Abstract
Background
Development of atherosclerosis, which is the leading cause of death in developed countries is due to persistent chronic inflammation in the artery wall. Exciting discoveries related to IL-1R-TLR signaling in development of atherosclerosis plaque have triggered intense interest in the molecular mechanisms by which innate immune signaling modulates the onset and development of atherosclerosis. Previous studies have clearly shown the definitive role of proinflammatory cytokine IL-1 in the development of atherosclerosis. Removal of IL-1 or IL-1R reduced vascular inflammation and atherosclerosis in ApoE−/− mice. Recent studies have provided direct evidence supporting a link between innate immunity and atherogenesis. While it is still controversial about whether infectious pathogens contribute to cardiovascular diseases, direct genetic evidence indicates the importance of TLR-IL-1R signaling in atherogenesis.
Methods and Results
To investigate the specific role of IRAK4 kinase in the development of atherosclerosis, IRAK4 kinase inactive knockin (IRAK4KI) mice were bred onto ApoE−/− mice to generate IRAK4KI/ApoE−/− mice. The aortic sinus lesion formation was impaired in IRAK4KI/ApoE−/− mice compared to that in ApoE−/− mice. Furthermore, proinflammatory cytokine production was reduced in the aortic sinus region of IRAK4KI/ApoE−/− mice compared to that in ApoE−/− mice. Importantly, the IRAK4 kinase activity was required for modified LDL-induced IκBα phosphorylation (NFκB activation) and expression of a subset of proinflammatory genes, but not for the activation of MAPKs in bonemarrow-derived macrophage (BMDM). Importantly, inactivation of IRAK4 kinase had no effect on modified LDL uptake and foam cell formation in BMDM.
Conclusions
Taken together, our results indicate that the IRAK4 kinase plays an important role in modified LDL-mediated signaling and the development of atherosclerosis, suggesting that pharmacological inhibition of IRAK4 kinase activity might be a feasible approach in the development of anti-atherosclerosis drugs.
Introduction
Development of atherosclerosis, which is the leading cause of death in developed countries is due to persistent chronic inflammation in the artery wall (1). Exciting discoveries related to IL-1R-TLR signaling in development of atherosclerosis plaque have triggered intense interest in the molecular mechanisms by which innate immune signaling modulates the onset and development of atherosclerosis. Previous studies have clearly shown the definitive role of proinflammatory cytokine IL-1 in the development of atherosclerosis. Removal of IL-1 or IL-1R reduced vascular inflammation and atherosclerosis in ApoE−/− mice (2;3). Recent studies have provided direct evidence supporting a link between innate immunity and atherogenesis. While it is still controversial about whether infectious pathogens contribute to cardiovascular diseases, direct genetic evidence indicates the importance of TLR-IL-1R signaling in atherogenesis (4). Elevated serum cholesterol is a major risk factor for the development of atherosclerosis in humans and genetically altered mice. Macrophage scavenger receptors (SR) that mediate the uptake of modified forms of LDL (low-density lipoproteins) cross-talk with TLRs to modulate macrophage apoptosis, modulating inflammatory response and atherosclerosis (5). A recent study demonstrated that modified LDL activates inflammatory signaling pathways through CD36-mediated activation of a new heterodimeric complex TLR4-TLR6 (6). TLR4 polymorphisms that map to extracellular domain of TLR4 and cause hyporesponsiveness to LPS is associated with reduced risk for carotid artery atherosclerosis (7). Importantly, TLR2-, TLR4- and IL-1R-deficient mice displayed reduced atherosclerosis, linking elevated serum cholesterol levels to activation of IL-1R-TLR signaling pathways (4;8;9).
Toll-like receptors (TLRs) are employed by the innate immune system to recognize conserved molecules associated with invading microorganisms, leading to inflammatory responses and linking to adaptive immunity (10–15). Upon binding the traditional TLR ligands (pathogens associated molecular patterns), all of the TLRs except TLR3 recruit the adaptor molecule MyD88 through the TIR domain, mediating MyD88-dependent pathways(16). MyD88 then recruits the serine-threonine kinases IL-1 receptor associated kinase4 (IRAK4) and IRAK1. IRAK4 phosphorylates IRAK1, which then mediates the recruitment of TRAF6 to the receptor complex (17). The IRAK1-TRAF6 complex then dissociates from the receptor to interact with and activate TAK1 and MEKK3, members of the MAP kinase kinase kinase (MAPKKK) family (18). The activation of TAK1 and MEKK3 eventually leads to the activation of NFkB and JNK (19), which in turn induce transcription of inflammatory cytokine and chemokine genes, such as those encoding TNFα, IL-1β, IL-6 and IL-8. TLR3 and TLR4 also employ a MyD88-indepenent pathway that uses TIR domain-containing adaptor inducing IFN-β (TRIF) to activate NFkB and IRF3. TLR4-mediated MyD88-independent activities are abolished in mice lacking TRAM, another adaptor in this pathway (20). While recent studies have shown that different forms of modified LDL can activate inflammatory signaling pathways through the activation of TLRs and scavenger receptors (SR) (6), the SR-TLR-mediated signaling pathways have not been well characterized.
IRAK4 has an essential role in Toll-like receptor (TLR)-mediated signaling (21;22). IRAK4 kinase-inactive knock-in mice were completely resistant to LPS- and CpG-induced shock, due to impaired TLR-mediated induction of pro-inflammatory cytokines and chemokines (23–26). While inactivation of IRAK4 kinase activity abolished TLR/IL-1R-mediated TAK1-dependent, but not MEKK3-dependent NFkB activation, a reduction of LPS-, R848- and IL-1-mediated mRNA stability contributed to the reduced cytokine and chemokine production in bone marrow (BM)-derived macrophages from IRAK4 kinase-inactive knock-in mice (24;26). These in vivo studies indicate that IRAK4 kinase activity plays a critical role in TLR-dependent immune responses (27). Much effort has been devoted towards the search for IRAK4 inhibitors, with the hope of developing better anti-inflammatory therapies. Therefore, it is critical to determine the importance of IRAK4 kinase activity in different chronic inflammatory diseases. To investigate the role of IRAK4 kinase activity in the development of atherosclerosis, IRAK4 kinase inactive knockin (IRAK4KI) mice were bred onto ApoE−/− mice to generate ApoE−/−/IRAK4KI mice. The aortic sinus lesion formation and proinflammatory cytokine production were reduced in the aortic sinus region of ApoE−/−/IRAK4KI mice compared to that in ApoE−/− mice. Importantly, acLDL-induced IκBα phosphorylation (NFκB activation) and expression of a subset of proinflammatory genes were impaired in bone marrow (BM)-derived macrophages from IRAK4 kinase-inactive knock-in mice. Taken together, our results indicate that the IRAK4 kinase activity plays an important role in acLDL-mediated signaling and the development of atherosclerosis, suggesting that pharmacological inhibition of IRAK4 kinase activity might be a feasible approach in the development of anti-atherosclerosis drugs.
Results
Impaired aortic sinus lesion formation in IRAK4 kinase-inactive knock-in ApoE−/− mice
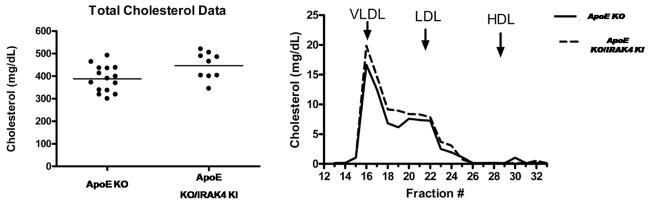
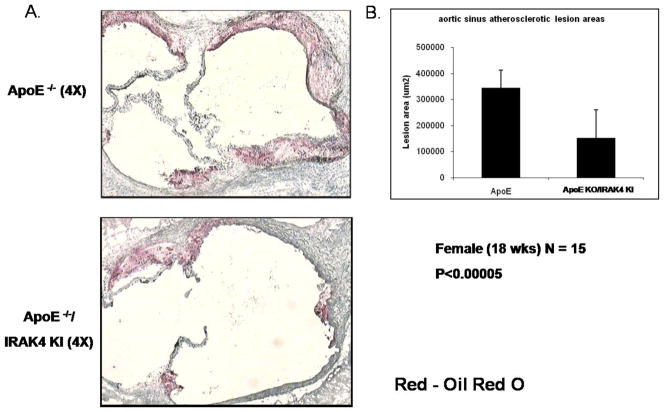
Our preliminary results indicated that the IRAK4 kinase activity is required for TLR/IL-1R-induced TAK1-dependent NFκB activation and mRNA stability. In this study, we examined the role of IRAK4 kinase activity in the development and pathogenesis of atherosclerosis by using a spontaneous mouse model of atherosclerosis, the ApoE-deficient mice (ApoE−/−), a well characterized mouse model of human atherosclerosis. ApoE is critical for normal metabolism of cholesterol-containing lipoproteins in mice and ApoE−/− mice spontaneously develop hypercholesterolemia and atherosclerosis, even when fed with a normal chow diet. To investigate the impact of IRAK4 kinase activity on atherosclerosis, IRAK4 kinase inactive knockin mice (IRAK4 KI) were crossed with ApoE−/− mice to generate ApoE−/−/IRAK4KI mice. We first examined whether the inactivation of kinase activity of IRAK4 affects total plasma cholesterol level. The plasma cholesterol (Fig 1 A) and lipoprotein levels (Fig 1 B) were similar between female 16-weeks old ApoE−/−/IRAK4KI and ApoE−/− mice fed a normal chow diet, indicating that the disruption of IRAK4 kinase activity did not substantially affect lipid biosynthesis or metabolism. Previously, others have shown that functional deficiency of IRAK4 inhibited formation of both early and advanced vascular lesions in a mouse model of physical vascular injury coupled with accelerated atherosclerosis in ApoE−/− mice (28). This prior study combined injury with the atherogenic milieu in ApoE−/− mice; however, in the current study we used a mouse model of spontaneous atherosclerosis by feeding ApoE−/− mice with a chow diet until 18 weeks of age. The impact of functional deficiency of IRAK4 on the development of spontaneous atherosclerosis was determined by comparing the severity of atherosclerosis of ApoE−/−/IRAK4KI with that of ApoE−/− mice. Atherosclerotic lesions were about 60% smaller in ApoE−/−/IRAK4KI compared to that of ApoE−/− mice (Fig. 2). The lesions, when present, consisted primarily of macrophages. Thus, functional deficiency of IRAK4 inhibited vascular lesion formation in IRAK4 kinase inactive knock-in ApoE−/− mice in a mouse model of spontaneous atherosclerosis.
Figure 1. Total cholesterol and plasma lipoprotein distribution in 16-week-old apoE −/− or IRAK4 KI/ApoE −/− mice.
A. Total cholesterol in 16-week-old apoE−/− or IRAK4KI/apoE−/− mice
B. FPLC elution profiles of cholesterol from apoE −/− and IRAK4 KI/apoE−/− mice. Arrows indicate the elution peak for each lipoprotein class: very low density lipoprotein (VLDL), low density lipoprotein (LDL) and high density lipoprotein (HDL).
Figure 2. Deficiency of IRAK4 kinase activity greatly reduced aortic root lesion area in Apoe −/− mice.
A. Representative photographs of aortic sinus plaques from IRAK4 KI/Apoe−/− (lower) compared with Apoe−/− (upper) mice. ApoE−/− and IRAK4 KI/ApoE−/− mice were fed with normal chow diet for 18 weeks after weaning. Cross sections were stained with Oil Red O for neutral lipid (4X objective lens).
B. Lesion area in ApoE−/− and IRAK4 KI/IRAK4 KI mice on 18 weeks of chow diet after weaning. Mean ± S.D.; p<0.00005
Functional deficiency of IRAK4 leads to reduced proinflammatory mRNA expression in arterial tissue
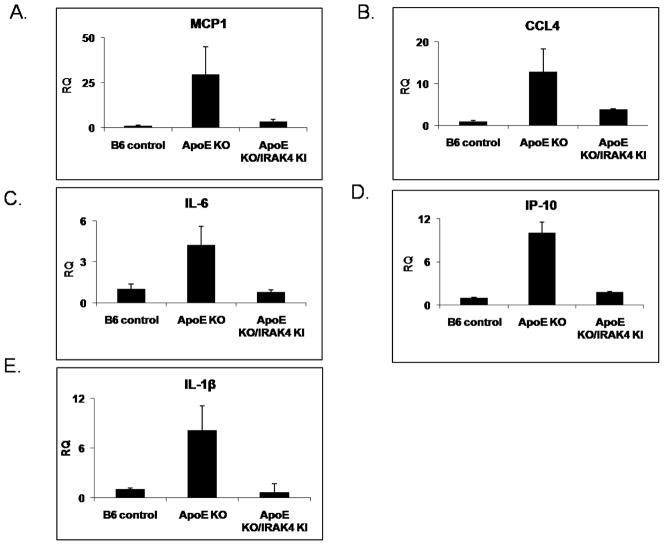
ApoE−/− mice spontaneously develop hypercholesterolemia and atherosclerosis. Modified lipoproteins accumulate in the artery wall during hypercholesterolemia, setting off a cascade of pro-inflammatory events, particularly the expression of cytokines and chemokines that further amplify the inflammatory cascade, including recruitment and activation of macrophages. To examine the mechanism by which IRAK4 impacts on the development of spontaneous atherosclerosis in ApoE−/− mice, we analyzed the expression of proinflammatory cytokines using RNA isolated from the total aorta of ApoE−/− and ApoE−/−/IRAK4KI mice fed a normal chow diet for 20 weeks. The expression of proinflammatory genes, such as MCP1, CCL4, IL-6, IP-10, and IL-1β was indeed significantly increased in the aortic sinus region of these aged ApoE−/− mice compared to C57BL/6 wild-type mice (Fig. 3). Consistent with the reduced vascular lesion formation in ApoE−/−/IRAK4KI mice, the chemokine/cytokine production was impaired in the absence of IRAK4 kinase activity, suggesting IRAK4 kinase activity is required for the production of inflammatory genes (Fig. 3). Importantly, the mRNA expression of IL-1R, Toll-like receptors (TLR2, TLR1, TLR4, and TLR6) and scavenger receptors (SR-A and CD36) was similarly up-regulated in both ApoE−/− and ApoE−/−/IRAK4KI aorta compared to that in C57BL/6 wild-type mice (data not shown). Thus, the reduced inflammatory gene expression in the absence of IRAK4 kinase activity in this model is not due to down-regulation of these innate immune receptors.
Figure 3. Reduced inflammatory gene expression in arterial tissue of ApoE/IRAK4 KI.
(A–E) At age 18 weeks, apoE−/− (n=6), IRAK4KI/apoE−/− (n=6), and control B6 mice (n=2) were sacrificed and aortas retrieved. Total RNA from aorta was analyzed by quantitative RT-PCR of specific primer pairs for MCP1 (A), CCL4 (B), IL-6 (C), IP-10 (D) and IL-1β (E). Shown is the expression of MCP1, CCL4, IL-6, IP-10 and IL-1β normalized to beta-actin expression. The -fold induction was calculated as compared with the expression of WT untreated cells (set as 1-fold).
IRAK4 kinase activity is required for the induction of a subset of pro-inflammatory genes in response to modified LDL
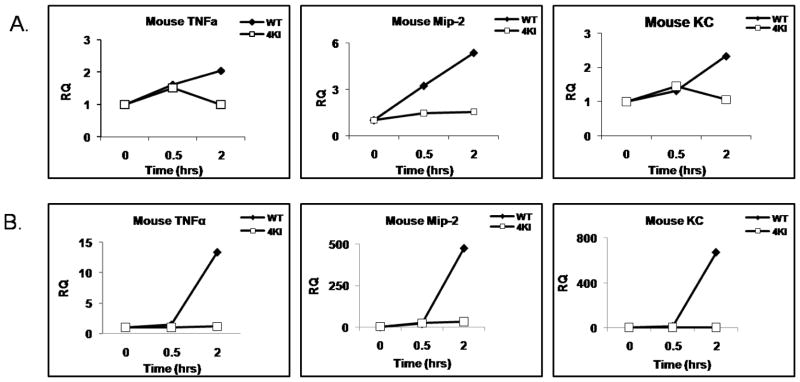
While IRAK4 kinase activity is required for TLR/IL-1R-mediated signaling, modified LDLs have been shown to activate inflammatory genes in macrophages through the co-activation of TLRs and SRs. Therefore, it is important to examine whether IRAK4 kinase activity is required for modified LDL-induced inflammatory response in macrophages. To identify the acLDL-induced-IRAK4-dependent target genes, we performed quantitative Real-time PCR to measure the expression of a subset of inflammatory genes using total RNAs isolated from wild-type and IRAK4KI BM macrophages with and without acLDL stimulation. Interestingly, acLDL-induced expression of proinflammatory cytokine/chemokine (TNFα, MIP-2 and KC) was greatly reduced in IRAK4 kinase-inactive knock-in macrophages compared to that in wild-type macrophages (Fig 4 A), indicating the critical role of IRAK4 kinase activity in acLDL-mediated signaling.
Figure 4. Impaired acLDL-mediated gene expression in macrophages from IRAK4 kinase-inactive knock-in mice.
A–B. Bonemarrow-derived macrophages of wild-type and IRAK4 kinase-inactive knock-in mice were untreated or treated with acLDL (100 μg/ml) (A) or NO2-LDL (B) for 30 and 120 min. Total RNA was subjected to RT-PCR in order to measure the relative expression of TNFα (A), MIP-2 (B) and KC (C). Shown is the expression of TNFα, MIP-2 and KC normalized to beta-actin expression. The fold induction was calculated as compared with the expression of WT untreated cells.
In addition, we also examined CD36-specific ligand, NO2-LDL-induced gene transcription in WT and IRAK4 kinase-inactive knock-in macrophage by quantitative Real-time PCR. NO2-LDL-medaited proinflammatory gene expression was also greatly reduced in IRAK4 kinase-inactive knock-in macrophage compared to WT macrophage (Fig 4 B).
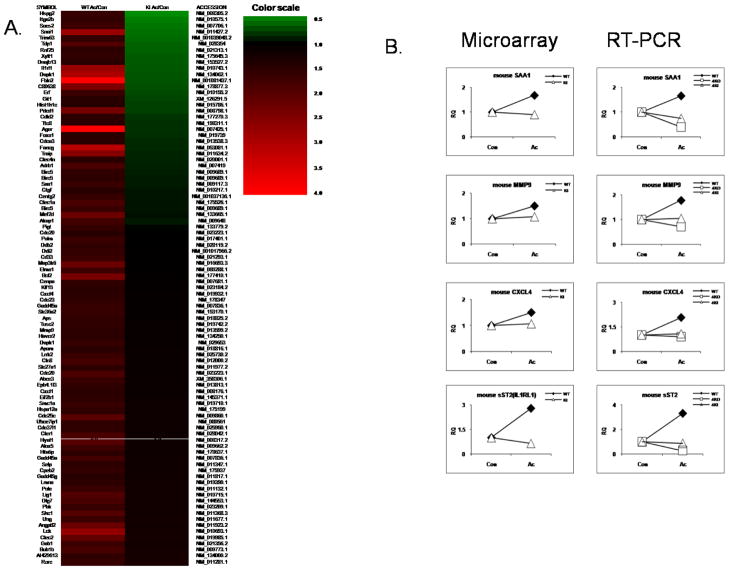
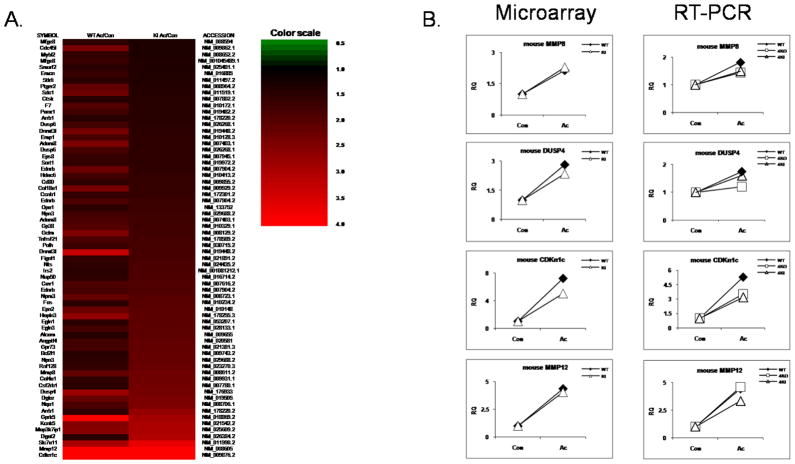
To identify global changes in gene expression, we examined gene expression profiles of macrophages from wild-type and IRAK4 kinase-inactive knock-in mice in response to acLDL stimulation using the Illumina microarray with probes for 23,000 transcripts. Bone marrow-derived macrophages from wild-type and IRAK4 kinase-inactive knock-in mice were treated with acLDL for 24 hours. Importantly, we have identified a group of genes (91 genes) that were induced only in wild-type but not in IRAK4 kinase-inactive knock-in macrophages at 24 hours of acLDL treatment, some of which were confirmed by real-time PCR (Fig. 5 A–B). For example, SAA1, MMP9, CXCL4 (PF4) and sST2 (IL-1RL1) mRNAs were induced by acLDL stimulation in wild-type, but not in IRAK4 KI macrophage (Fig 5 A–B). We also identified a set of IRAK4-independent genes that were similarly induced by 24 hours of acLDL treatment (>1.5 fold of induction) in both wild-type and IRAK4 kinase-inactive knockin macrophages (Fig 6 A–B). For example, MMP8, a neutrophil collagenase, CDKn1c, a cyclin-dependent kinase inhibitor (p57), DUSP4, a dual specific protein phosphatase and MMP12, a macrophage metalloelastase mRNAs were similarly upregulated in wild-type and IRAK4 kinase-inactive knock-in macrophages.
Figure 5. Illumina mRNA expression profiling of IRAK4 kinase activity-dependent genes.
A. Heatmap of the genes that were induced only in wild-type bonemarrow-derived macrophage, but not in the IRAK4 KI macrophage upon 24 hours of acLDL (100 μg/ml) stimulation.
B. Quantitative Real-time PCR of selected genes from Figure 6 A. Wild-type, IRAK4 kinase-inactive knock-in and IRAK4-deficient macrophages were either untreated or stimulated with acLDL for 24 hours. The fold change was calculated compared with the expression of untreated samples.
Figure 6. Illumina mRNA expression profiling of IRAK4 kinase activity-independent genes.
A. Heatmap of the genes that were induced similarly (>1.5 fold) in both wild-type and IRAK4 kinase inactive knock-in macrophage upon 24 hours of acLDL (100 μg/ml) stimulation.
B. Quantitative Real-time PCR of selected genes from Figure 6 A. Wild-type, IRAK4 kinase-inactive knock-in and IRAK4-deficient macrophages were either untreated or stimulated with acLDL for 24 hours. The fold change was calculated compared with the expression of untreated samples (set as value 1)
Inactivation of IRAK4 kinase activity has no effect on acLDL uptake and foam cell formation in macrophages
Endothelial cell injury and dysfunction is a characteristic of the formation of arterial lesions and is a prominent theory of atherosclerosis disease development. Circulating monocytes adhere to activated endothelium due to expression of adhesion molecules, where they differentiate into macrophages, and accumulate lipoproteins, leading to their characteristic foam cell phenotype. Foam cells contribute to the growth and vulnerability of the atherosclerosis plaque by producing growth factors, cytokines, and matrix-degrading metalloproteinases (MMPs), and by interacting with surrounding endothelium, lymphocytes and smooth muscle cells. Therefore macrophage foam cell formation is the hallmark of early atherosclerosis. Since IRAK4 kinase activity is required for acLDL-induced inflammatory response in macrophages, it is important to determine whether the inactivation of IRAK4 kinase activity has any impact on acLDL uptake and foam cell formation in macrophages. Bone marrow-derived macrophages from IRAK4 KI mice showed no difference in the number of acLDL-induced foam cells (detected by oil red O staining) compared to wild-type macrophages (data not shown), suggesting that the kinase activity of IRAK4 is dispensable for acLDL induced foam cell formation. We also measured cholesterol accumulation in macrophages upon acLDL stimulation. Consistent with foam cell formation results, bone marrow-derived macrophages from IRAK4 KI mice showed similar levels of cholesterol mass accumulation compared to that in wild-type cells (data not shown). These results are highly consistent with a previous study that MyD88 is required for early onset of atherosclerosis development, but does not play a critical role in foam cell formation (9).
TLR2 and TLR4 are partially required for acLDL-induced inflammatory gene expression
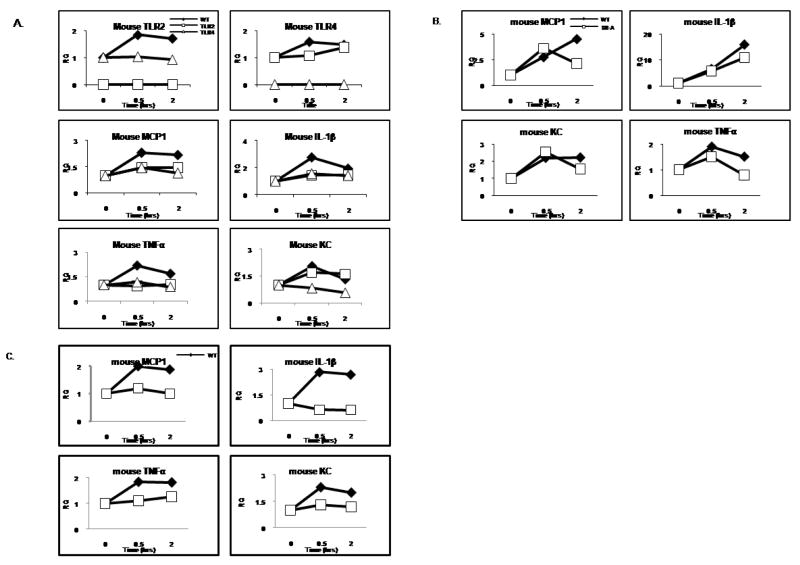
The fact that the impact of IRAK4 kinase activity on modified-LDL-mediated inflammatory response is not due to the ability of the macrophages to uptake or accumulate lipid suggests acLDL might directly activates the inflammatory signaling pathways through a specific receptor(s) which dependents on IRAK4 kinase activity to signal. It has been shown that TLRs, including TLR2 and TLR4 are involved in the development of atherosclerotic plaques through the endogenous ligands, including modified LDLs. However, how TLRs participate or regulate the development of atherosclerosis remains unclear. Intriguingly, we indeed found that acLDL-mediated gene induction was substantially reduced in TLR2- or TLR4-deficient macrophages, indicating that TLR2 and/or TLR4 may play an important role in acLDL-mediated signaling pathway (Fig. 7A). Therefore, consistent with the in vivo evidence that TLRs contribute to the pathogenesis of atherosclerosis, TLRs, especially TLR2 and TLR4-mediated signaling by endogenous ligands, such as a modified LDL, may indeed present a critical link between innate immune response and cardiovascular disease pathogenesis.
Figure 7. SR-A/CD36/TLR2/TLR4 is partially required for acLDL-mediated gene transcriptions.
A. Bonemarrow-derived macrophages of wild-type, TLR2, and TLR4 deficient mice were untreated or treated with acLDL (100 μg/ml) for 30 and 120 min. Total RNA was subjected to RT-PCR in order to measure the relative expression of TLR2, TLR4, MCP-1, TNFα, KC and IL-1β. Shown is the expression of TLR2, TLR4, MCP-1, TNFα, KC and IL-1β normalized to beta-actin expression. The -fold induction was calculated as compared with the expression of WT untreated cells.
B. Bonemarrow-derived macrophages of wild-type and SR-A deficient mice were untreated or treated with acLDL (100 μg/ml) for 30 and 120 min. Total RNA was subjected to RT-PCR in order to measure the relative expression of MCP-1, TNFα, KC and IL-1β. Shown is the expression of MCP-1, TNFα, KC and IL-1β normalized to beta-actin expression. The -fold induction was calculated as compared with the expression of WT untreated cells.
C. Bonemarrow-derived macrophages of wild-type and CD36 deficient mice were untreated or treated with acLDL (100 μg/ml) for 30 and 120 min. Total RNA was subjected to RT-PCR in order to measure the relative expression of MCP-1, TNFα, KC and IL-1β. Shown is the expression of MCP-1, TNFα, KC and IL-1β normalized to beta-actin expression. The -fold induction was calculated as compared with the expression of WT untreated cells.
We then tested whether scavenger receptors SR-A and CD36 are required for acLDL-mediated induction in four inflammatory response genes in macrophages, since both receptors have been implicated as receptors for the uptake of acLDL. Bone marrow-derived macrophages from wild-type, SR-A-, or CD36-deficient mice were treated with acLDL. Whereas acLDL-mediated induction of these four inflammatory genes was substantially reduced in CD36-deficient macrophages, we observed partial reduction in SR-A-deficient macrophages compared to that in wild-type cells (Fig. 7B–C). These results suggest that CD36 is required for acLDL-induced inflammatory gene induction through cross-talk with TLR2 and/or TLR4, while SR-A might play a modulatory role in acLDL-mediated signaling.
IRAK4 kinase activity is required for modified LDL induced NFκB activation, but not for MAPK pathway activation
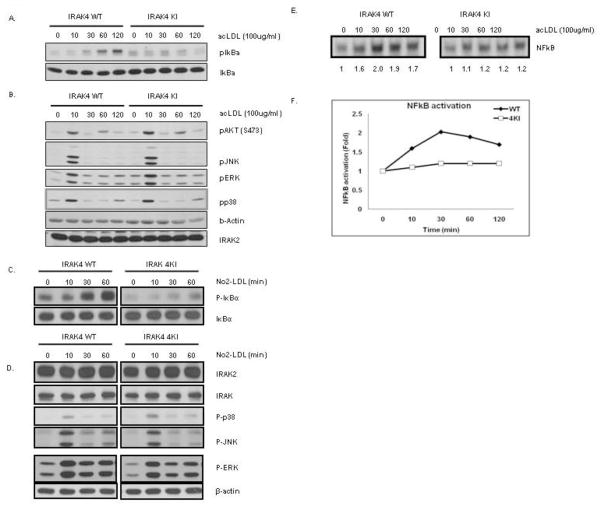
In order to further determine the role of the kinase activity of IRAK4 in modified LDL-mediated signaling, we examined the activation of the NFκB and MAPK pathways in wild-type and IRAK4 kinase-inactive knock-in bone marrow-derived macrophages in response to acLDL stimulation. As shown in Figure 8 A–B, although acLDL-mediated phosphorylation of ERK, JNK, p38 and AKT were comparable, acLDL-induced IκBα phosphorylation was greatly reduced in bone marrow-derived macrophages from IRAK4 kinase-inactive knock-in mice as compared to that in bone marrow-derived macrophages from wild-type mice.
Figure 8. The requirement of IRAK4 kinase activity in NFkB activation.
A. Cell lysates from WT and IRAK4 KI bone marrow-derived macrophages that were either untreated or treated with acLDL (100 μg/ml) for the indicated times were analyzed by Western analysis with antibodies against anti-IκBα and anti-p-IκBα
B. Cell lysates from WT and IRAK4 KI bone marrow-derived macrophages that were either untreated or treated with acLDL (100 μg/ml) for the indicated times were analyzed by Western analysis with antibodies against anti-p-AKT, anti-p-JNK, anti-p-ERK, anti-p-p38, anti-IRAK2, and anti-β-actin.
C. Cell lysates from WT and IRAK4 KI bone marrow-derived macrophages that were either untreated or treated with NO2-LDL (100 μg/ml) for the indicated times were analyzed by Western analysis with antibodies against anti-IκBα and anti-p-IκBα
D. Cell lysates from WT and IRAK4 KI bone marrow-derived macrophages that were either untreated or treated with acLDL (100 μg/ml) for the indicated times were analyzed by Western analysis with antibodies against anti-IRAK1, anti-p-JNK, anti-p-ERK, anti-p-p38, anti-IRAK2, and anti-GAPDH.
E. Cell lysates from WT and IRAK4 KI bone marrow-derived macrophages that were either untreated or treated with acLDL (100 μg/ml) for the indicated times were analyzed by electrophoretic mobility shift assay with an NFκB specific probe
F. Densitometry of NFκB electrophoretic mobility shift assay results in C using the NIH Image software package. Similar results were obtained in three separate experiments.
Moreover, CD36-specific ligand NO2-LDL activation of the MAPK pathway, including JNK, p38 and ERK were comparable, however, NO2-LDL-mediated IκBα phosphorylation was greatly attenuated in macrophages from IRAK4 kinase-inactive knock-in as compared to wild type mice (Fig 8 C–D). These results indicate that the kinase activity of IRAK4 is required for modified LDL-mediated NFκB activation. Intriguingly, many of the IRAK4 kinase-dependent genes we identified above are well-known NFκB-dependent genes (including SAA1, MMP9, sST2, and PF4), suggesting the critical role of modified LDL-induced IRAK4-dependent NFkB activation in induction of these genes.
AcLDL-mediated NFκB DNA binding activity was greatly attenuated in macrophages from IRAK4 kinase-inactive knock-in mice as compared to that in wild-type mice further supporting the importance of NFκB activation in induction of subset of proinflammatory mediator genes (Fig 8 E–F).
Discussion
The molecular mechanisms of atherosclerosis development are complex, and still poorly understood. Recent studies revealed that TLR/IL-1R signaling is involved in the development of atherosclerotic plaques. In this study, we investigated the role of IRAK4 kinase activity in the development of atherosclerosis. IRAK4 protein, especially its kinase activity, is required for the aortic plaque formation as well as pro-inflammatory cytokine production important for the development of atherosclerosis. Although the total cholesterol and plasma lipoprotein distribution are comparable, ApoE−/−/IRAK4 KI mice showed dramatic reduction in aortic sinus lesion size compared to that in ApoE−/− mice. NFκB activation and NFkB-dependent pro-inflammatory gene expression were abrogated in IRAK4 KI macrophage upon addition of putative atherogenic ligands acLDL and NO2-LDL, indicating that the critical role of IRAK4 kinase activity in the development of atherosclerosis through the regulation of NFκB-dependent target genes.
Emerging studies have indicated that in atherosclerosis, TLRs play a critical role in sensing deposition of modified LDLs and triggering a sterile inflammation. Removal of TLR2, TLR4 or adaptor MyD88 indeed reduced the development and pathogenesis of vascular inflammation and atherosclerosis in ApoE−/− or LDLR−/− mice (4;8;29). Consistent with these previous findings, we demonstrated here that IRAK4, a key kinase downstream of TLRs-MyD88, plays an essential role in the development and pathogenesis of atherosclerosis through its kinase activity. While it is clear that SR-A and CD36 are required for the uptake of modified LDLs and facilitate the activation and heterodimerization of TLRs (6), it is intriguing that CD36−/−SR-A−/−/ApoE−/− triple deficiency had minimum impact on overall lesion area in the aortic root as compared to that in ApoE−/− mice (30). However, these compound mutant mice had a substantial suppressive effect on inflammatory gene expression and plaque necrosis. These results implicate that other scavenger receptors (in addition to SR-A and CD36) might be involved in the detection of modified LDLs in vivo through their cross-talks with TLRs to trigger and participate in the initiation process of sterile inflammation in hyperlipidemic environment. The fact that ApoE−/−IRAK4KI mice displayed substantial reduction in overall lesion area in the aortic root indicates that pharmacologic inhibitors of IRAK4 kinase activity might be effective in blocking atherogenesis.
Importantly, we have clearly shown that IRAK4 is required for modified LDL-induced NFkB, but not MAPK activation. Consistent with this, we identified a subset of genes regulated in an IRAK4 kinase activity-dependent manner in modified LDL-stimulated macrophages, are mainly NFκB target genes. This group of genes contains several known proteins which have a critical role in cardiovascular disease such as an atherosclerosis. For example, MMP9 is a critical regulator of macrophage migration and differentiation, while CXCL4, aka platelet factor 4 (PF4) is a known chemoatractant for monocytes and promotes their differentiation to uptake modified LDL or cholesterol. We also identified a group of genes regulated in an IRAK4-independent manner in acLDL-stimulated macrophages. While some of these genes might be induced through TLR-dependent MyD88/IRAK4-independent manner, the IRAK4-independent genes might also be TLR-independent and are induced through the activation of scavenger receptor-mediated signaling.
Two parallel IL-1-mediated signaling pathways have been uncovered for IL-1R-TLR-mediated NFkB activation: TAK1-dependent and MEKK3-dependent, respectively. The TAK1-dependent pathway leads to IKKα/β phosphorylation and IKK βactivation, resulting in classical NFkB activation through IκBα phosphorylation and degradation. The TAK1-independent MEKK3-dependent pathway involves IKKγ phosphorylation and IKKα activation, resulting in NFκB activation through dissociation of phosphorylated IκBα from NFkB without IκBα degradation.
IRAK4 kinase-inactive mutant failed to mediate IL-1R-TLR-induced TAK1-dependent NFkB activation pathway, but mediated IL-1-induced TAK1-independent NFkB activation and retained the ability to activate substantial gene expression. One would predict that pharmacological blocking of IRAK4 kinase activity will leave intact some degree of host defence, while reducing the levels and duration of inflammatory responses. Future studies are required for investigate the detailed molecular mechanism for modified LDL-induced IRAK4-dependent NFkB activation. Although the detailed signaling mechanism and physiological impact of the modified LDL-induced IRAK4-depdendent or –independent pathway are still unclear, IRAK4 kinase activity is required for spontaneous development of atherosclerosis in ApoE−/− mouse. Therefore, it is likely that pharmacological inhibition of IRAK4 kinase activity will be a promising strategy for anti-atherosclerosis therapy.
Supplementary Material
Suppl. Figure 1. Hypothetical model of acLDL-mediated signaling pathway.
Suppl. Figure 2 Expression of TLRs in total aorta. At age 18 weeks, apoE−/− (n=6), IRAK4KI/apoE−/− (n=6), and control B6 mice (n=2) were sacrificed and aortas retrieved. Total RNA from aorta was analyzed by quantitative RT-PCR of specific primer pairs for TLR2, TLR1, TLR6, TLR4 and CD36.
Acknowledgments
The works was supported by PPG PO1 HL 029582-26 (to P. D., P. F., J. S., and X. L.).
Reference List
- 1.Lusis AJ. Atherosclerosis. Nature. 2000;407:233–241. doi: 10.1038/35025203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kirii H, Niwa T, Yamada Y, Wada H, Saito K, Iwakura Y, Asano M, Moriwaki H, Seishima M. Lack of interleukin-1beta decreases the severity of atherosclerosis in ApoE-deficient mice. Arterioscler Thromb Vasc Biol. 2003;23:656–660. doi: 10.1161/01.ATV.0000064374.15232.C3. [DOI] [PubMed] [Google Scholar]
- 3.Chi H, Messas E, Levine RA, Graves DT, Amar S. Interleukin-1 receptor signaling mediates atherosclerosis associated with bacterial exposure and/or a high-fat diet in a murine apolipoprotein E heterozygote model: pharmacotherapeutic implications. Circulation. 2004;110:1678–1685. doi: 10.1161/01.CIR.0000142085.39015.31. [DOI] [PubMed] [Google Scholar]
- 4.Michelsen KS, Wong MH, Shah PK, Zhang W, Yano J, Doherty TM, Akira S, Rajavashisth TB, Arditi M. Lack of Toll-like receptor 4 or myeloid differentiation factor 88 reduces atherosclerosis and alters plaque phenotype in mice deficient in apolipoprotein E. Proc Natl Acad Sci USA. 2004;101:10679–10684. doi: 10.1073/pnas.0403249101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Seimon TA, Obstfeld A, Moore KJ, Golenbock DT, Tabas I. Combinatorial pattern recognition receptor signaling alters the balance of life and death in macrophages. Proc Natl Acad Sci USA. 2006;103:19794–19799. doi: 10.1073/pnas.0609671104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stewart CR, Stuart LM, Wilkinson K, van Gils JM, Deng J, Halle A, Rayner KJ, Boyer L, Zhong R, Frazier WA, Lacy-Hulbert A, Khoury JE, Golenbock DT, Moore KJ. CD36 ligands promote sterile inflammation through assembly of a Toll-like receptor 4 and 6 heterodimer. Nat Immunol. 2010;11:155–161. doi: 10.1038/ni.1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cook DN, Pisetsky DS, Schwartz DA. Toll-like receptors in the pathogenesis of human disease. Nat Immunol. 2004;5:975–979. doi: 10.1038/ni1116. [DOI] [PubMed] [Google Scholar]
- 8.Mullick AE, Tobias PS, Curtiss LK. Modulation of atherosclerosis in mice by Toll-like receptor 2. J Clin Invest. 2005;115:3149–3156. doi: 10.1172/JCI25482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bjorkbacka H, V, Kunjathoor V, Moore KJ, Koehn S, Ordija CM, Lee MA, Means T, Halmen K, Luster AD, Golenbock DT, Freeman MW. Reduced atherosclerosis in MyD88-null mice links elevated serum cholesterol levels to activation of innate immunity signaling pathways. Nat Med. 2004;10:416–421. doi: 10.1038/nm1008. [DOI] [PubMed] [Google Scholar]
- 10.Medzhitov R, Preston-Hurlburt P, Janeway CA., Jr A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature. 1997;388:394–397. doi: 10.1038/41131. [DOI] [PubMed] [Google Scholar]
- 11.Rock FL, Hardiman G, Timans JC, Kastelein RA, Bazan JF. A family of human receptors structurally related to Drosophila Toll. Proc Natl Acad Sci USA. 1998;95:588–593. doi: 10.1073/pnas.95.2.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Takeuchi O, Hoshino K, Kawai T, Sanjo H, Takada H, Ogawa T, Takeda K, Akira S. Differential roles of TLR2 and TLR4 in recognition of gram-negative and gram-positive bacterial cell wall components. Immunity. 1999;11:443–451. doi: 10.1016/s1074-7613(00)80119-3. [DOI] [PubMed] [Google Scholar]
- 13.Chuang TH, Ulevitch RJ. Cloning and characterization of a sub-family of human toll-like receptors: hTLR7, hTLR8 and hTLR9. Eur Cytokine Netw. 2000;11:372–378. [PubMed] [Google Scholar]
- 14.Hemmi H, Takeuchi O, Kawai T, Kaisho T, Sato S, Sanjo H, Matsumoto M, Hoshino K, Wagner H, Takeda K, Akira S. A Toll-like receptor recognizes bacterial DNA. Nature. 2000;408:740–745. doi: 10.1038/35047123. [DOI] [PubMed] [Google Scholar]
- 15.Zhang D, Zhang G, Hayden MS, Greenblatt MB, Bussey C, Flavell RA, Ghosh S. A toll-like receptor that prevents infection by uropathogenic bacteria. Science. 2004;303:1522–1526. doi: 10.1126/science.1094351. [DOI] [PubMed] [Google Scholar]
- 16.Akira S, Takeda K, Kaisho T. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat Immunol. 2001;2:675–680. doi: 10.1038/90609. [DOI] [PubMed] [Google Scholar]
- 17.Cao Z, Xiong J, Takeuchi M, Kurama T, Goeddel DV. TRAF6 is a signal transducer for interleukin-1. Nature. 1996;383:443–446. doi: 10.1038/383443a0. [DOI] [PubMed] [Google Scholar]
- 18.Deng L, Wang C, Spencer E, Yang L, Braun A, You J, Slaughter C, Pickart C, Chen ZJ. Activation of the IkappaB kinase complex by TRAF6 requires a dimeric ubiquitin-conjugating enzyme complex and a unique polyubiquitin chain. Cell. 2000;103:351–361. doi: 10.1016/s0092-8674(00)00126-4. [DOI] [PubMed] [Google Scholar]
- 19.Ninomiya-Tsuji J, Kishimoto K, Hiyama A, Inoue J, Cao Z, Matsumoto K. The kinase TAK1 can activate the NIK-I kappaB as well as the MAP kinase cascade in the IL-1 signalling pathway. Nature. 1999;398:252–256. doi: 10.1038/18465. [DOI] [PubMed] [Google Scholar]
- 20.Yamamoto M, Sato S, Hemmi H, Uematsu S, Hoshino K, Kaisho T, Takeuchi O, Takeda K, Akira S. TRAM is specifically involved in the Toll-like receptor 4-mediated MyD88-independent signaling pathway. Nat Immunol. 2003;4:1144–1150. doi: 10.1038/ni986. [DOI] [PubMed] [Google Scholar]
- 21.Suzuki N, Suzuki S, Duncan GS, Millar DG, Wada T, Mirtsos C, Takada H, Wakeham A, Itie A, Li S, Penninger JM, Wesche H, Ohashi PS, Mak TW, Yeh WC. Severe impairment of interleukin-1 and Toll-like receptor signalling in mice lacking IRAK-4. Nature. 2002;416:750–756. doi: 10.1038/nature736. [DOI] [PubMed] [Google Scholar]
- 22.Picard C, Puel A, Bonnet M, Ku CL, Bustamante J, Yang K, Soudais C, Dupuis S, Feinberg J, Fieschi C, Elbim C, Hitchcock R, Lammas D, Davies G, Al Ghonaium A, Al Rayes H, Al Jumaah S, Al Hajjar S, Al Mohsen IZ, Frayha HH, Rucker R, Hawn TR, Aderem A, Tufenkeji H, Haraguchi S, Day NK, Good RA, Gougerot-Pocidalo MA, Ozinsky A, Casanova JL. Pyogenic bacterial infections in humans with IRAK-4 deficiency. Science. 2003;299:2076–2079. doi: 10.1126/science.1081902. [DOI] [PubMed] [Google Scholar]
- 23.Kawagoe T, Sato S, Jung A, Yamamoto M, Matsui K, Kato H, Uematsu S, Takeuchi O, Akira S. Essential role of IRAK-4 protein and its kinase activity in Toll-like receptor-mediated immune responses but not in TCR signaling. J Exp Med. 2007;204:1013–1024. doi: 10.1084/jem.20061523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koziczak-Holbro M, Gluck A, Tschopp C, Mathison JC, Gram H. IRAK-4 kinase activity-dependent and -independent regulation of lipopolysaccharide-inducible genes. Eur J Immunol. 2008;38:788–796. doi: 10.1002/eji.200737886. [DOI] [PubMed] [Google Scholar]
- 25.Koziczak-Holbro M, Joyce C, Gluck A, Kinzel B, Muller M, Tschopp C, Mathison JC, Davis CN, Gram H. IRAK-4 kinase activity is required for interleukin-1 (IL-1) receptor- and toll-like receptor 7-mediated signaling and gene expression. J Biol Chem. 2007;282:13552–13560. doi: 10.1074/jbc.M700548200. [DOI] [PubMed] [Google Scholar]
- 26.Kim TW, Staschke K, Bulek K, Yao J, Peters K, Oh KH, Vandenburg Y, Xiao H, Qian W, Hamilton T, Min B, Sen G, Gilmour R, Li X. A critical role for IRAK4 kinase activity in Toll-like receptor-mediated innate immunity. J Exp Med. 2007;204:1025–1036. doi: 10.1084/jem.20061825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li X. IRAK4 in TLR/IL-1R signaling: possible clinical applications. Eur J Immunol. 2008;38:614–618. doi: 10.1002/eji.200838161. [DOI] [PubMed] [Google Scholar]
- 28.Rekhter M, Staschke K, Estridge T, Rutherford P, Jackson N, Gifford-Moore D, Foxworthy P, Reidy C, Huang XD, Kalbfleisch M, Hui K, Kuo MS, Gilmour R, Vlahos CJ. Genetic ablation of IRAK4 kinase activity inhibits vascular lesion formation. Biochem Biophys Res Commun. 2008;367:642–648. doi: 10.1016/j.bbrc.2007.12.186. [DOI] [PubMed] [Google Scholar]
- 29.Mullick AE, Soldau K, Kiosses WB, Bell TA, III, Tobias PS, Curtiss LK. Increased endothelial expression of Toll-like receptor 2 at sites of disturbed blood flow exacerbates early atherogenic events. J Exp Med. 2008;205:373–383. doi: 10.1084/jem.20071096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Manning-Tobin JJ, Moore KJ, Seimon TA, Bell SA, Sharuk M, Alvarez-Leite JI, de Winther MP, Tabas I, Freeman MW. Loss of SR-A and CD36 activity reduces atherosclerotic lesion complexity without abrogating foam cell formation in hyperlipidemic mice. Arterioscler Thromb Vasc Biol. 2009;29:19–26. doi: 10.1161/ATVBAHA.108.176644. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Suppl. Figure 1. Hypothetical model of acLDL-mediated signaling pathway.
Suppl. Figure 2 Expression of TLRs in total aorta. At age 18 weeks, apoE−/− (n=6), IRAK4KI/apoE−/− (n=6), and control B6 mice (n=2) were sacrificed and aortas retrieved. Total RNA from aorta was analyzed by quantitative RT-PCR of specific primer pairs for TLR2, TLR1, TLR6, TLR4 and CD36.