The survival of motor neurons (SMN) protein plays an important role in the biogenesis of spliceosomal snRNPs and is one factor required for the integrity of nuclear Cajal bodies (CBs). CBs are enriched in small CB-specific (sca) RNAs, which guide the formation of pseudouridylated and 2′-O-methylated residues in the snRNAs. Because SMN-deficient cells lack typical CBs, the authors asked whether the modification of internal residues of major and minor snRNAs is defective in these cells. They found that modification of major and minor spliceosomal snRNAs is normal in SMN-deficient cells. These results show that the differential splicing defects in SMN-deficient cells are not due to failure of post-transcriptional modification of either major or minor snRNAs.
Keywords: SMN, snRNAs, Cajal body, minor spliceosomal snRNAs
Abstract
The survival of motor neuron (SMN) protein plays an important role in the biogenesis of spliceosomal snRNPs and is one factor required for the integrity of nuclear Cajal bodies (CBs). CBs are enriched in small CB-specific (sca) RNAs, which guide the formation of pseudouridylated and 2′-O-methylated residues in the snRNAs. Because SMN-deficient cells lack typical CBs, we asked whether the modification of internal residues of major and minor snRNAs is defective in these cells. We mapped modified nucleotides in the major U2 and the minor U4atac and U12 snRNAs. Using both radioactive and fluorescent primer extension approaches, we found that modification of major and minor spliceosomal snRNAs is normal in SMN-deficient cells. Our experiments also revealed a previously undetected pseudouridine at position 60 in human U2 and 2′-O-methylation of A1, A2, and G19 in human U4atac. These results confirm, and extend to minor snRNAs, previous experiments showing that scaRNPs can function in the absence of typical CBs. Furthermore, they show that the differential splicing defects in SMN-deficient cells are not due to failure of post-transcriptional modification of either major or minor snRNAs.
INTRODUCTION
The removal of introns from nuclear pre-mRNA molecules is an essential step in the expression of eukaryotic genes. Two different splicing machineries can be distinguished: The major or U2-dependent spliceosome excises U2-type introns, whereas the U12-dependent spliceosome removes U12-type introns (for review, see Will and Lührmann 2001, 2005; Patel and Steitz 2003). The U2-dependent spliceosome contains U1, U2, U4, and U6 snRNAs; the U12-dependent machinery contains the functional counterparts U11, U12, U4atac, and U6atac. The U5 snRNA is common to both spliceosomes.
The biogenesis of major and minor snRNPs proceeds through similar pathways. An important player in the production of both particles is the SMN protein, the product of the Survival of Motor Neuron genes (SMN1 and SMN2). Mutations in SMN1 are responsible for spinal muscular atrophy (SMA), an autosomal recessive neurodegenerative disease, primarily affecting α-motor neurons of the lower spinal cord (Roberts et al. 1970; Lefebvre et al. 1995). SMN protein is a ubiquitously expressed essential protein in all metazoans. It is enriched in nuclear Cajal bodies (CBs) along with RNA processing factors involved in the biogenesis of snRNPs (Gall 2003; Cioce and Lamond 2005). Interestingly, reduction in SMN protein expression in SMA is associated with failure of the SMN complex to assemble into typical CBs, both in cells derived from SMA patients and in an SMA mouse model (Coovert et al. 1997; Lefebvre et al. 1997; Frugier et al. 2000).
Numerous studies have shown that SMN is responsible for formation of the heptameric Sm protein core complex and its association with snRNA (Fisher et al. 1997; Liu et al. 1997; Meister et al. 2001; Pellizzoni et al. 2002). In addition, SMN deficiency alters the stoichiometry of major and minor snRNAs in SMN-deficient mouse tissues and causes widespread and tissue-specific pre-mRNA splicing defects in SMA mice models (Gabanella et al. 2007; Zhang et al. 2008). More recently, it has been shown that formation of the minor U4atac/U6atac/U5 tri-snRNPs is hindered in lymphoblasts derived from a patient suffering from severe SMA. In these cells, splicing of some, but not all minor U12-type introns is perturbed (Boulisfane et al. 2011). Altogether, these results demonstrate that SMN deficiency leads to CB disruption, defects in minor tri-snRNP formation, and inefficient splicing of minor introns.
These coordinated effects of SMN deficiency suggest that the underlying defect might lie in some molecular event(s) that takes place in the CB itself. A likely candidate for such an event would be modification of internal residues on the snRNAs, since the machinery that carries out these modifications is concentrated in CBs. Specifically, CBs are enriched in small CB-specific (sca) RNAs, which function as guides for 2′-O-methylation and pseudouridylation of spliceosomal snRNAs (Jády and Kiss 2001; Darzacq et al. 2002; Jády et al. 2003; Kiss et al. 2004). Furthermore, it is known that snRNA modifications guided by scaRNAs are important for efficient spliceosome assembly and splicing itself (Yu et al. 1998; Dönmez et al. 2004; Zhao and Yu 2004, 2007).
In this study we examined snRNA modifications in SMN-deficient human cells that lack typical CBs. We found that 2′-O-methylation and pseudouridylation are normal in the major U2 snRNA and in the U12 and U4atac minor snRNAs. Our results show that neither normal levels of SMN nor typical CBs are required for the production of modified residues in spliceosomal major and minor snRNAs. Thus, hypomodification of snRNAs is not responsible for the differential splicing defects observed in SMN-deficient cells.
RESULTS AND DISCUSSION
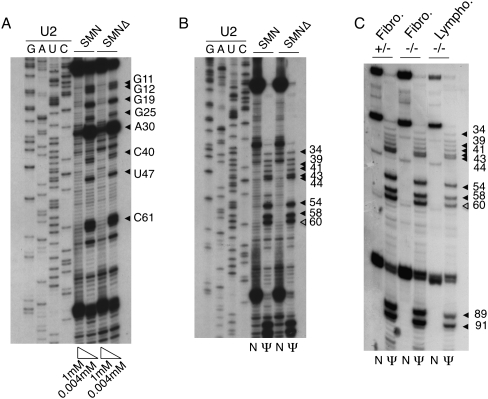
To determine whether SMN deficiency leads to defects in the modification of spliceosomal snRNAs, we first examined total RNA from control and SMN-depleted HeLa cells. In earlier experiments we reported that SMN could be reduced to ∼20%–25% of the control level using siRNAs (Girard et al. 2006; Lemm et al. 2006). To map modified residues in snRNAs, we used reverse transcriptase-based methods after chemical treatment of RNA with CMC (to map pseudouridines) and at low dNTP concentrations (to map 2′-O-methylation). Mapping of endogenous U2 snRNA was first performed using a 32P-labeled oligonucleotide complementary to bases 144–160 of human U2 snRNA (oligo B93). All of the known 2′-O-methylated positions (Massenet et al. 1998) were unambiguously detected in RNA from both control and SMN-deficient cells with the exception of Am1 and Um2 (Fig. 1A). Similarly, pseudouridines were detected in both samples with the exception of positions 6, 7, and 15 (Fig. 1B). A major stop was also found at position 61, showing that human U2 snRNA carries an additional pseudouridine at position 60.
FIGURE 1.
Mapping of 2′-O-methylated nucleotides (A) and pseudouridines (B,C) in human U2 snRNA using radioactive primer extension. Filled arrowheads indicate stop signals corresponding to known modified residues; open arrowheads in B and C indicate the newly recognized pseudouridine at position 60. (A) Primer extension was performed in the presence of 1 mM and 0.004 mM dNTP on RNA isolated from control HeLa cells (SMN) and from HeLa cells depleted for SMN using siRNA (SMNΔ). (Lanes GAUC) Dideoxy sequencing reactions. (B) Total RNA from control (SMN) and SMN-depleted (SMNΔ) HeLa cells was either treated with CMC and alkali buffer (lanes Ψ) or with alkali buffer alone as a control (lanes N). (Lanes GAUC) Sequencing reactions. (C) Primer extensions were performed as described in B using RNA isolated from SMN1−/− fibroblasts (−/−) and heterozygous controls (+/−) as well as from SMN1−/− lymphoblasts.
These results suggest that modification of U2 snRNA does occur in SMN-deficient HeLa cells and is not, therefore, dependent on normal levels of SMN complex nor on the presence of canonical CBs. However, we were concerned that siRNA depletion might give rise to a mixed population of cells, some of which might have higher SMN levels than others. To circumvent this problem, we examined cells derived from SMA patients (SMN1−/− fibroblasts and lymphoblasts). While these cells are not null for SMN, they do have reduced SMN levels (Gangwani et al. 2001; Wan et al. 2005) and represent a more homogeneous population than the siRNA-treated HeLa cells. In addition, these cells lack typical coilin-positive CBs. Instead, coilin is dispersed in smaller “residual nuclear bodies” (Coovert et al. 1997; Boulisfane et al. 2011). Total RNA was prepared from these cells and used in primer extension analysis. As shown in Figure 1C, SMN1−/− fibroblasts and lymphoblasts both show a pattern of pseudouridylated residues in U2 snRNA indistinguishable from that observed in control cells. The same additional stop at position 61 was detectable in all three RNA samples.
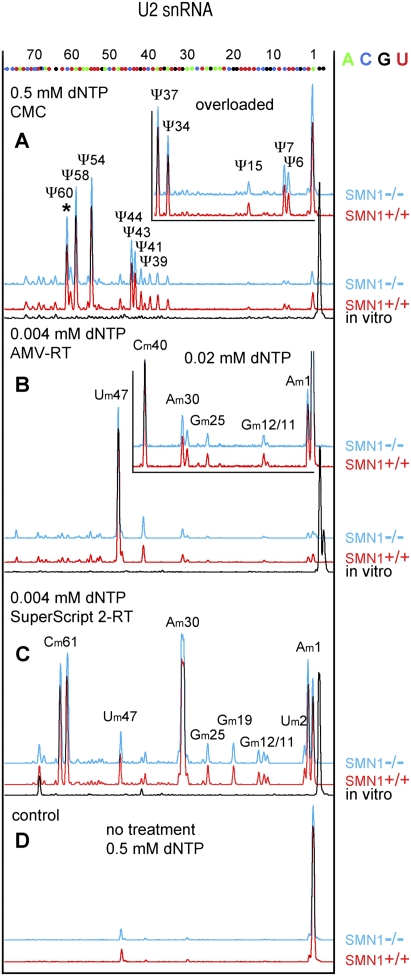
The presence of pseudouridine at all expected positions in U2 snRNA was confirmed by primer extension reactions using a fluorescently labeled oligonucleotide, followed by separation on capillary columns (Fig. 2A). This second approach showed the presence of a prominent peak at position 61, confirming that human U2 snRNA carries a previously unrecognized pseudouridine at position 60. A pseudouridine at the equivalent position 60 has also been reported in a survey of post-transcriptional modifications in the 5′ half of rat liver and brain snRNA (Branlant et al. 1982; Massenet et al. 1998), indicating that this position might indeed be modified in mammals. The fluorescent technique was also used to map 2′-O-methyl residues. All known methylated residues were detected with the exception of G19 and C61 when AMV-RT (New England Biolabs) was used to perform the assay (Fig. 2B). The failure to detect Gm19 and Cm61 in human U2 snRNA using the primer extension approach has been reported repeatedly (Jády et al. 2003; Motorin et al. 2007). The weak pauses at these positions do not depend on the labeled oligo used, but instead on the source and type of reverse transcription enzyme. When SuperScript 2-RT (Invitrogen) was used instead of AMV-RT, primer extension termination was obvious for mapping Cm61 and Gm19, but methylated C40 failed to arrest reverse transcription (Fig. 2C). Interestingly, an extra peak was detectable at the actual position of Cm61. It is possible that the 2′-O-methylated residue produced a double stop signal (Kiss and Jády 2004). However, a second stop opposite a modified residue is usually not a major one. Another possibility is that the newly identified pseudouridine at position 60 is methylated. Based on our observations, we encourage other researchers who use primer extension for mapping 2′-O-methylated residues, to test several reverse transcriptases to get a comprehensive map of post-transcriptional modifications. Altogether, our results demonstrate that the modification of major spliceosomal snRNAs is not dependent on normal levels of SMN and that scaRNPs are able to perform their guide function in cells that lack typical CBs.
FIGURE 2.
Mapping of modified nucleotides in U2 snRNA from SMN1−/− and control SMN1+/+ lymphoblasts using the fluorescent primer extension technique. Positions of nucleotides were determined by alignment with sequencing reactions run on in vitro-transcribed U2 snRNA (shown at top). (A) Twelve pseudouridines are detectable in human U2 snRNA from SMN1+/+ (red traces) and SMN1−/− lymphoblasts (blue traces) after CMC treatment (Ψ6, Ψ7, Ψ15, Ψ34, Ψ37, Ψ39, Ψ41, Ψ43, Ψ44, Ψ54, Ψ58, Ψ60). (*) The newly identified Ψ60. Peaks corresponding to Ψ6, Ψ7, and Ψ15 are more prominent when samples are overloaded (inset). CMC-treated in vitro-transcribed U2 shows no internal peaks (black). (B) Seven 2′-O-methylated residues are detectable in human U2 from SMN1+/+ (red traces) and SMN1−/− lymphoblasts (blue traces) when primer extension is performed at a low concentration of dNTPs using AMV-RT (Am1, Gm11, Gm12, Gm25, Am30, Cm40, Um47). No peaks are observed when primer extension is performed on in vitro-transcribed U2 snRNA (black). (C) Nine 2′-O-methylated residues are detectable unequivocally when SuperScript 2 RT was used for primer extension (Am1, Um2, Gm11, Gm12, Gm19, Gm25, Am30, Um47, Cm61). Note the double stop signal for Cm61: one typical stop preceding the 2′-O-methylated nucleotide and the second stop at the actual position of the modification. In total, all known 2′-O-methylated positions were detected in B and C. (D) Control reactions were performed at a high concentration of dNTPs using RNA from SMN1+/+ (red) and SMN1−/− (blue) lymphoblasts without any chemical treatment.
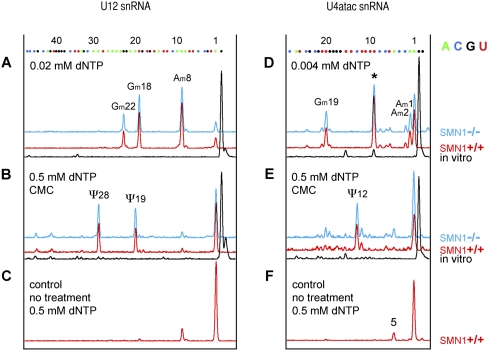
We recently demonstrated that formation of the minor U4atac/U6atac/U5 tri-snRNP is strongly disturbed in SMN1−/− lymphoblasts, whereas the major tri-snRNP is only slightly affected (Boulisfane et al. 2011). Based on this observation, we asked whether modification of the minor snRNAs might be defective in these cells. We characterized modified residues in U12 and U4atac snRNAs using the fluorescent primer extension approach. We chose these two snRNAs because they are known to carry pseudouridines and 2′-O-methyl residues (Massenet et al. 1998; Massenet and Branlant 1999; Tycowski et al. 2004). We did not analyze U11, in which no modified nucleotides have been described, nor U6atac, which has a single pseudouridine at position 83 near the 3′ end of the molecule, and which would be challenging to detect by the fluorescent technique because it would give rise to a short fragment that lies in a noisy region.
The analysis of U12 snRNA (Fig. 3A) demonstrated the expected 2′-O-methyl residues at Am8, Gm18, and Gm22 in RNA extracted from both SMN1+/+ and SMN1−/− lymphoblasts. These modified positions in U12 mirror the modifications in the 5′ terminal stem–loop structure of U2 snRNA, whose importance in pre-mRNA splicing competency is well established (Dönmez et al. 2004). Our analysis demonstrated that pseudouridylation of U12 also occurs in SMN-deficient cells, since strong reverse-transcriptase stops were detected at positions corresponding to Ψ19 and Ψ28 in both SMN1+/+ and SMN1−/− cells (Fig. 3B).
FIGURE 3.
Mapping of modified nucleotides in minor snRNAs U12 (left) and U4atac (right). (A) Three 2′-O-methyl groups are detectable in U12 snRNA from SMN1+/+ (red) and SMN1−/− (blue) lymphoblasts (Am8, Gm18, Gm22). (B) Two pseudouridines are found in U12 snRNA from SMN1+/+ (red) and SMN1−/− (blue) lymphoblasts (Ψ19 and Ψ28) and no peak is observed for in vitro-transcribed U12 snRNA (black). (C) Only a tiny peak for Am8 is detectable in control untreated RNA. (D) 2′-O-methylation is detectable in U4atac at positions A1, A2, an G19. A prominent stop is observed also at position 9 (star) suggesting a 2′-O-methyl group at C8, but the reaction is terminated at the same position when in vitro-transcribed RNA is used (black trace). (E) A pseudouridine at position 12 is detectable in U4atac from SMN1+/+ (red) and SMN1−/− (blue) lymphoblasts, but not in control in vitro-transcribed U4atac (black). (F) When control primer extension reactions are run on RNA samples isolated from lymphoblasts an extra peak appears at position 5. This might correspond to a different isoform of human U4atac. In fact, 19 different genes are reported for U4atac in the human genome databases (http://useast.ensembl.org/Homo_sapiens/) and the U4atac.14-201 variant fits the detected size.
We also found normal modification of U4atac in RNA derived from both SMN1+/+ and SMN1−/− cells (Fig. 3D,E). A strong reverse-transcriptase stop was observed at position 13 after CMC treatment (Fig. 3E), supporting the previous finding that position 12 is a pseudouridine, which probably corresponds to the pseudouridine at position 4 in vertebrate U4 snRNAs (Massenet and Branlant 1999). 2′-O-methylation was detected at positions A1, A2, and G19 (Fig. 3D). A peak at position 9 corresponding to methylation at C8, although prominent, is of uncertain significance, since a peak is also detectable at this position in the control in vitro-transcribed RNA (Fig. 3D). However, it is tempting to assume that C8 of U4atac is 2′-O-methylated, because a 2′-O-methyl group at the same position does exist in U4 snRNA.
In summary, our results demonstrate that modification of U2, U12, and U4atac snRNAs occurs in HeLa cells after SMN knockdown by siRNA and in SMN-deficient cultured cells derived from SMA patients. Since both types of cells have reduced levels of SMN, modification of these snRNAs does not depend on cells having a normal level of SMN protein.
Our results also have implications for the relationship between CBs and snRNA modification. Because scaRNAs are concentrated in CBs, and indeed derive their name from this localization (Darzacq et al. 2002), it has been commonly assumed that modification of snRNAs must occur there. However, coilin-null mice that lack typical CBs have properly modified snRNAs (Jády et al. 2003) and scaRNA levels and modification of U1, U2, U4, and U5 snRNAs are normal in Drosophila mutants that entirely lack CBs (Deryusheva and Gall 2009; Liu et al. 2009; Nizami et al. 2010). Furthermore, many cell types or individual cells in otherwise normal tissues lack CBs, yet must have modified snRNAs in order to carry out normal splicing. In this study we used three types of human cells with reduced levels of SMN: fibroblasts and lymphoblasts from SMN-deficient patients and HeLa cells in which the SMN level was reduced by siRNA. The fibroblasts and lymphoblasts used both lack typical CBs, yet clearly have modified major and minor snRNAs. After siRNA knockdown of SMN, HeLa cells lack typical coilin-positive CBs, but do exhibit smaller coilin-positive foci in their nuclei. These cells also have normally modified major and minor snRNAs. Our results thus support the view that modification of snRNAs can take place in the nucleoplasm in the absence of morphologically typical CBs. Further direct proof that CBs are not required for snRNA modification was demonstrated in experiments with Xenopus oocyte nuclei (Deryusheva and Gall 2009). In this case, an in vitro-transcribed scaRNA and an in vitro-transcribed (unmodified) snRNA were added to a sample of nucleoplasm that had been centrifuged to remove all nuclear bodies. After appropriate incubation of the extract, the snRNA was shown to have been specifically modified by the scaRNA. Modification occurred even when the scaRNA lacked the CAB box signal that targets guide RNAs to the CB (Richard et al. 2003).
In conclusion, our experiments demonstrate that modification of both major and minor snRNAs can take place in cells that are deficient for SMN. Since these cells either lack CBs entirely or have an unusual distribution of CB components, our results also demonstrate that modification of minor snRNAs can occur in the absence of typical CBs. Thus, the differential splicing defects observed in SMN-deficient cells (Zhang et al. 2008; Boulisfane et al. 2011) are not due to hypomodification of snRNAs. However, we cannot exclude formally that the modification process of internal residues in snRNAs might be defective (at least partially) in motor neurons of SMA patients. Given that the formation of CBs is directly related to the neuronal body size (Pena et al. 2001), motor neurons might be particularly dependent on typical CBs and more vulnerable to their disruption than other cells. It would, however, be difficult to analyze the modification status of snRNAs in motor neurons, because spinal cords contain additional cell types in which the snRNA modification process would not be altered, such as Schwann cells and glial cells. Further studies will be needed to uncover the mechanism responsible for the splicing perturbations of particular transcripts in motor neurons of SMA patients.
MATERIALS AND METHODS
Plasmid construction
Human U2 snRNA sequence was amplified from genomic DNA isolated from HeLa cells. The PCR fragment was cloned into pGEM-T Easy vector (Promega). The human U12 and U4atac genes were PCR amplified from plasmids. The blunted PCR fragments were cloned into pUC19 vector linearized with SmaI.
Oligonucleotides used were the following:
U2 forward, TAATTAACCCTCACTAAAGGATCGCTTCTCGGCCTTTTG;
U2 reverse, TGGGTGCACCGTTCCTGGA;
U12 forward, AATTAACCCTCACTAAAGGATGCCTTAAACTTATGAGTAAGG;
U12 reverse, TAATACGACTCACTATAGGGCGGGCAGATCGCAACTCCC;
U4atac forward, AATTAACCCTCACTAAAGGAACCATCCTTTTCTTGGGGT;
U4atac reverse, TAATACGACTCACTATAGGGTATTTTTCCAAAAATTGCACC.
T3 and T7 promoters were included in oligonucleotides (underlined) to make in vitro-transcribed control RNAs.
Cell culture and RNA preparation
Depletion of SMN by siRNA was performed in HeLa cells as described in Girard et al. (2006). A fibroblast cell line from an SMA patient (GM3813) and a control (GM3814) were studied, as well as a lymphoblast cell line (GM10684) derived from an SMA patient and a control. These lines were chosen because the amount of SMN protein is decreased in both cell types (Coovert et al. 1997; Gangwani et al. 2001; Wan et al. 2005). Cells from both mutant lines display “residual CBs,” whereas typical CBs are found in control cells. Cells were cultured using RPMI medium as described previously (Girard et al. 2006; Boulisfane et al. 2011).
Total RNA was purified from cells with Tri-Reagent (Sigma) according to the manufacturer's instructions and treated with RQ1 RNase-free DNase (Promega).
Mapping of modified residues
The modification mapping protocols were as described (Kiss and Jády 2004; Deryusheva and Gall 2009). Mapping of 2′-O-methylated nucleotides was performed by dNTP concentration-dependent primer extension analysis. Pseudouridines were visualized by primer extension after reacting the RNA with CMC (N-cyclohexyl-N′-[2-morpholinoethyl] carbodiimide metho-p-toluene sulfonate) followed by mild alkali treatment. Total RNA (5–10 μg) was used for analysis of the major U2 snRNA, whereas 25 μg of total RNA was used to determine modifications in minor U12 and U4atac snRNAs.
The oligonucleotides used were the following:
B93, U2 [144-160] TACCAGGTCGATGCGTG;
SD114, U2 [91-115] CTCCCTGTTCCAAAAATCCATTTAA;
SD170, U4atac [62-79] TGTGTTGTTCAGGCGTTA;
SD179, U12 [78-97] GCGACCTTTACCCGCTCAAA.
Primer B93 was 5′-end labeled with [γ32P]ATP and T4 polynucleotide kinase, whereas oligonucleotides SD114, SD170, and SD179 were terminally labeled with a 6-FAM fluorescent dye.
Radioactively labeled fragments were separated on 10% polyacrylamide-urea denaturing gels while fluorescently labeled fragments were separated on 50-cm capillary columns in POP4 acrylamide polymer on a capillary electrophoresis instrument (ABI Prism 3100 Geneteic Analyzer).
ACKNOWLEDGMENTS
We thank R. Padgett for the gift of plasmids encoding human minor snRNA genes, and Allison Pinder for technical help. We thank members of our laboratories for stimulating discussions. This work was supported by a grant from SMA Europe to R.B. and by NIH Grant GM33397 to J.G.G.
Footnotes
Article published online ahead of print. Article and publication date are at http://www.rnajournal.org/cgi/doi/10.1261/rna.030106.111.
REFERENCES
- Boulisfane N, Choleza M, Rage F, Neel H, Soret J, Bordonné R 2011. Impaired minor tri-snRNP assembly generates differential splicing defects of U12-type introns in lymphoblasts derived from a type I SMA patient. Hum Mol Genet 20: 641–648 [DOI] [PubMed] [Google Scholar]
- Branlant C, Krol A, Ebel JP, Lazar E, Haendler B, Jacob M 1982. U2 RNA shares a structural domain with U1, U4, and U5 RNAs. EMBO J 1: 1259–1265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cioce M, Lamond A 2005. Cajal bodies: a long history of discovery. Annu Rev Cell Dev Biol 21: 105–131 [DOI] [PubMed] [Google Scholar]
- Coovert DD, Le TT, McAndrew PE, Strasswimmer J, Crawford TO, Mendell JR, Coulson SE, Androphy EJ, Prior TW, Burghes AH 1997. The survival motor neuron protein in spinal muscular atrophy. Hum Mol Genet 6: 1205–1214 [DOI] [PubMed] [Google Scholar]
- Darzacq X, Jády BE, Verheggen C, Kiss AM, Bertrand E, Kiss T 2002. Cajal body-specific small nuclear RNAs: a novel class of 2′-O-methylation and pseudouridylation guide RNAs. EMBO J 21: 2746–2756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deryusheva S, Gall JG 2009. Small Cajal body–specific RNAs of Drosophila function in the absence of Cajal bodies. Mol Biol Cell 20: 5250–5259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dönmez G, Hartmuth K, Lührmann R 2004. Modified nucleotides at the 5′ end of human U2 snRNA are required for spliceosomal E-complex formation. RNA 10: 1925–1933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer U, Liu Q, Dreyfuss G 1997. The SMN-SIP1 complex has an essential role in spliceosomal snRNP biogenesis. Cell 90: 1023–1029 [DOI] [PubMed] [Google Scholar]
- Frugier T, Tiziano FD, Cifuentes-Diaz C, Miniou P, Roblot N, Dierich A, Le Meur M, Melki J 2000. Nuclear targeting defect of SMN lacking the C-terminus in a mouse model of spinal muscular atrophy. Hum Mol Genet 9: 849–858 [DOI] [PubMed] [Google Scholar]
- Gabanella F, Butchbach ME, Saieva L, Carissimi C, Burghes AH, Pellizzoni L 2007. Ribonucleoprotein assembly defects correlate with spinal muscular atrophy severity and preferentially affect a subset of spliceosomal snRNPs. PLoS ONE 2: e921 doi: 10.1371/journal.pone.0000921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gall JG 2003. The centennial of the Cajal body. Nat Rev Mol Cell Biol 4: 975–980 [DOI] [PubMed] [Google Scholar]
- Gangwani L, Mikrut M, Theroux S, Sharma M, Davis RJ 2001. Spinal muscular atrophy disrupts the interaction of ZPR1 with the SMN protein. Nat Cell Biol 3: 376–383 [DOI] [PubMed] [Google Scholar]
- Girard C, Neel H, Bertrand E, Bordonné R 2006. Depletion of SMN by RNA interference in HeLa cells induces defects in Cajal body formation. Nucleic Acids Res 34: 2925–2932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jády BE, Kiss T 2001. A small nucleolar guide RNA functions both in 2′-O-ribose methylation and pseudouridylation of the U5 spliceosomal RNA. EMBO J 20: 541–551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jády BE, Darzacq X, Tucker KE, Matera AG, Bertrand E, Kiss T 2003. Modification of Sm small nuclear RNAs occurs in the nucleoplasmic Cajal body following import from the cytoplasm. EMBO J 22: 1878–1888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiss T, Jády BE 2004. Functional characterization of 2′-O-methylation and pseudouridylation guide RNAs. Methods Mol Biol 265: 393–408 [DOI] [PubMed] [Google Scholar]
- Kiss AM, Jády BE, Bertrand E, Kiss T 2004. Human box H/ACA pseudouridylation guide RNA machinery. Mol Cell Biol 24: 5797–5807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefebvre S, Bürglen L, Reboullet S, Clermont O, Burlet P, Viollet L, Benichou B, Cruaud C, Millasseau P, Zeviani M, et al. 1995. Identification and characterization of a spinal muscular atrophy-determining gene. Cell 80: 155–165 [DOI] [PubMed] [Google Scholar]
- Lefebvre S, Burlet P, Liu Q, Bertrandy S, Clermont O, Munnich A, Dreyfuss G, Melki J 1997. Correlation between severity and SMN protein level in spinal muscular atrophy. Nat Genet 16: 265–269 [DOI] [PubMed] [Google Scholar]
- Lemm I, Girard C, Kuhn AN, Watkins NJ, Schneider M, Bordonné R, Lührmann R 2006. Ongoing U snRNP biogenesis is required for the integrity of Cajal bodies. Mol Biol Cell 17: 3221–3231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Fischer U, Wang F, Dreyfuss G 1997. The spinal muscular atrophy disease gene product, SMN, and its associated protein SIP1 are in a complex with spliceosomal snRNP proteins. Cell 90: 1013–1021 [DOI] [PubMed] [Google Scholar]
- Liu JL, Wu Z, Nizami Z, Deryusheva S, Rajendra TK, Gao H, Beumer KJ, Carroll D, Matera AG, Gall JG 2009. Coilin is essential for Cajal body organization in Drosophila melanogaster. Mol Biol Cell 20: 1661–1670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massenet S, Branlant C 1999. A limited number of pseudouridine residues in the human atac spliceosomal UsnRNAs as compared to human major spliceosomal UsnRNAs. RNA 5: 1495–1503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massenet S, Mougin A, Branlant C 1998. Posttranscriptional modifications in the U small nuclear RNAs. In The modification and editing of RNA (ed. Grosjean H Benne R), pp. 201–228 ASM Press, Washington, DC [Google Scholar]
- Meister G, Bühler D, Pillai R, Lottspeich F, Fischer U 2001. A multiprotein complex mediates the ATP-dependent assembly of spliceosomal U snRNPs. Nat Cell Biol 3: 945–949 [DOI] [PubMed] [Google Scholar]
- Motorin Y, Muller S, Behm-Ansmant I, Branlant C 2007. Identification of modified residues in RNAs by reverse transcription-based methods. Methods Enzymol 425: 21–53 [DOI] [PubMed] [Google Scholar]
- Nizami ZF, Deryusheva S, Gall JG 2010. Cajal bodies and histone locus bodies in Drosophila and Xenopus. Cold Spring Harb Symp Quant Biol 75: 313–320 [DOI] [PubMed] [Google Scholar]
- Patel AA, Steitz JA 2003. Splicing double: insights from the second spliceosome. Nat Rev Mol Cell Biol 4: 960–970 [DOI] [PubMed] [Google Scholar]
- Pellizzoni L, Yong J, Dreyfuss G 2002. Essential role for the SMN complex in the specificity of snRNP assembly. Science 298: 1775–1779 [DOI] [PubMed] [Google Scholar]
- Pena E, Berciano MT, Fernandez R, Ojeda JL, Lafarga M 2001. Neuronal body size correlates with the number of nucleoli and Cajal bodies, and with the organization of the splicing machinery in rat trigeminal ganglion neurons. J Comp Neurol 430: 250–263 [DOI] [PubMed] [Google Scholar]
- Richard P, Darzacq X, Bertrand E, Jády BE, Verheggen C, Kiss T 2003. A common sequence motif determines the Cajal body-specific localisation of box H/ACA scaRNAs. EMBO J 22: 4283–4293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts DG, Chavez J, Court SDM 1970. The genetic component in child mortality. Arch Dis Child 45: 33–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tycowski KT, Aab A, Steitz JA 2004. Guide RNAs with 5′ caps and novel box C/D snoRNA-like domains for modification of snRNAs in metazoa. Curr Biol 14: 1985–1995 [DOI] [PubMed] [Google Scholar]
- Wan L, Battle DJ, Yong J, Gubitz AK, Kolb SJ, Wang J, Dreyfuss G 2005. The survival of motor neurons protein determines the capacity for snRNP assembly: biochemical deficiency in spinal muscular atrophy. Mol Cell Biol 25: 5543–5551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Will CL, Lührmann R 2001. Spliceosomal UsnRNP biogenesis, structure and function. Curr Opin Cell Biol 13: 290–301 [DOI] [PubMed] [Google Scholar]
- Will CL, Lührmann R 2005. Splicing of a rare class of introns by the U12-dependent spliceosome. Biol Chem 386: 713–724 [DOI] [PubMed] [Google Scholar]
- Yu YT, Shu MD, Steitz JA 1998. Modifications of U2 snRNA are required for snRNP assembly and pre-mRNA splicing. EMBO J 17: 5783–5795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Lotti F, Dittmar K, Younis I, Wan L, Kasim M, Dreyfuss G 2008. SMN deficiency causes tissue-specific perturbations in the repertoire of snRNAs and widespread defects in splicing. Cell 133: 585–600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, Yu YT 2004. Pseudouridines in and near the branch site recognition region of U2 snRNA are required for snRNP biogenesis and pre-mRNA splicing in Xenopus oocytes. RNA 10: 681–690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, Yu YT 2007. Incorporation of 5-fluorouracil into U2 snRNA blocks pseudouridylation and pre-mRNA splicing in vivo. Nucleic Acids Res 35: 550–558 [DOI] [PMC free article] [PubMed] [Google Scholar]