perception of circles, t(7) = 0.246, n.s. (Figure 4A). The trend of a greater aspect-ratio-repulsion index for flat than tall ellipses that we obtained in Experiments 1 and 2 was significant in this experiment, t(7) = 2.550, p < 0.04, d = 0.902. The indices did not significantly differ between the within-hemifield (M = 0.343, SEM = 0.136) and between-hemifield (M = 0.363, SEM = 0.081) conditions, t(7) = 0.377, n.s. Thus, as in Experiments 1 and 2, the aspect-ratio-repulsion effect influenced the perception of both tall and flat ellipses (with greater influences on flat ellipses), equivalently in the within- and between-hemifield conditions, but did not affect the perception of circles. Unlike Experiment 1, but as in Experiment 2, the aspect-ratio-repulsion index did not differ between the outward (M = 0.386, SEM = 0.132) and inward (M = 0.340, SEM = 0.093) directions, t(7) = 0.273, n.s. The overall magnitude of the aspect-ratio-repulsion effect slightly increased in this experiment (Figure 4A) compared to Experiments 1 (Figure 2A) and 2 (Figure 3A), but the increase was not statistically significant (Experiment 3 vs. Experiment 1, t[14] = 0.568, n.s.; Experiment 3 vs. Experiment 2, t[14] = 0.351, n.s.).
Hierarchical aspect-ratio interaction
As in Experiments 1 and 2, observers underestimated aspect ratios as indicated by the slope of the perceived-vs.-actual-aspect-ratio function being significantly less than one (M = 0.735, SEM = 0.053), t(7) = 13.785, p < 0.01, d = 4.874. The magnitude of underestimation, however, decreased in this experiment as the slope (M = 0.735; Figure 4B) was significantly steeper than in Experiment 1 (M = 0.471; Figure 2B), t(14) = 3.187, p < 0.01, d = 1.599, and also steeper than in Experiment 2 (M = 0.662; Figure 3B), though the latter difference was not significant, t(14) = 1.079, n.s. Overall, allowing additional processing time increased the perceptual veridicality of aspect ratio.
As evident in Figure 4B, the global assimilation effect disappeared with longer processing time. A three-way ANOVA with global organization (vertical or horizontal), aspect ratio of the rated ellipse (seven values), and aspect ratio of the paired ellipse (same or different) as the independent variables and aspect-ratio rating as the dependent variable yielded no main effect of global organization, F(1, 7) = 0.0003, n.s., and global organization did not interact with any of the remaining variables (all F-values < 1.787, and all p-values > 0.172).
Thus, longer processing time made the perception of aspect ratios more veridical and slightly increased the aspect-ratio-repulsion effect but eliminated the global assimilation effect.
Discussion
Using briefly and simultaneously presented pairs of ellipses, we have demonstrated two types of spatial interactions in the processing of aspect ratio. One is simultaneous aspect-ratio repulsion in which the aspect ratios of two ellipses in a pair appear to be distorted away from each other (e.g., when a tall ellipse and a taller ellipse are presented, the less tall ellipse appears flatter and the taller ellipse appears even taller). The other is global assimilation in which the global spatial organization of a pair of ellipses is assimilated into the perceived aspect ratio of each ellipse (e.g., a tall ellipse appears taller when in a vertical arrangement with another ellipse). These two effects demonstrate that during early stages of coding multiple images, shape representations are susceptible to interactions through lateral (underlying simultaneous aspect-ratio repulsion) and hierarchical (underlying global assimilation) neural connections.
Lateral aspect-ratio interactions
Visual features interact both temporally and spatially. Temporal interactions are generally repulsive (i.e., viewing the first stimulus distorts the feature value of the second stimulus so that it appears more dissimilar to the first stimulus). Spatial interactions can result in either repulsive (i.e., simultaneously presented stimuli appear more dissimilar in their feature values) or averaging (i.e., simultaneously presented stimuli appear more similar in their feature values) effects.
Temporal and spatial feature interactions have been extensively studied for low-level coding of local orientation. It is typically assumed that perceived orientation is coded by the population activity (e.g., a centroid) of neurons tuned to different orientations (e.g., Regan & Beverley, 1985; Suzuki, 2005; Vogels, 1990; Westheimer, Shimamura, & McKee, 1976). The standard explanation for the temporal and spatial repulsive interactions in perceived orientation, known as the tilt aftereffect (temporal) and the tilt illusion (spatial), is based on orientation-specific inhibition in time and space (see Schwartz et al., 2007, for a review). For example, when a slightly right-tilted grating is viewed, neurons that are responsive to that orientation get adapted (i.e., temporarily suppressed), so that when a vertical grating is subsequently viewed, the adapted right-tilt-preferring neurons make a reduced contribution, skewing the population activity toward left-tilt-preferring neurons, making the vertical grating appear slightly left tilted. Similarly, when a small vertical grating is surrounded by a larger slightly right-tilted grating, the right-tilt-preferring neurons strongly responding to the surrounding grating laterally inhibit the right-tilt-preferring neurons weakly responding to the central vertical grating, skewing the population response to the central grating toward the left-tilt-preferring neurons, making the central vertical grating appear slightly left tilted. Similar temporal and spatial interactions have been demonstrated for the perception of spatial frequency (e.g., Blakemore, Nachmias, & Sutton, 1970; Blakemore & Sutton, 1969; Klein et al., 1974), presumably coded in low-level processing, and curvature (e.g., Bell, Gheorghiu, & Kingdom, 2009; Gheorghiu & Kingdom, 2007; Gibson, 1933), presumably coded in low/intermediate-level processing.
In addition to these repulsive interactions, orientation averaging occurs when gratings are presented in close proximity (e.g., Parkes, Lund, Angelucci, Solomon, & Morgan, 2001). A potential explanation of perceptual averaging is the within-receptive-field neural averaging that is characteristic of ventral visual neurons. When two stimuli are briefly and simultaneously presented within a receptive field of a ventral visual neuron in the absence of selective attention to one stimulus, the neuron responds as if averaging its responses to the individual stimuli presented alone (e.g., Chelazzi et al., 1998; Kastner et al., 2001; Miller et al., 1993; Reynolds et al., 1999; Rolls & Tovée, 1995; Sato, 1989; Zoccolan et al., 2005). Because the gratings used by Parkes et al. (2001) were peripherally presented and only 0.47° apart, it is possible that adjacent gratings fell within the same receptive fields of orientation-tuned neurons in V1 and V2 (e.g., Dow, Snyder, Vautin, & Bauer, 1981; Hubel & Wiesel, 1974; Schiller, Finlay, & Volman, 1976). Thus, for low-level orientation processing, the extant results suggest that orientation-specific inhibitory interactions in time and space produce the tilt aftereffect and tilt illusion, respectively, and within-receptive-field neural averaging may produce perceptual averaging in crowded displays.
Temporal and spatial interactions have also been investigated for high-level face processing. Many studies have demonstrated temporal repulsive interactions (aftereffects) for various facial attributes (e.g., identity, gender, expression, attractiveness, feature spacing, and race; Fox & Barton, 2007; Jaquet, Rhodes, & Hayward, 2007; Leopold, Rhodes, Müller, & Jeffery, 2005; Little, DeBruine, & Jones, 2005; Rhodes, Jeffery, Watson, Clifford, & Nakayama; 2003; Rhodes et al., 2004; Webster & MacLin, 1999). Spatial averaging has also been demonstrated for the perception of facial expressions; averaging occurs when two faces are briefly presented within the same visual hemifield (Sweeny et al., 2009), that is, when both faces are simultaneously presented within single receptive fields of high-level face-tuned neurons. Interestingly, spatial repulsive interactions do not occur when two faces are presented in separate visual hemifields (Sweeny et al., 2009), that is, when faces fall in separate receptive fields for most high-level face-tuned neurons. Note that the receptive fields of high-level ventral visual neurons are large but mostly contralateral (e.g., Boussaoud et al., 1991; Desimone & Gross, 1979; DiCarlo & Maunsell, 2003; Kastner et al., 2001), especially when two stimuli are simultaneously presented in separate hemifields (Chelazzi et al., 1998). Thus, for high-level face processing, feature-specific inhibitory interactions that cause perceptual repulsion seem to occur in time but not in space, while within-receptive-field neural averaging produces perceptual averaging within each hemifield.
How do temporal and spatial interactions influence the coding of intermediate-level features? Aspect ratio is a simple but versatile two-dimensional feature. Unlike low-level features such as local orientation, spatial frequency, and curvature, aspect ratio is defined by closed contours that contribute to the perception of figure versus ground (e.g., Elder & Zucker, 1992; Koffka, 1935). Closed contours (as opposed to open curves) appear to be represented in V3/VP and V4 (e.g., Dumoulin & Hess, 2007) and figure information is represented in LOC (e.g., Appelbaum, Wade, Vildavski, Pettet, & Norcia, 2006) in humans. Neural tuning for aspect ratio (width and height) emerges in V4 in macaque (e.g., Desimone & Schein, 1987). Aspect-ratio coding also distinguishes itself from curvature coding (of open contours) based on behavioral aftereffects. Whereas curvature aftereffects (on open contours) can occur without awareness of the adaptor, aspect-ratio aftereffects require awareness of the adaptor (Sweeny et al., 2010). Behavioral and neural results also suggest that processing of aspect ratio contributes to perception of common objects and faces as well as perception of 3D surfaces (e.g., Biederman, 2001; Biederman & Kalocsai, 1997; Knill, 1998a, 1998b; Rhodes, 1988; Young & Yamane, 1992). Thus, aspect ratio is a bona fide intermediate-level feature in terms of both level of neural processing and pattern complexity. Although temporal repulsive interactions have been demonstrated for aspect ratio (e.g., Regan & Hamstra, 1992; Suzuki, 2003, 2005; Suzuki & Cavanagh, 1998), spatial interactions have not been investigated. The current results clearly demonstrate spatial repulsive interactions in aspect ratio, implicating lateral inhibitory interactions in the coding of a 2D feature. This simultaneous aspect-ratio-repulsion effect is robust in that it repulsively distorted perceived aspect ratios both toward and away from the neutral aspect ratio (circle), whether the ellipse-to-mask SOA was 40 ms or 140 ms, and whether the interacting ellipses were aligned or randomly misaligned (or diagonally arranged based on a pilot study).
The simultaneous aspect-ratio-repulsion effect was statistically equivalent whether ellipses were presented within the same visual hemifield or in separate visual hemifields, indicating that the underlying lateral inhibitory interactions can occur through long-range neural connections across the two cerebral hemispheres. Because perceptual averaging (presumably mediated by within-receptive-field averaging) analogous to that found for facial expression (Sweeny et al., 2009) did not occur when ellipses were presented in separate quadrants in our experiments, it is reasonable to speculate that the receptive fields of aspect-ratio-tuned neurons are no larger than a quadrant of the visual field.
The characteristics of temporal and spatial repulsive interactions and spatial averaging in low-, intermediate-, and high-level visual processing suggest two general principles. First, temporal repulsive interactions appear to be robust across all levels of processing, demonstrated, for example, by strong temporal repulsive aftereffects for orientation, spatial frequency, curvature, aspect ratio, skew, taper, convexity, and facial attributes (see Suzuki, 2005 for a review). This may be related to the fact that detecting change is always important. It is unnecessary to pay attention to things that do not change over time, but when a change occurs, it would be beneficial for the change to be exaggerated so that it is more noticeable (e.g., Clifford et al., 2007). Second, spatial repulsive interactions appear to diminish in higher level pattern processing. Spatial repulsive interactions are strong in the perception of local orientation (involving low-level processing) as exemplified by tilt illusion effects that are evident even in static viewing. Although the aspect-ratio-repulsion effect (reflecting intermediate-level processing) was robust when ellipses were briefly presented in the current study, a slightly tall ellipse statically presented next to a circle, for example, does not appear any taller. Spatial repulsive interactions do not occur in high-level facial-expression processing (Sweeny et al., 2009).
Strong spatial repulsion in lower level processing may contribute to image parsing because differences in low-level local features (such as orientation) demarcate texture boundaries (e.g., Beck, 1966a, 1966b, 1983). Our results demonstrate that spatial repulsion is also operative in intermediate-level processing of aspect ratio. This may be related to the fact that aspect ratio contributes to perception of surface properties (Knill, 1998a, 1998b). For example, spatial repulsive interactions in aspect ratio might accentuate places where surface orientations change. In contrast, in higher level processing of facial expressions, spatial averaging rather than repulsion might be more useful under brief viewing because it would be more important to rapidly grasp the overall sentiment of a crowd rather than to notice differences among faces.
Hierarchical aspect-ratio interactions
Although our primary finding was lateral inhibitory interactions in processing aspect ratio, we also demonstrated a hierarchical interaction demonstrating that a global organization can influence the coding of individual shapes. Specifically, a tall ellipse appeared taller when it was a part of a vertical organization than when it was a part of a horizontal organization. It is as if the global organization was assimilated into individual ellipses' aspect ratios. The global assimilation effect selectively influenced the perception of tall ellipses (Figures 2B and 3B). Although future experiments are necessary to explain the origin of this selectivity, the fact that the aspect-ratio-repulsion effect tended to show the opposite selectivity (more strongly influencing the perception of flat than tall ellipses; Figures 2A–4A) suggests that the assimilation and repulsion effects are mediated by different mechanisms. As discussed below, the temporal characteristics of the two effects suggest that rapid feedback from high-level processing of global organization to intermediate-level processing of aspect ratio mediates the global assimilation effect, whereas persistent lateral inhibition within aspect-ratio processing underlies the repulsion effect.
The aspect-ratio-repulsion effect was slightly strengthened when the ellipse-to-mask SOA was increased from 40 to 140 ms, whereas the global assimilation effect disappeared. This temporal dissociation provided evidence against the possibility that our results could be a general artifact of using a post-cueing procedure. As we described in the Methods section for Experiment 1, we used post-cueing (rather than concurrent cueing) in order to encourage observers to fully attend to both ellipses during their brief presentation. Consequently, observers had to hold both ellipses in memory during the 500-ms masking period until the post-cue (indicating which ellipse to report) was presented and to hold the shape of the target ellipse until the matching screen appeared. It is possible that memory for aspect ratios degraded during this period. Effects of memory degradation should be greater when stimulus encoding is weaker. This is consistent with our results that the aspect-ratio-repulsion effect was slightly (not significantly) weaker with the shorter ellipse-to-mask SOA where the stimulus signal was weaker (Experiments 1 and 2, vs. 3). This explanation, however, does not hold for the global assimilation effect, which was eliminated by the longer ellipse-to-mask SOA where the stimulus signal was stronger and its visual memory should have been less degraded.
Although we cannot conclusively rule out potential contributions of visual memory to the aspect-ratio-repulsion and global assimilation effects, our results are more consistently explained by lateral inhibitory interactions and by the rapid global-to-local progression of perceptual information postulated by reverse hierarchy theory (Hochstein & Ahissar, 2002). It is reasonable to assume that aspect ratio is coded by populations of neurons in an intermediate-level visual area (e.g., Desimone & Schein, 1987; Dumoulin & Hess, 2007), which are tuned to different aspect ratios, and the perceived aspect ratio is represented by a central tendency of their population activity (e.g., Deneve, Latham, & Pouget, 1999; Lee, Rohrer, & Sparks, 1988; Vogels, 1990; Young & Yamane, 1992). Suppose a horizontally organized pair of tall and flat ellipses was presented. Each ellipse would activate an aspect-ratio-tuned neural population with receptive fields covering its location. Consider the neural population responding to the tall ellipse where tall-tuned neurons would be responding relatively more strongly than flat-tuned neurons. We postulate facilitative connections between high-level and intermediate-level visual neurons that respond to similar feature values, similar direction of elongation in this case. Rapid feedback from high-level neurons that code the global horizontal elongation would selectively boost responses of the intermediate-level flat-tuned neurons, making the tall ellipse appear flatter, producing the global assimilation effect. In parallel, the flat-tuned neurons strongly responding to the neighboring flat ellipse would inhibit the flat-tuned neurons weakly responding to the tall ellipse via lateral inhibition, skewing the population response to the tall ellipse to be more strongly dominated by the tall-tuned neurons. This would make the tall ellipse appear even taller, producing the aspect-ratio-repulsion effect.
Reverse hierarchy theory (Hochstein & Ahissar, 2002) postulates that perception is initially dominated by high-level visual processing and then proceeds backward to be subsequently dominated by lower level processing. Consistent with this theory, the global assimilation effect was strong when bottom-up processing was rapidly truncated by backward masking at 40-ms SOA, allowing high-level processing that initially dominates perception to strongly modulate intermediate-level aspect-ratio-tuned neurons via feedback. Increasing the ellipse-to-mask SOA to 140 ms (~50 ms beyond the time when feedback reaches V1; e.g., Lee et al., 1998; Zipser et al., 1996) dissipated the global assimilation effect presumably by allowing bottom-up processing to continue until perception was subsequently dominated by lower level processing. In contrast, lateral inhibitory interactions among aspect-ratio-tuned neural populations should remain robust (or even become stronger) with increased processing of the ellipses. Accordingly, the aspect-ratio-repulsion effect became slightly stronger with increased ellipse-to-mask SOA. Thus, a parsimonious account of our results is that the global assimilation effect reflects transient excitatory feedback from high-level global-elongation-tuned neurons (potentially in the inferotemporal cortex; e.g., Kayaert et al., 2003) to intermediate-level aspect-ratio-tuned neurons (potentially in V4; e.g., Desimone & Schein, 1987), whereas the aspect-ratio-repulsion effect reflects persistent lateral inhibitory interactions among the aspect-ratio-tuned neurons.
It is unclear what behavioral benefit a global assimilation effect might confer. Because this effect occurs only momentarily (it is gone by 140 ms), it is unlikely that the global assimilation effect influences typical perceptual experience where objects are fixated for 200–400 ms (e.g., Yarbus, 1967). Nevertheless, the global assimilation effect provides a behavioral window into the rapid initial hierarchical neural interactions involved in forming representations of multiple shapes.
In summary, previous research has demonstrated (1) temporal repulsive interactions for low-level (orientation and spatial frequency), low/intermediate-level (curvature), intermediate-level (aspect ratio, skew, taper, and convexity), and high-level (various facial attributes) visual features, (2) spatial repulsive interactions for low-level (orientation and spatial frequency) and low/intermediate-level (curvature) features, and (3) spatial averaging for a high-level feature (facial expression; but also for orientation in a highly crowded display). Here we investigated spatial interactions for the intermediate-level feature of aspect ratio. We demonstrated spatial repulsive interactions for aspect ratio even across the vertical meridian, indicative of long-range lateral inhibitory interactions in the mechanisms that code aspect ratio. We also demonstrated a novel effect where a global organization is initially assimilated into the perceived aspect ratios of constituent ellipses, indicative of transient reverse hierarchical influences from high-level to intermediate-level processing. These results suggest that aspect ratio coding is influenced by both lateral inhibitory and hierarchical excitatory neural interactions. From a broader perspective, we suggested that the robust temporal repulsive interactions across all levels of pattern coding, stronger spatial repulsive interactions in lower level pattern coding, and stronger spatial averaging in higher level pattern coding are consistent with functional demands.
Acknowledgments
We thank Kevin Price for help with testing observers. This study was supported by the National Institutes of Health Research Grants R01 EY018197 and T32 EY007043 and by the National Science Foundation Research Grant BCS0643191.
Footnotes
Commercial relationships: none.
References
- Appelbaum LG, Wade AR, Vildavski VY, Pettet MW, Norcia AM. Cue-invariant networks for figure and background processing in human visual cortex. Journal of Neuroscience. 2006;26:11695–11708. doi: 10.1523/JNEUROSCI.2741-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar M. A cortical mechanism for triggering top-down facilitation in visual object recognition. Journal of Cognitive Neuroscience. 2003;15:600–609. doi: 10.1162/089892903321662976. [DOI] [PubMed] [Google Scholar]
- Beck J. Effect of orientation and of shape similarity on perceptual grouping. Perception & Psychophysics. 1966a;1:300–302. [Google Scholar]
- Beck J. Perceptual grouping produced by changes in orientation and shape. Science. 1966b;154:538–540. doi: 10.1126/science.154.3748.538. [DOI] [PubMed] [Google Scholar]
- Beck J. Textural segmentation, second-order statistics, and textural elements. Biological Cybernetics. 1983;48:125–130. doi: 10.1007/BF00344396. [DOI] [PubMed] [Google Scholar]
- Bell J, Gheorghiu E, Kingdom FAA. Orientation tuning of curvature adaptation reveals both curvature-polarity-selective and non-selective mechanisms. Journal of Vision. 2009;9(12):3, 1–11. doi: 10.1167/9.12.3. http://www.journalofvision.org/content/9/12/3, doi:10.1167/9.12.3. [PubMed] [Article] [DOI] [PubMed] [Google Scholar]
- Biederman I. Recognizing depth-rotated objects: A review of recent research and theory. Spatial Vision. 2001;13:241–253. doi: 10.1163/156856800741063. [DOI] [PubMed] [Google Scholar]
- Biederman I, Kalocsai P. Neurocomputational bases of object and face recognition. Philosophical Transactions of the Royal Society of London B. 1997;352:1203–1219. doi: 10.1098/rstb.1997.0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake R, Fox R. Adaptation to invisible gratings and the site of binocular rivalry suppression. Nature. 1974;249:488–490. doi: 10.1038/249488a0. [DOI] [PubMed] [Google Scholar]
- Blakemore C, Carpenter RHS, Georgeson MA. Lateral inhibition between orientation detectors in the human visual system. Nature. 1970;228:37–39. doi: 10.1038/228037a0. [DOI] [PubMed] [Google Scholar]
- Blakemore C, Nachmias J, Sutton P. The perceived spatial frequency shift: Evidence for frequency-selective neurons in the human brain. The Journal of Physiology. 1970;210:727–750. doi: 10.1113/jphysiol.1970.sp009238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore C, Sutton P. Size adaptation: A new after-effect. Science. 1969;166:245–247. doi: 10.1126/science.166.3902.245. [DOI] [PubMed] [Google Scholar]
- Boussaoud D, Desimone R, Ungerleider LG. Visual topography of area TEO in the macaque. The Journal of Comparative Neurology. 1991;306:554–575. doi: 10.1002/cne.903060403. [DOI] [PubMed] [Google Scholar]
- Carpenter RHS, Blakemore C. Interactions between orientations in human vision. Experimental Brain Research. 1973;18:287–303. doi: 10.1007/BF00234599. [DOI] [PubMed] [Google Scholar]
- Chelazzi L, Duncan J, Miller EK, Desimone R. Responses of neurons in inferior temporal cortex during memory-guided visual search. Journal of Neurophysiology. 1998;80:2918–2940. doi: 10.1152/jn.1998.80.6.2918. [DOI] [PubMed] [Google Scholar]
- Clifford CW, Webster MA, Stanley GB, Stocker AA, Kohn A, Sharpee TO, et al. Visual adaptation: Neural, psychological and computational aspects. Vision Research. 2007;47:3125–3131. doi: 10.1016/j.visres.2007.08.023. [DOI] [PubMed] [Google Scholar]
- Deneve S, Latham PE, Pouget A. Reading population codes: A neural implementation of ideal observers. Nature Neuroscience. 1999;2:740–745. doi: 10.1038/11205. [DOI] [PubMed] [Google Scholar]
- Desimone R, Gross CG. Visual areas in the temporal cortex of the macaque. Brain Research. 1979;178:363–380. doi: 10.1016/0006-8993(79)90699-1. [DOI] [PubMed] [Google Scholar]
- Desimone R, Schein SJ. Visual properties of neurons in area V4 of the macaque: Sensitivity to stimulus form. Journal of Neurophysiology. 1987;57:835–868. doi: 10.1152/jn.1987.57.3.835. [DOI] [PubMed] [Google Scholar]
- De Valois RL, Albrecht DG, Thorell LG. Spatial frequency selectivity of cells in macaque visual cortex. Vision Research. 1982;22:545–559. doi: 10.1016/0042-6989(82)90113-4. [DOI] [PubMed] [Google Scholar]
- DiCarlo JJ, Maunsell JHR. Anterior inferotemporal neurons of monkeys engaged in object recognition can be highly sensitive to object retinal position. Journal of Neurophysiology. 2003;89:3265–3278. doi: 10.1152/jn.00358.2002. [DOI] [PubMed] [Google Scholar]
- Dow BM, Snyder AZ, Vautin RG, Bauer R. Magnification factor and receptive field size in foveal striate cortex of the monkey. Experimental Brain Research. 1981;44:213–228. doi: 10.1007/BF00237343. [DOI] [PubMed] [Google Scholar]
- Dumoulin SO, Hess RF. Cortical specialization for concentric shape processing. Vision Research. 2007;47:1608–1613. doi: 10.1016/j.visres.2007.01.031. [DOI] [PubMed] [Google Scholar]
- Elder J, Zucker S. The effect of contour closure on the rapid discrimination of two-dimensional shapes. Vision Research. 1992;33:981–991. doi: 10.1016/0042-6989(93)90080-g. [DOI] [PubMed] [Google Scholar]
- Felleman DJ, Van Essen DC. Distributed hierarchical processing in the primate cerebral cortex. Cerebral Cortex. 1991;1:1–47. doi: 10.1093/cercor/1.1.1-a. [DOI] [PubMed] [Google Scholar]
- Fox CJ, Barton JJS. What is adapted in face adaptation? The neural representations of expression in the human visual system. Brain Research. 2007;1127:80–89. doi: 10.1016/j.brainres.2006.09.104. [DOI] [PubMed] [Google Scholar]
- Gattass R, Sousa APB, Gross CG. Visuotopic organization and extent of V3 and V4 or the macaque. Journal of Neuroscience. 1988;8:1831–1845. doi: 10.1523/JNEUROSCI.08-06-01831.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gheorghiu E, Kingdom FAA. The spatial feature underlying the shape-frequency and shape-amplitude after-effects. Vision Research. 2007;47:834–844. doi: 10.1016/j.visres.2006.11.023. [DOI] [PubMed] [Google Scholar]
- Gibson JJ. Adaptation, after-effect and contrast in the perception of curved lines. Journal of Experimental Psychology. 1933;16:1–31. [Google Scholar]
- Gibson JJ. Adaptation, after-effect and contrast in the perception of tilted lines: II. Simultaneous contrast and areal restriction of the aftereffect. Journal of Experimental Psychology. 1937;20:553–569. [Google Scholar]
- Gilbert CD. Horizontal integration and cortical dynamics. Neuron. 1992;9:1–13. doi: 10.1016/0896-6273(92)90215-y. [DOI] [PubMed] [Google Scholar]
- He S, MacLeod DI. Orientation-selective adaptation and tilt after-effect from invisible patterns. Nature. 2001;411:473–476. doi: 10.1038/35078072. [DOI] [PubMed] [Google Scholar]
- Hegdé J, Van Essen DC. Selectivity for complex shapes in primate visual area V2. Journal of Neuroscience. 2000;20:RC61. doi: 10.1523/JNEUROSCI.20-05-j0001.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegdé J, Van Essen DC. A comparative study of shape representation in macaque visual areas V2 and V4. Cerebral Cortex. 2007;17:1100–1116. doi: 10.1093/cercor/bhl020. [DOI] [PubMed] [Google Scholar]
- Hikosaka K. Tolerances of responses to visual patterns in neurons of the posterior inferotemporal cortex in the macaque against changing stimulus size and orientation, and deleting patterns. Behavioral Brain Research. 1999;100:67–76. doi: 10.1016/s0166-4328(98)00114-4. [DOI] [PubMed] [Google Scholar]
- Hochstein S, Ahissar M. View from the top: Hierarchies and reverse hierarchies in the visual system. Neuron. 2002;36:791–804. doi: 10.1016/s0896-6273(02)01091-7. [DOI] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN. Receptive fields and functional architecture of monkey striate cortex. The Journal of Physiology. 1968;195:215–243. doi: 10.1113/jphysiol.1968.sp008455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN. Uniformity of monkey striate cortex: A parallel relationship between field size, scatter, and magnification factor. Journal of Computational Neuroscience. 1974;158:295–306. doi: 10.1002/cne.901580305. [DOI] [PubMed] [Google Scholar]
- Jaquet E, Rhodes G, Hayward WG. Opposite aftereffects for Chinese and Caucasian faces are selective for social category information and not just physical face differences. Quarterly Journal of Experimental Psychology. 2007;60:1457–1467. doi: 10.1080/17470210701467870. [DOI] [PubMed] [Google Scholar]
- Kastner S, De Weerd P, Pinsk MA, Elizondo MI, Desimone R, Ungerleider LG. Modulation of sensory suppression: Implications for receptive field sizes in the human visual cortex. Journal of Neurophysiology. 2001;86:1398–1411. doi: 10.1152/jn.2001.86.3.1398. [DOI] [PubMed] [Google Scholar]
- Kayaert G, Biederman I, Vogels R. Shape tuning in macaque inferior temporal cortex. Journal of Neuroscience. 2003;23:3016–3027. doi: 10.1523/JNEUROSCI.23-07-03016.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein S, Stromeyer CF, III, Ganz L. The simultaneous spatial frequency shift: A dissociation between the detection and perception of gratings. Vision Research. 1974;14:1421–1432. doi: 10.1016/0042-6989(74)90017-0. [DOI] [PubMed] [Google Scholar]
- Knill DC. Discriminating surface slant from texture: Comparing human and ideal observers. Vision Research. 1998a;38:1683–1711. doi: 10.1016/s0042-6989(97)00325-8. [DOI] [PubMed] [Google Scholar]
- Knill DC. Surface orientation from texture: Ideal observers, generic observers and the information content of texture cues. Vision Research. 1998b;38:1655–1682. doi: 10.1016/s0042-6989(97)00324-6. [DOI] [PubMed] [Google Scholar]
- Koffka K. Principles of Gestalt psychology. New York. Harcourt, Brace & World; 1935. [Google Scholar]
- Lamme VAF, Roelfsema PR. The distinct modes of vision offered by feedforward and recurrent processing. Trends in Neuroscience. 2000;23:571–579. doi: 10.1016/s0166-2236(00)01657-x. [DOI] [PubMed] [Google Scholar]
- Lamme VAF, Supèr H, Spekreijse H. Feedforward, horizontal, and feedback processing in the visual cortex. Current Opinion in Neurobiology. 1998;8:529–535. doi: 10.1016/s0959-4388(98)80042-1. [DOI] [PubMed] [Google Scholar]
- Lee C, Rohrer WH, Sparks DL. Population coding of saccadic eye movements by neurons in the superior colliculus. Nature. 1988;332:357–360. doi: 10.1038/332357a0. [DOI] [PubMed] [Google Scholar]
- Lee TS, Mumford D, Romero R, Lamme VAF. The role of the primary visual cortex in higher level vision. Vision Research. 1998;38:2429–2454. doi: 10.1016/s0042-6989(97)00464-1. [DOI] [PubMed] [Google Scholar]
- Leopold DA, Rhodes G, Müller KM, Jeffrey L. The dynamics of visual adaptation to faces. Proceedings of the Royal Society of London B: Biological Sciences. 2005;272:897–904. doi: 10.1098/rspb.2004.3022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little AC, DeBruine LM, Jones BC. Sex-contingent face after-effects suggest distinct neural populations code male and female faces. Proceedings of the Royal Society of London B: Biological Sciences. 2005;272:2283–2287. doi: 10.1098/rspb.2005.3220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnussen S, Kurtenbach W. Linear summation of tilt illusion and tilt aftereffect. Vision Research. 1980;20:39–42. doi: 10.1016/0042-6989(80)90139-x. [DOI] [PubMed] [Google Scholar]
- Miller EK, Gochin PM, Gross CG. Suppression of visual responses of neurons in inferior temporal cortex of the awake macaque by addition of a second stimulus. Brain Research. 1993;616:25–29. doi: 10.1016/0006-8993(93)90187-r. [DOI] [PubMed] [Google Scholar]
- Moradi F, Koch C, Shimojo S. Face adaptation depends on seeing the face. Neuron. 2005;45:169–175. doi: 10.1016/j.neuron.2004.12.018. [DOI] [PubMed] [Google Scholar]
- Müller JR, Metha AB, Krauskopf J, Lennie P. Rapid adaptation in visual cortex to the structure of images. Science. 1999;285:1405–1408. doi: 10.1126/science.285.5432.1405. [DOI] [PubMed] [Google Scholar]
- Navon D. Forest before the trees: The precedence of global features in visual perception. Cognitive Psychology. 1977;9:353–383. [Google Scholar]
- Paquet L, Merikle PM. Global precedence in attended and nonattended objects. Journal of Experimental Psychology: Human Perception and Performance. 1988;14:89–100. doi: 10.1037//0096-1523.14.1.89. [DOI] [PubMed] [Google Scholar]
- Parkes L, Lund J, Angelucci A, Solomon J, Morgan M. Compulsory averaging of crowded orientation signals in human vision. Nature Neuroscience. 2001;4:739–744. doi: 10.1038/89532. [DOI] [PubMed] [Google Scholar]
- Pasupathy A, Connor CE. Responses to contour features in macaque area V4. Journal of Neurophysiology. 1999;82:2490–2502. doi: 10.1152/jn.1999.82.5.2490. [DOI] [PubMed] [Google Scholar]
- Pasupathy A, Connor CE. Shape representation in area V4: Position-specific tuning for boundary conformation. Journal of Neurophysiology. 2001;86:2505–2519. doi: 10.1152/jn.2001.86.5.2505. [DOI] [PubMed] [Google Scholar]
- Pasupathy A, Connor CE. Population coding of shape in area V4. Nature Neuroscience. 2002;5:1332–1338. doi: 10.1038/nn972. [DOI] [PubMed] [Google Scholar]
- Regan D, Beverley KI. Postadaptation orientation discrimination. Journal of the Optical Society of America. 1985;2:147–155. doi: 10.1364/josaa.2.000147. [DOI] [PubMed] [Google Scholar]
- Regan D, Hamstra SJ. Shape discrimination and the judgment of perfect symmetry: Dissociation of shape from size. Vision Research. 1992;32:1845–1864. doi: 10.1016/0042-6989(92)90046-l. [DOI] [PubMed] [Google Scholar]
- Reynolds JH, Chelazzi L, Desimone R. Competitive mechanisms subserve attention in macaque areas V2 and V4. Journal of Neuro-science. 1999;19:1736–1753. doi: 10.1523/JNEUROSCI.19-05-01736.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes G. Looking at faces: First-order and second-order features as determinates of facial appearance. Perception. 1988;17:43–63. doi: 10.1068/p170043. [DOI] [PubMed] [Google Scholar]
- Rhodes G, Jeffery L, Watson TL, Clifford CWG, Nakayama K. Fitting the mind to the world: Face adaptation and attractiveness aftereffects. Psychological Science. 2003;14:558–566. doi: 10.1046/j.0956-7976.2003.psci_1465.x. [DOI] [PubMed] [Google Scholar]
- Rhodes G, Jeffery L, Watson TL, Jaquet E, Winkler C, Clifford CWG. Orientation-contingent face aftereffects and implications for face-coding mechanisms. Current Biology. 2004;14:2119–2123. doi: 10.1016/j.cub.2004.11.053. [DOI] [PubMed] [Google Scholar]
- Rockland KS, Van Hoesen GW. Direct temporal-occipital feedback connections to striate cortex (V1) in the macaque monkey. Cerebral Cortex. 1994;4:300–313. doi: 10.1093/cercor/4.3.300. [DOI] [PubMed] [Google Scholar]
- Rolls ET, Tovée MJ. The responses of single neurons in the temporal visual cortical areas of the macaque when more than one stimulus is present in the receptive field. Experimental Brain Research. 1995;103:409–420. doi: 10.1007/BF00241500. [DOI] [PubMed] [Google Scholar]
- Rolls ET, Tovée MJ, Panzeri S. The neurophysiology of backward visual masking: Information analysis. Journal of Cognitive Neuroscience. 1999;11:300–311. doi: 10.1162/089892999563409. [DOI] [PubMed] [Google Scholar]
- Rovamo J, Virsu V. An estimation and application of the human cortical magnification factor. Experimental Brain Research. 1979;37:495–510. doi: 10.1007/BF00236819. [DOI] [PubMed] [Google Scholar]
- Sato T. Interactions of visual stimuli in the receptive fields of inferior temporal neurons in awake macaques. Experimental Brain Research. 1989;77:23–30. doi: 10.1007/BF00250563. [DOI] [PubMed] [Google Scholar]
- Sato T, Kawamura T, Iwai E. Responsiveness of inferotemporal single units to visual pattern stimuli in monkeys performing discrimination. Experimental Brain Research. 1980;39:313–319. doi: 10.1007/BF00236651. [DOI] [PubMed] [Google Scholar]
- Schiller PH, Finlay BL, Volman SF. Quantitative studies of single-cell properties in monkey striate cortex: II. Orientation specificity and ocular dominance. Journal of Neurophysiology. 1976;39:1320–1333. doi: 10.1152/jn.1976.39.6.1320. [DOI] [PubMed] [Google Scholar]
- Schwartz O, Hsu A, Dayan P. Space and time in visual context. Nature Reviews, Neuroscience. 2007;8:522–535. doi: 10.1038/nrn2155. [DOI] [PubMed] [Google Scholar]
- Sugase Y, Yamane S, Ueno S, Kawano K. Global and fine information coded by single neurons in the temporal visual cortex. Nature. 1999;400:869–873. doi: 10.1038/23703. [DOI] [PubMed] [Google Scholar]
- Suzuki S. Attentional selection of overlapped shapes: A study using brief shape aftereffects. Vision Research. 2003;43:549–561. doi: 10.1016/s0042-6989(02)00683-1. [DOI] [PubMed] [Google Scholar]
- Suzuki S. High-level pattern coding revealed by brief shape aftereffects. In: Clifford C, Rhodes G, editors. Fitting the mind to the world: Adaptation and aftereffects in high-level vision. Advances in visual cognition series. vol. 2. Oxford University Press; New York, NY: 2005. pp. 135–172. [Google Scholar]
- Suzuki S, Cavanagh P. A shape-contrast effect for briefly presented stimuli. Journal of Experimental Psychology: Human Perception and Performance. 1998;24:1315–1341. doi: 10.1037//0096-1523.24.5.1315. [DOI] [PubMed] [Google Scholar]
- Sweeny TD, Grabowecky M, Suzuki S. Shape aftereffects require awareness. The 10th Annual Meeting of the Vision Sciences Society.2010. [Google Scholar]
- Sweeny TD, Paller KA, Grabowecky M, Suzuki S. Within-hemifield perceptual averaging of facial expressions predicted by neural averaging. Journal of Vision. 2009;9(3):2, 1–11. doi: 10.1167/9.3.2. http://www.journalofvision.org/content/9/3/2, doi:10.1167/9.3.2. [PubMed] [Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura H, Tanaka K. Visual response properties of cells in the ventral and dorsal parts of the macaque inferotemporal cortex. Cerebral Cortex. 2001;11:384–399. doi: 10.1093/cercor/11.5.384. [DOI] [PubMed] [Google Scholar]
- Vogels R. Population coding of stimulus orientation by striate cortical cells. Biological Cybergenetics. 1990;64:25–31. doi: 10.1007/BF00203627. [DOI] [PubMed] [Google Scholar]
- Webster MA, MacLin OH. Figural aftereffects in the perception of faces. Psychonomic Bulletin & Review. 1999;6:647–653. doi: 10.3758/bf03212974. [DOI] [PubMed] [Google Scholar]
- Westheimer G, Shimamura K, McKee SP. Interference with line orientation sensitivity. Journal of the Optical Society of America. 1976;66:332–338. doi: 10.1364/josa.66.000332. [DOI] [PubMed] [Google Scholar]
- Yarbus AL. Eye movements and vision. Plenum Press; New York: 1967. [Google Scholar]
- Young MP, Yamane S. Sparse population coding of faces in the inferotemporal cortex. Science. 1992;256:1327–1331. doi: 10.1126/science.1598577. [DOI] [PubMed] [Google Scholar]
- Zipser K, Lamme VAF, Schiller PH. Contextual modulation in primary visual cortex. Journal of Neuroscience. 1996;16:7376–7389. doi: 10.1523/JNEUROSCI.16-22-07376.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoccolan D, Cox DD, DiCarlo JJ. Multiple object response normalization in monkey inferotemporal cortex. Journal of Neuroscience. 2005;25:8150–8164. doi: 10.1523/JNEUROSCI.2058-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]