Abstract
Low-level laser light therapy (LLLT) exerts beneficial effects on motor and histopathological outcomes after experimental traumatic brain injury (TBI), and coherent near-infrared light has been reported to improve cognitive function in patients with chronic TBI. However, the effects of LLLT on cognitive recovery in experimental TBI are unknown. We hypothesized that LLLT administered after controlled cortical impact (CCI) would improve post-injury Morris water maze (MWM) performance. Low-level laser light (800 nm) was applied directly to the contused parenchyma or transcranially in mice beginning 60–80 min after CCI. Injured mice treated with 60 J/cm2 (500 mW/cm2×2 min) either transcranially or via an open craniotomy had modestly improved latency to the hidden platform (p<0.05 for group), and probe trial performance (p<0.01) compared to non-treated controls. The beneficial effects of LLLT in open craniotomy mice were associated with reduced microgliosis at 48 h (21.8±2.3 versus 39.2±4.2 IbA-1+ cells/200×field, p<0.05). Little or no effect of LLLT on post-injury cognitive function was observed using the other doses, a 4-h administration time point and 7-day administration of 60 J/cm2. No effect of LLLT (60 J/cm2 open craniotomy) was observed on post-injury motor function (days 1–7), brain edema (24 h), nitrosative stress (24 h), or lesion volume (14 days). Although further dose optimization and mechanism studies are needed, the data suggest that LLLT might be a therapeutic option to improve cognitive recovery and limit inflammation after TBI.
Key words: cognition, controlled cortical impact, inflammation, low-level laser light therapy, mice, microglia
Introduction
Traumatic brain injury (TBI) affects an estimated 1.4 million Americans every year (Langlois and Sattin, 2005; Rutland-Brown et al., 2006). Many of those who survive will join the approximately 5.3 million Americans currently living with TBI-related disabilities (Selassie et al., 2008; Zaloshnja et al., 2008). Despite advances in emergency and critical care of TBI patients, no specific therapy exists to attenuate the post-injury cognitive deficits that persistently challenge TBI survivors (Dikmen et al., 2009; Salmond and Sahakian, 2005; Salmond et al., 2006).
Low-level laser light therapy (LLLT) is the application of light in the red or near-infrared spectrum (600–1000 nm) at a power density between 1 and 5 W/cm2. LLLT facilitates wound healing by proliferative effects on dividing cells (Conlan et al., 1996), and exerts potent anti-inflammatory effects outside the central nervous system (Wong and Wilder-Smith, 2002). In acute injury paradigms, LLLT reduces myocardial infarction (Ad and Oron, 2001), improves neurological deficits after stroke (Oron et al., 2006), and promotes regeneration and functional recovery after peripheral nerve injury (Anders et al., 2004). Pilot studies and case reports have also shown promise for LLLT as a therapeutic agent for clinical recovery in stroke (Lampl et al., 2007; Zivin et al., 2009), nerve injuries (Rochkind, 2009), and TBI (Naeser et al., 2010).
In the first study of LLLT reported in a weight-drop TBI model, Oron and associates (2007) showed a beneficial effect of LLLT on post-injury motor function and brain tissue atrophy following a single application 4 h after injury. However, the effects of LLLT on cognitive outcomes were not reported. Here we hypothesize that treatment with an appropriate dose of LLLT would attenuate cognitive dysfunction after controlled cortical impact (CCI) in mice.
Methods
Animals
Mice (n=239) were housed in 12-h day/night cycles in a pathogen-free facility at Massachusetts General Hospital in accordance with the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals. Food and water was given ad libitum. Male C57BL/6 mice 3 months of age weighing 25–30 g (Jackson Laboratories, Bar Harbor, ME) were used. For all experiments including CCI, the investigators were blinded to study group.
Experimental protocols
Two experimental groups received LLLT or no treatment. In the open craniotomy group, LLLT was applied directly to the contused brain. In the transcranial group, LLLT was applied after CCI transcranially with the scalp sutured closed and the craniotomy bone replaced. The open craniotomy group received LLLT at five different doses: 30, 60, 105, 120, or 210 J/cm2, whereas the transcranial group received only 60 J/cm2 at various times after CCI. For all experiments, the day of CCI was considered day 0. Motor testing (wire grip test) was performed on post-injury days 1, 3, 5, and 7. Morris water maze (MWM) testing was performed on days 7–10. Lesion size analysis was performed on mice killed 14 days after injury. Brain edema and nitrotyrosine enzyme-linked immunosorbent assay (ELISA) were performed at 24 h after injury, whereas microglia immunohistochemistry was done at 48 h.
Controlled cortical impact
The CCI model was used as previously described (Mannix et al., 2010). The Massachusetts General Hospital Institutional Animal Care and Use Committee approved the trauma protocol. Anesthesia was induced using a Fluotec 3 vaporizer (Colonial Medical, Amherst, NH), and 70% nitrous oxide, 4–5% isoflurane (Anaquest, Memphis, TN), and the balance was oxygen. Following induction, the mice were removed from the chamber, positioned on a stereotaxic frame with the nose inside an open plastic tube carrying anesthesia from the chamber to the animal, and then out into a negative pressure hood. Isoflurane was reduced to 3.5–4%. Considerable side-stream ventilation of room air mixed with the 3.5–4% isoflurane. This level of anesthesia produces unresponsiveness to tail and toe pinch and to surgical procedures, and maintains blood pressure and blood gases within normal limits (except for occasional mild hyperventilation), as reported previously by our group (Khuman et al., 2011; Yager et al., 2008; Yang et al., 2010). The total duration under anesthesia was typically 2–3 min. A 5-mm craniotomy was made with a portable drill and trephine over the left parieto-temporal cortex. The bone flap was removed and the dura was left intact. Impact was delivered using a 3-mm flat-tipped pneumatic piston at a velocity of 6 m/sec, for a duration of 100 msec and a depth of 0.6 mm. The bone flap was discarded and the scalp incision was sutured closed. The mice were returned to their cages to recover from anesthesia in room air. Sham-injured mice (n=12/group) underwent craniotomy, LLLT (60 J/cm2) or no treatment, but not CCI.
Low-level laser light therapy
The injured mice were randomly assigned to non-treated (control) or LLLT-treated groups. At various times after CCI or sham injury, the mice were re-anesthetized and placed on a stereotaxic frame. Using an 800-nm laser device (Thor Photomedicine Ltd., Chesham, Buckinghamshire, U.K.) mounted 1 cm above the head of the mouse, LLLT was applied using a 13-mm aperture (beam size=1.32 cm2). In the open craniotomy groups, 60–80 min after CCI the scalp sutures were removed, exposing the craniotomy site. Prior to treatment, the mice were randomly allocated to various LLLT groups based on energy level and irradiance time as follows: 30 J/cm2 (250 mW/cm2×2 min, n=7), 60 J/cm2 (500 mW/cm2×2 min, n=22), 105 J/cm2 (250 mW/cm2×7 min, n=7), 120 J/cm2 (1000 mW/cm2×2 min, n=10), 210 J/cm2 (500 mW/cm2×7 min, n=10), or 0 J (control, n=43). The laser beam was placed such that it illuminated the contused brain (exposed through the craniotomy), as well as the contralateral uninjured hemisphere (covered by the skull bone). After treatment the scalp was sutured closed and the mice were allowed to recover from anesthesia in their cages. Injured non-treated mice (controls) were anesthetized for the same duration as the LLLT-treated mice, and their sutures were similarly reopened and closed.
For transcranial LLLT only 60 J/cm2 (500 mW/cm2×2 min) was used. The head was shaved, exposing the scalp over the right and left parieto-temporal region. Using the same distance to the laser and beam size as in the open craniotomy groups, LLLT (or no treatment) was applied such that the beam illuminated the entire area of the top of the scalp. Transcranial LLLT was given as a single dose of 60 J/cm2 at 60–80 min (n=12/group), or 4 h (n=9/group) after CCI, or one treatment per day for 7 consecutive days beginning 60–80 min after CCI (n=10/group). Following LLLT treatment the mice were placed back in their cages to recover from anesthesia. Table 1 summarizes the LLLT parameters used in all experiments.
Table 1.
Low-Level Laser Light Treatment Paradigms Used following Injury
| 250 mW/cm22 min | 500 mW/cm22 min | 1000 mW/cm22 min | 250 mW/cm27 min | 500 mW/cm27 min | |
|---|---|---|---|---|---|
| Average power (W) | 0.33 | 0.65 | 1.30 | 0.33 | 0.65 |
| Illuminated area (cm2) | 1.3 | 1.3 | 1.3 | 1.3 | 1.3 |
| Irradiance (W/cm2 | 0.250 | 0.500 | 1.000 | 0.250 | 0.500 |
| Irradiation time (sec) | 120 | 120 | 120 | 420 | 420 |
| Energy density (J/cm2) | 30 | 60 | 120 | 105 | 210 |
| Energy (Joules) | 39 | 78 | 156 | 137 | 273 |
Morris water maze
The Morris water maze (MWM) was used to evaluate spatial learning and memory beginning 7 days after sham-injury or CCI (Bermpohl et al., 2007). A total of 159 animals were subjected to MWM testing using the same paradigm for each experimental group. The apparatus consisted of a white pool 83 cm in diameter and 60 cm deep, filled with water to a depth of 29 cm. Visible cues were positioned on the walls of the tank and around the room. Water temperature was maintained at approximately 25°C. A clear acrylic glass goal platform 10 cm in diameter was positioned 0.5 cm below the surface of the water (hidden platform), approximately 15 cm from the southwest wall (target quadrant) of the tank. Each mouse was subjected to two trials per day beginning on post-injury day 7, and ending on post-injury day 9, with a single hidden platform trial (number 5), and a probe trial. On post-injury day 10 two sets of visible platform trials were performed. For each trial, the mice were randomized to one of four starting locations (north, south, east, and west), and placed in the pool facing the wall. The maximum time allotted to reach the platform was 90 sec. If the mouse failed to reach the platform within the allotted time, it was placed on the platform by the experimenter and allowed to remain there for 10 sec. In the probe trial, the goal platform was removed, the mice were placed in the tank opposite the target quadrant, and the time spent in the target quadrant was assessed over 60 sec. The probe score was time in seconds (sec) spent by the mouse in the target quadrant. To control for possible differences in visual acuity or sensorimotor function, two sets of visible platform trials were performed on the day following the probe trial. For the visible trials the goal platform was raised 0.5 cm above the water and clearly marked with red tape (visible platform). Performance in the hidden and visible platform trials was quantified as latency to the platform in seconds (sec).
Wire grip test
Vestibular-motor function in open craniotomy mice (n=44 mice total) was assessed using the wire grip test. Testing was performed on days 1, 3, 5, and 7 after CCI. Briefly, the mice were placed on a metal wire (45 cm) suspended between two upright poles 45 cm above a table. The animals were scored based on the manner in which they held onto the wire during a 60-sec period (Mannix et al., 2010).
Assessment of microglia activation
For immunohistochemical detection of microglia in open craniotomy LLLT, the mice were transcardially perfused with 4% paraformaldehyde 48 h after injury. The brains were post-fixed for 24 h in 4% paraformaldehyde and cryoprotected in 30% sucrose for another 24 h. Coronal sections were cut (16–20 μm) and mounted on poly-L-lysine-coated slides. The sections were washed in PBS, blocked in 3% normal goat serum in PBS for 1 h, and incubated overnight at 4°C with rabbit anti-Iba-1 (ionized calcium binding adaptor molecule 1) antibody (1:250; Wako Pure Chemical Industries Ltd., Osaka, Japan). The slides were then washed in PBS and incubated with the appropriate Cy3-conjugated secondary antibody (1:300; Jackson ImmunoResearch, West Grove, PA) for 60 min, washed in PBS, and cover-slipped for analysis using excitation/emission spectra of 568/585 nm. Microglia were assessed quantitatively (n=4/group) in 200×fields taken from 6–8 sections per mouse. Regions of interest included the ipsilateral cortex inferior and lateral to the contusion, the ipsilateral dentate gyrus, and the ipsilateral corpus callosum directly below the contusion. Overall, 12 200×microscopic fields (1100×1100 μm) were assessed per mouse.
Assessment of brain edema
Brain edema was assessed at 24 h (the time of peak edema in our CCI model) by measuring brain water content using the (wet – dry)/(wet brain weight) method. Mice treated with open craniotomy and 60 J/cm2 (n=6), 120 J/cm2 (n=6), or controls (n=6), were killed 24 h after injury and the brains were bisected into injured and non-injured hemispheres. Each hemisphere was immediately weighed (wet weight), then dried at 99°C for 72 h, and dry weights were obtained. Percentage brain water content was expressed as (wet – dry weight)/(wet weight)×100%.
Assessment of lesion volume
Injured mice treated via open craniotomy and 60 J/cm2 (n=16) or controls (n=16), after MWM training were sacrificed and brain tissue was isolated for assessment of lesion volume. The brains were sectioned in the coronal plane (12 μm) at intervals of 0.5 mm from the anterior to the posterior end. The sections were mounted on poly-L-lysine-coated slides and stained with hematoxylin. Area of the uninjured and injured hemisphere per section was measured using image analysis software (NIS Elements BR 3.0, Tokyo, Japan). The hemispheric volume was obtained by summing area of each section and multiplying it by 0.5. Lesion volume (mm3) was expressed as the difference between the uninjured and injured hemisphere volumes.
Nitrotyrosine ELISA
Mice were killed at 24 h following sham injury (n=8) or CCI (n=16) with open craniotomy LLLT or no treatment. The brains were removed, rapidly dissected on ice, and contused parenchyma was isolated. Brain tissue was homogenized on ice using radio immunoprecipitation assay (RIPA; Boston Bioproducts, Worcester, MA) buffer. The homogenates were centrifuged at 12,000g for 15 min at 4°C and supernatants were subjected to protein assay (DC protein assay; BioRad Laboratories, Hercules, CA). Nitrotyrosine levels in supernatants were assayed using a competitive ELISA according to the instructions of the manufacturer (catalog # 17-376; Millipore, Billerica, MA).
Brain temperature monitoring
To assess the effect of LLLT on brain temperature in the open craniotomy and transcranial protocols, mice (n=4–5/group) were anesthetized with isoflurane and a brain temperature probe (30×1 mm, temperature range 0–55.5°C; FHC Inc., Brunswick, ME) was inserted underneath the left (injured) parietal cortex. For open craniotomy assessments, the scalp was incised, craniotomy was done, and the bone flap removed. LLLT was applied directly to the exposed cortex and the rest of the cranium for 2 min, as in the CCI experiments. For assessment in the transcranial protocol, the bone flap was replaced and the scalp sutured closed prior to the initiation of LLLT.
Statistical analysis
Data are mean±standard error of the mean (SEM). Data were analyzed using GraphPad Prism V (GraphPad Software Inc., La Jolla, CA). Wire grip test and MWM results were analyzed using two-factor repeated-measures analysis of variance (RM ANOVA, group×time). Probe trial data, brain water content, lesion volume, and ELISA data were analyzed by a rank sum test. With n=22 mice per group (44 mice total), and a treatment effect of 25–30%, alpha=0.05 and sigma=0.3, the power to detect a difference between means in any given trial of the MWM is above 0.8. For probe trials, the power to detect differences between mean values with 25% treatment effect is 0.7 with n=8/group. For all comparisons, p<0.05 was considered significant.
Results
All mice survived CCI and the experimental period including LLLT and behavioral testing. In the open craniotomy protocol, LLLT increased brain temperature by 0.2±0.1°C (30 J/cm2), 2.5±0.4°C (60 J/cm2), and 4.1±0.3°C (120 J/cm2), over the 2-min application period. In the transcranial protocol, LLLT (60 J/cm2) increased brain temperature by 1.8±0.1°C. In all cases, brain temperature returned to baseline within 3–5 min.
Effect of LLLT on motor function after controlled cortical impact
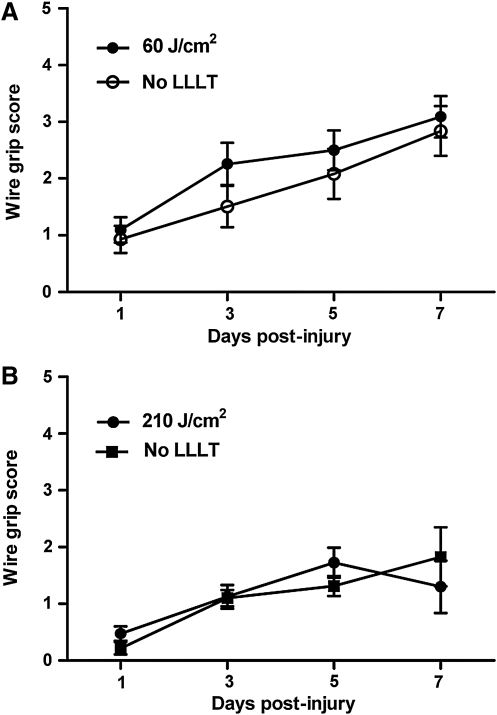
Motor recovery after CCI in mice treated with 60 J/cm2 or 210 J/cm2 did not differ from that of injured control mice (p=0.36 and p=0.85, respectively; Fig. 1A and B). LLLT did not affect motor performance in sham-injured mice (not shown), and CCI produced robust motor deficits compared to sham injury (p<0.01 for group, data not shown).
FIG. 1.
Effect of low-level laser light therapy (LLLT) on wire grip test performance after controlled cortical impact. Motor function was assessed using the wire grip test on post-injury days 1, 3, 5, and 7. (A) Motor recovery did not differ between mice treated with 60 J/cm2 compared to injured control mice (p=0.35 for group; n=18–20/group). (B) Injured mice treated with 210 J/cm2 had no difference in their motor recovery compared to control mice (p=0.85 for group; n=7–8/group). Wire grip performance in all mice improved over the experimental period (p<0.0001 for time).
Effect of LLLT on cognitive function after controlled cortical impact
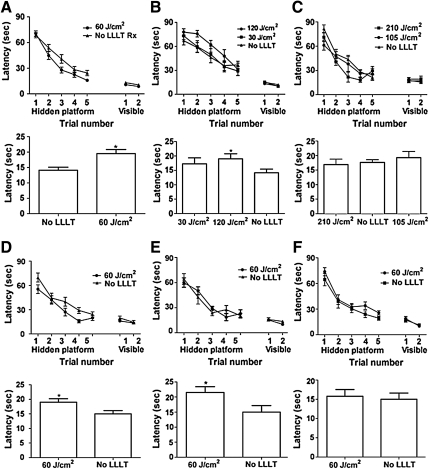
All sham-injured and CCI-injured groups showed a time-dependent (p<0.0001) improvement in latency to the hidden platform, indicating some degree of learning ability after CCI. In the open craniotomy group, mice treated with 60 J/cm2 performed significantly better than controls in the hidden platform (p=0.03 for group; Fig. 2A) and probe trials (p=0.004; Fig. 2A), suggesting a beneficial effect of this level of LLLT on post-injury cognitive function. Sham-injured non-treated mice performed similarly to sham-injured animals treated with 60 J/cm2 (p>0.05 for group in the hidden platform trials and probe trials), suggesting that LLLT does not improve baseline learning ability with the doses used. Injured mice treated with doses of 30, 105, 120, and 210 J/cm2 (Fig. 2B and C) did not show robust effects on the hidden platform trials. Mice treated with 120 J/cm2 had improved probe trial scores compared to controls (p=0.02; Fig. 2B).
FIG. 2.
Effect of low-level laser light therapy (LLLT) on recovery of cognitive function after controlled cortical impact (CCI). Cognitive function was assessed using the Morris water maze. In the open craniotomy group, (A) mice treated with 60 J/cm2 performed significantly better than controls in the hidden platform (p=0.03 for group; n=22; upper panel) and probe trials (*p=0.004; lower panel). (B) Treatment with doses of 30 or 120 J/cm2 did not improve hidden platform trials (p>0.1 for group; n=7–10/group; upper panel), but mice treated with 120 J/cm2 had improved probe trial scores compared to controls (*p=0.02; lower panel). (C) Injured mice treated with 105 J/cm2 performed similarly to controls (p>0.05 for group effect on the hidden platform and probe trials; n=7/group). Mice treated with 210 J/cm2 had improved performance in the hidden platform (p=0.039 for group; n=8; upper panel), but not in the probe trials (p=0.95; lower panel). In mice treated with transcranial LLLT, (D) a single dose of 60 J/cm2 given 60–80 min post-injury improved hidden platform trial performance (p=0.018 for group; n=12–13/group; upper panel), and probe trial latency (*p=0.021 versus controls; lower panel). (E) Daily application of transcranial LLLT for 7 days after CCI had no benefit on the hidden platform trials (p=0.935 for group; n=10/group; upper panel); however, this regimen improved probe trial performance (*p<0.03 versus controls; lower panel). (F) Transcranial LLLT (60 J/cm2) administered at 4 h post-injury did not improve hidden platform (p=0.13 for group; n=9/group; upper panel) or probe trial performance (p=0.6 versus controls; lower panel). No significant differences among treatment groups were observed in the visible platform trials. All LLLT-treated and control (non-treated) mice showed progressive improvement in the hidden platform trials (p<0.0001 for time), indicating learning.
In the transcranial LLLT groups, injured mice administered a single dose of LLLT (60 J/cm2) given 60–80 min post-injury had significant improvement in hidden platform trials (p=0.018 for group effect; Fig. 2D) and probe trials (p=0.021; Fig. 2D) compared to controls. Beneficial effects of LLLT on hidden platform trials were lost when LLLT was applied daily for 7 days after CCI; however, this regimen improved probe trial performance (p<0.03 versus controls; Fig. 2E). Transcranial LLLT (60 J/cm2) administered at 4 h post-injury did not improve hidden platform or probe trial performance (Fig. 2F).
Effect of LLLT on histopathology after CCI in the open craniotomy groups
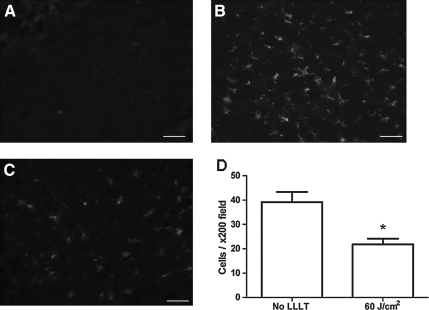
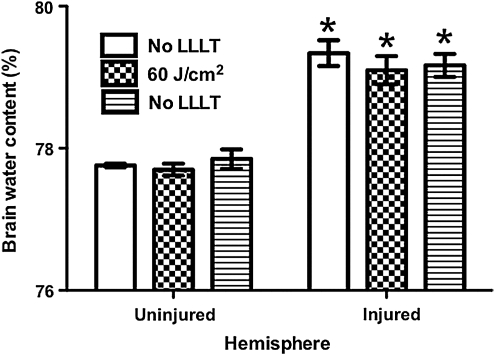
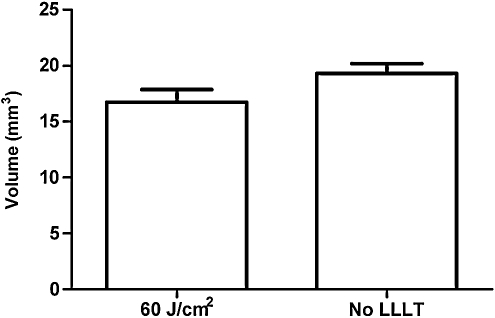
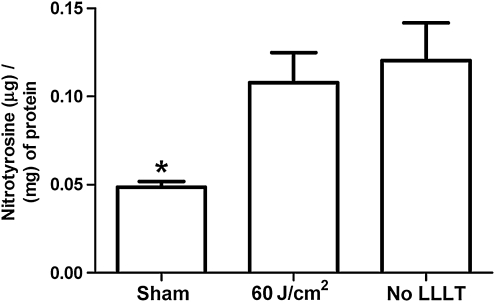
Quantitative analyses of microglial activation revealed a significant reduction of activated microglia in LLLT-treated (60 J/cm2) mice compared to injured non-treated mice (Fig. 3), suggesting a robust anti-inflammatory effect of LLLT. CCI produced a robust increase in injured hemispheric brain water content versus the uninjured hemisphere (p<0.01); however, LLLT (60 or 120 J/cm2) did not influence the magnitude of brain edema assessed at 24 h, the time of peak edema in our CCI model (Fig. 4). Likewise, brain tissue loss did not differ between the 60 J/cm2-treated group and controls (p=0.12; Fig. 5). Protein nitrosylation was significantly increased in CCI versus sham injury (p<0.01); however, LLLT (60 J/cm2) did not reduce brain nitrotyrosine compared to controls (p=0.87; Fig. 6).
FIG. 3.
Effect of low-level laser light therapy (LLLT) on microglial activation after controlled cortical impact. Representative photomicrographs made at 48 h following injury show (A) normal resting microglia in the contralateral hemisphere, (B) robust microgliosis in the ipsilateral hemisphere of injured control (non-treated) mice, and (C) reduced microgliosis in the ipsilateral cortex following 60 J/cm2 laser treatment (magnification 200×). (D) Quantitative analysis of microgliosis assessed at 48 h showing significant reduction with 60 J/cm2 LLLT (*p=0.03 versus controls; scale bars=10 μm).
FIG. 4.
Effect of low-level laser light therapy (LLLT) on post-injury brain edema. Brain water content in injured hemispheres was significantly greater than that of uninjured hemispheres in all groups (*p<0.01); however, brain water content in injured hemispheres did not differ among treatment groups.
FIG. 5.
Effect of low-level laser light therapy (LLLT) on post-injury lesion volume. Brain tissue loss measured on day 14 post-injury did not differ between open craniotomy mice treated with 60 J/cm2 (n=16) versus controls (p=0.12; n=16).
FIG. 6.
Effect of low-level laser light therapy (LLLT) on protein nitrosylation in mouse brain at 24 h after controlled cortical impact (CCI). The total concentration of nitrosylated protein was significantly higher at 24 h post-CCI injury in LLLT-treated and non-treated controls compared to sham animals (*p<0.0001; n=8/group). Nitrotyrosine levels did not differ at 24 h after CCI between the LLLT (60 J/cm2) and non-treated control groups (p=0.65).
Discussion
To our knowledge this is the first study to report efficacy of LLLT in reducing cognitive deficits following experimental TBI. We found that 60 J/cm2 (500 mW/cm2×2 min) of LLLT given 60–80 min post-CCI modestly improved spatial learning and memory as assessed by a Morris water maze paradigm. Cognitive benefits of LLLT were not observed in sham-injured mice, suggesting that the beneficial effects of LLLT that we observed are specific to post-injury mechanisms. A single application of 60 J/cm2 was also associated with reduced microgliosis at 48 h, the peak time of microglial activation in our CCI model. LLLT induced a brief increase in brain temperature that returned to baseline within 2–5 min, suggesting that the beneficial effects of LLLT were not due to changes in brain temperature during its application (Mochizuki-Oda et al., 2002). The results suggest that LLLT may have clinical utility as a specific therapy for cognitive deficits after focal TBI.
Our study extends the beneficial effects of LLLT reported by Oron and associates (2007) to cognitive outcomes (assessed after the resolution of motor deficits) after focal TBI. In contrast to their study, we found no beneficial effects of LLLT on post-injury motor function (assessed on days 1–7 because motor deficits typically resolve within a week after CCI) or histopathology (lesion size, assessed at day 14 to ensure a stable cavitary lesion) after CCI. These divergent results may be explained in part by model differences (CCI produces a greater amount of tissue damage compared to the closed-head injury model used by Oron and colleagues), and differences in the sensitivity of motor tests to detect motor impairment, as the neurological severity score used by Oron and associates is likely a more sensitive indicator of neurological dysfunction compared to the wire grip test (Fujimoto et al., 2004). Differences in delivered LLLT energy level (1.2–2.4 J/cm2 used by Oron and associates versus 60 J/cm2 in the current study) also may have contributed to the different outcomes.
Pre-clinical and clinical studies of LLLT in stroke (Lapchak et al., 2007,2010) and TBI (Naeser et al., 2010) showed beneficial effects of light in the range of 800 nm, therefore we chose this wavelength in our studies. We performed a limited number of dose-response experiments modulating power level and duration of LLLT, and found efficacy at discrete power levels, with the best therapeutic effect on cognitive outcome seen by using 60 J/cm2 in both the open craniotomy and transcranial groups. Although fewer or no beneficial effects were observed at other doses, no adverse effects were observed at higher power levels. Prior reports describe significant beneficial effects of LLLT in experimental stroke and TBI using 1.2–2.4 J/cm2 (Lapchak et al., 2007; Oron et al., 2006), and in a pilot study in stroke patients using 1 J/cm2 applied to multiple scalp regions for 2 min (Lampl et al., 2007).
LLLT is sensitive to irradiance and total energy delivered. The Arndt-Schultz law is frequently quoted as a suitable model to describe dose-dependent effects of LLLT (Sommer et al., 2001). The Arndt-Schultz law states that “weak stimuli increases physiologic activity, moderate stimuli inhibit activity, and very strong stimuli abolish activity.” In the context of LLLT the increasing “stimulus” may be irradiation time, or it may be increased beam intensity (irradiance). Also, the Bunsen-Roscoe rule of reciprocity, which predicts that if the products of time of exposure and irradiance are equal, then the quantities of material undergoing change will be equal, has failed in LLLT studies, and a non-linear, s-curve response has been demonstrated (Lanzafame et al., 2007; Lubart, 2006). Thus, longer irradiation times at lower power densities than those employed in the current study may prove even more beneficial in future studies. This is a critical issue in the field because clinical studies of near-infrared light therapy are ongoing in patients, and optimal dosing parameters to improve cognitive outcome have yet to be established in experimental TBI models.
One limitation of our study is that it is not possible to determine precisely the power density of light at the level of individual brain regions. Our unpublished data show that transmitted LLLT (800 nm, 500 mW/cm2) is reduced by approximately 15% by scalp tissue, and 46% by the cranium. Mice in the current study were shaved because black fur only allows approximately 2% transmittance of LLLT (unpublished observations). Furthermore, power measurements done in our lab show that 1–4% of applied LLLT (500 mW/cm2×2 min) is transmitted through the entire scalp, skull, and brain of naive anesthetized mice, suggesting that the entire brain was subjected to some degree of LLLT in our studies.
We found group effects in MWM testing in both the open craniotomy and transcranial LLLT (60 J/cm2) groups, as well as effects of LLLT on probe trials only with other doses. These data indicate that suboptimal doses of LLLT may affect recall of spatial memory, even in the absence of an effect on acquisition of spatial learning (as assessed in part by hidden platform trials), or acquisition of non-spatial procedural learning (hippocampus-independent), as assessed by visible platform trials. The probe trial is the gold standard of recall of hippocampus-dependent spatial learning, which is separable from non-hippocampus-dependent aspects of learning assessed by hidden and visible platform trials (D'Hooge and De Deyn, 2001; Morris et al., 1982; Yau et al., 2002). Thus the beneficial effects of LLLT on probe trials are biologically significant and imply improved hippocampal function after TBI.
A number of in vitro and in vivo experimental studies suggest that mitochondria are the primary target of LLLT (Eells et al., 2004; Karu et al., 2005; Pastore et al., 2000). Absorption of photons by cytochrome c releases bound nitric oxide, increases cytochrome c oxidase activity, and improves efficiency of electron transport; the resulting change in cellular redox state leads to increased ATP production and reduced reactive oxygen species (Karu et al., 2005; Tafur et al., 2010). LLLT may also induce redox-sensitive transcription factors such as nuclear factor-kappa B (NF-κB), that promote gene transcription leading to reduced cell death and enhanced neurological function (Lubart et al., 2005; Schreck et al., 1992; Tafur and Mills, 2008). The cellular response to LLLT is not linear, however, and increasing energy density may exacerbate injury by paradoxically increasing oxidative stress and reducing NF-κB activity (Lanzafame et al., 2007; Lubart, 2006). Biphasic dose effects of LLLT have also been reported in some studies, which are generally explained by excessive generation of reactive oxygen species (Streifler et al., 2007), nitric oxide (NO), activation of cytotoxic pathways, and decreased NF-κB activation at higher doses (Huang et al., 2009). Thus, interpretation of the negative data regarding modulation of protein nitrosylation by LLLT (assessed here at 24 h due to the robust increase seen in control CCI mice versus sham injury) in the current study is limited since only one energy level at one time point was studied. A second limitation is that the nitrotyrosine ELISA employed may be insensitive to changes in nitrosative stress in discrete populations of cells, particularly those involved in learning and memory in the hippocampus.
We found a beneficial effect of transcranial LLLT delivered at 60–80 min, but not 4 h after CCI. A therapeutic window of 60–80 min is well within the response time of emergency personnel in the field or medics on the battlefield, and is therefore clinically relevant. Oron and colleagues (2007) reported at least a 4-h therapeutic window for efficacy of LLLT to improve motor deficits and reduce brain tissue atrophy in focal TBI, and therapeutic windows of 6–24 h have been reported in experimental stroke, depending on the species and the model used (Lapchak et al., 2007; Oron et al., 2006). Given the modest improvement in MWM performance seen with LLLT in the current study, we believe that more work is needed to determine optimal dosing to better interpret therapeutic window studies.
We found a robust reduction in microglial activation 48 h post-CCI, the peak of microgliosis in our CCI model and other experimental TBI models (Harting et al., 2008; Ziebell and Morganti-Kossmann, 2010). To our knowledge these are the first data to show an anti-inflammatory effect of LLLT in the immune response to TBI. Immunomodulation with suppression of immune cell activation and cytokine/chemokine expression with light therapy has also been reported following experimental spinal cord injury (Byrnes et al., 2005). In a cryogenic brain injury model LLLT reduced levels of tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), and IL-6 in the first 24 h (Moreira et al., 2009). LLLT reduced inducible nitric oxide synthase (iNOS) expression and increased TGF-β (an anti-inflammatory response) in zymosan-induced arthritis in rats (de Morais et al., 2010; Moriyama et al., 2005). Reduced levels of salivary TNF-α and IL-6 were observed in patients who received laser phototherapy for denture stomatitis (Simunovic-Soskic et al., 2010). The lack of a reduction in overall tissue damage by LLLT in the current study suggests that its effect on microglial activation is not a function of reduced necrosis or overall brain tissue damage, but rather represents a specific effect of LLLT on the initiating mechanisms of microglial activation.
Whether or not the anti-inflammatory effect of LLLT observed in the current study contributes to the improved cognitive function is unknown. Several studies from our group and others have shown an association between inhibition of acute microglial activation and improved functional outcome after experimental TBI (Homsi et al., 2010; Shein et al., 2008; You et al., 2008), but other studies do not show such an association (Bermpohl et al., 2007). To date no study has convincingly demonstrated whether microglia enhance or inhibit functional recovery after TBI, in part because specific reagents targeting microglia are lacking.
Randomized controlled clinical trials suggest that LLLT improves functional outcome after stroke (Lampl et al., 2007), and reduces chronic radicular pain (Konstantinovic et al., 2010). Recently, case reports by Naeser and associates (2010) showed improvement of cognitive function with transcranial non-coherent light therapy administered to two patients in the chronic phase of TBI. Our study differs from these reports in that we applied LLLT during the acute period of TBI. Our data are translationally relevant to TBI patients in the field or emergency department. Our data may also be relevant to patients undergoing decompressive craniectomy, who may have worse cognitive outcomes associated with this procedure (Cooper et al., 2011). Our data showing loss of benefit of LLLT in hidden platform trials in mice treated for 7 days versus 1 day after CCI highlight the need for further studies to establish rational dosing paradigms, determine the therapeutic window, and provide evidence of the safety of LLLT applied acutely after TBI. Such studies are needed to optimize clinical trials of LLLT in patients with TBI, and perhaps other forms of acute brain injury.
Acknowledgments
This work was supported by grants from the Massachusetts General Hospital Center for the Integration of Medicine and Technology (CIMIT) (M.J.W.), and RO1NS047447 (M.J.W.).
Author Disclosure Statement
No competing financial interests exist.
References
- Ad N. Oron U. Impact of low level laser irradiation on infarct size in the rat following myocardial infarction. Int. J. Cardiol. 2001;80:109–116. doi: 10.1016/s0167-5273(01)00503-4. [DOI] [PubMed] [Google Scholar]
- Anders J.J. Geuna S. Rochkind S. Phototherapy promotes regeneration and functional recovery of injured peripheral nerve. Neurol. Res. 2004;26:233–239. doi: 10.1179/016164104225013914. [DOI] [PubMed] [Google Scholar]
- Bermpohl D. You Z. Lo E.H. Kim H.H. Whalen M.J. TNF alpha and Fas mediate tissue damage and functional outcome after traumatic brain injury in mice. J. Cereb. Blood Flow Metab. 2007;27:1806–1818. doi: 10.1038/sj.jcbfm.9600487. [DOI] [PubMed] [Google Scholar]
- Byrnes K.R. Waynant R.W. Ilev I.K. Wu X. Barna L. Smith K. Heckert R. Gerst H. Anders J.J. Light promotes regeneration and functional recovery and alters the immune response after spinal cord injury. Lasers Surg. Med. 2005;36:171–185. doi: 10.1002/lsm.20143. [DOI] [PubMed] [Google Scholar]
- Conlan M.J. Rapley J.W. Cobb C.M. Biostimulation of wound healing by low-energy laser irradiation. A review. J. Clin. Periodontol. 1996;23:492–496. doi: 10.1111/j.1600-051x.1996.tb00580.x. [DOI] [PubMed] [Google Scholar]
- Cooper D.J. Rosenfeld J.V. Murray L. Arabi Y.M. Davies A.R. D'Urso P. Kossmann T. Ponsford J. Seppelt I. Reilly P. Wolfe R. Decompressive craniectomy in diffuse traumatic brain injury. N. Engl. J. Med. 2011;364:1493–1502. doi: 10.1056/NEJMoa1102077. [DOI] [PubMed] [Google Scholar]
- D'Hooge R. De Deyn P.P. Applications of the Morris water maze in the study of learning and memory. Brain Res. Brain Res. Rev. 2001;36:60–90. doi: 10.1016/s0165-0173(01)00067-4. [DOI] [PubMed] [Google Scholar]
- de Morais N.C. Barbosa A.M. Vale M.L. Villaverde A.B. de Lima C.J. Cogo J.C. Zamuner S.R. Anti-inflammatory effect of low-level laser and light-emitting diode in zymosan-induced arthritis. Photomed. Laser Surg. 2010;28:227–232. doi: 10.1089/pho.2008.2422. [DOI] [PubMed] [Google Scholar]
- Dikmen S.S. Corrigan J.D. Levin H.S. Machamer J. Stiers W. Weisskopf M.G. Cognitive outcome following traumatic brain injury. J. Head Trauma Rehabil. 2009;24:430–438. doi: 10.1097/HTR.0b013e3181c133e9. [DOI] [PubMed] [Google Scholar]
- Eells J.T. Wong-Riley M.T. VerHoeve J. Henry M. Buchman E.V. Kane M.P. Gould L.J. Das R. Jett M. Hodgson B.D. Margolis D. Whelan H.T. Mitochondrial signal transduction in accelerated wound and retinal healing by near-infrared light therapy. Mitochondrion. 2004;4:559–567. doi: 10.1016/j.mito.2004.07.033. [DOI] [PubMed] [Google Scholar]
- Fujimoto S.T. Longhi L. Saatman K.E. Conte V. Stocchetti N. McIntosh T.K. Motor and cognitive function evaluation following experimental traumatic brain injury. Neurosci. Biobehav. Rev. 2004;28:365–378. doi: 10.1016/j.neubiorev.2004.06.002. [DOI] [PubMed] [Google Scholar]
- Harting M.T. Jimenez F. Adams S.D. Mercer D.W. Cox C.S., Jr. Acute, regional inflammatory response after traumatic brain injury: Implications for cellular therapy. Surgery. 2008;144:803–813. doi: 10.1016/j.surg.2008.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homsi S. Piaggio T. Croci N. Noble F. Plotkine M. Marchand-Leroux C. Jafarian-Tehrani M. Blockade of acute microglial activation by minocycline promotes neuroprotection and reduces locomotor hyperactivity after closed head injury in mice: a twelve-week follow-up study. J. Neurotrauma. 2010;27:911–921. doi: 10.1089/neu.2009.1223. [DOI] [PubMed] [Google Scholar]
- Huang Y.Y. Chen A.C. Carroll J.D. Hamblin M.R. Biphasic dose response in low level light therapy. Dose Response. 2009;7:358–383. doi: 10.2203/dose-response.09-027.Hamblin. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karu T.I. Pyatibrat L.V. Afanasyeva N.I. Cellular effects of low power laser therapy can be mediated by nitric oxide. Lasers Surg. Med. 2005;36:307–314. doi: 10.1002/lsm.20148. [DOI] [PubMed] [Google Scholar]
- Khuman J. Meehan W.P. Zhu X. Qiu J. Hoffmann U. Zhang J. Giovannone E. Lo E.H. Whalen M.J. Tumor necrosis factor and Fas receptor contribute to cognitive deficits independent of cell death after concussive traumatic brain injury in mice. J. Cereb. Blood Flow Metab. 2011;31:778–789. doi: 10.1038/jcbfm.2010.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konstantinovic L.M. Cutovic M.R. Milovanovic A.N. Jovic S.J. Dragin A.S. Letic M. Miler V.M. Low-level laser therapy for acute neck pain with radiculopathy: a double-blind placebo-controlled randomized study. Pain Med. 2010;11:1169–1178. doi: 10.1111/j.1526-4637.2010.00907.x. [DOI] [PubMed] [Google Scholar]
- Lampl Y. Zivin J.A. Fisher M. Lew R. Welin L. Dahlof B. Borenstein P. Andersson B. Perez J. Caparo C. Ilic S. Oron U. Infrared laser therapy for ischemic stroke: a new treatment strategy: results of the NeuroThera Effectiveness and Safety Trial-1 (NEST-1) Stroke. 2007;38:1843–1849. doi: 10.1161/STROKEAHA.106.478230. [DOI] [PubMed] [Google Scholar]
- Langlois J.A. Sattin R.W. Traumatic brain injury in the United States: research and programs of the Centers for Disease Control and Prevention (CDC) J. Head Trauma Rehabil. 2005;20:187–188. doi: 10.1097/00001199-200505000-00001. [DOI] [PubMed] [Google Scholar]
- Lanzafame R.J. Stadler I. Kurtz A.F. Connelly R. Peter T.A., Sr. Brondon P. Olson D. Reciprocity of exposure time and irradiance on energy density during photoradiation on wound healing in a murine pressure ulcer model. Lasers Surg. Med. 2007;39:534–542. doi: 10.1002/lsm.20519. [DOI] [PubMed] [Google Scholar]
- Lapchak P.A. Taking a light approach to treating acute ischemic stroke patients. Transcranial near-infrared laser therapy translational science. Ann. Med. 2010;42:576–586. doi: 10.3109/07853890.2010.532811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapchak P.A. Salgado K.F. Chao C.H. Zivin J.A. Transcranial near-infrared light therapy improves motor function following embolic strokes in rabbits: an extended therapeutic window study using continuous and pulse frequency delivery modes. Neuroscience. 2007;148:907–914. doi: 10.1016/j.neuroscience.2007.07.002. [DOI] [PubMed] [Google Scholar]
- Lubart R. Eichler M. Lavi R. Friedman H. Shainberg A. Low-energy laser irradiation promotes cellular redox activity. Photomed. Laser Surg. 2005;23:3–9. doi: 10.1089/pho.2005.23.3. [DOI] [PubMed] [Google Scholar]
- Lubart R. Low level laser therapy is not low. Photomed. Laser Surg. 2006;24:532. doi: 10.1089/pho.2006.24.532. author reply 532–533. [DOI] [PubMed] [Google Scholar]
- Mannix R.C. Zhang J. Park J. Zhang X. Bilal K. Walker K. Tanzi R.E. Tesco G. Whalen M.J. Age-dependent effect of apolipoprotein E4 on functional outcome after controlled cortical impact in mice. J. Cereb. Blood Flow Metab. 2010;31:351–361. doi: 10.1038/jcbfm.2010.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochizuki-Oda N. Kataoka Y. Cui Y. Yamada H. Heya M. Awazu K. Effects of near-infrared laser irradiation on adenosine triphosphate and adenosine diphosphate contents of rat brain tissue. Neurosci. Lett. 2002;323:207–210. doi: 10.1016/s0304-3940(02)00159-3. [DOI] [PubMed] [Google Scholar]
- Moreira M.S. Velasco I.T. Ferreira L.S. Ariga S.K. Barbeiro D.F. Meneguzzo D.T. Abatepaulo F. Marques M.M. Effect of phototherapy with low intensity laser on local and systemic immunomodulation following focal brain damage in rat. J. Photochem. Photobiol. B. 2009;97:145–151. doi: 10.1016/j.jphotobiol.2009.09.002. [DOI] [PubMed] [Google Scholar]
- Moriyama Y. Moriyama E.H. Blackmore K. Akens M.K. Lilge L. In vivo study of the inflammatory modulating effects of low-level laser therapy on iNOS expression using bioluminescence imaging. Photochem. Photobiol. 2005;81:1351–1355. doi: 10.1562/2005-02-28-RA-450. [DOI] [PubMed] [Google Scholar]
- Morris R.G. Garrud P. Rawlins J.N. O'Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature. 1982;297:681–683. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- Naeser M.A. Saltmarche A. Krengel M.H. Hamblin M.R. Knight J.A. Improved cognitive function after transcranial, light-emitting diode treatments in chronic, traumatic brain injury: Two case reports. Photomed Laser Surg. 2010;29:351–358. doi: 10.1089/pho.2010.2814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oron A. Oron U. Chen J. Eilam A. Zhang C. Sadeh M. Lampl Y. Streeter J. DeTaboada L. Chopp M. Low-level laser therapy applied transcranially to rats after induction of stroke significantly reduces long-term neurological deficits. Stroke. 2006;37:2620–2624. doi: 10.1161/01.STR.0000242775.14642.b8. [DOI] [PubMed] [Google Scholar]
- Oron A. Oron U. Streeter J. de Taboada L. Alexandrovich A. Trembovler V. Shohami E. Low-level laser therapy applied transcranially to mice following traumatic brain injury significantly reduces long-term neurological deficits. J. Neurotrauma. 2007;24:651–656. doi: 10.1089/neu.2006.0198. [DOI] [PubMed] [Google Scholar]
- Pastore D. Greco M. Passarella S. Specific helium-neon laser sensitivity of the purified cytochrome c oxidase. Int. J. Radiat. Biol. 2000;76:863–870. doi: 10.1080/09553000050029020. [DOI] [PubMed] [Google Scholar]
- Rochkind S. Phototherapy in peripheral nerve injury for muscle preservation and nerve regeneration. Photomed. Laser Surg. 2009;27:219–220. doi: 10.1089/pho.2009.9955. [DOI] [PubMed] [Google Scholar]
- Rutland-Brown W. Langlois J.A. Thomas K.E. Xi Y.L. Incidence of traumatic brain injury in the United States, 2003. J. Head Trauma Rehabil. 2006;21:544–548. doi: 10.1097/00001199-200611000-00009. [DOI] [PubMed] [Google Scholar]
- Salmond C.H. Sahakian B.J. Cognitive outcome in traumatic brain injury survivors. Curr. Opin. Crit. Care. 2005;11:111–116. doi: 10.1097/01.ccx.0000155358.31983.37. [DOI] [PubMed] [Google Scholar]
- Salmond C.H. Menon D.K. Chatfield D.A. Williams G.B. Pena A. Sahakian B.J. Pickard J.D. Diffusion tensor imaging in chronic head injury survivors: correlations with learning and memory indices. Neuroimage. 2006;29:117–124. doi: 10.1016/j.neuroimage.2005.07.012. [DOI] [PubMed] [Google Scholar]
- Schreck R. Albermann K. Baeuerle P.A. Nuclear factor kappa B: an oxidative stress-responsive transcription factor of eukaryotic cells (a review) Free Radic. Res. Commun. 1992;17:221–237. doi: 10.3109/10715769209079515. [DOI] [PubMed] [Google Scholar]
- Selassie A.W. Zaloshnja E. Langlois J.A. Miller T. Jones P. Steiner C. Incidence of long-term disability following traumatic brain injury hospitalization, United States, 2003. J. Head Trauma Rehabil. 2008;23:123–131. doi: 10.1097/01.HTR.0000314531.30401.39. [DOI] [PubMed] [Google Scholar]
- Shein N.A. Grigoriadis N. Horowitz M. Umschwief G. Alexandrovich A.G. Simeonidou C. Grigoriadis S. Touloumi O. Shohami E. Microglial involvement in neuroprotection following experimental traumatic brain injury in heat-acclimated mice. Brain Res. 2008;1244:132–141. doi: 10.1016/j.brainres.2008.09.032. [DOI] [PubMed] [Google Scholar]
- Simunovic-Soskic M. Pezelj-Ribarić S. Brumini G. Glažar I. Gržić R. Miletić I. Salivary levels of TNF-α and IL-6 in patients with denture stomatitis before and after laser phototherapy. Photomed. Laser Surg. 2010;28:189–193. doi: 10.1089/pho.2008.2420. [DOI] [PubMed] [Google Scholar]
- Sommer A.P. Pinheiro A.L. Mester A.R. Franke R.P. Whelan H.T. Biostimulatory windows in low-intensity laser activation: lasers, scanners, and NASA's light-emitting diode array system. J. Clin. Laser Med. Surg. 2001;19:29–33. doi: 10.1089/104454701750066910. [DOI] [PubMed] [Google Scholar]
- Streifler J.Y. Tanne D. Gross B. Lampl Y. Bornstein N.M. [Guidelines for the management of stroke—2007 prevention of ischemic stroke—general approach and medical treatment] Harefuah. 2007;146:373–379. 405. [PubMed] [Google Scholar]
- Tafur J. Mills P.J. Low-intensity light therapy: exploring the role of redox mechanisms. Photomed. Laser Surg. 2008;26:323–328. doi: 10.1089/pho.2007.2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tafur J. Van Wijk E.P. Van Wijk R. Mills P.J. Biophoton detection and low-intensity light therapy: a potential clinical partnership. Photomed. Laser Surg. 2010;28:23–30. doi: 10.1089/pho.2008.2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong S.F. Wilder-Smith P. Pilot study of laser effects on oral mucositis in patients receiving chemotherapy. Cancer J. 2002;8:247–254. doi: 10.1097/00130404-200205000-00008. [DOI] [PubMed] [Google Scholar]
- Yager P.H. You Z. Qin T. Kim H.H. Takahashi K. Ezekowitz A.B. Stahl G.L. Carroll M.C. Whalen M.J. Mannose binding lectin gene deficiency increases susceptibility to traumatic brain injury in mice. J. Cereb. Blood Flow Metab. 2008;28:1030–1039. doi: 10.1038/sj.jcbfm.9600605. [DOI] [PubMed] [Google Scholar]
- Yang J. You Z. Kim H.H. Hwang S.K. Khuman J. Guo S. Lo E.H. Whalen M.J. Genetic analysis of the role of tumor necrosis factor receptors in functional outcome after traumatic brain injury in mice. J. Neurotrauma. 2010;27:1037–1046. doi: 10.1089/neu.2009.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yau J.L. Noble J. Hibberd C. Rowe W.B. Meaney M.J. Morris R.G. Seckl J.R. Chronic treatment with the antidepressant amitriptyline prevents impairments in water maze learning in aging rats. J. Neurosci. 2002;22:1436–1442. doi: 10.1523/JNEUROSCI.22-04-01436.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You Z. Savitz S.I. Yang J. Degterev A. Yuan J. Cuny G.D. Moskowitz M.A. Whalen M.J. Necrostatin-1 reduces histopathology and improves functional outcome after controlled cortical impact in mice. J. Cereb. Blood Flow Metab. 2008;28:1564–1573. doi: 10.1038/jcbfm.2008.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaloshnja E. Miller T. Langlois J.A. Selassie A.W. Prevalence of long-term disability from traumatic brain injury in the civilian population of the United States, 2005. J. Head Trauma Rehabil. 2008;23:394–400. doi: 10.1097/01.HTR.0000341435.52004.ac. [DOI] [PubMed] [Google Scholar]
- Ziebell J.M. Morganti-Kossmann M.C. Involvement of pro- and anti-inflammatory cytokines and chemokines in the pathophysiology of traumatic brain injury. Neurotherapeutics. 2010;7:22–30. doi: 10.1016/j.nurt.2009.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zivin J.A. Albers G.W. Bornstein N. Chippendale T. Dahlof B. Devlin T. Fisher M. Hacke W. Holt W. Ilic S. Kasner S. Lew R. Nash M. Perez J. Rymer M. Schellinger P. Schneider D. Schwab S. Veltkamp R. Walker M. Streeter J. Effectiveness and safety of transcranial laser therapy for acute ischemic stroke. Stroke. 2009;40:1359–1364. doi: 10.1161/STROKEAHA.109.547547. [DOI] [PubMed] [Google Scholar]