Abstract
Visual pattern processing becomes increasingly complex along the ventral pathway, from the low-level coding of local orientation in the primary visual cortex to the high-level coding of face identity in temporal visual areas. Previous research using pattern aftereffects as a psychophysical tool to measure activation of adaptive feature coding has suggested that awareness is relatively unimportant for the coding of orientation, but awareness is crucial for the coding of face identity. We investigated where along the ventral visual pathway awareness becomes crucial for pattern coding. Monoptic masking, which interferes with neural spiking activity in low-level processing while preserving awareness of the adaptor, eliminated open-curvature aftereffects but preserved closed-curvature aftereffects. In contrast, dichoptic masking, which spares spiking activity in low-level processing while wiping out awareness, preserved open-curvature aftereffects but eliminated closed-curvature aftereffects. This double dissociation suggests that adaptive coding of open and closed curvatures straddles the divide between weakly and strongly awareness-dependent pattern coding.
Keywords: awareness, pattern adaptation, visual perception
Visual feature processing becomes progressively more complex along the ventral pathway: Local orientation and spatial frequency are coded in V1 and V2; curvature, configurations of curved contours, convexity, aspect ratio, and texture are coded in V2 and V4; and complex geometric features, object parts, and faces are coded in inferotemporal cortex (for reviews, see Loffler, 2008; Orban, 2008; Suzuki, 2005). Visual aftereffects provide a psychophysical tool to behaviorally probe the neural population activity that adaptively codes a given feature. For example, prolonged viewing of a specific feature value (e.g., a 40° tilt) may distort the coding of a subsequently presented stimulus with a slightly different value (e.g., a 30° tilt) and cause it to appear more dissimilar to the adapted value (e.g., it may appear to have a 25° tilt). Repulsive aftereffects such as this provide evidence of adaptation of the underlying feature-coding (e.g., orientation-coding) mechanisms that accentuate changes in feature values (e.g., Clifford et al., 2007; Schwartz, Hsu, & Dayan, 2007; Suzuki & Cavanagh, 1998; Suzuki, 2005).
Using aftereffects as a measure of adaptive feature coding, researchers have shown that coding of facial identity (a feature coded in high-level visual areas) strongly depends on awareness, whereas coding of orientation and spatial frequency (features coded in low-level visual areas) occurs relatively independently of awareness, as long as the images are of sufficiently high contrast (e.g., Blake, Tadin, Sobel, Raissian, & Chong, 2006; Moradi, Koch, & Shimojo, 2005; Yang, Hong, & Blake, 2010). Thus, awareness is relatively unimportant for adaptive coding at the lower end of visual processing (for high-contrast patterns), but it becomes crucial at the higher end of ventral visual processing. The goal of the current study was to determine the level of processing at which adaptive pattern coding becomes strongly dependent on awareness.
We focused on curvature, because it is an intermediate-level visual feature likely to be coded by neural population activity in low- to intermediate-level visual areas (V1, V2, and V4; e.g., Hegdé & Van Essen, 2007; Pasupathy & Connor, 2002). It is important to note that the same curved contour can be presented in isolation (an open arc) or as a part of a closed shape (an ellipse). It has been shown that closed contours contribute to figure-ground segregation and rapid shape discrimination (e.g., Elder & Zucker, 1992; Koffka, 1935), and they also elicit responses in higher-level visual areas (e.g., V3/VP and V4; Dumoulin & Hess, 2007). Thus, processing of open versus closed curvature may mark the boundary between weak and strong dependence on awareness.
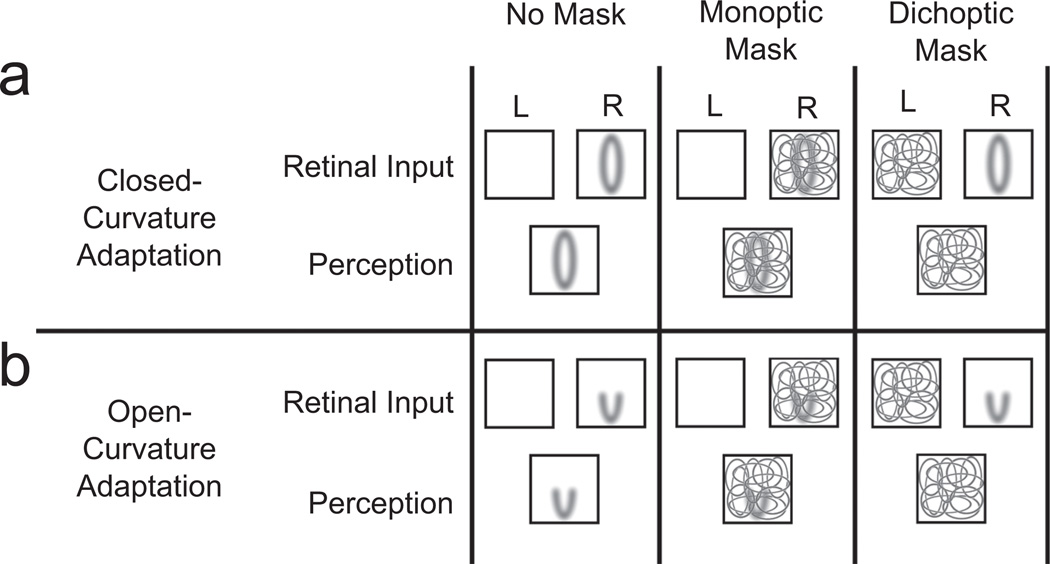
In the experiments reported here, we used dichoptic masking to disrupt visual awareness and monoptic masking to interfere with low-level visual processing (Fig. 1). In dichoptic masking, a masking pattern is presented to a different eye than the adaptor stimulus is. This renders the adaptor invisible (this process is also known as continuous flash suppression; e.g., Moradi et al., 2005; Tsuchiya & Koch, 2005), but may spare lateral geniculate nucleus (LGN) and V1 spiking responses to the adaptor (e.g., Fries, Roelfsema, Engel, Konig, & Singer, 1997; Wilke, Logothetis, & Leopold, 2006). In contrast, monoptic masking involves superimposing a masking pattern on an adaptor stimulus and presenting it to the same eye. The adaptor is often clearly visible, but monoptic masking may interfere with LGN and V1 spiking responses to the adaptor more strongly than does dichoptic masking (effects of dichoptic and monoptic masking on LGN and V1 spiking responses are inferred from neurophysiological results on transient masking; Macknik & Martinez-Conde, 2004; see details in Discussion).
Fig. 1.
Illustration of the three masking conditions in Experiment 1 (a; closed-curvature stimuli) and in Experiment 2 (b; open-curvature stimuli). Stimuli were presented to the left eye (L; not illustrated here), the right eye (R), or both eyes, depending on condition. In the no-mask condition, the adaptor was presented to one eye (with no mask) and was nearly always clearly visible. In the monoptic-mask condition, the adaptor was presented to one eye, and the mask was superimposed over the adaptor in the same eye; both mask and adaptor were nearly always clearly visible. In the dichoptic-mask condition, the adaptor and mask were presented to different eyes; in almost all cases, only the mask was visible.
Thus, if an aftereffect is eliminated by monoptic masking but survives dichoptic masking during the adaptation period, such a result would suggest that adaptive coding of the corresponding feature depends on intact low-level spiking responses (in LGN and V1), but it does not depend on the mechanisms that generate awareness. In contrast, if an aftereffect survives monoptic masking but is eliminated by dichoptic masking during the adaptation period, such a result would suggest that adaptive coding of the corresponding feature is resistant to low-level interference (in LGN and V1) but is crucially dependent on the mechanisms that generate awareness. Our experiments were designed to determine whether adaptive coding of open and closed curvature straddles this divide between dependence on low-level spiking responses and dependence on awareness-related mechanisms.
Experiment 1: Closed-Curvature Aftereffects
We examined closed-curvature aftereffects by measuring aspect-ratio aftereffects on ellipses under three conditions. In the no-mask condition, the adaptor was presented with no masking. In the monoptic-mask condition, the adaptor was visible but degraded by monoptic masking (in which the mask was presented to the same eye as the adaptor); such masking potentially generated interference that began in low-level processing. In the dichoptic-mask condition, the adaptor was rendered invisible by dichoptic masking (in which the mask was presented to the opposite eye); this type of masking potentially spared low-level spiking activity but interfered with the mechanisms that generate visual awareness.
Method
Observers
Twelve students from Northwestern University gave informed consent to participate in the experiment. All had normal or corrected-to-normal visual acuity and normal stereo vision.
Stimuli
Stimuli were displayed within binocularly presented frames (2.29° × 2.29°, luminance = 52.4 cd/m2) defined by 1.0°-thick checkerboard borders consisting of 0.5° × 0.5° white squares (luminance = 94.5 cd/m2) and black squares (luminance = 3.9 cd/m2). These frames were surrounded by a light gray background (luminance = 52.4 cd/m2), and both background and frames remained on screen throughout the entire trial sequence. The binocular frames were used to promote stable binocular fusion. A stereoscope with four front-surface mirrors was used to present stimuli to separate eyes. All adapting ellipses were drawn with dark gray lines (thickness = 0.23°, luminance = 43.4 cd/m2). Adapting ellipses were either vertically stretched (i.e., tall; 0.8° × 2.18°, log aspect ratio = .419) or horizontally stretched (i.e., wide; 2.18° × 0.8°, log aspect ratio = −.419). Test ellipses were either slightly tall (1.09° × 1.32°, log aspect ratio = .043), circular (1.43° × 1.43°, log aspect ratio = 0), or slightly wide (1.32° × 1.09°, log aspect ratio = −.043). We varied the aspect ratios of the test stimuli so that observers would respond to different aspect ratios even in the absence of an aftereffect. For the response stimulus that appeared in the adjustment procedure at the end of each trial, we used ellipses with a broad range of aspect ratios but with equivalent areas (log aspect ratios—tall: .419, .374, .343, .311, .285, .221, .176, .131, .087, .043; circular: 0.0; wide: −.043, −.087, −.131, −.176, −.221, −.285, −.311, −.343, −.374, −.419).
A high-contrast dynamic pattern was used as the mask because such a pattern presented to one eye renders a static image presented to the other eye invisible (e.g., Fang & He, 2005; Moradi et al., 2005; Tsuchiya & Koch, 2005). The dynamic mask consisted of an array of 11 overlapping high-contrast ellipses composed of solid lines (line thickness = 0.06°, luminance = 33.8 cd/m2). The mask was made dynamic by rotating the entire array by 90° every 20 ms. The contour of the adapting ellipse was Gaussian-blurred (radius = 3.5 pixels) and reduced in contrast compared with the mask, and the adaptor was faded in through four luminance steps (84.5 cd/m2, 65.3 cd/m2, 52.1 cd/m2, and 43.4 cd/m2). All stimuli were presented against a white background (luminance = 94.5 cd/m2) within the binocular frame, at a viewing distance of 115 cm.
Procedure
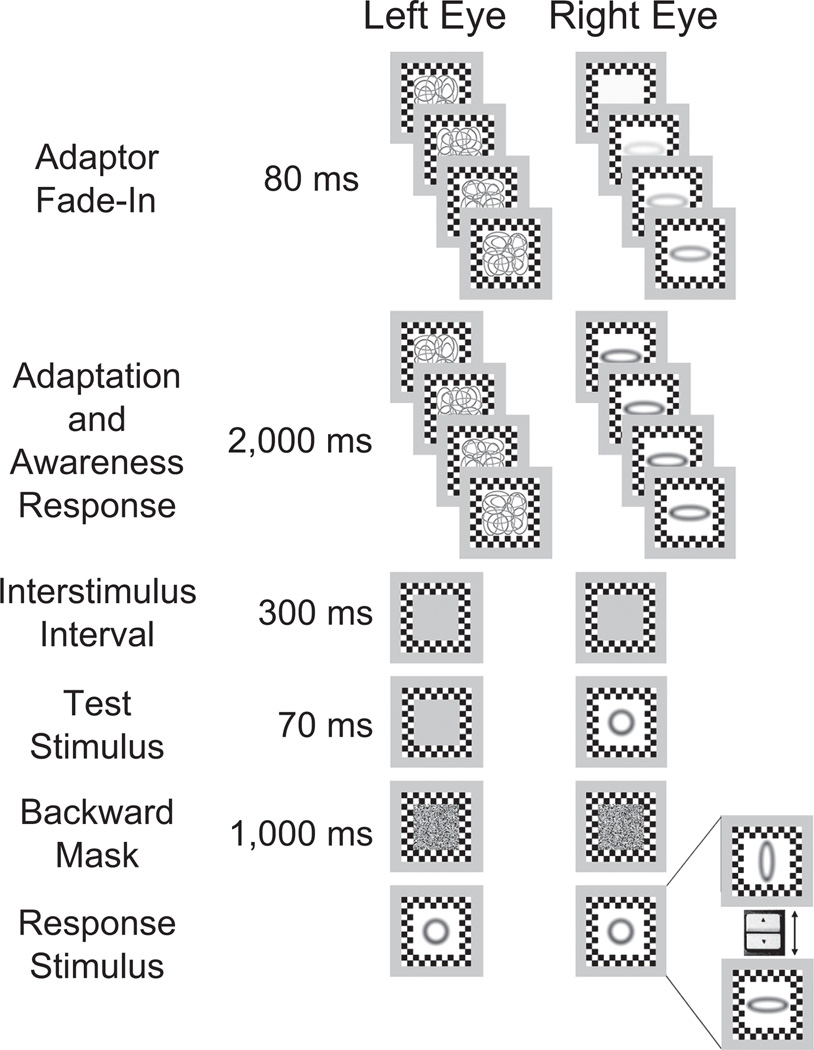
Observers were tested individually in a dimly lit room, and they initiated each trial. In the monoptic-mask and dichoptic-mask conditions, the mask appeared on screen at the start of the trial, and the adaptor faded in across four steps, each lasting 20 ms (Fig. 2). (In the no-mask condition, the adaptor appeared in exactly the same way, but no mask was present.) We used this procedure because it nearly always suppressed the adapting ellipse from awareness when the dynamic mask was dichoptically presented; at the same time, the spatial-frequency difference between the adaptor and the mask made it easy to perceptually differentiate the two when the mask was monoptically superimposed over the adaptor (Fig. 1a).
Fig. 2.
Example trial sequence from a dichoptic-mask trial inducing adaptation to a closed-curvature ellipse with a wide aspect ratio. A dynamic mask rotating 90° every 20 ms was presented to one eye. An adaptor (which faded in across four steps lasting 80 ms total) was presented to the other eye. Both mask and adaptor then remained on-screen for 2,000 ms. During this time, observers reported whether the adaptor stimulus was visible and, if it was, whether it was tall or wide. The adaptor and mask were followed by an interstimulus interval lasting 300 ms, and then the test stimulus was presented for 70 ms to the same eye that was presented with the adaptor stimulus (the test stimulus is shown here as a circle, but it could also be a slightly tall or slightly wide ellipse). A backward mask was then presented to both eyes for 1,000 ms, after which observers saw a response stimulus. The response stimulus always started out as a circle, and observers adjusted it to the perceived aspect ratio of the test stimulus using the up and down arrow keys on the keyboard. The adjusted response stimuli shown here represent the tall and wide ends of the range of selection options.
After the adaptor faded in, it (and the mask, when present) remained on the screen for 2,000 ms (Fig. 2). Observers were instructed to maintain fixation at the center of the stimulus window. During the adaptation period, observers verbally indicated whether the adaptor ellipse was visible. They were encouraged to report any perception of an adaptor ellipse, no matter how faint or brief. When the ellipse was visible, observers reported its aspect ratio (tall or wide). The adaptation period was followed by a binocularly presented gray field (300 ms), which was then followed by one of the three test ellipses, briefly presented for 70 ms1 to one eye. The test ellipse was always presented to the same eye as the adaptor had been (to maximize the aftereffect) and was followed by a Gaussian-noise mask (with pixel-luminance values ranging from 1.1 cd/m2 to 88.2 cd/m2) presented to both eyes for 1,000 ms. Observers then adjusted the aspect ratio of a response ellipse (presented to both eyes) by pressing the up or down arrow key to select among 21 aspect-ratio choices ranging from very tall to very wide. Options always began with a circle, which observers adjusted until it matched the perceived aspect ratio of the test ellipse.
The monoptic-mask and dichoptic-mask trials were randomly intermixed within the same block (24 trials per mask type). The no-mask trials were run in a subsequent block (24 trials) because the magnitude of the closed-curvature (aspectratio) aftereffect was expected to be largest in the no-mask condition. We wanted to guard against the possibility that observers might expect to experience equivalent closed-curvature aftereffects in the mask conditions on the basis of prior exposure to large aftereffects in the no-mask condition. In each block, tall and wide adaptors were randomly (and equiprobably) paired with a slightly tall, circular, or slightly wide test ellipse across trials.
Results
Trial categorization
To categorize trials, we used each observer’s trial-by-trial accounts of whether they were aware of the adapting stimulus. Trials in each condition were categorized as aware if an observer correctly reported the aspect ratio (tall vs. wide) of the adaptor and as unaware if observers reported seeing no adaptor ellipse or incorrectly reported the aspect ratio of the adaptor. On average, 97.1% (SEM = 1.29%) of no-mask trials were categorized as aware (wide adaptor: 97.1% of trials, SEM = 1.2%, tall adaptor: 97.2% of trials, SEM = 1.6%), 100% of monoptic-mask trials were categorized as aware, and 92.0% (SEM = 3.8%) of dichoptic-mask trials were categorized as unaware (wide adaptor: 89.2% of trials, SEM = 5.3%; tall adaptor: 94.8% of trials, SEM = 2.9%). Observers were thus almost always aware of the adaptor on the no-mask trials, always aware of the adaptor on the monoptic-mask trials, and almost always unaware of the adaptor on the dichoptic-mask trials. The small number of trials on which observers were unaware of the adaptor with no mask or aware of the adaptor with the dichoptic mask were excluded from further analyses.
Closed-curvature aftereffects with and without awareness
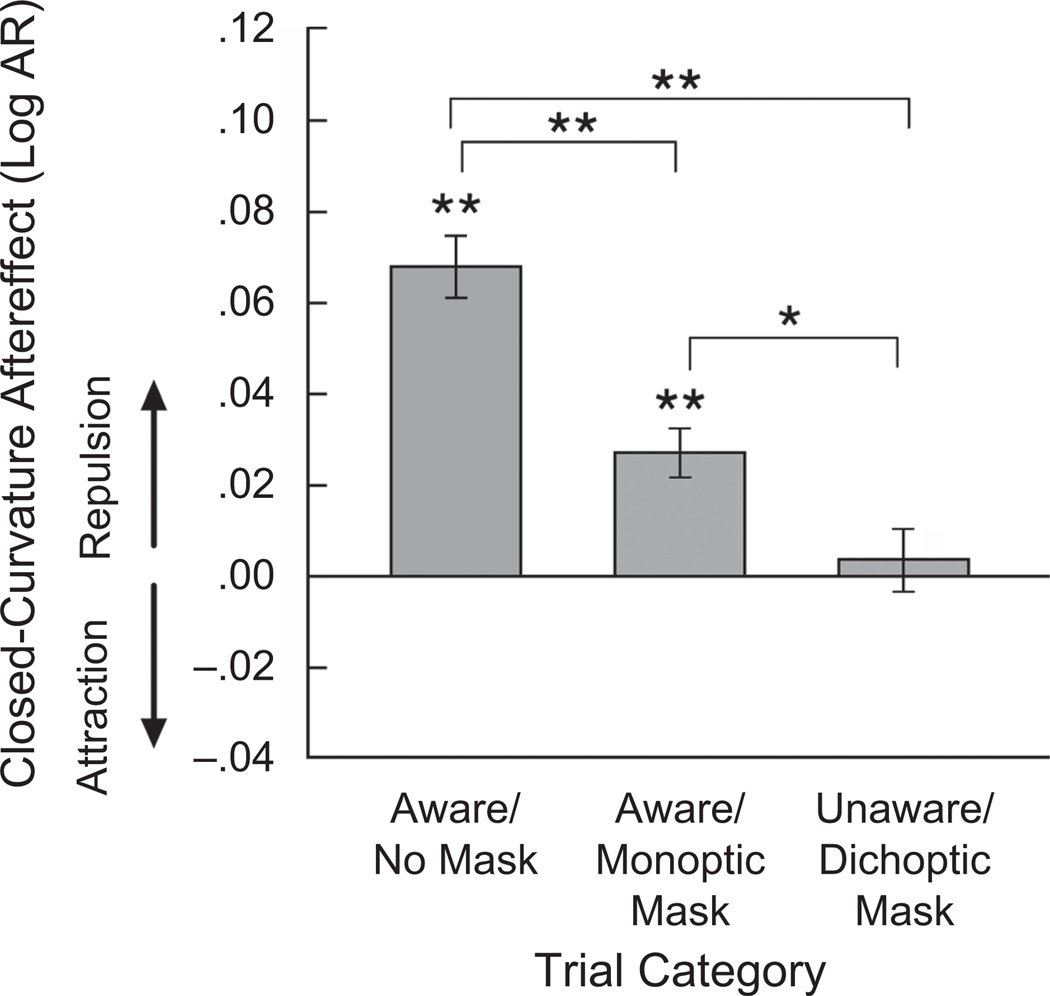
We computed an aftereffect index by subtracting the mean aspect-ratio rating of each test ellipse following adaptation to a wide ellipse from its mean aspect-ratio rating following adaptation to a tall ellipse (in log-aspect-ratio units) for each observer for each condition. A positive value indicated a repulsive aftereffect (e.g., a wide adaptor made a test ellipse appear taller), a negative value indicated an attractive aftereffect (e.g., a wide adaptor made a test ellipse appear wider), and zero indicated the absence of an aftereffect.
A two-factor analysis of variance (ANOVA) was conducted with trial category (aware/no-mask, aware/monoptic mask, unaware/dichoptic mask) and test-ellipse aspect ratio (slightly tall, circular, slightly wide) as the independent variables and aftereffect index as the dependent variable. It yielded a significant main effect of trial category, F(2, 22) = 18.006, p < .0001, ηp2 = .621, but no main effect of test-ellipse aspect ratio, F(2, 22) = 0.533, n.s., nor an interaction between trial category and test-ellipse aspect ratio, F(4, 44) = 1,268, n.s. Thus, the three masking conditions significantly modulated closed-curvature aftereffects, but the aftereffects were equivalent for the test ellipses of different aspect ratios.
Follow-up analyses showed that closed-curvature aftereffects occurred only when observers were aware of the adaptor (Fig. 3). Aftereffect indices were significantly greater than zero in both the aware/no-mask category, t(11) = 6.316, p < .001, d = 1.823, and the aware/monoptic-mask category, t(11) = 4.177, p < .01, d = 1.206, though monoptic masking weakened the aftereffect, t(11) = 4.196, p < .01, d = 1.212. In contrast, closed-curvature aftereffects disappeared when observers were unaware of the adaptor (in the unaware/dichoptic-mask category), t(11) = 0.836, n.s. Thus, when a curved contour was presented as a part of a closed ellipse, interference in the mechanisms that generate visual awareness disrupted adaptive coding of curvature, but the coding appears to have been resistant to interference in low-level spiking activity.
Fig. 3.
Results from Experiment 1: closed-curvature aftereffect as a function of trial category. Aftereffects were measured in log aspect ratio (AR). A positive value indicates a repulsive aftereffect (e.g., a wide adaptor making a test ellipse appear taller), a negative value indicates an attractive aftereffect (e.g., a wide adaptor making a test ellipse appear wider), and zero indicates an absence of an aftereffect. Error bars represent ±1 SEM (adjusted for within-observer comparisons). Asterisks indicate statistical significance (*p < .05, **p < .01).
Experiment 2: Open-Curvature Aftereffects
We next determined the effects of low-level interference and awareness on adaptive coding of open curvature. We used curved contours that were identical to the lower halves of the ellipses used in Experiment 1, so that local features of the stimuli were matched across the two experiments.
Method
Observers
Twenty-four students from Northwestern University gave informed consent to participate in the experiment. All had normal or corrected-to-normal visual acuity and normal stereo-vision.
Stimuli and procedure
All stimuli and procedures were identical to those used in Experiment 1, except that the adaptor and test stimuli consisted of open contours instead of closed contours. Each adaptor, test, and matching contour was identical to the lower half of the corresponding ellipse from Experiment 1 (cf. Fig. 1b with Fig. 1a). Using the bottom halves of the ellipses should not have biased our results, because we found no differences in the magnitude or direction of curvature aftereffects (with no mask during adaptation) when we used the top, bottom, left, or right halves of the ellipses in a pilot experiment. Because the masking conditions and procedure were identical to those used in Experiment 1, and because the open contours were identical to the component contours of the adaptor, test, and matching ellipses used in Experiment 1, any differences between the results of Experiments 1 and 2 should be attributable to engaging adaptive coding of open curvature instead of closed curvature.
Results
Trial categorization
Trials were categorized into three types—aware/no mask, aware/monoptic mask, and unaware/dichoptic mask—using the same method as in Experiment 1. On average, 97.1% (SEM = 0.78%) of no-mask trials were categorized as aware (wide adaptor: 97.8% of trials, SEM = 0.78%; tall adaptor: 96.4% of trials, SEM = 1.2%), 99.4% (SEM = 0.32%) of monoptic-mask trials were categorized as aware (wide adaptor: 99.3% of trials, SEM = 0.52%; tall adaptor: 99.6% of trials, SEM = 0.37%), and 89.4% (SEM = 2.3%) of dichoptic-mask trials were categorized as unaware (wide adaptor: 86.0% of trials, SEM = 3.0%; tall adaptor: 92.6% of trials, SEM = 2.7%). Thus, as in Experiment 1, observers were almost always aware of the adaptor on the no-mask and monoptic-mask trials, and observers were unaware of the adaptor on the majority of the dichoptic-mask trials. The small number of trials on which observers were unaware of the adaptor with no mask, unaware of the adaptor with the monoptic mask, or aware of the adaptor with the dichoptic mask were excluded from further analyses.
Open-curvature aftereffects with and without awareness
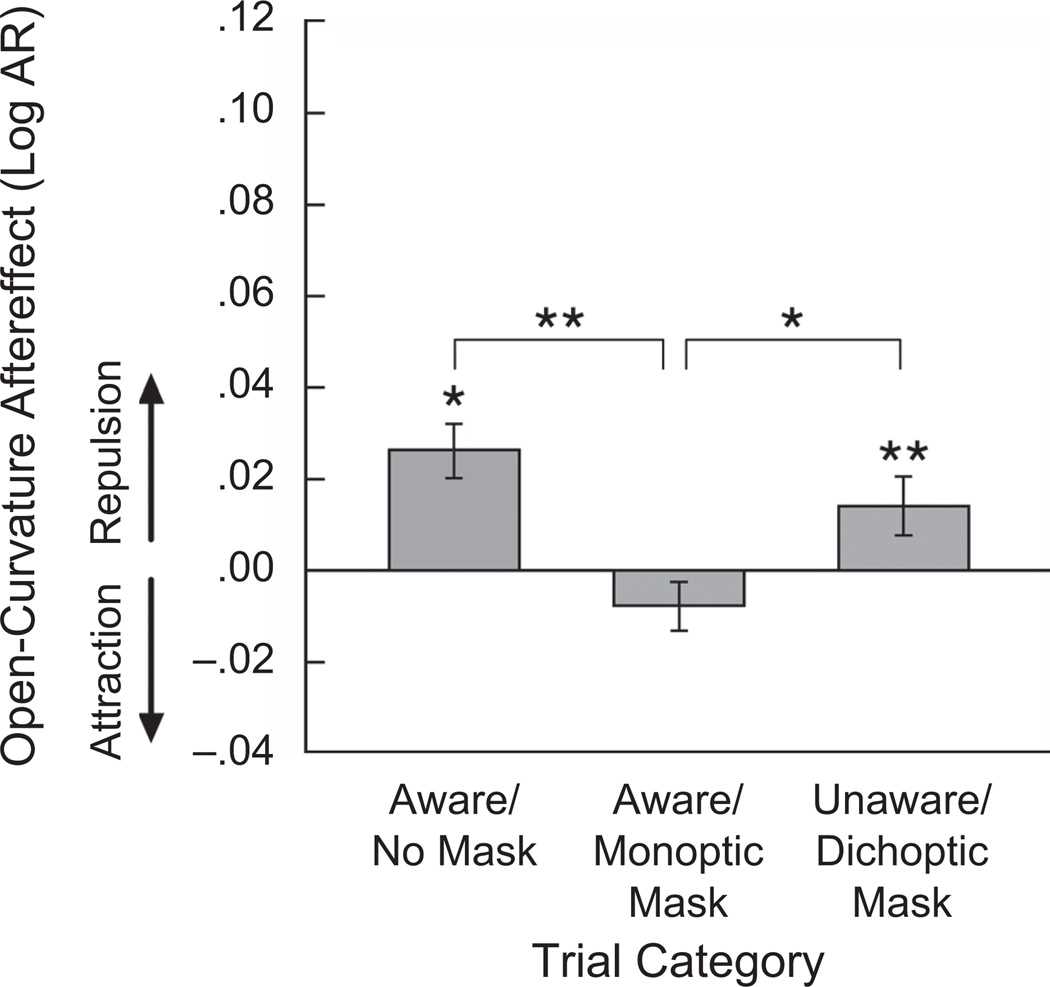
A two-factor ANOVA was conducted with trial category (aware/no mask, aware/monoptic mask, unaware/dichoptic mask) and test-contour curvature (slightly more curved, half circle, slightly less curved) as the independent variables and aftereffect index (with positive and negative values indicating repulsive and attractive aftereffects, respectively) as the dependent variable. The ANOVA yielded a main effect of trial category, F(2, 46) = 7.145, p < .01, ηp2 = .237, and a main effect of test-contour curvature, F(2, 46) = 3.341, p < .05, ηp2 = .127, but no interaction between trial category and test-contour curvature, F(4, 92) = 0.814, n.s. The three masking conditions thus significantly modulated open-curvature aftereffects. The significant main effect of test-contour curvature reflected that the repulsive open-curvature aftereffect was larger for the slightly-more-curved test contour compared with both the half-circle test contour, t(23) = 2.564, p < .05, d = 0.523, and the slightly-less-curved test contour, t(23) = 2.268, p < .05, d = 0.462. This could be related to the fact that curvature perception is less precise for larger open curvatures (e.g., Wilson, 1985), and uncertainty in coding might increase influences from aftereffects. The effect of test curvature, however, does not confound our evaluation of the trial-category effects because the two effects did not interact.
Follow-up analyses showed that, unlike closed-curvature aftereffects, which depended on awareness of the adaptor, open-curvature aftereffects were disrupted by the monoptic mask, which did not impair awareness (Fig. 4). Aftereffect indices were significantly greater than zero in the aware/no-mask category, t(23) = 2.461, p < .05, d = 0.502, and the unaware/dichoptic-mask category, t(23) = 3.293, p < .01, d = 0.672, but not significantly different from zero in the aware/monoptic-mask category, t(23) = 0.798, n.s. It is important to note that open-curvature aftereffects were undiminished in the unaware/dichoptic-mask category compared with the aware/no-mask category, t(23) = 1.198, n.s., but were significantly reduced in the aware/monoptic-mask category compared with both the aware/no-mask category, t(23) = 3.914, p < .001, d = 0.798, and the unaware/dichoptic-mask category, t(23) = 2.365, p < .05, d = 0.482. Thus, the dichoptic mask, which interfered with awareness of the adaptor, had no effect on open-curvature adaptation, whereas the monoptic mask, which may have interfered with low-level spiking activity, eliminated open-curvature adaptation. These results suggest that adaptive coding of open curvature is relatively independent of the neural mechanisms that generate visual awareness, but it depends on clean spiking signals in low-level processing.
Fig. 4.
Results from Experiment 2: open-curvature aftereffect as a function of trial category. Aftereffects were measured in log aspect ratio (AR). A positive value indicates a repulsive aftereffect (e.g., a more curved adaptor making a test contour appear less curved), a negative value indicates an attractive aftereffect (e.g., a more curved adaptor making a test contour appear more curved), and zero indicates an absence of an aftereffect. Error bars represent ±1 SEM (adjusted for within-observer comparisons). Asterisks indicate statistical significance (*p < .05, **p < .01)
Discussion
In the ventral visual pathway thought to mediate object perception (e.g., Ishai, Ungerleider, Martin, Schouten, & Haxby, 1999), progressively more complex pattern features are coded in higher-level visual areas (e.g., Loffler, 2008; Orban, 2008; Suzuki, 2005). Previous research has shown that awareness plays a relatively minor role in low-level feature adaptation, whereas it plays a crucial role in high-level feature adaptation (e.g., Blake et al., 2006; Moradi et al., 2005; Yang et al., 2010). This suggests that neural mechanisms that generate awareness become more relevant to adaptive feature coding in higher-level visual areas. Our primary goal was to determine at what stage of ventral visual processing the divide occurs between weak and strong dependence on awareness.
Our results showing a double dissociation in visual pattern adaptation suggest that this divide occurs between the coding of open and closed curvatures. Monoptic masking (presented to the same eye as the adaptor) left the adaptor visible and eliminated open-curvature aftereffects, but it only reduced closed-curvature aftereffects. In contrast, the same mask presented dichoptically (to the opposite eye to the adaptor) rendered the adaptor invisible and eliminated closed-curvature aftereffects but left open-curvature aftereffects intact. What neural mechanisms might account for this double dissociation?
Continuous dichoptic masking potentially impairs visual awareness either by disrupting synchronous oscillatory neural responses in the gamma frequency range (e.g., Fries et al., 1997) and the mid-to-lower frequency range (< 30 Hz; e.g., Gail, Brinksmeyer, & Eckhorn, 2004; Wilke et al., 2006) in primary visual cortex (V1), by disrupting the phase-locking of gamma oscillations to lower-frequency oscillations (e.g., Doesburg, Green, McDonald, & Ward, 2009), or by a combination of these mechanisms. These disruptive effects of dichoptic masking on the coherence of neural responses do not alter the spiking activity in V1 (e.g., Fries et al., 1997; Wilke et al., 2006), but they substantially reduce the spiking activity in high-level visual areas (e.g., Leopold & Logothetis, 1999; Logothetis, 1998). Because synchronization of neural responses increases their effect on postsynaptic targets (e.g., Azouz & Gray, 2000), disrupting synchronization in low-level visual areas might reduce the spiking activity in higher-level visual areas.
The neural effects of continuous monoptic masking are less clear. Nevertheless, Macknik and Martinez-Conde (2004) compared monoptic with dichoptic neural interactions in V1 associated with transient masking effects (in which the target and mask were presented sequentially but in close temporal proximity). They found that monoptic masking interfered with spiking activity in V1 substantially more than did dichoptic masking. If continuous and transient neural interactions associated with monoptic and dichoptic masking are assumed to be similar, then it could be inferred that our monoptic masking more strongly interfered with V1 spiking activity than did our dichoptic masking. Because spiking activity of high-level visual neurons is closely associated with stimulus visibility (e.g., Leopold & Logothetis, 1999; Logothetis, 1998), we assume that our monoptic masking, which left the adaptor visible, did not substantially interfere with spiking activity in high-level visual areas. This may sound like a paradox, because if our monoptic masking interfered with spiking activity in V1, it should also have interfered with spiking activity in high-level visual areas. The fact that our monoptic masking reduced the closed-curvature aftereffect is indicative of some degree of high-level interference. Nevertheless, we speculate that our monoptic masking did not eliminate the closed-curvature aftereffect for two reasons: First, it left neural synchrony in V1 relatively undisturbed, and, second, high-level global processes that pool signals from lower-level local processes across a relatively large spatial region may have averaged out some of the noise arising in low-level processing (e.g., Sweeny, Grabowecky, Kim, & Suzuki, 2011).
Taking these assumptions together, we can reasonably speculate that our dichoptic masking disrupted neural processes that are closely associated with visual awareness, including neural response synchrony, across-frequency phase locking, and spiking activity in high-level visual areas, but without substantially interfering with spiking activity in V1. In contrast, our monoptic masking interfered with spiking activity in V1 but left neural synchrony, phase locking, and high-level spiking activity relatively unaffected. On the basis of these considerations, we suggest that adaptive coding of open and closed curvatures straddles the divide between relatively awareness-independent feature coding (which is sensitive to spiking activity in low-level visual areas) and strongly awareness-dependent feature coding (which is sensitive to neural synchronization in low-level visual areas and spiking activity in high-level visual areas).
Note that because our adaptor and test stimuli were presented in overlapping regions, it is likely that the aftereffects we obtained included contributions from local orientation adaptation (e.g., Dickinson, Almeida, Bell, & Badcock, 2010). Local orientation adaptation, however, would have contributed equivalently to the aftereffects for open and closed curves, and thus it cannot account for the double dissociation that we obtained. Furthermore, previous results have demonstrated aftereffects for both open and closed curves over and above any contributions from local orientation aftereffects (e.g., Gheorghiu & Kingdom, 2007; Regan & Hamstra, 1992; Suzuki & Cavanagh, 1998).
It might be argued that closed-curvature aftereffects might depend on attention to, rather than awareness of, an adaptor because attention and awareness are closely associated, as exemplified by the phenomenon of inattentional blindness (e.g., Simons, 2000). Recent studies, however, have demonstrated that the mechanisms of attention and awareness are dissociable; for example, images suppressed from awareness can guide attention (e.g., Jiang, Costello, Fang, Huang, & He, 2006), and attended images can fade from awareness (e.g., Bonneh, Cooperman, & Sagi, 2001). Furthermore, it is likely that our observers attended to the central region in which the adaptor was presented on both the unaware/dichoptic-mask trials and aware/monoptic-mask trials. This is probable for at least five reasons. First, observers were given the task of detecting the adaptor to report its shape. Second, adaptors were always presented at the same central location. Third, the dichoptic-mask trials were randomly intermixed with the monoptic-mask trials (on which an adaptor was virtually always visible). Fourth, a dichoptic-masking display appeared similar to a monoptic-masking display when an adaptor broke through and became visible during dichoptic masking (this occurred on ~10% of the trials). Finally, observers correctly reported the aspect ratio of an adaptor ellipse on the vast majority of the monoptic-mask trials and also on the dichoptic-mask trials when the adaptor broke through. Moreover, a hypothesis that attention might have increased adaptation to closed curvature could not explain why attention would have reduced adaptation to open curvature.
Thus, all things considered, the most likely explanation of our double dissociation is that adaptive coding of open and closed curvatures marks the transition in visual pattern coding between sensitivity to spiking activity in low-level processing and sensitivity to the mechanisms that generate visual awareness (including response synchronization in low-level visual areas and spiking activity in high-level visual areas).
A question remains as to why the divide occurs between the coding of open and closed curvatures. One possibility is that contour closure marks a distinct stage in ventral visual processing (e.g., Kovács & Julesz, 1993; Saarinen & Levi, 1999). Local curvatures inform surface properties such as figure-ground stratification (e.g., Pao, Geiger, & Rubin, 1999) and bumpiness (e.g., Stevens & Brookes, 1987). Rapid processing of surface properties, unconstrained by explicit awareness, would facilitate planning and execution of action. Furthermore, continuous adaptation to surface regularity would be important for marking deviations that require behavioral modifications (e.g., Clifford et al., 2007). It is thus possible that the relatively awareness-independent adaptive coding of local curvature is utilized by the dorsal visual pathway that mediates action (e.g., Fang & He, 2005; Goodale & Westwood, 2004). In contrast, closed contours signal the presence of objects (e.g., Elder & Zucker, 1992; Koffka, 1935). In particular, the aspect ratio of a closed shape (e.g., an ellipse) is a basic two-dimensional feature (e.g., Regan & Hamstra, 1992; Suzuki & Cavanagh, 1998) associated with face perception (Young & Yamane, 1992) and also with any object analyses that require information about directions of elongation (e.g., Biederman, 1987). It is possible that, unlike the case of adapting to environmental regularity for action, it might be beneficial to adapt to (i.e., deemphasize) object-related features only when objects are consciously perceived.
Acknowledgments
Funding
This research was supported by National Institutes of Health Grants R01 EY018197-02S1 and EY018197 and National Science Foundation Grant BCS 0643191.
Footnotes
Declaration of Conflicting Interests
The authors declared that they had no conflicts of interest with respect to their authorship or the publication of this article.
Because of a technical problem, the test duration deviated from 70 ms on 10.2% of the trials in Experiment 1 and 10.3% of the trials in Experiment 2; those trials were removed from the analyses. However, this did not bias our results, because the deletions were randomly distributed across trials, and there were no empty cells; that is, we had data for every stimulus combination from each observer.
References
- Azouz R, Gray CM. Dynamic spike threshold reveals a mechanism for synaptic coincidence detection in cortical neurons in vivo. Proceedings of the National Academy of Sciences, USA. 2000;97:8110–8115. doi: 10.1073/pnas.130200797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biederman I. Recognition-by-components: A theory of human image understanding. Psychological Review. 1987;94:115–147. doi: 10.1037/0033-295X.94.2.115. [DOI] [PubMed] [Google Scholar]
- Blake R, Tadin D, Sobel KV, Raissian TA, Chong SC. Strength of early visual adaptation depends on visual awareness. Proceedings of the National Academy of Sciences, USA. 2006;103:4783–4788. doi: 10.1073/pnas.0509634103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonneh YS, Cooperman A, Sagi D. Motion-induced blindness in normal observers. Nature. 2001;411:798–801. doi: 10.1038/35081073. [DOI] [PubMed] [Google Scholar]
- Clifford CWG, Webster MA, Stanley GB, Stocker AA, Kohn A, Sharpee TO, Schwartz O. Visual adaptation: Neural, psychological and computational aspects. Vision Research. 2007;47:3125–3131. doi: 10.1016/j.visres.2007.08.023. [DOI] [PubMed] [Google Scholar]
- Dickinson JE, Almeida RA, Bell J, Badcock DR. Global shape aftereffects have a local substrate: A tilt aftereffect field. Journal of Vision. 2010;10(13) doi: 10.1167/10.13.5. Article 5. Retrieved from http://www.journalofvision.org/content/10/13/5.full. [DOI] [PubMed] [Google Scholar]
- Doesburg SM, Green JJ, McDonald JJ, Ward LM. Rhythms of consciousness: Binocular rivalry reveals large-scale oscillatory network dynamics mediating visual perception. PLoS ONE. 2009;4(7):e6142. doi: 10.1371/journal.pone.0006142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumoulin SO, Hess RF. Cortical specialization for concentric shape processing. Vision Research. 2007;47:1608–1613. doi: 10.1016/j.visres.2007.01.031. [DOI] [PubMed] [Google Scholar]
- Elder J, Zucker S. The effect of contour closure on the rapid discrimination of two-dimensional shapes. Vision Research. 1992;33:981–991. doi: 10.1016/0042-6989(93)90080-g. [DOI] [PubMed] [Google Scholar]
- Fang F, He S. Cortical responses to invisible objects in the human dorsal and ventral pathways. Nature Neuroscience. 2005;8:1380–1385. doi: 10.1038/nn1537. [DOI] [PubMed] [Google Scholar]
- Fries P, Roelfsema PR, Engel AK, Konig P, Singer W. Synchronization of oscillatory responses in visual cortex correlates with perception in interocular rivalry. Proceedings of the National Academy of Sciences, USA. 1997;94:12699–12704. doi: 10.1073/pnas.94.23.12699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gail A, Brinksmeyer HJ, Eckhorn R. Perception-related modulations of local field potential power and coherence in primary visual cortex of awake monkey during binocular rivalry. Cerebral Cortex. 2004;14:300–313. doi: 10.1093/cercor/bhg129. [DOI] [PubMed] [Google Scholar]
- Gheorghiu E, Kingdom FA. The spatial feature underlying the shape-frequency and shape-amplitude after-effects. Vision Research. 2007;47:834–844. doi: 10.1016/j.visres.2006.11.023. [DOI] [PubMed] [Google Scholar]
- Goodale MA, Westwood DA. An evolving view of duplex vision: Separate but interacting cortical pathways for perception and action. Current Opinion in Neurobiology. 2004;14:203–211. doi: 10.1016/j.conb.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Hegdé J, Van Essen DC. A comparative study of shape representation in macaque visual areas V2 and V4. Cerebral Cortex. 2007;17:1100–1116. doi: 10.1093/cercor/bhl020. [DOI] [PubMed] [Google Scholar]
- Ishai A, Ungerleider LG, Martin A, Schouten JL, Haxby JV. Distributed representation of objects in the human ventral visual pathway. Proceedings of the National Academy of Sciences, USA. 1999;96:9379–9384. doi: 10.1073/pnas.96.16.9379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y, Costello P, Fang F, Huang M, He S. A gender- and sexual orientation-dependent spatial attentional effect of invisible images. Proceedings of the National Academy of Sciences, USA. 2006;103:17048–17052. doi: 10.1073/pnas.0605678103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koffka K. Principles of Gestalt psychology. New York, NY: Harcourt, Brace, & World; 1935. [Google Scholar]
- Kovács I, Julesz B. A closed curve is much more than an incomplete one: Effect of closure in figure-ground segmentation. Proceedings of the National Academy of Sciences, USA. 1993;90:7495–7497. doi: 10.1073/pnas.90.16.7495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leopold DA, Logothetis NK. Multistable phenomena: Changing views in perception. Trends in Cognitive Sciences. 1999;3:254–264. doi: 10.1016/s1364-6613(99)01332-7. [DOI] [PubMed] [Google Scholar]
- Loffler G. Perception of contours and shapes: Low and intermediate stage mechanisms. Vision Research. 2008;48:2106–2127. doi: 10.1016/j.visres.2008.03.006. [DOI] [PubMed] [Google Scholar]
- Logothetis NK. Single units and conscious vision. Philosophical Transactions of the Royal Society B: Biological Sciences. 1998;353:1801–1818. doi: 10.1098/rstb.1998.0333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macknik SL, Martinez-Conde S. Dichoptic visual masking reveals that early binocular neurons exhibit weak interocular suppression: Implications for binocular vision and visual awareness. Journal of Cognitive Neuroscience. 2004;16:1049–1059. doi: 10.1162/0898929041502788. [DOI] [PubMed] [Google Scholar]
- Moradi F, Koch C, Shimojo S. Face adaptation depends on seeing the face. Neuron. 2005;45:169–175. doi: 10.1016/j.neuron.2004.12.018. [DOI] [PubMed] [Google Scholar]
- Orban GA. Higher order visual processing in macaque extrastriate cortex. Physiological Reviews. 2008;88:59–89. doi: 10.1152/physrev.00008.2007. [DOI] [PubMed] [Google Scholar]
- Pao H, Geiger D, Rubin N. Measuring convexity for figure/ground separation; Proceedings of the Seventh IEEE International Conference on Computer Vision; 1999. pp. 948–955. (Available from IEEE Xplore Digital Library: http://ieeexplore.ieee.org/xpl/freeabs_all.jsp?arnumber=790350) [Google Scholar]
- Pasupathy A, Connor CE. Population coding of shape in area V4. Nature Neuroscience. 2002;5:1332–1338. doi: 10.1038/nn972. [DOI] [PubMed] [Google Scholar]
- Regan D, Hamstra SJ. Shape discrimination and the judgement of perfect symmetry: Dissociation of shape from size. Vision Research. 1992;32:1845–1864. doi: 10.1016/0042-6989(92)90046-l. [DOI] [PubMed] [Google Scholar]
- Saarinen J, Levi DM. Effect of contour closure on shape perception. Spatial Vision. 1999;12:227–238. doi: 10.1163/156856899x00139. [DOI] [PubMed] [Google Scholar]
- Schwartz O, Hsu A, Dayan P. Space and time in visual context. Nature Reviews Neuroscience. 2007;8:522–535. doi: 10.1038/nrn2155. [DOI] [PubMed] [Google Scholar]
- Simons DJ. Attentional capture and inattentional blindness. Trends in Cognitive Sciences. 2000;4:147–155. doi: 10.1016/s1364-6613(00)01455-8. [DOI] [PubMed] [Google Scholar]
- Stevens KA, Brookes A. Detecting structure by symbolic constructions on tokens. Computer Vision, Graphics, and Image Processing. 1987;37:238–260. [Google Scholar]
- Suzuki S. High-level pattern coding revealed by brief shape aftereffects. In: Clifford C, Rhodes G, editors. Advances in visual cognition: Vol. 2. Fitting the mind to the world: Adaptation and aftereffects in high-level vision. Oxford, England: Oxford University Press; 2005. pp. 135–172. [Google Scholar]
- Suzuki S, Cavanagh P. A shape-contrast effect for briefly presented stimuli. Journal of Experimental Psychology: Human Perception and Performance. 1998;24:1315–1341. doi: 10.1037//0096-1523.24.5.1315. [DOI] [PubMed] [Google Scholar]
- Sweeny TD, Grabowecky M, Kim YJ, Suzuki S. Internal curvature signal and noise in low- and high-level vision. Journal of Neurophysiology. 2011;105:1236–1257. doi: 10.1152/jn.00061.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchiya N, Koch C. Continuous flash suppression reduces negative afterimages. Nature Neuroscience. 2005;8:1096–1101. doi: 10.1038/nn1500. [DOI] [PubMed] [Google Scholar]
- Wilke M, Logothetis NK, Leopold DA. Local field potential reflects perceptual suppression in monkey visual cortex. Proceedings of the National Academy of Sciences, USA. 2006;103:17507–17512. doi: 10.1073/pnas.0604673103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson HR. Discrimination of contour curvature: Data and theory. Journal of the Optical Society of America A. 1985;2:1191–1199. doi: 10.1364/josaa.2.001191. [DOI] [PubMed] [Google Scholar]
- Yang E, Hong S-W, Blake R. Adaptation aftereffects to facial expressions suppressed from visual awareness. Journal of Vision. 2010;10(12) doi: 10.1167/10.12.24. Article 24. Retrieved from http://www.journalofvision.org/content/10/12/24.full. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young MP, Yamane S. Sparse population coding of faces in the inferotemporal cortex. Science. 1992;256:1327–1331. doi: 10.1126/science.1598577. [DOI] [PubMed] [Google Scholar]