Abstract
Traumatic brain injury (TBI), a leading cause of death and disability in the United States, causes potentially preventable damage in part through the dysregulation of neural calcium levels. Calcium dysregulation could affect the activity of the calcium-sensitive phosphatase calcineurin (CaN), with serious implications for neural function. The present study used both an in vitro enzymatic assay and Western blot analyses to characterize the effects of lateral fluid percussion injury on CaN activity and CaN-dependent signaling in the rat forebrain. TBI resulted in an acute alteration of CaN phosphatase activity and long-lasting alterations of its downstream effector, cofilin, an actin-depolymerizing protein. These changes occurred bilaterally in the neocortex and hippocampus, appeared to persist for hours after injury, and coincided with synapse degeneration, as suggested by a loss of the excitatory post-synaptic protein PSD-95. Interestingly, the effect of TBI on cofilin in some brain regions was blocked by a single bolus of the CaN inhibitor FK506, given 1 h post-TBI. Overall, these findings suggest a loss of synapse stability in both hemispheres of the laterally-injured brain, and offer evidence for region-specific, CaN-dependent mechanisms.
Key words: calcineurin, cofilin, excitatory post-synaptic protein PSD-95, lateral fluid percussion injury, spine-associated Rap guanosine triphosphatase activating protein
Introduction
A traumatic brain injury (TBI) occurs every 19 sec in the United States (Faul et al., 2010) and is a leading cause of death and disability. TBI typically involves both an immediate, mechanical destruction of tissue, as well as prolonged reactive processes, such as excitotoxicity, which develop over a period of hours to days. This secondary injury can affect cellular function well beyond the site of impact, and so may be responsible for many of the long-lasting neurological deficits associated with TBI (Ghajar, 2000; Siesjo and Siesjo, 1996). However, the secondary injury may be treatable or even preventable, given the apparent window for therapeutic intervention.
Calcium-regulated systems have been implicated in the spread of neuronal damage following ischemia and TBI (Deshpande et al., 2008; Sun et al., 2008; Tymianski and Tator, 1996). One such system involves the calcium-regulated phosphatase calcineurin (CaN; Kurz et al., 2005a,2005b; Pallen and Wang, 1985). CaN mediates many cellular responses to brain injury, including the expression of cytokines (Fernandez et al., 2007) and neuronal nitric oxide synthase (Dawson et al., 1993), and glial apoptosis (Szydlowska et al., 2006) and activation (Norris et al., 2005). CaN also regulates synaptic plasticity under normal conditions (Groth et al., 2003; Park et al., 2006) and in various pathological states, including prolonged seizure (Kurz et al., 2008), amyloid beta exposure (Wu et al., 2010), and TBI (Campbell et al., 2011). Given its regulation by calcium and its role in cellular responses to injury, CaN and its signaling pathways may contribute to the pathology of TBI.
Previous research by our laboratory found both an increase in CaN activity and its re-distribution to synapses in the rat forebrain after fluid percussion TBI (Kurz et al., 2005a,2005b). More recently, we found that these changes coincide with a CaN-dependent loss of dendritic spines in the rat neocortex and hippocampus (Campbell et al., 2011). It is unclear how CaN is involved in this spine loss, though several signaling pathways that connect CaN activity to spine loss have been characterized in vitro (Campbell et al., 2009). One pathway involves remodeling the spine's actin-rich cytoskeleton by the actin-binding protein cofilin (Kurz et al., 2008; Shankar et al., 2007; Zhou et al., 2004). Another pathway involves the targeted proteolysis of the spine-associated Rap guanosine triphosphatase activating protein (SPAR; Pak and Sheng, 2003; Wu et al., 2007). While these pathways have been demonstrated in other injury models, it is unclear whether they can explain the spine loss we observed in the TBI model (Campbell et al., 2011). Therefore, the present study examined the activity and temporal profile of these CaN-dependent pathways in the fluid percussion model of TBI. We report significant changes in CaN activity and in its downstream substrate, cofilin. Along with our companion article (Campbell et al., 2011), these experiments characterize cellular mechanisms through which TBI can cause dendritic spine loss.
Methods
All animal use procedures were performed in strict accordance with the Guide for the Care and Use of Laboratory Animals described by the National Institutes of Health, and was approved by the Virginia Commonwealth University Institutional Animal Care and Use Committee. Animal subjects received ad libitum access to food and water and were maintained on a 12-h light/dark cycle throughout the experiment. A total of 105 adult male Sprague-Dawley rats (90 days old; 350–400 g) were used in this study. These rats were randomly distributed among the following treatment groups: controls (naïve, age-matched; n=20), FK-506-only (n=4), TBI-only (n=76), and TBI+FK-506 (n=5).
Fluid percussion injury
The TBI and TBI+FK-506 groups were surgically prepared for TBI as follows. First, the rats were anesthetized with sodium pentobarbital (54 mg/kg IP) or isoflurane (3% isoflurane in a carrier gas mixture of 30% N2O and 70% O2) and placed in a stereotactic frame. The scalp was then incised and a 4.8-mm hole was made over the left hemisphere using a manual trephine centered 4 mm caudal to the bregma and −3 mm lateral to the sagittal suture. An anchor screw was inserted into the skull overlying the opposite hemisphere. A modified female Luer-Lok syringe hub (2.6 mm inside diameter) was placed over the exposed dura, affixed with cyanoacrylate adhesive, and then secured with dental acrylic. The hub was filled with sterile saline and the scalp was sutured closed. On the following day, the subjects were anesthetized with isoflurane (4 min of 4% isoflurane in a carrier mixture of 30% N2O and 70% O2), and subjected to fluid percussion of the intact dura over the left parietal cortex. The fluid percussion device used in these experiments is identical to that described by Dixon and colleagues (Dixon et al., 1987). Immediately after fluid percussion injury, the Luer-Lok fitting, screw, and dental cement were removed from the skull and the scalp was sutured closed. The subjects were then placed in a supine position, and the time at which they righted themselves was recorded. Upon recovery of righting reflexes, the subjects were returned to their home cages and monitored daily. At 1 h post-TBI, some rats (n=5; TBI+FK-506) received a single injection of FK-506 (5 mg/kg IP; Astellas Pharma Inc., Tokyo, Japan). As a drug control group, additional age-matched Sprague-Dawley rats (FK-506 only; n=4) were administered FK-506 (5 mg/kg IP), and then sacrificed 23 h after drug administration.
Isolation and homogenization of brain regions
The rats were briefly anesthetized with isoflurane and quickly decapitated at the following time points after TBI: 1 h (n=7); 6 h (n=4); 12 h (n=5); 18 h (n=13); 24 h (n=18); 24 h+FK-506 (n=5); 48 h (n=4); 1 week (n=5); 2 weeks (n=6); and 4 weeks (n=5). Age-matched control rats and FK-506-only rats were sacrificed along with TBI rats in the same manner. The brains were rapidly dissected on an iced Petri dish to reduce post-mortem enzyme activity. Ipsilateral and contralateral hemispheres of neocortex and hippocampus were dissected whole and separately homogenized with 10 strokes (up and down) at 12,000 rpm using a motorized homogenizer (Tri-R Instruments, Inc., Rockville Center, NY). Brain regions were homogenized in ice-cold homogenization buffer containing 7 mM EGTA, 5 mM EDTA, 1 mM dithiothreitol, 0.3 mM phenylmethylsulfonylfluoride, and 300 mM sucrose. Neocortical hemispheres and hippocampal hemispheres were homogenized in 4 mL and 2 mL of buffer, respectively. Homogenates were aliquotted and stored at −80°C until use. Homogenates from the same animals were used for multiple experiments whenever possible. To control for storage artifact in the Western analyses of phosphorylated cofilin, samples from post-TBI animals were normalized to equivalently-stored control samples.
pNPP assay of CaN activity
CaN activity was measured using the procedure of Pallen and Wang (1985), as optimized by Kurz and associates (Kurz et al., 2001,2005b). Briefly, all reaction tubes were prepared on ice and contained the following: 25 mM MOPS (pH 7.0), 1 mM DTT, 2 mM p-nitrophenyl phosphate (pNPP; Sigma-Aldrich, St. Louis, MO). Tubes used to measure basal CaN activity also contained 2 mM EGTA and 2 mM EDTA. Tubes used to measure maximal CaN activity contained the same reagents as for basal reactions, with the addition of 2 mM MnCl2. Manganese activates CaN much more strongly than calcium in the pNPP assay, and is therefore used to reveal changes in maximal CaN activity (Kurz et al., 2005b). Final reaction volume was 1 mL. Prior to use, the protein concentrations of all homogenate samples were determined using the method of Bradford (Bradford, 1976). Reactions were initiated by the addition of 50 μg/mL brain homogenate. The reactions were incubated at 37°C for 30 min in a shaking water bath. The tubes were then removed from the water bath and placed in ice to stop the reaction. Absorbance of the reaction mixture was immediately measured at 405 nm in a spectrophotometer (UV-2101; Shimadzu Scientific Instruments, Inc., Columbia, MD). Absorbance units were converted to nmol of pNP produced by comparison to a pNP concentration standard absorption curve.
Western blot analysis
Brain samples were normalized using the Bradford method, resolved by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE; Mini-Protean II system; Bio-Rad, Hercules, CA), and transferred to nitrocellulose membranes using the Trans-blot system (Bio-Rad). Transfer quality was confirmed by staining the blots with a reversible protein stain, Ponceau S (Sigma-Aldrich), according to the manufacturer's instructions. After de-staining, the blots were twice immersed for 15 min in blocking solution containing phosphate-buffered saline (PBS, pH 7.4), 0.05% (v/v) polyoxyethylene sorbitan monolaurate (Tween 20), and 2.5% blotting grade dry milk (Bio-Rad). The blots were then incubated overnight with the appropriate antibody in blocking solution at 4°C. Antibodies were used at the following dilutions: rabbit anti-cofilin (1:500, catalog no. 3842; Chemicon International, Temecula, CA); rabbit anti-Ser3 phosphorylated cofilin (1:500, catalog no. 3831; Chemicon International); rabbit anti-PSD-95 (1:1000, catalog no. 51-9600; Invitrogen, San Diego, CA); or goat anti-E6TP1 (SPAR, 1:1000, catalog no. sc-20846; Santa Cruz Biotechnology, Santa Cruz, CA). The membranes were then washed in 0.05% Tween 20-PBS three times for 10 min each. Next, the nitrocellulose was incubated for 45 min in the appropriate horseradish peroxidase-conjugated secondary antibody (Thermo Scientific, Waltham, MA), diluted 1:4000 in blocking solution. The blots were then washed three times in PBS for 10 min each. Immunoreactive bands were revealed by chemiluminescence (Pierce Femto or Pico Luminol Substrate; Pierce, Rockford, IL) and exposed to x-ray film (Kodak X-OMAT), which were then developed with a Kodak X-OMAT developer. The mean optical densities of immunoreactive bands were measured by computer-assisted densitometry (GS-800 Calibrated Densitometer; Bio-Rad), and compared to a linear concentration curve as previously described (Churn et al., 1992). In the SPAR and PSD-95 experiments, data were normalized to whole-lane optical densities obtained from the same Ponceau S-stained blots (Romero-Calvo et al., 2010).
Statistical analysis
Group means were statistically analyzed with GraphPad Prism 4.0 (Graph-Pad Software, San Diego, CA). All comparisons were made by one-way analysis of variance (ANOVA). Post-hoc analyses were conducted with either Tukey's test (only data related to Fig. 7) or Dunnett's test (all other data). Groups were considered significantly different if p<0.05. All data are presented as group mean±standard deviation. Sample sizes (n values) are shown on the figures.
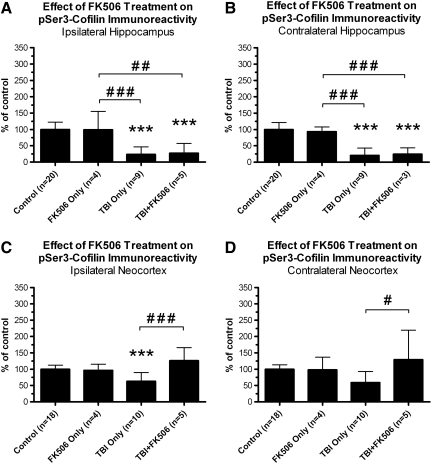
FIG. 7.
A 1-h post-traumatic brain injury (TBI) administration of FK-506 prevented the 24 h post-TBI loss of pSer3-cofilin immunoreactivity in the neocortex but not in the hippocampus. Adult rats received either lateral fluid percussion TBI alone (TBI only,), TBI followed by a 1-h post-TBI injection of FK-506 (5 mg/kg IP; TBI+FK-506), FK-506 injection but no TBI (FK-506 only), or no treatment (control). All groups were sacrificed at 24 h post-TBI (or 23 h post-injection), along with equivalently-aged control rats. The ipsilateral and contralateral hemispheres of the hippocampus and neocortex were dissected, separately homogenized, and subjected to immunoblotting with antibodies recognizing the serine 3-phosphorylated cofilin (pSer3-cofilin). Statistical analysis identified region-specific effects of FK-506 treatment on pSer3-cofilin phosphorylation. For example, FK-506 treatment did not prevent the loss of pSer3-cofilin at 24 h post-TBI, in either the ipsilateral hippocampus (A) or contralateral hippocampus (B). In the neocortex, however, FK-506 treatment completely blocked the loss of pSer3-cofilin at 24 h post-TBI in both the ipsilateral (C) and contralateral (D) hemispheres. All comparisons were made by one-way analysis of variance with Tukey's post-hoc test (***p<0.001 versus controls; #p<0.05 versus the other bracketed group; ##p<0.01 versus the other bracketed group; ###p<0.001 versus the other bracketed group).
Results
Lateral fluid percussion injury (LFPI) TBI
Adult male rats received a TBI by fluid percussion of the dura overlying the left parietal cortex. The mean fluid percussion pressure was 2.22±0.11 atm and did not differ significantly across groups [F(9,64)=1.111, p=0.3678]. A common correlate of injury severity is the latency between injury and the recovery of the righting reflex (righting time; Thompson et al., 2005). In the present study, the mean righting time was 7.00±3.19 min. This mean righting time corresponds to an injury of moderate severity (Hallam et al., 2004). Righting time did not differ significantly across groups [F(9,63)=0.9287, p=0.5068]. Some subjects (n=3) exhibited apnea immediately following injury and were mechanically ventilated until spontaneous respirations resumed (mean duration 25±5 sec). The acute mortality rate associated with LFPI in this study was approximately 3% (2 of 76 rats died within 7 days of LFPI and so were excluded from all data analyses).
Lateral TBI enhanced the enzymatic activity of calcineurin
To determine the effect of lateral TBI on CaN activity, the ipsilateral and contralateral hemispheres of the neocortex and hippocampus were dissected, separately homogenized, and subjected to a well-characterized pNPP assay (Kurz et al., 2001,2003,2005b,2008; see methods section). Using this approach, CaN activity was measured either in the absence of stimulating cations (basal CaN activity), or in the presence of stimulating cations (maximal CaN activity).
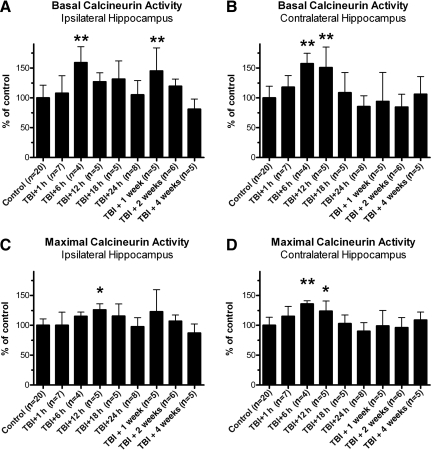
Lateral TBI had a significant effect on basal CaN activity in the ipsilateral hippocampus, compared to analogous tissue from control animals [F(8,56)=5.617, p<0.0001; Fig. 1A]. Post-hoc analysis indicates that this effect was delayed, as basal CaN activity at 1 h post-TBI did not differ significantly from controls (p>0.05). By 6 h post-TBI, however, basal CaN activity in this region had increased significantly over control levels (p<0.01). The increase was transient, as basal CaN activity did not differ significantly from controls at 12 h, 18 h, or 24 h post-TBI (p>0.05 for each time point). Basal CaN activity increased again at 1 week post-TBI relative to controls (p<0.01). This increase also appeared to be transient, as basal CaN activity at 2 weeks and 4 weeks post-TBI did not differ significantly from controls (p>0.05 for each time point).
FIG. 1.
Lateral traumatic brain injury (TBI) enhanced calcineurin phosphatase activity in the hippocampus. Hippocampal tissue was isolated from adult rats at specific time points after lateral fluid percussion injury, homogenized, and subjected to an in vitro assay of calcineurin (CaN) enzymatic activity. CaN activity was measured either in the absence of stimulating cations (basal CaN activity), or in the presence of stimulating cations (maximal CaN activity). In the ipsilateral hippocampus, these experiments revealed increases in basal CaN activity at 6 h and 1 week post-TBI, relative to controls (A). Similarly, in the contralateral hippocampus, increases in basal CaN activity were detected at 6 h and 12 h post-TBI, relative to controls (B). Lateral TBI also affected the maximal activity of CaN, causing increases at 12 h post-TBI in the ipsilateral hippocampus (C), and at 6 h and 12 h post-TBI in the contralateral hippocampus (D), compared to controls. All comparisons were made by one-way analysis of variance with Dunnett's post hoc test (*p<0.05, **p<0.01).
The contralateral hippocampus also showed a significant change in basal CaN activity after lateral TBI, compared to controls [F(8,56)=5.524, p<0.0001; Fig. 1B]. Like the ipsilateral hippocampus, basal CaN activity in the contralateral hippocampus was unchanged at 1 h post-TBI (p>0.05), but increased significantly by 6 h post-TBI, relative to controls (p<0.01). Basal CaN activity at 12 h post-TBI was also significantly elevated over control levels (p<0.01). This increase was transient, as basal CaN activity returned to control levels by 18 h post-TBI and remained near control levels at the subsequent time points (p>0.05 for each time point). Overall, lateral TBI caused delayed, transient increases in basal CaN activity in the ipsilateral hippocampus and in the contralateral hippocampus.
The maximal enzymatic activity of CaN was also altered by lateral TBI. In the ipsilateral hippocampus, for example, TBI had a significant effect on maximal CaN activity, relative to controls [F(8,56)=3.283, p=0.0039; Fig. 1C]. The effect was delayed though, as maximal CaN activity at 1 h and 6 h post-TBI did not differ significantly from controls (p>0.05, each time point). By 12 h post-TBI, however, ipsilateral hippocampus showed a significant increase in maximal CaN activity over control levels (p<0.05). This increase did not persist, as maximal CaN activity was not significantly altered at 18 h post-TBI, nor at the later time points, relative to controls (p>0.05, each time point).
The contralateral hippocampus also showed significant changes in maximal CaN activity after lateral TBI, compared to controls [F(8,56)=4.791, p=0.0002; Fig. 1D]. As with basal CaN activity, maximal CaN activity was unchanged at 1 h post-TBI (p>0.05) but increased significantly at 6 h and 12 h post-TBI, relative to controls (p<0.01 and p<0.05, respectively). The increase did not persist though, as maximal CaN activity had returned to control levels by 18 h post-TBI and remained near control levels at the subsequent time points (p>0.05, each time point). Thus, lateral TBI caused delayed but transient increases in maximal CaN activity in the ipsilateral hippocampus and in the contralateral hippocampus.
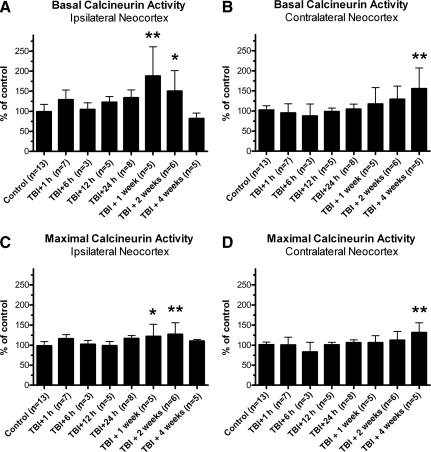
Lateral TBI altered CaN phosphatase activity in the neocortex as well. In the ipsilateral neocortex, for example, lateral TBI had a significant effect on basal CaN activity and maximal CaN activity relative to controls [basal CaN activity, F(7,43)=5.519, p=0.0001, Fig. 2A; maximal CaN activity, F(7,43)=3.264, p=0.0072, Fig. 2C]. The effect was delayed though, as neither basal nor maximal CaN activity differed significantly from controls at the acute time points of 1 h, 6 h, 12 h, and 24 h post-TBI (p>0.05 for each time point). By 1 week post-TBI, however, the ipsilateral neocortex showed significant increases in both basal CaN activity and maximal CaN activity, compared to control levels (basal CaN activity, p<0.01, Fig. 2A; maximal CaN activity, p<0.05, Fig. 2C). Basal and maximal CaN activity were also increased above control levels at 2 weeks post-TBI (basal CaN activity, p<0.05, Fig. 2A; maximal CaN activity, p<0.01, Fig. 2C), but returned to control levels by 4 weeks post-TBI (p>0.05; Fig. 2A and C).
FIG. 2.
Lateral traumatic brain injury (TBI) enhanced calcineurin phosphatase activity in the neocortex. Neocortical tissue was isolated from adult rats at specific time points after lateral fluid percussion injury, homogenized, and subjected to an in vitro assay of calcineurin (CaN) enzymatic activity. CaN activity was measured either in the absence (basal CaN activity) or presence (maximal CaN activity) of stimulating cations. In the ipsilateral neocortex, basal CaN activity increased significantly above control levels by 1 week and 2 weeks post-TBI (A). The contralateral neocortex also showed an increase in basal CaN activity, but weeks later, at 4 weeks post-TBI, compared to controls (B). The maximal activity of CaN also changed after lateral TBI, increasing significantly over control levels at 1 week and 2 weeks post-TBI in the ipsilateral neocortex (C), and at 4 weeks post-TBI in the contralateral neocortex (D). All comparisons were made by one-way analysis of variance with Dunnett's post-hoc test (*p<0.05, **p<0.01).
The contralateral neocortex also underwent significant changes in basal and maximal CaN activity after lateral TBI, compared to control tissue [basal CaN activity, F(7,43)=3.483, p=0.0048, Fig. 2B; maximal CaN activity, F(7,43)=3.088, p=0.0100, Fig. 2D]. These too were delayed changes, as neither basal nor maximal CaN activity differed significantly from controls at 1 h, 6 h, 12 h, 24 h, 1 week, or 2 weeks post-TBI (p>0.05 for each time point; Fig. 2B and D). By 4 weeks post-TBI, both basal and maximal CaN activity had increased significantly above control levels (p<0.01; Fig. 2B and D). The data thus demonstrate delayed but transient increases in CaN activity in the ipsilateral neocortex, followed weeks later by an increase in CaN activity in the contralateral neocortex.
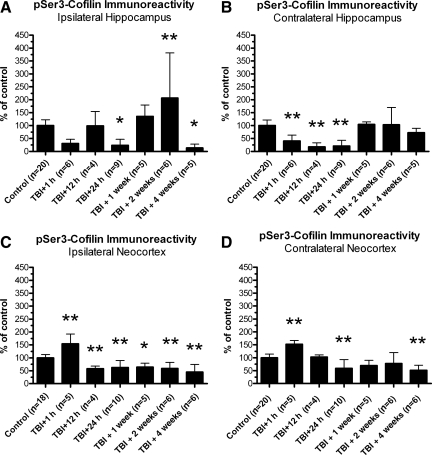
Lateral TBI caused time- and region-dependent changes in pSer3-cofilin immunoreactivity
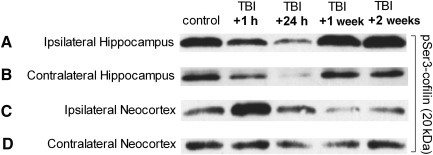
To further characterize the TBI-induced changes in CaN activity, we examined the effect of TBI on cofilin, an endogenous protein that is activated downstream of CaN (Meberg et al., 1998; Wang et al., 2005). Cofilin is an actin-binding and -depolymerizing protein critically involved in both functional and structural remodeling of excitatory synapses (Calvo et al., 2010; Morishita et al., 2005; Romero- Zhou et al., 2004). CaN activation leads to de-phosphorylation of a key serine 3 (Ser3) residue on cofilin, resulting in an increase in cofilin activity (Meberg et al., 1998; Wang et al., 2005). To test whether lateral TBI affects cofilin Ser3 phosphorylation, ipsilateral and contralateral hemispheres of the neocortex and hippocampus were dissected after LFPI, separately homogenized, and subjected to Western blot analysis with a phospho-specific antibody recognizing the inactivated, serine 3-phosphorylated cofilin (pSer3-cofilin). This analysis revealed apparent, time-dependent changes in pSer3-cofilin immunoreactivity in hippocampal and neocortical structures (Fig. 3). In both the ipsilateral and contralateral hippocampi, for example, pSer3-cofilin immunoreactivity appeared to decrease acutely within 1 h of TBI, and remain decreased at 24 h post-TBI (Fig. 3A and B). However, in animals allowed to recover for 1 week after injury, pSer3-cofilin immunoreactivity appeared to return to control levels. A different trend was observed in the neocortex, where pSer3-cofilin immunoreactivity appeared to increase or remain unchanged at 1 h post-TBI, but then decreased at the subsequent time points (Fig. 3C and D).
FIG. 3.
Lateral traumatic brain injury (TBI) caused apparent changes in serine 3-phosphorylated cofilin (pSer3-cofilin) immunoreactivity. Western blot analysis was performed on crude homogenates of whole ipsilateral and contralateral hemispheres of hippocampus and neocortex dissected from adult rats 1 h, 24 h, 1 week, or 2 weeks after lateral TBI, and from age-matched control rats. The immunoreactivity of pSer3-cofilin was detected with a phospho-specific antibody. Results suggest that the hippocampus and neocortex underwent time- and region-dependent changes in pSer3-immunoreactivity after lateral TBI. In both the ipsilateral and contralateral hippocampi, for example, pSer3-cofilin immunoreactivity appeared to decrease below control levels by 1 h post-TBI, and remained decreased at 24 h post-TBI (A and B). However, by 1 week post-TBI, pSer3-cofilin immunoreactivity appeared to have increased to control levels or beyond. A different trend was observed in the neocortex, where pSer3-cofilin immunoreactivity seemed to initially increase (C), or remain unchanged (D) at 1 h post-TBI, but then decreased at later time points (C and D).
To further investigate the apparent changes in pSer3-cofilin immunoreactivity, additional Western blots were performed for quantitative analysis (Fig. 4). These experiments confirmed that lateral TBI caused time- and region-dependent changes in pSer3-cofilin immunoreactivity. For example, lateral TBI significantly altered pSer3-cofilin immunoreactivity in the ipsilateral hippocampus, compared to analogous tissue from controls [F(6,48)=7.953, p<0.0001; Fig. 4A]. At 1 h post-TBI in the ipsilateral hippocampus, pSer3-cofilin immunoreactivity appeared to decrease relative to control levels, though post-hoc analysis indicated that this decrease was not statistically significant (1 h post-TBI versus controls, p>0.05). Nor did pSer3-cofilin immunoreactivity at 12 h post-TBI differ significantly from controls (p>0.05). However, a significant decrease in pSer3-cofilin immunoreactivity was observed at 24 h post-TBI, relative to controls (p<0.05). This decrease did not persist, as pSer3-cofilin immunoreactivity had returned to control levels by 1 week post-TBI (p>0.05), and was elevated above control levels at 2 weeks post-TBI (p<0.01). Surprisingly, at 4 weeks post-TBI, pSer3-cofilin immunoreactivity again fell below control levels (p<0.05).
FIG. 4.
Lateral traumatic brain injury (TBI) caused time- and region-dependent changes in serine 3-phosphorylated cofilin (pSer3-cofilin) immunoreactivity. The ipsilateral and contralateral hemispheres of the hippocampus and neocortex were dissected from post-TBI rats and their age-matched controls, separately homogenized, and subjected to immunoblotting with antibodies recognizing pSer3-cofilin. The results indicated significant changes in pSer3-cofilin immunoreactivity following lateral TBI, relative to controls. In the ipsilateral hippocampus, for example, pSer3-cofilin decreased significantly at 24 h post-TBI, increased at 2 weeks post-TBI, but then decreased again at 4 weeks post-TBI, relative to controls (A). The contralateral hippocampus also underwent an acute decrease in pSer3-cofilin immunoreactivity at 1 h, 12 h, and 24 h post-TBI, compared to controls (B). The neocortex showed a different pattern of changes in pSer3-cofilin immunoreactivity, with an increase at 1 h post-TBI, followed by decreases at subsequent time points, in both the ipsilateral neocortex (C), and contralateral neocortex (D), relative to controls. Specifically, pSer3-cofilin immunoreactivity decreased below control levels at 12 h post-TBI and all later time points in the ipsilateral neocortex (C), and at 24 h and 4 weeks post-TBI in the contralateral neocortex (D). All comparisons were made by one-way analysis of variance with Dunnett's post-hoc test (*p<0.05, **p<0.01).
The contralateral hippocampus also underwent significant changes in pSer3-cofilin immunoreactivity after lateral TBI, relative to controls [F(6,48)=13.60, p<0.0001; Fig. 4B]. Like the ipsilateral hippocampus, the contralateral hippocampus showed an acute, TBI-induced decrease in pSer3-cofilin immunoreactivity. Specifically, at 1 h, 12 h, and 24 h post-TBI in the contralateral hippocampus, pSer3-cofilin immunoreactivity was significantly below control levels (p<0.01 for each time point). As observed in the ipsilateral hemisphere, pSer3-cofilin immunoreactivity returned to control levels by 1 week post-TBI, and did not change significantly thereafter (1 week, 2 weeks, or 4 weeks post-TBI, p>0.05). Overall, the data demonstrate an acute decrease in pSer3-cofilin immunoreactivity in both hemispheres of the hippocampus.
Lateral TBI also caused significant changes in pSer3-cofilin immunoreactivity in the neocortex, though these changes differed from those observed in the hippocampus. In the ipsilateral neocortex, TBI had a significant effect on pSer3-cofilin immunoreactivity, compared to controls [F(6,47)=17.27, p<0.0001; Fig. 4C]. However, in contrast to what was observed in the hippocampal fractions, lateral TBI caused an initial increase in pSer3-cofilin immunoreactivity in the ipsilateral neocortex. Post-hoc analysis shows that at 1 h post-TBI, pSer3-cofilin immunoreactivity in the ipsilateral neocortex was significantly increased above control levels (p<0.01). This initial increase was followed by a decrease, as pSer3-cofilin fell substantially below control levels by 12 h post-TBI (p<0.01). pSer3-cofilin immunoreactivity was significantly decreased at all subsequent time points as well, relative to controls (24 h post-TBI, p<0.01; 1 week post-TBI, p<0.05; 2 weeks post-TBI, p<0.01; 4 weeks post-TBI, p<0.01).
The contralateral neocortex also showed changes in pSer3-cofilin immunoreactivity after lateral TBI [F(6,47)=12.61, p<0.0001; Fig. 4D], changes which were similar to those observed in the ipsilateral neocortex. As in the ipsilateral neocortex, pSer3-cofilin immunoreactivity in the contralateral neocortex increased over control levels by 1 h post-TBI (p<0.01). However, pSer3-cofilin immunoreactivity returned to control levels by 12 h post-TBI (p>0.05). The downward trend continued at 24 h post-TBI, when pSer3-cofilin immunoreactivity decreased substantially below control levels (p<0.01). The pSer3-cofilin immunoreactivity at 1 week and 2 weeks post-TBI appeared reduced compared to controls, but not to a statistically significant extent (p>0.05 for each time point). At 4 weeks post-TBI though, pSer3-cofilin immunoreactivity was significantly below control levels (p<0.01), similar to what was observed on the ipsilateral side. In general, lateral TBI caused acute but transient increases in pSer3-cofilin immunoreactivity, followed by delayed but persistent decreases, bilaterally in the neocortex.
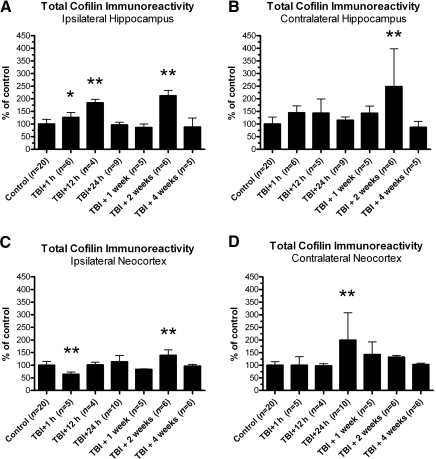
Lateral TBI generally caused transient increases in total cofilin protein
To determine whether TBI-induced changes in pSer3-cofilin immunoreactivity were due to quantitative changes in cofilin protein, Western blot analyses were repeated with an antibody recognizing total cofilin protein, regardless of Ser3 phosphorylation. This antibody was generated using the same peptide sequence as the pSer3-specific antibody, but without the phosphate at the Ser3 residue. These analyses revealed significant changes in total cofilin immunoreactivity, though nearly all of these changes were increases.
Lateral TBI significantly altered total cofilin immunoreactivity in the ipsilateral hippocampus relative to analogous tissue from control animals [F(6,48)=40.10, p<0.0001; Fig. 5A]. Post hoc analysis indicated that total cofilin immunoreactivity at 1 h and 12 h post-TBI in the ipsilateral hippocampus was significantly above control levels (p<0.05 and p<0.01, respectively). The increase was transient, as total cofilin immunoreactivity at 24 h and 1 week post-TBI did not differ significantly from controls (p>0.05 for each time point). Total cofilin immunoreactivity increased above control levels again at 2 weeks post-TBI (p<0.01), but returned to control levels by 4 weeks post-TBI (p>0.05).
FIG. 5.
Lateral traumatic brain injury (TBI) generally caused transient increases in total cofilin immunoreactivity. Western blot analyses were repeated using an antibody recognizing cofilin regardless of serine 3 (Ser3) phosphorylation status (total cofilin). Analyses revealed time- and region-dependent changes in total cofilin immunoreactivity, relative to controls. In the ipsilateral hippocampus, for example, total cofilin immunoreactivity was increased at 1 h, 12 h, and 2 weeks post-TBI, relative to controls (A). In the contralateral hippocampus, total cofilin immunoreactivity also increased above control levels, but only weeks later, at 2 weeks post-TBI (B). Unlike all other brain regions tested, the ipsilateral neocortex showed a decrease in total cofilin immunoreactivity at 1 h post-TBI, relative to controls. However, total cofilin immunoreactivity returned to control levels by 12 h post-TBI, and then increased above control levels weeks later, at 2 weeks post-TBI (C). The contralateral neocortex underwent a delayed increase in total cofilin immunoreactivity at 24 h post-TBI, relative to controls (D). All comparisons were made by one-way analysis of variance with Dunnett's post-hoc test (*p<0.05, **p<0.01).
The contralateral hippocampus also underwent significant changes in total cofilin immunoreactivity after lateral TBI, relative to controls [F(6,49)=6.342, p<0.0001; Fig. 5B]. In contrast to the ipsilateral hippocampus, acute changes in total cofilin immunoreactivity were not observed in the contralateral hippocampus, as total cofilin immunoreactivity did not differ significantly from controls at 1 h or 12 h post-TBI (p>0.05 for each time point). Nor did total cofilin immunoreactivity change significantly at 24 h or 1 week post-TBI, relative to controls (p>0.05 for each time point). As observed in the ipsilateral hippocampus, however, total cofilin immunoreactivity increased above control levels at 2 weeks post-TBI (p<0.01), but then returned to control levels by 4 weeks post-TBI (p>0.05). Overall, lateral TBI caused acute and delayed increases in total cofilin immunoreactivity in each hemisphere of the hippocampus, but no significant decrease at any time point tested.
Lateral TBI altered total cofilin immunoreactivity in the neocortex as well. In the ipsilateral neocortex, TBI had a significant effect on total cofilin immunoreactivity compared to controls [F(6,49)=11.20, p<0.0001; Fig. 5C]. In contrast to the hippocampal data, post-hoc analysis revealed a decrease in total cofilin immunoreactivity in the ipsilateral neocortex at 1 h post-TBI (p<0.01). This decrease was transient, as total cofilin immunoreactivity had returned to control levels by 12 h post-TBI (p>0.05). Total cofilin immunoreactivity remained near control levels at 24 h and 1 week post-TBI (p>0.05 for each time point), but then increased at 2 weeks post-TBI, relative to controls (p<0.01). Similar to what was observed in the hippocampus, the increase at 2 weeks post-TBI did not persist, as total cofilin immunoreactivity at 4 weeks post-TBI in the ipsilateral neocortex did not differ significantly from controls (p>0.05).
The contralateral neocortex, like the hippocampus, did not show a significant loss of total cofilin immunoreactivity after lateral TBI at any time point tested. However, TBI did have a significant effect on total cofilin immunoreactivity in the contralateral neocortex relative to controls [F(6,49)=5.228, p=0.0003; Fig. 5D]. In contrast to the ipsilateral neocortex, total cofilin immunoreactivity at 1 h post-TBI in the contralateral neocortex did not differ significantly from controls (p>0.05). Nor was there a significant change in total cofilin immunoreactivity at 12 h post-TBI, relative to controls (p>0.05). At 24 h post-TBI, however, total cofilin immunoreactivity increased significantly above control levels (p<0.01). This increase did not last, as total cofilin immunoreactivity had returned to control levels by 1 week post-TBI (p>0.05), and did not differ significantly from controls at 2 weeks or 4 weeks post-TBI (p>0.05). In general, lateral TBI caused delayed but transient increases in total cofilin immunoreactivity in each hemisphere of the neocortex, as a decrease was only seen at 1 h post-TBI in the ipsilateral hemisphere.
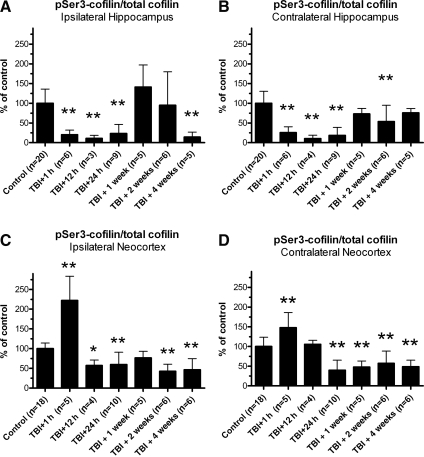
Lateral TBI resulted in time- and region-specific changes in the percentage of Ser3 phosphorylated cofilin
To correct for TBI-induced changes in cofilin protein expression, pSer3-cofilin immunoreactivity data were normalized to total cofilin immunoreactivity data for each tissue sample. This yielded a ratio of pSer3-cofilin to total cofilin (p/t cofilin) for each animal, allowing us to determine whether TBI affected the proportion of Ser3-phosphorylated cofilin. Statistical analysis of these data revealed significant changes in p/t cofilin after lateral TBI, as detailed below.
Lateral TBI significantly altered p/t cofilin in the ipsilateral hippocampus, compared to controls [F(6,47)=10.71, p<0.0001; Fig. 6A]. Post-hoc analysis indicates that p/t cofilin was significantly decreased at 1 h, 12 h, and 24 h post-TBI, relative to controls (p<0.01 for each time point). This decrease did not persist, as p/t cofilin at 1 week and 2 weeks post-TBI did not differ significantly from controls (p>0.05 for each time point). At 4 weeks post-TBI, however, p/t cofilin again decreased below control levels (p<0.01).
FIG. 6.
Lateral traumatic brain injury (TBI) caused time- and region-dependent changes in the proportion of Ser3-phosphorylated cofilin (pSer3-cofilin). To determine whether TBI affected the proportion of Ser3 phosphorylated cofilin, pSer3-cofilin immunoreactivity data were normalized to total cofilin immunoreactivity data for each sample, yielding a ratio of pSer3-cofilin to total cofilin (p/t cofilin). Analysis of these data identified time- and region-dependent changes in p/t cofilin. In the ipsilateral hippocampus, for example, p/t cofilin was significantly below control levels at 1 h, 12 h, and 24 h post-TBI, and again at 4 weeks post-TBI (A). In the contralateral hippocampus, a similar reduction in p/t cofilin was observed at 1 h, 12 h, and 24 h post-TBI, and then at 2 weeks post-TBI, relative to controls (B). The ipsilateral neocortex showed an increase in p/t cofilin at 1 h post-TBI, followed by decreases in p/t cofilin at 12 h, 24 h, 2 weeks, and 4 weeks post-TBI, relative to controls (C). A similar pattern was observed in the contralateral neocortex, where p/t cofilin was significantly above control levels at 1 h post-TBI, but then dropped below control levels at 24 h, 1 week, 2 weeks, and 4 weeks post-TBI (D). All comparisons were made by one-way analysis of variance with Dunnett's post-hoc test (*p<0.05, **p<0.01).
The contralateral hippocampus also underwent significant changes in p/t cofilin after lateral TBI, relative to controls [F(6,48)=16.76, p<0.0001; Fig. 6B]. Like the ipsilateral hippocampus, the contralateral hippocampus showed acute and delayed decreases in p/t cofilin. At 1 h, 12 h, and 24 h post-TBI in the contralateral hippocampus, for example, p/t cofilin was substantially below control levels (p<0.01 for each time point). The decrease appeared to be transient, as p/t cofilin did not differ significantly from controls at 1 week post-TBI (p>0.05). However, p/t cofilin decreased again by 2 weeks post-TBI (p<0.01), relative to controls, before returning to control levels by 4 weeks post-TBI (p>0.05). Overall, lateral TBI resulted in significant alterations in p/t cofilin in both the ipsilateral and the contralateral hippocampus.
Lateral TBI altered p/t cofilin immunoreactivity in the neocortex as well. In the ipsilateral neocortex, for instance, TBI had a significant effect on p/t cofilin, compared to controls [F(6,47)=28.98, p<0.0001; Fig. 6C]. Post-hoc analysis shows that p/t cofilin was increased above control levels at 1 h post-TBI (p<0.01). This increase in p/t cofilin was followed by decreases at 12 h and 24 h post-TBI, relative to controls (p<0.05 and p<0.01, respectively). By 1 week post-TBI, p/t cofilin had recovered to control levels (p>0.05). However, additional decreases in p/t cofilin were observed at 2 weeks and 4 weeks post-TBI, relative to controls (p<0.01 for each time point).
The contralateral neocortex also showed significant changes in p/t cofilin after lateral TBI, relative to controls [F(6,47)=17.37, p<0.0001; Fig. 6D]. As in the ipsilateral neocortex, p/t cofilin at 1 h post-TBI in the contralateral neocortex was significantly above control levels (p<0.01). This increase was transient though, as p/t cofilin had returned to control levels by 12 h post-TBI (p>0.05). The downward trend continued at 24 h post-TBI, when p/t cofilin decreased substantially below control levels (p<0.01). Similar decreases in p/t cofilin were observed at all subsequent time points, 24 h, 1 week, 2 weeks, and 4 weeks post-TBI, relative to controls (p<0.01 for each time point). In general, both the ipsilateral and contralateral hemispheres of the neocortex showed acute but transient increases in p/t cofilin, followed by delayed but apparently persistent decreases in p/t cofilin, after lateral TBI.
Post-TBI treatment with FK-506 prevented cofilin de-phosphorylation in neocortex but not hippocampus
In vitro studies have shown that CaN negatively regulates the phosphorylation of the cofilin Ser3 residue (Meberg et al., 1998), which could explain how pSer3-cofilin immunoreactivity decreased after TBI in the absence of any overt loss of cofilin protein (see Figs. 3 and 4). To test whether CaN caused the apparent de-phosphorylation of cofilin after TBI, we administered a selective CaN inhibitor, FK-506 (5 mg/kg IP) to rats at 1 h post-TBI, and sacrificed them at 24 h post-TBI (TBI+FK-506). To control for drug effects, an age-matched group of uninjured rats (n=5) were also injected with FK-506 (5 mg/kg IP), and then sacrificed 23 h post-injection (FK-506-only). Whole hemispheres of neocortex and hippocampus were dissected, separately homogenized, and subjected to Western blot analysis with a phospho-specific antibody recognizing Ser3-phosphorylated cofilin. Data were then compared across the following groups, using one-way ANOVA and Tukey's post-hoc test: controls, FK-506-only, TBI-only, and TBI+FK-506. These analyses indicate that FK-506 treatment had region-specific effects on pSer3-cofilin immunoreactivity after lateral TBI.
In the ipsilateral hippocampus, for example, pSer3-cofilin immunoreactivity differed significantly between the treated groups and controls [F(3,34)=20.97, p<0.001; Fig. 7A]. Post-hoc comparisons revealed a significant reduction in pSer3-cofilin immunoreactivity in the TBI+FK-506 group relative to the FK-506-only group (p<0.001) or controls (p<0.001). In addition, pSer3-cofilin immunoreactivity did not differ significantly between the TBI-only and FK-506 groups (p>0.05).
As in the ipsilateral hippocampus, pSer3-cofilin immunoreactivity in the contralateral hippocampus differed significantly between the treated groups and controls [F(3,32)=36.24, p<0.0001; Fig. 7B]. Also like the ipsilateral hippocampus, the contralateral hippocampus showed a significant loss of pSer3-cofilin immunoreactivity in the FK-506+TBI relative to the FK-506-only and control groups (p<0.001 for each), but no significant difference between the FK-506+TBI and TBI-only groups (p>0.05). The data thus suggest that the FK-506 treatment failed to prevent the 24-h post-TBI loss of pSer3-cofilin immunoreactivity in either hemisphere of the hippocampus.
The ipsilateral neocortex also showed significant differences in pSer3-cofilin immunoreactivity between the control and treated groups [F(3,33)=10.35, p<0.0001; Fig. 7C]. In contrast to the hippocampus, however, pSer3-cofilin immunoreactivity in the ipsilateral neocortex did not differ significantly between the TBI+FK-506 group and the FK-506-only group (p>0.05), nor between the TBI+FK-506 group and controls (p>0.05). In addition, pSer3-cofilin immunoreactivity in the FK-506+TBI group was increased substantially over that of the TBI-only group (p<0.001). This increase in pSer3-cofilin immunoreactivity was not likely due to an increase in cofilin protein expression, as one-way ANOVA found no significant difference in total cofilin immunoreactivity between the control and treated groups [control, n=20, 100±15% of control; FK-506-only, n=4, 97±37% of control; TBI-only, n=10, 113±26% of control; TBI+FK-506, n=5, 92±27% of control; F(3,35)=1.320, p=0.2835].
As in the ipsilateral neocortex, pSer3-cofilin immunoreactivity in the contralateral neocortex differed significantly between the control and treated groups [F(3,33)=4.109, p=0.0139; Fig. 7D]. Also like the ipsilateral side, there was no significant difference in pSer3-cofilin immunoreactivity between the TBI+FK-506 group and the control group (p>0.05), or between the TBI+FK-506 group and the FK-506-only groups (p>0.05). Again, however, there was a significant increase in pSer3-cofilin immunoreactivity in the TBI+FK-506 group relative to the TBI-only group (p<0.05), which did not appear to be due to an increase in cofilin protein [total cofilin immunoreactivity: control, n=20, 100±14% of control; FK-506-only, n=4, 102±32% of control; TBI-only, n=10, 200±108% of control; TBI+FK-506, n=5, 118±42% of control; F(3,35)=6.893, p=0.0009; TBI-only versus FK-506+TBI, p>0.05]. The data thus strongly suggest that the FK-506 treatment prevented the 24-h post-TBI loss of pSer3-cofilin immunoreactivity in both the ipsilateral neocortex and contralateral neocortex.
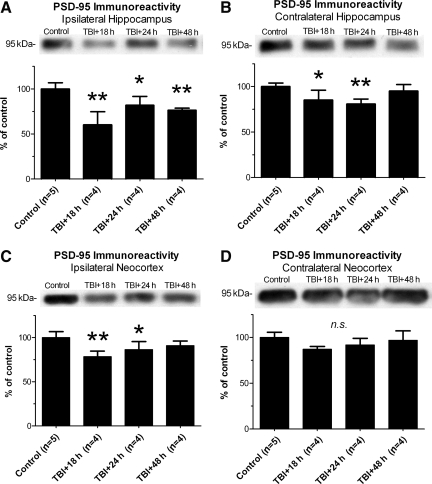
Lateral TBI caused a decrease in PSD-95 immunoreactivity
Pathological activation of CaN and cofilin are associated with the degeneration of excitatory synapses in vitro (Pak and Sheng, 2003; Shankar et al., 2007; Zhou et al., 2004). To test whether synapse degeneration occurred in our TBI model, Western blots were used to measure the immunoreactivity of a post-synaptic marker protein, PSD-95, in neocortical and hippocampal homogenates obtained 18 h, 24 h, and 48 h after LFPI. As described below, TBI caused a significant loss of PSD-95 immunoreactivity in the ipsilateral neocortex and bilateral hippocampus.
Lateral TBI had a significant effect on PSD-95 immunoreactivity in the ipsilateral hippocampus, compared to analogous tissue from control animals [F(3,13)=13.75, p=0.0003; Fig. 8A]. Post-hoc analysis indicates that PSD-95 immunoreactivity at 18 h, 24 h, and 48 h post-TBI in the ipsilateral hippocampus was significantly below control levels (p<0.01, p<0.05, and p<0.01, respectively). The contralateral hippocampus also showed significant changes in PSD-95 immunoreactivity after lateral TBI, relative to controls [F(3,13)=6.890, p=0.0051; Fig. 8B]. As in the ipsilateral hippocampus, PSD-95 immunoreactivity in the contralateral hippocampus decreased below control levels at 18 h and 24 h post-TBI (p<0.05 and p<0.01, respectively). Unlike in the ipsilateral hippocampus, however, PSD-95 immunoreactivity in the contralateral hippocampus returned to control levels by 48 h post-TBI (p>0.05). Overall, lateral TBI caused an acute loss of PSD-95 immunoreactivity bilaterally in the hippocampus, though the loss appeared to persist longer in the ipsilateral hemisphere.
FIG. 8.
Lateral traumatic brain injury (TBI) caused a loss of PSD-95 immunoreactivity in forebrain. The ipsilateral and contralateral hemispheres of the hippocampus and neocortex were dissected from adult rats at 18 h, 24 h, and 48 h after lateral fluid percussion TBI, separately homogenized, and subjected to immunoblotting with antibody recognizing the post-synaptic protein PSD-95. Lateral TBI caused a significant loss of PSD-95 immunoreactivity at 18 h, 24 h, and 48 h post-TBI in the ipsilateral hippocampus (A), and at 18 h and 24 h post-TBI in the contralateral hippocampus (B), relative to controls. In the ipsilateral neocortex, PSD-95 immunoreactivity also decreased significantly below control levels at 18 h and 24 h post-TBI (C). In the contralateral neocortex, however, PSD-95 immunoreactivity did not differ significantly from controls at any time point tested (D). All comparisons were made by one-way analysis of variance with Dunnett's post-hoc test (*p<0.05; **p<0.01; n.s., no significant differences).
Lateral TBI also affected PSD-95 immunoreactivity in the ipsilateral neocortex, compared to controls [F(3,14)=8.328, p=0.002; Fig. 8C]. Post-hoc analysis revealed a significant loss of PSD-95 immunoreactivity in the ipsilateral neocortex at 18 h and 24 h post-TBI, relative to controls (p<0.01 and p<0.05, respectively). This loss did not persist though, as PSD-95 immunoreactivity at 48 h post-TBI did not differ significantly from controls (p>0.05). Unlike the ipsilateral neocortex, the contralateral neocortex showed no significant change in PSD-95 immunoreactivity after TBI, compared to controls [F(3,13)=2.921, p=0.0739; Fig. 8D]. The data thus demonstrate an acute but transient loss of PSD-95 immunoreactivity in the ipsilateral neocortex after lateral TBI.
Effect of lateral TBI on SPAR immunoreactivity
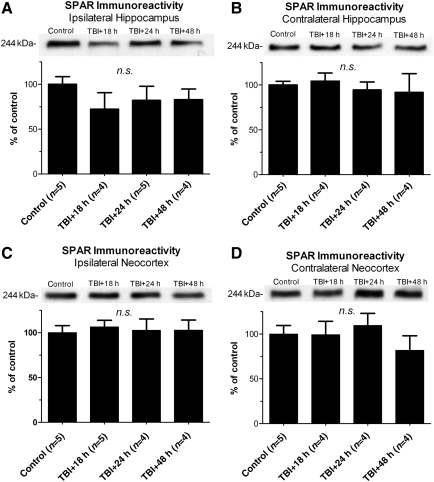
Injury to hippocampal neurons in vitro can cause a CaN-dependent induction of serum-induced kinase (Snk), resulting in increased proteolysis of SPAR, a protein which is critical to post-synaptic stability (Pak and Sheng, 2003; Seeburg and Sheng, 2008; Seeburg et al., 2008). To determine whether SPAR proteolysis occurred after lateral TBI, SPAR immunoreactivity was measured using Western blots of neocortical and hippocampal homogenates obtained 18 h, 24 h, and 48 h after LFPI. No significant change in SPAR immunoreactivity was detected in either hemisphere of the neocortex or hippocampus, at any post-TBI time point tested, relative to controls [ipsilateral hippocampus, F(3,14)=3.134, p=0.0593; contralateral hippocampus, F(3,13)=0.8802, p=0.4767; ipsilateral neocortex, F(3,14)=0.3530, p=0.7877; contralateral neocortex, F(3,13)=2.850, p=0.0784; Fig. 9]. The data therefore suggest that SPAR proteolysis is unlikely to be a mechanism of dendritic spine loss in the neocortex and dentate gyrus at 24 h post-TBI (Campbell et al., 2011).
FIG. 9.
Effect of lateral traumatic brain injury (TBI) on spine-associated Rap guanosine triphosphatase activating protein (SPAR) immunoreactivity. The ipsilateral and contralateral hemispheres of the hippocampus and neocortex were dissected from adult rats at 18 h, 24 h, and 48 h after lateral fluid percussion TBI, separately homogenized, and subjected to immunoblotting with an antibody recognizing SPAR. No change in SPAR immunoreactivity was detected at any time point tested, in the ipsilateral hippocampus (A), contralateral hippocampus (B), ipsilateral neocortex (C), or contralateral neocortex (D), relative to controls. All comparisons were made by one-way analysis of variance with Dunnett's post-hoc test (n.s., no significant differences).
Discussion
In the present study we examined the effects of lateral fluid percussion on CaN activity and CaN-dependent signaling in the rat forebrain. An in vitro assay revealed significant, time-dependent changes in CaN phosphatase activity in the hippocampus and neocortex after lateral TBI. Changes in CaN activity were further characterized by Western blot analyses. These analyses measured the effect of lateral TBI on two signaling pathways through which CaN regulates the stability of dendritic spines. The results implicate cellular pathways through which lateral TBI could alter dendritic spine stability. Together with its companion study (Campbell et al., 2011), the present study demonstrates a specific cellular mechanism through which lateral TBI can cause a loss of dendritic spines. This spine loss may be clinically relevant if it alters inter-neuronal communication and so contributes to cognitive impairment.
Analogous to what our group observed in a midline TBI model (Kurz et al., 2005a,2005b), lateral TBI resulted in a delayed, transient change in CaN phosphatase activity in the hippocampus and neocortex. The increase in CaN activity could be explained by an acute increase in CaN protein, as has been reported in a different model of lateral TBI (Bales et al., 2010). An alternative hypothesis is that CaN activity is enhanced through some post-translational modification of the enzyme (e.g., a partial proteolytic cleavage that disinhibits its phosphatase activity; Manalan and Klee, 1983), as has been proposed in models of central fluid percussion injury (Kurz et al., 2005b) and status epilepticus (Kurz et al., 2001). Recently, D'Amelio and colleagues reported evidence of a caspase-3-cleaved, constitutively-active fragment of CaN in a mouse model of Alzheimer's disease (D'Amelio et al., 2010). Enhancement of CaN activity in this manner would agree with our findings from the midline TBI model, wherein no change in CaN holoenzyme expression was observed (Kurz et al., 2005b). However, it is noteworthy that the changes in CaN activity induced by lateral TBI were not as long-lasting as those induced by midline TBI. Specifically, the increase in CaN activity lasted for hours after lateral TBI, but for weeks after midline TBI (Kurz et al., 2005b).
CaN activity can also be influenced by endogenous factors, such as Ca2+ binding, that cannot be measured by our in vitro assay. These endogenous changes in CaN activity can, however, be measured by the downstream effects on CaN substrates. In the present study we therefore examined a downstream substrate of CaN, cofilin. CaN activity leads to de-phosphorylation of a key Ser3 residue on cofilin (Wang et al., 2005), and the removal of this Ser3 phosphate enables cofilin to bind and de-polymerize actin (Moriyama et al., 1996). The breakdown of actin filaments by cofilin is critical to synaptic plasticity and a number of other cellular processes (for review, see Bernstein and Bamburg, 2010). Therefore, this choice of substrate allowed us not only to indirectly measure CaN activity, but to directly assess the cellular consequences of CaN activity.
In the present study we found that lateral TBI caused time- and region-dependent changes in pSer3-cofilin immunoreactivity in both the ipsilateral and contralateral hemispheres of the hippocampus and neocortex. These changes could be due to changes in cofilin protein expression or Ser3 phosphorylation or both. While total cofilin immunoreactivity did change at some post-TBI time points relative to controls, only once did it correspond to a change in pSer3-cofilin immunoreactivity (i.e., at 2 weeks post-TBI in the ipsilateral hippocampus, pSer3-cofilin increased and total cofilin increased). It is therefore reasonable to conclude that all of the decreases in pSer3-cofilin immunoreactivity we observed were due to decreased Ser3 phosphorylation and not due to decreased cofilin protein expression. Likewise, all but one of the increases in pSer3-cofilin immunoreactivity appeared to be due to increased Ser3 phosphorylation, not to increased cofilin protein expression. A noteworthy example is the loss of pSer3-cofilin that occurred bilaterally in the neocortex at 24 h post-TBI. This loss cannot be attributed to a decrease in cofilin protein alone, because total cofilin immunoreactivity was unchanged in the ipsilateral neocortex at 24 h post-TBI, and actually increased in the contralateral neocortex at this time point, relative to controls. Therefore, the loss of pSer3-cofilin most likely represents Ser3 de-phosphorylation. Previous studies have shown that this de-phosphorylation can occur downstream of CaN activity (Wang et al., 2005). Though our in vitro assay found no significant change in CaN phosphatase activity at 24 h post-TBI, it is possible that some endogenous (e.g., Ca2+-mediated) increase in CaN activity led to the cofilin de-phosphorylation we observed. Consistent with this possibility, the present study found that the CaN inhibitor FK-506, administered 1 h post-TBI, prevented the loss of pSer3-cofilin immunoreactivity bilaterally in the neocortex at 24 h post-TBI. The data thus suggest that a TBI-induced increase in CaN activity leads to cofilin de-phosphorylation in the neocortex at 24 h post-TBI.
The results of the present study strongly suggest that TBI causes cofilin de-phosphorylation bilaterally in the rat forebrain. The resulting increase in cofilin activity could degrade the actin-rich cytoskeleton of dendritic spines, causing the spines to shrink or collapse (Kurz et al., 2008; Shankar et al., 2007; Zhou et al., 2004). Since dendritic spines form most of the excitatory synapses in the brain, their collapse may physically de-stabilize and disrupt synapses. Indeed, lateral TBI is associated with synapse degeneration, as is evident by the loss of synaptic proteins reported in previous studies (Ansari et al., 2008a,2008b), and the loss of PSD-95 immunoreactivity observed in the present study. Thus, a pathological increase in cofilin activity may result in not only a loss of dendritic spines, but a loss of spine synapses (Shankar et al., 2007).
Interestingly, the apparent de-phosphorylation of cofilin observed in the present study coincides with a loss of dendritic spines reported in our companion study (Campbell et al., 2011). Specifically, the ipsilateral neocortex at 24 h post-TBI shows cofilin de-phosphorylation (Fig. 3) and spine loss (Campbell et al., 2011), both of which are prevented by a 1-h post-TBI administration of FK-506. These studies, along with the evidence from other models (e.g., Kurz et al., 2008; Shankar et al., 2007; Zhou et al., 2004), thus implicate cofilin de-phosphorylation as a mechanism of dendritic spine loss in the neocortex (Fig. 10, cofilin pathway).
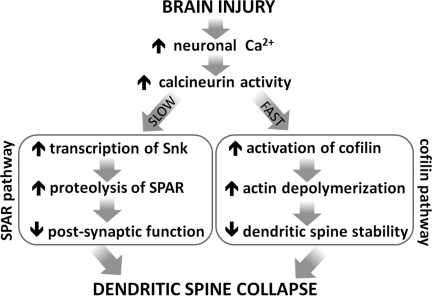
FIG. 10.
Calcineurin-dependent mechanisms by which brain injury could cause dendritic spine collapse. Brain injury can cause an increase in the activity of the calcium-sensitive phosphatase calcineurin (CaN). CaN-dependent signaling can then lead to dendritic spine collapse through different signaling pathways. In the neocortex, for example, CaN activity may lead to a rapid de-phosphorylation/activation of the actin-depolymerizing protein cofilin. Excessive cofilin activity could disrupt the spine's actin-rich cytoskeleton, resulting in spine shrinkage or de-stabilization (e.g., Zhou et al., 2004). In the hippocampus, however, a different mechanism may be involved, a mechanism involving CaN but not cofilin. For example, an injury-induced increase in CaN activity can lead to a transcriptional upregulation of serum-induced kinase (Snk), ultimately resulting in the targeted proteolysis of a spine-stabilizing protein, spine-associated Rap guanosine triphosphatase activating protein (SPAR; Pak and Sheng, 2003). SPAR loss is associated with degeneration of post-synaptic function and structure, including spine loss (Pak and Sheng, 2003; Pak et al., 2001; Seeburg et al., 2008). Therefore, cofilin activation and SPAR proteolysis represent different mechanisms by which CaN may cause spine loss. Evidence from the present study favors cofilin activation as a regional mechanism of spine loss after TBI, but does not rule out focal involvement of SPAR proteolysis.
Surprisingly, the same FK-506 treatment failed to block cofilin de-phosphorylation in the hippocampus. We suspect that different timing or mechanisms are involved in cofilin de-phosphorylation in the hippocampus. For example, CaN may have been activated in the hippocampus earlier than in the neocortex after lateral TBI. Indeed, cofilin was significantly de-phosphorylated at 1 h post-TBI in the hippocampus, though it is unclear from the present study whether this de-phosphorylation is CaN-dependent. However, if CaN is responsible for this acute de-phosphorylation of cofilin, then FK-506 may need to be administered earlier than 1 h post-TBI to fully prevent CaN activation in the hippocampus. By 1 h post-TBI, CaN may have already conveyed the signal to de-phosphorylate cofilin, or may have been cleaved into a catalytically-active fragment that resists inhibition by FK-506 (Kay et al., 1989).
Though FK-506 administration failed to block cofilin de-phosphorylation in the hippocampus, it fully prevented a concurrent loss of dendritic spines in the dentate gyrus (Campbell et al., 2011). One possible explanation is that FK-506 inhibits CaN to varying degrees across the subfields of the hippocampus, perhaps due to differential expression of FK-506's partner in inhibiting CaN, FKBP12 (Kato et al., 2000). A more plausible explanation is that a different mechanism underlies spine loss in the hippocampus, a mechanism that is CaN-dependent but cofilin-independent. The present study considered one such mechanism: the proteolysis of SPAR. SPAR is a post-synaptic protein which stabilizes dendritic spines in hippocampal neurons (Pak et al., 2001; Fig. 10, SPAR pathway). In cultured hippocampal neurons, excitotoxic injury triggers a CaN-dependent increase in Snk protein, resulting in increased proteolysis of SPAR and a corresponding loss of dendritic spines (Pak and Sheng, 2003). In contrast to these in vitro findings, however, in the present study we detected no significant change in SPAR immunoreactivity at 18 h, 24 h, or 48 h post-TBI in the hippocampus, nor in the neocortex. One could thus conclude that SPAR proteolysis did not occur after lateral TBI, and so is not a major mechanism of spine loss in this model. However, our data cannot exclude the possibility that SPAR proteolysis occurred focally (e.g., within the dentate gyrus, the only hippocampal subfield that showed spine loss; Campbell et al., 2011). Such a spatially-limited change in SPAR protein would not likely be detected in a Western blot analysis of a whole hippocampal hemisphere. Future studies using immunohistochemistry or micro-dissection could detect changes in SPAR protein with better spatial resolution.
Considering the findings of the present study and its companion (Campbell et al., 2011), we hypothesize that different CaN-dependent mechanisms underlie spine loss in the neocortex and dentate gyrus following lateral TBI. In the neocortex, CaN may cause spine loss through activation (de-phosphorylation) of cofilin, whereas in the dentate gyrus, CaN may cause spine loss through a cofilin-independent pathway. These studies do not, however, exclude the possibility of other CaN-dependent mechanisms of spine loss in this model. Further investigation is needed to definitively characterize the mechanisms of spine loss following lateral TBI.
Another interesting parallel between the present study and its companion is found at the 1-week post-TBI time point. By 1 week post-TBI in the present study, cofilin phosphorylation had returned to control levels bilaterally in the hippocampus. This recovery represented a substantial increase in cofilin phosphorylation relative to the preceding time point. At the same 1-week post-TBI time point, our companion study found an increase in dendritic spine density bilaterally in the hippocampus (Campbell et al., 2011). The increase in cofilin phosphorylation and spine density may be related, as the phosphorylation/inactivation of cofilin would favor the cytoskeletal expansion necessary for spine growth. Therefore, the increase in cofilin phosphorylation during recovery from TBI may play a role in the increase in spine density.
The present study and its companion (Campbell et al., 2011) identify potential mechanisms of dendritic remodeling in the traumatically-injured forebrain. This remodeling could disrupt neuronal circuits, or facilitate aberrant re-wiring, and so contribute to cognitive impairment and other TBI sequelae. CaN inhibition may help to prevent these sequelae by preserving synaptic circuits following TBI, and so could be a new option for treating a widespread medical problem that currently has few treatment options.
Acknowledgments
The authors are grateful to Drs. Bob Hamm and Tom Reeves for use of the fluid percussion injury device, and to Drs. Hamm and Leroy Thacker for guidance with statistics. The authors would also like to acknowledge David Register for his technical assistance with these studies. This work was supported by Commonwealth Neurotrauma Initiative grant 07-302E (to S.B.C.), and an Alzheimer's and Related Diseases Research Award Fund, award number 11-1 (to S.B.C.).
Author Disclosure Statement
No competing financial interests exist.
References
- Ansari M.A. Roberts K.N. Scheff S.W. A time course of contusion-induced oxidative stress and synaptic proteins in cortex in a rat model of TBI. J. Neurotrauma. 2008b;25:513–526. doi: 10.1089/neu.2007.0451. [DOI] [PubMed] [Google Scholar]
- Ansari M.A. Roberts K.N. Scheff S.W. Oxidative stress and modification of synaptic proteins in hippocampus after traumatic brain injury. Free Radic. Biol. Med. 2008a;45:443–452. doi: 10.1016/j.freeradbiomed.2008.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bales J.W. Ma X. Yan H.Q. Jenkins L.W. Dixon C.E. Regional calcineurin subunit B isoform expression in rat hippocampus following a traumatic brain injury. Brain Res. 2010;1358:211–220. doi: 10.1016/j.brainres.2010.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein B.W. Bamburg J.R. ADF/cofilin: a functional node in cell biology. Trends Cell Biol. 2010;20:187–195. doi: 10.1016/j.tcb.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protien-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Campbell J.N. Churn S.B. Register D. Traumatic brain injury causes an FK506-sensitive loss and an overgrowth of dendritic spines in rat forebrain. J. Neurotrauma. 2011 2011 Apr 25; doi: 10.1089/neu.2011.1761. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Campbell J.N. Kurz J.E. Churn S.B. Pathological remodeling of dendritic spines. In: Baylog L.R., editor. Dendritic Spines: Biochemistry, Modeling and Properties. Nova Science Publishers; New York: 2009. pp. 45–66. [Google Scholar]
- Churn S.B. Taft W.C. Billingsley M.S. Sankaran B. DeLorenzo R.J. Global forebrain ischemia induces a posttranslational modification of multifunctional calcium- and calmodulin-dependent kinase II. J. Neurochem. 1992;59:1221–1232. doi: 10.1111/j.1471-4159.1992.tb08431.x. [DOI] [PubMed] [Google Scholar]
- D'Amelio M. Cavallucci V. Middei S. Marchetti C. Pacioni S. Ferri A. Diamantini A. De Zio D. Carrara P. Battistini L. Moreno S. Bacci A. Ammassari-Teule M. Marie H. Cecconi F. Caspase-3 triggers early synaptic dysfunction in a mouse model of Alzheimer's disease. Nat. Neurosci. 2010;14:69–76. doi: 10.1038/nn.2709. [DOI] [PubMed] [Google Scholar]
- Dawson T.M. Steiner J.P. Dawson V.L. Dinerman J.L. Uhl G.R. Snyder S.H. Immunosuppressant FK506 enhances phosphorylation of nitric oxide synthase and protects against glutamate neurotoxicity. Proc. Natl. Acad. Sci. USA. 1993;90:9808–9812. doi: 10.1073/pnas.90.21.9808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshpande L.S. Sun D.A. Sombati S. Baranova A. Wilson M.S. Attkisson E. Hamm R.J. DeLorenzo R.J. Alterations in neuronal calcium levels are associated with cognitive deficits after traumatic brain injury. Neurosci. Lett. 2008;441:115–119. doi: 10.1016/j.neulet.2008.05.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon C.E. Lyeth B.G. Povlishock J.T. Findling R.L. Hamm R.J. Marmarou A. Young H.F. Hayes R.L. A fluid percussion model of experimental brain injury in the rat. J. Neurosurg. 1987;67:110–119. doi: 10.3171/jns.1987.67.1.0110. [DOI] [PubMed] [Google Scholar]
- Faul M. Xu L. Wald M. Coronado V. Traumatic brain injury in the United States: emergency department visits, hospitalizations, and deaths. Centers for Disease Control and Prevention, National Center for Injury Prevention and Control; Atlanta, GA: 2010. [Google Scholar]
- Fernandez A.M. Fernandez S. Carrero P. Garcia-Garcia M. Torres-Aleman I. Calcineurin in reactive astrocytes plays a key role in the interplay between proinflammatory and anti-inflammatory signals. J. Neurosci. 2007;27:8745–8756. doi: 10.1523/JNEUROSCI.1002-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghajar J. Traumatic brain injury. Lancet. 2000;356:923–929. doi: 10.1016/S0140-6736(00)02689-1. [DOI] [PubMed] [Google Scholar]
- Groth R.D. Dunbar R.L. Mermelstein P.G. Calcineurin regulation of neuronal plasticity. Biochem. Biophys. Res. Commun. 2003;311:1159–1171. doi: 10.1016/j.bbrc.2003.09.002. [DOI] [PubMed] [Google Scholar]
- Hallam T.M. Floyd C.L. Folkerts M.M. Lee L.L. Gong Q.Z. Lyeth B.G. Muizelaar J.P. Berman R.F. Comparison of behavioral deficits and acute neuronal degeneration in rat lateral fluid percussion and weight-drop brain injury models. J. Neurotrauma. 2004;21:521–539. doi: 10.1089/089771504774129865. [DOI] [PubMed] [Google Scholar]
- Kato H. Oikawa T. Otsuka K. Takahashi A. Itoyama Y. Postischemic changes in the immunophilin FKBP12 in the rat brain. Brain Res. Mol. Brain Res. 2000;84:58–66. doi: 10.1016/s0169-328x(00)00210-2. [DOI] [PubMed] [Google Scholar]
- Kay J.E. Doe S.E. Benzie C.R. The mechanism of action of the immunosuppressive drug FK-506. Cell Immunol. 1989;124:175–181. doi: 10.1016/0008-8749(89)90121-4. [DOI] [PubMed] [Google Scholar]
- Kurz J.E. Hamm R.J. Singleton R.H. Povlishock J.T. Churn S.B. A persistent change in subcellular distribution of calcineurin following fluid percussion injury in the rat. Brain Res. 2005a;1048:153–160. doi: 10.1016/j.brainres.2005.04.062. [DOI] [PubMed] [Google Scholar]
- Kurz J.E. Moore B.J. Henderson S.C. Campbell J.N. Churn S.B. A cellular mechanism for dendritic spine loss in the pilocarpine model of status epilepticus. Epilepsia. 2008;49:1696–1710. doi: 10.1111/j.1528-1167.2008.01616.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurz J.E. Parsons J.T. Rana A. Gibson C.J. Hamm R.J. Churn S.B. A significant increase in both basal and maximal calcineurin activity following fluid percussion injury in the rat. J. Neurotrauma. 2005b;22:476–490. doi: 10.1089/neu.2005.22.476. [DOI] [PubMed] [Google Scholar]
- Kurz J.E. Rana A. Parsons J.T. Churn S.B. Status epilepticus-induced changes in the subcellular distribution and activity of calcineurin in rat forebrain. Neurobiol. Dis. 2003;14:483–493. doi: 10.1016/j.nbd.2003.08.018. [DOI] [PubMed] [Google Scholar]
- Kurz J.E. Sheets D. Parsons J.T. Rana A. Delorenzo R.J. Churn S.B. A significant increase in both basal and maximal calcineurin activity in the rat pilocarpine model of status epilepticus. J. Neurochem. 2001;78:304–315. doi: 10.1046/j.1471-4159.2001.00426.x. [DOI] [PubMed] [Google Scholar]
- Manalan A.S. Klee C.B. Activation of calcineurin by limited proteolysis. Proc. Natl. Acad. Sci. USA. 1983;80:4291–4295. doi: 10.1073/pnas.80.14.4291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meberg P.J. Ono S. Minamide L.S. Takahashi M. Bamburg J.R. Actin depolymerizing factor and cofilin phosphorylation dynamics: response to signals that regulate neurite extension. Cell Motil. Cytoskeleton. 1998;39:172–190. doi: 10.1002/(SICI)1097-0169(1998)39:2<172::AID-CM8>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Morishita W. Marie H. Malenka R.C. Distinct triggering and expression mechanisms underlie LTD of AMPA and NMDA synaptic responses. Nat. Neurosci. 2005;8:1043–1050. doi: 10.1038/nn1506. [DOI] [PubMed] [Google Scholar]
- Moriyama K. Iida K. Yahara I. Phosphorylation of Ser-3 of cofilin regulates its essential function on actin. Genes Cells. 1996;1:73–86. doi: 10.1046/j.1365-2443.1996.05005.x. [DOI] [PubMed] [Google Scholar]
- Norris C.M. Kadish I. Blalock E.M. Chen K.C. Thibault V. Porter N.M. Landfield P.W. Kraner S.D. Calcineurin triggers reactive/inflammatory processes in astrocytes and is upregulated in aging and Alzheimer's models. J. Neurosci. 2005;25:4649–4658. doi: 10.1523/JNEUROSCI.0365-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pak D.T. Sheng M. Targeted protein degradation and synapse remodeling by an inducible protein kinase. Science. 2003;302:1368–1373. doi: 10.1126/science.1082475. [DOI] [PubMed] [Google Scholar]
- Pak D.T. Yang S. Rudolph-Correia S. Kim E. Sheng M. Regulation of dendritic spine morphology by SPAR, a PSD-95-associated RapGAP. Neuron. 2001;31:289–303. doi: 10.1016/s0896-6273(01)00355-5. [DOI] [PubMed] [Google Scholar]
- Pallen C.J. Wang J.H. A multifunctional calmodulin-stimulated phosphatase. Arch. Biochem. Biophys. 1985;237:281–291. doi: 10.1016/0003-9861(85)90279-6. [DOI] [PubMed] [Google Scholar]
- Park K.S. Mohapatra D.P. Misonou H. Trimmer J.S. Graded regulation of the Kv2.1 potassium channel by variable phosphorylation. Science. 2006;313:976–979. doi: 10.1126/science.1124254. [DOI] [PubMed] [Google Scholar]
- Romero-Calvo I. Ocon B. Martinez-Moya P. Suarez M.D. Zarzuelo A. Martinez-Augustin O. de Medina F.S. Reversible Ponceau staining as a loading control alternative to actin in Western blots. Anal. Biochem. 2010;401:318–320. doi: 10.1016/j.ab.2010.02.036. [DOI] [PubMed] [Google Scholar]
- Seeburg D.P. Sheng M. Activity-induced Polo-like kinase 2 is required for homeostatic plasticity of hippocampal neurons during epileptiform activity. J. Neurosci. 2008;28:6583–6591. doi: 10.1523/JNEUROSCI.1853-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeburg D.P. Feliu-Mojer M. Gaiottino J. Pak D.T. Sheng M. Critical role of CDK5 and Polo-like kinase 2 in homeostatic synaptic plasticity during elevated activity. Neuron. 2008;58:571–583. doi: 10.1016/j.neuron.2008.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankar G.M. Bloodgood B.L. Townsend M. Walsh D.M. Selkoe D.J. Sabatini B.L. Natural oligomers of the Alzheimer amyloid-beta protein induce reversible synapse loss by modulating an NMDA-type glutamate receptor-dependent signaling pathway. J. Neurosci. 2007;27:2866–2875. doi: 10.1523/JNEUROSCI.4970-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siesjo B.K. Siesjo P. Mechanisms of secondary brain injury. Eur. J. Anaesthesiol. 1996;13:247–268. [PubMed] [Google Scholar]
- Sun D.A. Deshpande L.S. Sombati S. Baranova A. Wilson M.S. Hamm R.J. DeLorenzo R.J. Traumatic brain injury causes a long-lasting calcium (Ca2+)-plateau of elevated intracellular Ca levels and altered Ca2+ homeostatic mechanisms in hippocampal neurons surviving brain injury. Eur. J. Neurosci. 2008;27:1659–1672. doi: 10.1111/j.1460-9568.2008.06156.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szydlowska K. Zawadzka M. Kaminska B. Neuroprotectant FK506 inhibits glutamate-induced apoptosis of astrocytes in vitro and in vivo. J. Neurochem. 2006;99:965–975. doi: 10.1111/j.1471-4159.2006.04136.x. [DOI] [PubMed] [Google Scholar]
- Thompson H.J. Lifshitz J. Marklund N. Grady M.S. Graham D.I. Hovda D.A. McIntosh T.K. Lateral fluid percussion brain injury: a 15-year review and evaluation. J. Neurotrauma. 2005;22:42–75. doi: 10.1089/neu.2005.22.42. [DOI] [PubMed] [Google Scholar]
- Tymianski M. Tator C.H. Normal and abnormal calcium homeostasis in neurons: a basis for the pathophysiology of traumatic and ischemic central nervous system injury. Neurosurgery. 1996;38:1176–1195. doi: 10.1097/00006123-199606000-00028. [DOI] [PubMed] [Google Scholar]
- Wang Y. Shibasaki F. Mizuno K. Calcium signal-induced cofilin dephosphorylation is mediated by Slingshot via calcineurin. J. Biol. Chem. 2005;280:12683–12689. doi: 10.1074/jbc.M411494200. [DOI] [PubMed] [Google Scholar]
- Wu H.Y. Hudry E. Hashimoto T. Kuchibhotla K. Rozkalne A. Fan Z. Spires-Jones T. Xie H. Arbel-Ornath M. Grosskreutz C.L. Bacskai B.J. Hyman B.T. Amyloid beta induces the morphological neurodegenerative triad of spine loss, dendritic simplification, and neuritic dystrophies through calcineurin activation. J. Neurosci. 2010;30:2636–2649. doi: 10.1523/JNEUROSCI.4456-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L.X. Sun C.K. Zhang Y.M. Fan M. Xu J. Ma H. Zhang J. Involvement of the Snk-SPAR pathway in glutamate-induced excitotoxicity in cultured hippocampal neurons. Brain Res. 2007;1168:38–45. doi: 10.1016/j.brainres.2007.06.082. [DOI] [PubMed] [Google Scholar]
- Zhou Q. Homma K.J. Poo M.M. Shrinkage of dendritic spines associated with long-term depression of hippocampal synapses. Neuron. 2004;44:749–757. doi: 10.1016/j.neuron.2004.11.011. [DOI] [PubMed] [Google Scholar]