Abstract
The effects of slight variations in brain temperature on the pathophysiological consequences of acute brain injury have been extensively described in models of moderate and severe traumatic brain injury (TBI). In contrast, limited information is available regarding the potential consequences of temperature elevations on outcome following mild TBI (mTBI) or concussions. One potential confounding variable with mTBI is the presence of elevated body temperature that occurs in the civilian or military populations due to hot environments combined with exercise or other forms of physical exertion. We therefore determined the histopathological effects of pre- and post-traumatic hyperthermia (39°C) on mTBI. Adult male Sprague-Dawley rats were divided into 3 groups: pre/post-traumatic hyperthermia, post-traumatic hyperthermia alone for 2 h, and normothermia (37°C). The pre/post-hyperthermia group was treated with hyperthermia starting 15 min before mild parasagittal fluid-percussion brain injury (1.4–1.6 atm), with the temperature elevation extending for 2 h after trauma. At 72 h after mTBI, the rats were perfusion-fixed for quantitative histopathological evaluation. Contusion areas and volumes were significantly larger in the pre/post-hyperthermia treatment group compared to the post-hyperthermia and normothermic groups. In addition, pre/post-traumatic hyperthermia caused the most severe loss of NeuN-positive cells in the dentate hilus compared to normothermia. These neuropathological results demonstrate that relatively mild elevations in temperature associated with peri-traumatic events may affect the long-term functional consequences of mTBI. Because individuals exhibiting mildly elevated core temperatures may be predisposed to aggravated brain damage after mTBI or concussion, precautions should be introduced to target this important physiological variable.
Key words: concussion, dentate hilus, fluid-percussion brain injury, hyperthermia, temperature, traumatic brain injury
Introduction
Mild traumatic brain injuries (mTBIs) represent the greatest proportion of all treated brain injuries worldwide, and are associated with a wide range of symptoms with variable rates and degrees of recovery (Tay et al., 2010). Approximately 300,000 sports-related TBIs occur in the United States each year, and most of them are reported to be mTBI-like concussions (Thurman et al., 1998). In addition, a significant proportion of military personnel deployed in Operation Enduring Freedom and Operation Iraqi Freedom have been exposed to blast injuries resulting in concussion (Terrio et al., 2009; Vasterling et al., 2009). Although these TBIs are relatively mild, concern has emerged regarding the long term consequences of mTBI such as learning disabilities, post-traumatic stress disorder, and other post-concussive symptoms, including headache, dizziness, irritability, and memory problems (Collins et al., 1999). Another growing concern in reference to mTBI is the incidence of repeated concussions observed in the military, and with recreational and professional sports (Collins et al., 1999; Lange et al., 2010; Terrio et al., 2009). Thus the clarification of the neuropathological signatures of mTBI and the mechanisms underlying the pathogenesis of the resulting functional deficits is an area of active investigation (Babikian et al., 2011; DeWitt and Prough, 2009; Dikranian et al., 2008; Mayer et al., 2010,2011; Shitaka et al., 2011; Smits et al., 2010).
Previous studies from a number of laboratories have reported the importance of small variations in body or central nervous system (CNS) temperature on histopathological and functional outcomes in various brain and spinal cord injury models. While mild reductions in temperature following an ischemic or traumatic insult protects vulnerable brain regions from injury, mild elevations in temperature can worsen outcome (Dietrich and Bramlett, 2010). These experimental findings have resulted in several clinical studies showing the beneficial effects of early cooling or temperature management strategies limiting periods of reactive hyperthermia in specific patient populations (Childs et al., 2006; Clifton et al., 2001; Jiang, 2009; Marion and Bullock, 2009; Polderman, 2008; Sinclair and Andrews, 2010). Taken together, these studies emphasize the need to evaluate the importance of spontaneous temperature fluctuations in individuals prior to or following a neurological insult in terms of altering patterns of vulnerability and outcome (Sinclair and Andrews, 2010).
Post-traumatic pyrexia is a common occurrence in patients with head injury (Jiang et al., 2002; Kilpatrick et al., 2000; Natale et al., 2000; Stocchetti et al., 2002). Observational studies have found that the occurrence of fever after severe TBI is associated with prolonged intensive care unit (ICU) stays (Kilpatrick et al., 2000; Natale et al., 2000). Jiang and colleagues (2002) reported a strong relationship between the incidence of fever and outcome in a study of 846 patients with TBI. Experimentally, post-traumatic hyperthermia has also been reported to worsen outcome after moderate and severe TBI (Dietrich et al., 1996b). In a study by Dietrich and colleagues (1996b), a 3-h period of systemic hyperthermia (39°C) induced 24 h after moderate fluid-percussion injury (FPI) to the brain led to increased mortality, significantly larger contusion volumes with aggravated blood–brain barrier dysfunction, and robust inflammatory responses. In a more recent study, Suzuki and associates (2004) demonstrated that post-traumatic hyperthermia also increased the vulnerability of cortical neurons and the severity of diffuse axonal injury. These clinical and experimental findings emphasize that hyperthermia initiated after a more severe brain injury may worsen functional and structural outcomes (Dietrich and Bramlett, 2007). In contrast to these studies, limited data are available regarding the consequences of hyperthermia on mTBI and how pre-existing temperature elevations could alter the histopathological consequences of this more common form of brain injury.
To investigate the effects of mild hyperthermia on concussive-like insults, we determined whether hyperthermia induced before and after mTBI would aggravate histopathological outcomes. For this study we used a well-described model of parasagittal FPI that has previously been shown to be sensitive to post-traumatic temperature modifications (Dietrich et al., 1996b; Suzuki et al., 2004). Quantitative neuropathological outcome measures for contusion volume and neuronal cell counts demonstrated a significant effect of this temperature manipulation on pathology after mTBI.
Methods
Animal groups
Thirty-two adult male Sprague-Dawley rats (290–360 g, Charles Rivers Laboratories, Wilmington, MA) were used and divided into 4 treatment groups (n=8 in each group). These included pre- and post-traumatic hyperthermia (pre/post-H), post-traumatic hyperthermia (post-H), normothermia (N), and sham-operated (S). The pre/post-H group was treated with hyperthermia (39°C) starting 15 min before mild parasagittal FPI (1.4–1.6 atm). Both the pre/post-H and post-H groups underwent hyperthermia for a 2-h period after mTBI. The N group was maintained at normothermia (37°C) throughout the same surgical procedures that the other mTBI groups received. Sham-operated animals were treated with normothermia in this study because previously published data demonstrated no histopathological consequences of an induced period of hyperthermia on any of the sham procedures (Dietrich et al., 1996b; Suzuki et al., 2004). At 2 h after mTBI or sham surgery, the animals were returned to their cages with free access to water and food until 72 h after surgery, when they were perfusion-fixed for quantitative histopathological analysis.
Surgical procedures
The animals were maintained on a 12-h/12-h light/dark cycle and given food ad libitum. All animal procedures followed the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals, and were approved by the University of Miami's Animal Care and Use Committee. The animals were anesthetized (70% nitrous oxide, 1–3% isoflurane, and 30% oxygen) 24 h prior to injury and surgically prepared for parasagittal FPI as described previously (Atkins et al., 2010). Briefly, a craniotomy (4.8 mm) was performed 3.8 mm posterior to the bregma and 2.5 mm lateral to the midline. A plastic injury tube was placed over the exposed dura and affixed to the skull with adhesive and dental acrylic. The scalp was then sutured closed, and the animals were allowed to recover before returning to their home cages. After fasting overnight, the animals were anesthetized (70% nitrous oxide, 1–3% isoflurane, and 30% oxygen), intubated, and subjected to a pressure pulse of mild (1.4–1.6 atm) intensity (Dixon et al., 1987). Prior to the FPI, catheters were placed in the tail artery to monitor arterial blood pressure and blood gases. Blood gas levels were calculated from arterial samples 15 min prior to temperature manipulations, and 15 min prior to and after mild FPI at 15 min, 1 h, and 2 h. Brain temperature was measured indirectly by a thermistor probe placed in the right temporalis muscle, and core temperature was measured by rectal thermistor probe. In an attempt to mimic the clinical situation, both brain and core temperatures were elevated by placing two automatic feedback heating lamps over the animal's head and body.
Histopathological analyses
TBI- and sham-operated animals were anesthetized (3% isoflurane, 70% nitrous oxide, and 30% oxygen for 5 min) and perfused transcardially with isotonic saline for 2 min (75 mL), and then for 30 min with 4% paraformaldehyde in 0.1 M sodium phosphate buffer (PB), pH 7.4 (350 mL). The brains were embedded in paraffin and sectioned (7 μm thick) to collect 5 series. The sections were stained with hematoxylin and eosin (H&E) and alternate sections were immunostained with NeuN. For immunostaining, the sections were deparaffinized, rehydrated, and incubated overnight at 4°C with anti-NeuN antibody (1:500; Millipore, Temecula, CA). The sections were washed with 0.1 M PBS (pH 7.4) and 0.4% Triton, and then incubated with secondary anti-mouse IgG antibody (1:200; Vector Laboratories, Burlingame, CA) for 2 h at room temperature. After rinsing, ABC Elite (Vector Laboratories) was applied for 2 h, and then the slides were washed with PBS followed by acetate-imidazole buffer (pH 7.2), and reacted with NiDAB (2.5% nickel ammonium sulfate acetate-imidazole buffer, 0.05% DAB, and 0.001% H2O2). The DAB reaction was stopped with acetate-imidazole buffer. The slides were then dehydrated, cleared, and cover-slipped for analysis.
Cortical contusion volumes were determined by tracing the entire extent of the contusion in H&E sections (5 μm thick after shrinkage, 140 μm apart) with a 5× objective on an Axiophot microscope (Carl Zeiss MicroImaging, Inc., Thornwood, NY) using the Neurolucida 7.50.1 software program (MicroBrightField Inc., Williston, VT). To identify the cortical contusion boundaries, we used evidence of pyknotic neurons, reactive astrocytes, hemorrhage, edema, and shearing at the gray/white matter interface between the cortex and external capsule. Contusion areas were also calculated for 5 coronal levels at and around the epicenter (−3.3, −4.3, −5.8, −6.8, and −7.3 mm posterior from the bregma).
To determine neuron survival in the dentate hilus, serial sections (5 μm thick after shrinkage, 140 μm apart) from −3.6 to −4.3 mm posterior to the bregma were quantified in an unbiased, systematic manner by a blind observer using the physical fractionator method following the workflow in StereoInvestigator 7.50.1 software (MicroBrightField, Inc.) with an Axiophot microscope (Carl Zeiss MicroImaging, Inc.). The dentate hilus was contoured at 5×, and then a counting grid of 60×200 μm was placed over the hilus. Using a 35×35-μm counting frame, NeuN-positive cells were counted in 20 randomly-placed sampling sites with a 63× objective. The Q values ranged from 130–262, and the average CE2 values were 0.0053±0.0011, 0.0079±0.0005, and 0.0057±0.0003, for the pre/post-H, post-H, and N groups, respectively.
Images were taken with a 20× objective on an Axiophot microscope and montaged using the virtual slice module in the Neurolucida 7.50.1 software program.
Statistical analysis
Data are expressed as mean±standard error of the mean (SEM). Contusion volumes and NeuN-positive cell counts were analyzed with a one-way analysis of variance (ANOVA), followed by a post-hoc Fisher's test. Physiological data and contusion areas were compared using a repeated-measures ANOVA followed by a post-hoc Fisher's test. Differences were considered significant at p<0.05.
Results
Physiological parameters
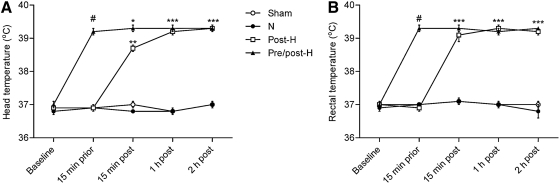
Physiological parameters of weight, atmospheric pressure of the FP pulse (atm), mean arterial blood pressure (MABP), Po2, Pco2, and blood pH are given in Table 1. Physiological variables were taken pre-hyperthermia, and prior to and after TBI at 15 min, 1 h, and 2 h. All physiological values were within the normal range, and there were no significant differences between the various experimental groups in terms of weight, atm, MABP, Po2, Pco2, and blood pH. As expected, head and rectal temperatures were significantly higher (p<0.001) in the pre/post-hyperthermic group than in the other three groups at 15 min prior to TBI, and in the post-hyperthermic and pre/post-hyperthermic groups compared to the sham and normothermic groups at 15 min, 1 h, and 2 h after mTBI (Fig. 1).
Table 1.
Physiological Parameters
| Parameter | Groups | Baseline | 15 min prior | 15 min post | 1 h post | 2 h post |
|---|---|---|---|---|---|---|
| Weight (g) | Sham | 324.2±10.2 | ||||
| N | 328.7±11.4 | |||||
| Post-H | 325.8±11.8 | |||||
| Pre/post-H | 325.0±9.7 | |||||
| atm | N | 1.50±0.02 | ||||
| Post-H | 1.51±0.03 | |||||
| Pre/post-H | 1.53±0.02 | |||||
| MABP | Sham | 116.5±11.8 | 119.2±9.1 | 121.8±9.3 | 104.4±8.2 | 114.7±7.7 |
| N | 102.0±11.1 | 103.3±6.0 | 112.9±9.0 | 97.4±7.0 | 92.2±5.5 | |
| Post-H | 112.2±10.4 | 109.5±12.8 | 132.2±10.2 | 122.7±10.5 | 116.6±11.8 | |
| Pre/post-H | 124.1±5.8 | 132.4±5.4 | 131.8±7.4 | 126.2±8.4 | 117.6±7.4 | |
| Blood Po2 | Sham | 152.8±9.8 | 154.8±9.2 | 142.3±8.4 | 137.3±6.6 | 143.7±9.5 |
| N | 138.5±6.3 | 139.2±4.4 | 127.2±5.9 | 125.0±3.7 | 121.8±6.1 | |
| Post-H | 156.3±8.2 | 159.5±10.6 | 133.5±8.8 | 131.6±9.9 | 131.9±13.5 | |
| Pre/post-H | 164.0±19.5 | 146.9±17.1 | 133.6±13.5 | 139.4±11.5 | 117.3±13.0 | |
| Blood Pco2 | Sham | 37.5±0.9 | 36.3±0.3 | 38.2±1.0 | 39.0±0.6 | 38.4±1.1 |
| N | 37.9±1.5 | 37.6±0.2 | 37.8±0.8 | 40.1±2.2 | 38.9±1.5 | |
| Post-H | 38.3±1.5 | 37.8±0.5 | 37.7±0.4 | 38.8±0.9 | 38.4±1.1 | |
| Pre/post-H | 39.4±1.0 | 37.8±0.5 | 38.5±0.6 | 38.9±0.8 | 37.8±1.0 | |
| Blood pH | Sham | 7.46±0.00 | 7.48±0.00 | 7.45±0.02 | 7.43±0.01 | 7.44±0.01 |
| N | 7.47±0.00 | 7.47±0.00 | 7.44±0.01 | 7.43±0.01 | 7.43±0.01 | |
| Post-H | 7.46±0.00 | 7.47±0.00 | 7.45±0.01 | 7.43±0.01 | 7.42±0.01 | |
| Pre/post-H | 7.44±0.00 | 7.44±0.00 | 7.43±0.01 | 7.43±0.01 | 7.43±0.01 |
Values are mean±standard error of the mean.
atm, atmospheric pressure; MABP, mean arterial blood pressure; N, normothermia; Post-H, post-TBI hyperthermia; Pre/post-H, pre- and post-TBI hyperthermia; Po2, partial arterial oxygen pressure; Pco2, partial arterial carbon dioxide pressure.
FIG. 1.
Temperature manipulations. Both head (A) and body (B) temperature were significantly higher before mTBI in the pre/post-H group, and after mTBI in the post-H and pre/post-H groups (#p<0.001 compared with the sham, N, and post-H groups; *p<0.05, **p<0.01, ***p<0.001 compared with the sham and N groups; mTBI, mild traumatic brain injury; pre/post-H, pre- and post-traumatic hyperthermia; post-H, post-traumatic hyperthermia; N, normothermia).
Cortical contusion areas and volumes
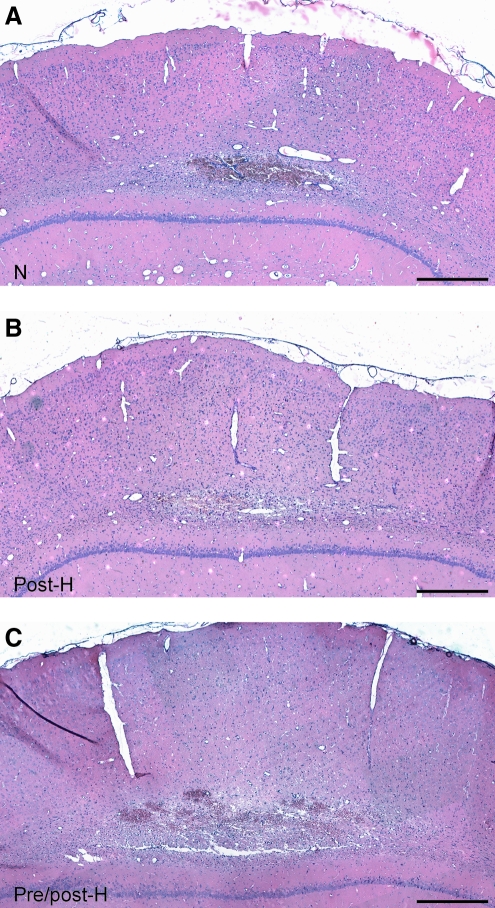
Following normothermic mTBI, a relatively small yet consistent hemorrhagic contusion was observed in the parietal cerebral cortex bordering the external capsule and lower cortical layer (Fig. 2A). Mild patterns of cellular inflammation were seen associated with the contused site. A similar pattern of contusion formation was seen in post-hyperthermic animals, for which temperature was elevated immediately after mTBI (Fig. 2B). In contrast to these findings, larger contusions were observed in animals that had mild hyperthermia induced prior to and after the TBI (Fig. 2C). Clear evidence of hemorrhagic sites and inflammatory cell infiltration were associated with the well-defined contusion. Also, swollen white matter tracts were apparent lying adjacent to the contused site.
FIG. 2.
Comparison of cortical contusions. There were no significant differences in pathology between the normothermic (A, N) and post-hyperthermic groups (B, post-H) in hematoxylin and eosin-stained sections. However, larger cortical contusions were noted in the pre/post-hyperthermic (C, pre/post-H) group. Images were taken at bregma level −5.8 mm (scale bars=300 μm).
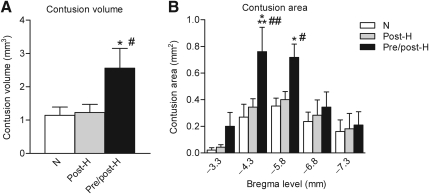
Quantitative assessment of contusion areas and volumes confirmed the increased damage associated with the pre/post-hyperthermic insult (Fig. 3). Compared to both normothermic and post-hyperthermic animals, pre/post-hyperthermic animals demonstrated significantly larger contusion volumes that were increased approximately twofold in size. Also, significant differences in contusion areas were observed at levels −4.3 and −5.8 mm posterior from the bregma between the pre/post-hyperthermic group and the normothermic and post-hyperthermic mTBI groups.
FIG. 3.
Contusion volume and area quantification. (A) Cortical contusion volume was significantly larger in the pre/post-hyperthermia group (Pre/post-H) compared to either the normothermic (N) group (*p<0.05) or the post-hyperthermic (Post-H) group (#p<0.05). (B) Contusion areas were significantly larger in the pre/post-hyperthermia group than the normothermia group (***p<0.001, *p<0.05) and the post-hyperthermia group (##p<0.01, #p<0.05) at bregma levels −4.3 and −5.8 mm.
Dentate hilus
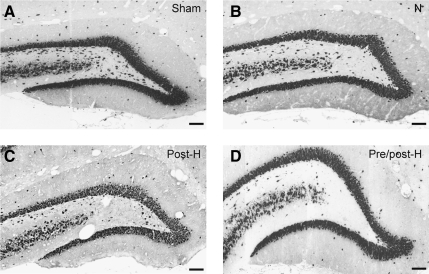
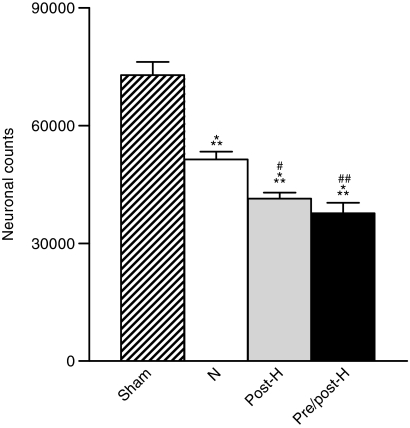
Immunocytochemical assessment of the dentate hilus also demonstrated significant differences between the various experimental groups (Fig. 4). Compared to sham-operated control animals, normothermic mTBI resulted in a significant loss of NeuN-positive cells in this vulnerable area of the ipsilateral hippocampus. An even further significant decrease in the number of NeuN-positive cells was seen in both the post-hyperthermic and pre/post-hyperthermic mTBI groups (Fig. 5).
FIG. 4.
Images of NeuN-immunostained sections were taken at bregma level −4.0 mm. Compared to sham animals (A, Sham), mTBI resulted in loss of neurons in normothermic animals (B, N), and this was more pronounced in both post-hyperthermic (C, Post-H), and pre/post-hyperthermic (D, Pre/post-H) animals (scale bars=100 μm).
FIG. 5.
Stereology results of NeuN-positive cell counts in the dentate hilus. When quantified using unbiased stereology, we found that neuronal numbers for all mTBI groups were significantly decreased compared to the sham group (***p<0.001, Sham). Dentate hilar neuronal counts in the post-hyperthermia group (#p<0.05, Post-H), and the pre/post-hyperthermia group (##p<0.001, Pre/post-h) were significantly decreased from the normothermia group.
Discussion
In this study of mTBI, we found that raising brain and body temperature to 39°C for 15 min prior to and after trauma significantly aggravated the histopathological consequences of this mild injury. Overall contusion volume and dentate hilar neuron vulnerability were significantly increased compared to TBI animals that were held at normothermic temperatures. These findings support previously published findings indicating that mild elevations in temperature may worsen outcomes in experimental and clinical situations following moderate and severe levels of brain trauma. The present findings are novel in that they provide evidence for clinically relevant elevations in pre-traumatic temperature, significantly influencing the histopathological consequences of a mild traumatic insult. These findings may have significant implications for the treatment and prevention of the detrimental consequences of mTBI and concussion.
The human body utilizes several thermoregulatory responses including vasomotor, hemodynamic, and altered sweat rates that maintain body temperature in a relatively narrow physiological range despite the temperature of the environment (Cabanac, 1975; Hayward and Baker, 1968; Sukstanskii and Yablonskiy, 2006). However, during periods of extreme exercise or other types of physical exertion, body temperatures may be mildly elevated (Goosey-Tolfrey et al., 2008; Özgünen et al., 2010). In a study by Özgünen and colleagues (2010), body temperature was reported to rise to 39°C in football players during periods of activity. In another study, Chinevere and associates (2008) reported elevations in core (rectal) temperature readings above 38°C in healthy volunteers wearing body armor during walking in a hot environment. In that study, the degree of exercise-induced heat stress was related to environmental temperature and relative humidity. Thus it is possible that in individuals that experience a mTBI or concussion in hot climates and/or during strenuous periods of activity, mildly elevated temperatures could play a role in the vulnerability of their CNS to acute injury (Hermstad and Adams, 2010).
Several studies have reported that post-injury hyperthermia can enhance the degree of brain damage and worsen long-term functional consequences (Dietrich et al., 1996b; Suzuki et al., 2004; Whalen et al., 1997). The mechanisms underlying this detrimental effect appear to be multifactorial and have been investigated in several models of trauma and ischemia (Chatzipanteli et al., 2000; Chopp et al., 1988; Kinoshita et al., 2002; Vitarbo et al., 2004; Dietrich and Bramlett, 2007). In a study by Suehiro and colleagues (1999), extracellular levels of glutamate were significantly elevated under hyperthermic ischemic conditions. In another study by Sharma (2006), glutamate and aspartate levels were elevated in selective brain regions after periods of heat stress. Hyperthermia during a cerebral ischemic insult was also reported to increase the magnitude of extracellular glutamate release (Takagi et al., 1994). With regard to the present discussion, excitotoxic events have been hypothesized to participate in the vulnerability of the dentate hilar interneurons to ischemic, traumatic, and seizure events (Atkins et al., 2010; Avignone et al., 2005; Buckmaster and Dudek, 1997; Grady et al., 2003; Hunt et al., 2011; Lowenstein et al., 1992). Thus hyperthermia-aggravated and/or prolonged elevations in extracellular glutamate and intracellular Ca2+ levels after mTBI may be responsible for the enhanced vulnerability of this neuronal population to mTBI. The fact that post-traumatic hyperthermia alone did not aggravate histopathological outcomes emphasizes that pathomechanisms initiated concurrently with the initial insult are most sensitive to the temperature elevations produced under the present experimental conditions.
Augmented blood–brain barrier breakdown and increased inflammatory processes and cytokine elevations are also seen with some hyperthermic insults (Chatzipanteli et al., 2000; Dietrich and Bramlett, 2007; Dietrich et al., 1996b). The fact that in this study design, brain and body temperatures were elevated prior to the mTBI would potentially maximize the augmentation of these injury cascades and promote tissue destruction and secondary injury processes. Prolonged periods of exercise have also been reported to reduce cerebral blood flow (CBF) and elevate the cerebral metabolic rate of oxygen (CMRO2) in humans (Nybo et al., 2002; Rasmussen et al., 2010). In experimental studies, CBF and CMRO2 increased with induced periods of hyperthermia (Carlsson et al., 1976; Katsumura et al., 1995; Ohmoto et al., 1996). In addition, the normal autoregulatory CBF response to varying blood pressure levels may be impaired by hyperthermia (Katsumura et al., 1995). Although the severity of various metabolic and hemodynamic disturbances would be expected to be dependent on the level and duration of hyperthermia, similar abnormalities have been described to occur after TBI (Bullock et al., 1992; Ginsberg et al., 1997). It is therefore possible that mismatches in CBF/CMRO2 and impairments of autoregulation that occur with mTBI are aggravated by the addition of the hyperthermic insult. These abnormal cerebrovascular and metabolic events could aggravate the pathogenesis of contusion formation and subsequent traumatic events.
The coagulation system is activated during periods of heatstroke, and can potentially progress to severe conditions including vascular coagulation resulting in local areas of severe ischemia (al-Mashhadani et al., 1994; Bouchama et al., 1996; Dematte et al., 1998). In one experimental study, heat stress-induced activation of coagulation led to histopathological changes in the CNS (Bouchama et al., 2005). The fact that elevations in temperature may induce coagulopathy in the intact brain is important since previous TBI studies have identified multifocal sites of intravascular coagulation in human brain specimens and experimental animals (Greuters et al., 2011; Stein et al., 2002). Kaufman and colleagues (1984) reported evidence for intravascular microthrombosis and abnormal coagulation in cases of fatal head injury. Microthrombosis was also observed in brains of surgical specimens of human cerebral contusions, after FPI, and after head rotational acceleration injuries (Dietrich et al., 1996a; Stein and Smith, 2004; Stein et al., 2002). In this regard, coagulation abnormalities can be an independent predictor of clinical outcome (Saggar et al., 2009; Sun et al., 2011). In the present study, pre/post-traumatic hyperthermia may induce subclinical changes in the coagulation system that could be enhanced by the mild traumatic insult. This vascular response to hyperthermia and mTBI may promote micro-circulatory failure and clot formation, resulting in subsequent ischemic neuronal damage. Future studies are required to investigate these possibilities.
In addition to these injury mechanisms, damage to white matter structures including diffuse and less severe degrees of axonal damage is thought to underlie many of the functional consequences of TBI (Adams et al., 1989; Povlishock et al., 1992). Evidence for diffuse axonal injury after mild, moderate, or severe TBI is commonly observed using a variety of immunohistochemical approaches, including amyloid precursor protein or silver stains to identify patterns of axonal degeneration (Bramlett et al., 1997; Carbonell and Grady, 1999; Hall et al., 2008). Most recently, new imaging modalities including diffusion tensor imaging and MR spectroscopic imaging have been used to detect evidence of axonal and parenchymal injury after concussion or blast injury (Belanger et al., 2007; Donald et al., 2011; Inglese et al., 2005; Levin et al., 2010; Mac Mayer et al., 2010). In a study by Mayer and colleagues (2010), white matter abnormalities as indicated by greater fractional anisotropy in the corpus callosum and specific hemispheric tracts was observed in patients with sub-acute mTBI. In another clinical investigation that assessed changes in brain metabolites by MR spectroscopic imaging, widespread alterations were reported to occur after mTBI that correlated with measures of cognitive performance (Govind et al., 2010). In contrast, Levin and colleagues (2010) failed to show evidence for white matter injury with mild-to-moderate blast-related TBI in veterans despite long-lasting deficits in verbal memory. In reference to the effects of hyperthermia on axonal damage after TBI, a previous study by Suzuki and colleagues (2004) reported that post-traumatic hyperthermia aggravated axonal damage as indicated by the frequency of amyloid precursor protein-immunoreactive axonal profiles after moderate FPI. Thus, future studies are required with mTBI to determine whether white matter structures are also highly vulnerable to the effects of pre-traumatic hyperthermia.
In conclusion, we have demonstrated the increased vulnerability of the mildly traumatized brain to periods of elevated temperature. Elevating the brain and core temperature immediately prior to mTBI produced more severe changes in contusion volume and damage to dentate hilar neurons. This finding would suggest that strategies that include the critical measurement and management of temperatures before or following an mTBI or concussion could be beneficial in limiting tissue vulnerability and subsequent behavioral deficits. Future studies are required to clarify the cellular and molecular mechanisms underlying these temperature-related responses and the testing of therapeutic interventions to limit these structural and functional consequences.
Acknowledgments
The authors wish to thank Jeremy Lytle for editorial assistance and David Sequeira and Yuan Kang for technical support. This study was supported in part by NIH grants R01 NS 042133, R01 NS 056072, and P50 NS 030291.
Author Disclosure Statement
No competing financial interests exist.
References
- Adams J.H. Doyle D. Ford I. Gennarelli T.A. Graham D.I. McLellan D.R. Diffuse axonal injury in head injury: definition, diagnosis and grading. Histopathology. 1989;15:49–59. doi: 10.1111/j.1365-2559.1989.tb03040.x. [DOI] [PubMed] [Google Scholar]
- al-Mashhadani S.A. Gader A.G. al Harthi S.S. Kangav D. Shaheen F.A. Bogus F. The coagulopathy of heat stroke: alterations in coagulation and fibrinolysis in heat stroke patients during the pilgrimage (Haj) to Makkah. Blood Coagul. Fibrinolysis. 1994;5:731–736. doi: 10.1097/00001721-199410000-00009. [DOI] [PubMed] [Google Scholar]
- Atkins C.M. Truettner J.S. Lotocki L. Sanchez-Molano J. Kang Y. Alonso O.F. Sick T.J. Dietrich W.D. Bramlett H.M. Post-traumatic seizure susceptibility is attenuated by hypothermia therapy. Eur. J. Neurosci. 2010;28:35–42. doi: 10.1111/j.1460-9568.2010.07467.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avignone E. Frenguelli B.G. Irving A.J. Differential responses to NMDA receptor activation in rat hippocampal interneurons and pyramidal cells may underlie enhanced pyramidal cell vulnerability. Eur. J. Neurosci. 2005;22:3077–3090. doi: 10.1111/j.1460-9568.2005.04497.x. [DOI] [PubMed] [Google Scholar]
- Babikian T. Satz P. Zaucha K. Light R. Lewis R.S. Asarnow R.F. The UCLA longitudinal study of neurocognitive outcomes following mild pediatric traumatic brain injury. J. Int. Neuropsychol. Soc. 2011;17:886–895. doi: 10.1017/S1355617711000907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belanger H.G. Vanderploeg R.D. Curtiss G. Warden D.L. Recent neuroimaging techniques in mild traumatic brain injury. J. Neuropsychiatry Clin. Neurosci. 2007;19:5–20. doi: 10.1176/jnp.2007.19.1.5. [DOI] [PubMed] [Google Scholar]
- Bouchama A. Bridey F. Hammami M.M. Lacombe C. al-Shail E. al-Ohali Y. Combe F. al-Sedairy S. de Prost D. Activation of coagulation and fibrinolysis in heatstroke. Thromb. Haemost. 1996;76:909–915. [PubMed] [Google Scholar]
- Bouchama A. Roberts G. Al Mohanna F. El-Sayed R. Lach B. Chollet-Martin S. Ollivier V. Al Baradei R. Loualich A. Nakeeb S. Eldali A. de Prost D. Inflammatory, hemostatic, and clinical changes in a baboon experimental model for heatstroke. J. Appl. Physiol. 2005;98:697–705. doi: 10.1152/japplphysiol.00461.2004. [DOI] [PubMed] [Google Scholar]
- Bramlett H.M. Kraydieh S. Green E.J. Dietrich W.D. Temporal and regional patterns of axonal damage following traumatic brain injury: A b-amyloid precursor protein immunocytochemical study in rats. J. Neuropathol. Exp. Neurol. 1997;56:1132–1141. doi: 10.1097/00005072-199710000-00007. [DOI] [PubMed] [Google Scholar]
- Buckmaster P.S. Dudek F.E. Neuron loss, granule cell axon reorganization, and functional changes in the dentate gyrus of epileptic kainate-treated rats. J. Comp. Neurol. 1997;385:385–404. [PubMed] [Google Scholar]
- Bullock R. Sakas D. Patterson J. Wyper D. Hadley D. Maxwell W. Teasdale G.M. Early post-traumatic cerebral blood flow mapping: correlation with structural damage after focal injury. Acta Neurochir. Suppl. (Wien.) 1992;55:14–17. doi: 10.1007/978-3-7091-9233-7_5. [DOI] [PubMed] [Google Scholar]
- Cabanac M. Temperature regulation. Annu. Rev. Physiol. 1975;37:415–439. doi: 10.1146/annurev.ph.37.030175.002215. [DOI] [PubMed] [Google Scholar]
- Carbonell W.S. Grady M.S. Regional and temporal characterization of neuronal, glial, and axonal response after traumatic brain injury in the mouse. Acta Neuropathol. (Berl.) 1999;98:396–406. doi: 10.1007/s004010051100. [DOI] [PubMed] [Google Scholar]
- Carlsson C. Hagerdal M. Siesjo B.K. The effect of hyperthermia upon oxygen consumption and upon organic phosphates, glycolytic metabolites, citric and cycle intermediates and associated amino acids in rat cerebral cortex. J. Neurochem. 1976;26:1001–1006. doi: 10.1111/j.1471-4159.1976.tb06484.x. [DOI] [PubMed] [Google Scholar]
- Chatzipanteli K. Alonso O.F. Kraydieh S. Dietrich W.D. Importance of posttraumatic hypothermia and hyperthermia on the inflammatory response after fluid percussion brain injury: Biochemical and immunocytochemical studies. J. Cereb. Blood Flow Metab. 2000;20:531–542. doi: 10.1097/00004647-200003000-00012. [DOI] [PubMed] [Google Scholar]
- Childs C. Vail A. Leach P. Rainey T. Protheroe R. King A. Brain temperature and outcome after severe traumatic brain injury. Neurocrit. Care. 2006;5:10–14. doi: 10.1385/NCC:5:1:10. [DOI] [PubMed] [Google Scholar]
- Chinevere T.D. Cadarette B.S. Goodman D.A. Ely B.R. Cheuvront S.N. Sawka M.N. Efficacy of body ventilation system for reducing strain in warm and hot climates. Eur. J. Appl. Physiol. 2008;103:307–314. doi: 10.1007/s00421-008-0707-9. [DOI] [PubMed] [Google Scholar]
- Chopp M. Welch K.M. Tidwell C.D. Knight R. Helpern J.A. Effect of mild hyperthermia on recovery of metabolic function after global cerebral ischemia in cats. Stroke. 1988;19:1521–1525. doi: 10.1161/01.str.19.12.1521. [DOI] [PubMed] [Google Scholar]
- Clifton G.L. Miller E.R. Choi S.C. Levin H.S. McCauley S. Smith K.R., Jr. Muizelaar J.P. Wagner F.C., Jr. Marion D.W. Luerssen T.G. Chesnut R.M. Schwartz M. Lack of effect of induction of hypothermia after acute brain injury. N. Engl. J. Med. 2001;344:556–563. doi: 10.1056/NEJM200102223440803. [DOI] [PubMed] [Google Scholar]
- Collins M.W. Grindel S.H. Lovell M.R. Dede D.E. Moser D.J. Phalin B.R. Nogle S. Wasik M. Cordry D. Daugherty K.M. Sears S.F. Nicolette G. Indelicato P. McKeag D.B. Relationship between concussion and neuropsychological performance in college football players. JAMA. 1999;282:964–970. doi: 10.1001/jama.282.10.964. [DOI] [PubMed] [Google Scholar]
- Dematte J.E. O'Mara K. Buescher J. Whitney C.G. Forsythe S. McNamee T. Adiga R.B. Ndukwu I.M. Near-fatal heat stroke during the 1995 heat wave in Chicago. Ann. Intern. Med. 1998;129:173–181. doi: 10.7326/0003-4819-129-3-199808010-00001. [DOI] [PubMed] [Google Scholar]
- DeWitt D.S. Prough D.S. Blast-induced brain injury and posttraumatic hypotension and hypoxemia. J. Neurotrauma. 2009;26:877–887. doi: 10.1089/neu.2007.0439. [DOI] [PubMed] [Google Scholar]
- Dietrich W.D. Bramlett H.M. Hyperthermia and central nervous system injury. Prog. Brain Res. 2007;162:201–217. doi: 10.1016/S0079-6123(06)62011-6. [DOI] [PubMed] [Google Scholar]
- Dietrich W.D. Bramlett H.M. The evidence for hypothermia as a neuroprotectant in traumatic brain injury. Neurotherapeutics. 2010;7:43–50. doi: 10.1016/j.nurt.2009.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich W.D. Alonso O. Busto R. Prado R. Dewanjee S. Dewanjee M.K. Ginsberg M.D. Widespread hemodynamic depression and focal platelet accumulation after fluid percussion brain injury: A double-label autoradiographic study in rats. J. Cereb. Blood Flow Metab. 1996a;16:481–489. doi: 10.1097/00004647-199605000-00015. [DOI] [PubMed] [Google Scholar]
- Dietrich W.D. Alonso O. Halley M. Busto R. Delayed posttraumatic brain hyperthermia worsens outcome after fluid percussion brain injury: a light and electron microscopic study in rats. Neurosurgery. 1996b;38:533–541. doi: 10.1097/00006123-199603000-00023. [DOI] [PubMed] [Google Scholar]
- Dikranian K. Cohen R. Mac Donald C. Pan Y. Brakefield D. Bayly P. Parsadanian A. Mild traumatic brain injury to the infant mouse causes robust white matter axonal degeneration which precedes apoptotic death of cortical and thalamic neurons. Exp. Neurol. 2008;211:551–560. doi: 10.1016/j.expneurol.2008.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon C.E. Lyeth B.G. Povlishock J.T. Findling R.L. Hamm R.J. Marmarou A. Young H.F. Hayes R.L. A fluid percussion model of experimental brain injury in the rat. J. Neurosurg. 1987;67:110–119. doi: 10.3171/jns.1987.67.1.0110. [DOI] [PubMed] [Google Scholar]
- Ginsberg M.D. Zhao W. Alonso O.F. Loor-Estades J.Y. Dietrich W.D. Busto R. Uncoupling of local cerebral glucose metabolism and blood flow after acute fluid-percussion injury in rats. Am. J. Physiol. 1997;272:H2859–H2868. doi: 10.1152/ajpheart.1997.272.6.H2859. [DOI] [PubMed] [Google Scholar]
- Goosey-Tolfrey V. Swainson M. Boyd C. Atkinson G. Tolfrey K. The effectiveness of hand cooling at reducing exercise-induced hyperthermia and improving distance-race performance in wheelchair and able-bodied athletes. J. Appl. Physiol. 2008;105:37–43. doi: 10.1152/japplphysiol.01084.2007. [DOI] [PubMed] [Google Scholar]
- Govind V. Gold S. Kaliannan K. Saigal G. Falcone S. Arheart K.L. Harris L. Jagid J. Maudsley A.A. Whole-brain proton MR spectroscopic imaging of mild-to-moderate traumatic brain injury and correlation with neuropsychological deficits. J. Neurotrauma. 2010;27:483–496. doi: 10.1089/neu.2009.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady M.S. Charleston J.S. Maris D. Witgen B.M. Lifshitz J. Neuronal and glial cell number in the hippocampus after experimental traumatic brain injury: Analysis by stereological estimation. J. Neurotrauma. 2003;20:929–941. doi: 10.1089/089771503770195786. [DOI] [PubMed] [Google Scholar]
- Greuters S. van den Berg A. Franschman G. Viersen V.A. Beishuizen A. Peerdeman S.M. Boer C. Acute and delayed mild coagulopathy are related to outcome in patients with isolated traumatic brain injury. Crit. Care. 2011;15:R2. doi: 10.1186/cc9399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall E.D. Bryant Y.D. Cho W. Sullivan P.G. Evolution of post-traumatic neurodegeneration after controlled cortical impact traumatic brain injury in mice and rats as assessed by the de Olmos silver and fluorojade staining methods. J. Neurotrauma. 2008;25:235–247. doi: 10.1089/neu.2007.0383. [DOI] [PubMed] [Google Scholar]
- Hayward J.N. Baker M.A. Role of cerebral arterial blood in the regulation of brain temperature in the monkey. Am. J. Physiol. 1968;215:389–403. doi: 10.1152/ajplegacy.1968.215.2.389. [DOI] [PubMed] [Google Scholar]
- Hermstad E. Adams B. Traumatic brain injury complicated by environmental hyperthermia. J. Emerg. Trauma Shock. 2010;3:66–69. doi: 10.4103/0974-2700.58660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt R.F. Scheff S.W. Smith B.N. Synaptic reorganization of inhibitory hilar interneuron circuitry after traumatic brain injury in mice. J. Neurosci. 2011;31:6880–6890. doi: 10.1523/JNEUROSCI.0032-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inglese M. Makani S. Johnson G. Cohen B.A. Silver J.A. Gonen O. Grossman R.I. Diffuse axonal injury in mild traumatic brain injury: a diffusion tensor imaging study. J. Neurosurg. 2005;103:298–303. doi: 10.3171/jns.2005.103.2.0298. [DOI] [PubMed] [Google Scholar]
- Jiang J.Y. Clinical study of mild hypothermia treatment for severe traumatic brain injury. J. Neurotrauma. 2009;26:399–406. doi: 10.1089/neu.2008.0525. [DOI] [PubMed] [Google Scholar]
- Jiang J.Y. Gao G.Y. Li W.P. Yu M.K. Zhu C. Early indicators of prognosis in 846 cases of severe traumatic brain injury. J. Neurotrauma. 2002;19:869–874. doi: 10.1089/08977150260190456. [DOI] [PubMed] [Google Scholar]
- Katsumura H. Kabuto M. Hosotani K. Handa Y. Kobayashi H. Kubota T. The influence of total body hyperthermia on brain haemodynamics and blood-brain barrier in dogs. Acta Neurochir. (Wien.) 1995;135:62–69. doi: 10.1007/BF02307416. [DOI] [PubMed] [Google Scholar]
- Kaufman H.H. Hui K.S. Mattson J.C. Borit A. Childs T.L. Hoots W.K. Bernstein D.P. Makela M.E. Wagner K.A. Kahan B.D. Gildenberg P.L. Clinicopathological correlations of disseminated intravascular coagulation in patients with head injury. Neurosurgery. 1984;15:34–42. doi: 10.1227/00006123-198407000-00008. [DOI] [PubMed] [Google Scholar]
- Kilpatrick M.M. Lowry D.W. Firlik A.D. Yonas H. Marion D.W. Hyperthermia in the neurosurgical intensive care unit. Neurosurgery. 2000;47:850–855. doi: 10.1097/00006123-200010000-00011. [DOI] [PubMed] [Google Scholar]
- Kinoshita K. Chatzipanteli K. Alonso O.F. Howard M. Dietrich W.D. The effect of brain temperature on hemoglobin extravasation after traumatic brain injury. J. Neurosurg. 2002;97:945–953. doi: 10.3171/jns.2002.97.4.0945. [DOI] [PubMed] [Google Scholar]
- Lange R.T. Iverson G.L. Rose A. Depression strongly influences postconcussion symptom reporting following mild traumatic brain injury. J. Head Trauma Rehabil. 2010;26:127–137. doi: 10.1097/HTR.0b013e3181e4622a. [DOI] [PubMed] [Google Scholar]
- Levin H.S. Wilde E. Troyanskaya M. Petersen N.J. Scheibel R. Newsome M. Radaideh M. Wu T. Yallampalli R. Chu Z. Li X. Diffusion tensor imaging of mild to moderate blast-related traumatic brain injury and its sequelae. J. Neurotrauma. 2010;27:683–694. doi: 10.1089/neu.2009.1073. [DOI] [PubMed] [Google Scholar]
- Lowenstein D.H. Thomas M.J. Smith D.H. McIntosh T.K. Selective vulnerability of dentate hilar neurons following traumatic brain injury: A potential mechanistic link between head trauma and disorders of the hippocampus. J. Neurosci. 1992;12:4846–4853. doi: 10.1523/JNEUROSCI.12-12-04846.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mac Donald C.L. Johnson A.M. Cooper D. Nelson E.C. Werner N.J. Shimony J.S. Snyder A.Z. Raichle M.E. Witherow J.R. Fang R. Flaherty S.F. Brody D.L. Detection of blast-related traumatic brain injury in U.S. military personnel. N. Engl. J. Med. 2011;364:2091–2100. doi: 10.1056/NEJMoa1008069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marion D. Bullock M.R. Current and future role of therapeutic hypothermia. J. Neurotrauma. 2009;26:455–467. doi: 10.1089/neu.2008.0582. [DOI] [PubMed] [Google Scholar]
- Mayer A.R. Ling J. Mannell M.V. Gasparovic C. Phillips J.P. Doezema D. Reichard R. Yeo R.A. A prospective diffusion tensor imaging study in mild traumatic brain injury. Neurology. 2010;74:643–650. doi: 10.1212/WNL.0b013e3181d0ccdd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer A.R. Mannell M.V. Ling J. Gasparovic C. Yeo R.A. Functional connectivity in mild traumatic brain injury. Hum. Brain Mapp. 2011 doi: 10.1002/hbm.21151. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natale J.E. Joseph J.G. Helfaer M.A. Shaffner D.H. Early hyperthermia after traumatic brain injury in children: risk factors, influence on length of stay, and effect on short-term neurologic status. Crit. Care Med. 2000;28:2608–2615. doi: 10.1097/00003246-200007000-00071. [DOI] [PubMed] [Google Scholar]
- Nybo L. Moller K. Volianitis S. Nielsen B. Secher N.H. Effects of hyperthermia on cerebral blood flow and metabolism during prolonged exercise in humans. J. Appl. Physiol. 2002;93:58–64. doi: 10.1152/japplphysiol.00049.2002. [DOI] [PubMed] [Google Scholar]
- Ohmoto Y. Fujisawa H. Ishikawa T. Koizumi H. Matsuda T. Ito H. Sequential changes in cerebral blood flow, early neuropathological consequences and blood-brain barrier disruption following radiofrequency-induced localized hyperthermia in the rat. Int. J. Hyperthermia. 1996;12:321–334. doi: 10.3109/02656739609022521. [DOI] [PubMed] [Google Scholar]
- Özgünen K.T. Kurdak S.S. Maughan R.J. Zeren C. Korkmaz S. Yazici Z. Ersoz G. Shirreffs S.M. Binnet M.S. Dvorak J. Effect of hot environmental conditions on physical activity patterns and temperature response of football players. Scand. J. Med. Sci. Sports. 2010;20(Suppl. 3):140–147. doi: 10.1111/j.1600-0838.2010.01219.x. [DOI] [PubMed] [Google Scholar]
- Polderman K.H. Induced hypothermia and fever control for prevention and treatment of neurological injuries. Lancet. 2008;371:1955–1969. doi: 10.1016/S0140-6736(08)60837-5. [DOI] [PubMed] [Google Scholar]
- Povlishock J.T. Erb D.E. Astruc J. Axonal response to traumatic brain injury: reactive axonal change, deafferentation, and neuroplasticity. J. Neurotrauma. 1992;9(Suppl. 1):S189–S200. [PubMed] [Google Scholar]
- Rasmussen P. Nybo L. Volianitis S. Moller K. Secher N.H. Gjedde A. Cerebral oxygenation is reduced during hyperthermic exercise in humans. Acta Physiol. (Oxf.) 2010;199:63–70. doi: 10.1111/j.1748-1716.2010.02084.x. [DOI] [PubMed] [Google Scholar]
- Saggar V. Mittal R.S. Vyas M.C. Hemostatic abnormalities in patients with closed head injuries and their role in predicting early mortality. J. Neurotrauma. 2009;26:1665–1668. doi: 10.1089/neu.2008.0799. [DOI] [PubMed] [Google Scholar]
- Sharma H.S. Hyperthermia influences excitatory and inhibitory amino acid neurotransmitters in the central nervous system. An experimental study in the rat using behavioural, biochemical, pharmacological, and morphological approaches. J. Neural Transm. 2006;113:497–519. doi: 10.1007/s00702-005-0406-1. [DOI] [PubMed] [Google Scholar]
- Shitaka Y. Tran H.T. Bennett R.E. Sanchez L. Levy M.A. Dikranian K. Brody D.L. Repetitive closed-skull traumatic brain injury in mice causes persistent multifocal axonal injury and microglial reactivity. J. Neuropathol. Exp. Neurol. 2011;70:551–567. doi: 10.1097/NEN.0b013e31821f891f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair H.L. Andrews P.J. Bench-to-bedside review: Hypothermia in traumatic brain injury. Crit. Care. 2010;14:204. doi: 10.1186/cc8220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smits M. Houston G.C. Dippel D.W. Wielopolski P.A. Vernooij M.W. Koudstaal P.J. Hunink M.G. van der Lugt A. Microstructural brain injury in post-concussion syndrome after minor head injury. Neuroradiology. 2010;53:553–563. doi: 10.1007/s00234-010-0774-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein S.C. Smith D.H. Coagulopathy in traumatic brain injury. Neurocrit. Care. 2004;1:479–488. doi: 10.1385/NCC:1:4:479. [DOI] [PubMed] [Google Scholar]
- Stein S.C. Chen X.H. Sinson G.P. Smith D.H. Intravascular coagulation: a major secondary insult in nonfatal traumatic brain injury. J. Neurosurg. 2002;97:1373–1377. doi: 10.3171/jns.2002.97.6.1373. [DOI] [PubMed] [Google Scholar]
- Stocchetti N. Rossi S. Zanier E.R. Colombo A. Beretta L. Citerio G. Pyrexia in head-injured patients admitted to intensive care. Intensive Care Med. 2002;28:1555–1562. doi: 10.1007/s00134-002-1513-1. [DOI] [PubMed] [Google Scholar]
- Suehiro E. Fujisawa H. Ito H. Ishikawa T. Maekawa T. Brain temperature modifies glutamate neurotoxicity in vivo. J. Neurotrauma. 1999;16:285–297. doi: 10.1089/neu.1999.16.285. [DOI] [PubMed] [Google Scholar]
- Sukstanskii A.L. Yablonskiy D.A. Theoretical model of temperature regulation in the brain during changes in functional activity. Proc. Natl. Acad. Sci. USA. 2006;103:12144–12149. doi: 10.1073/pnas.0604376103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y. Wang J. Wu X. Xi C. Gai Y. Liu H. Yuan Q. Wang E. Gao L. Hu J. Zhou L. Validating the incidence of coagulopathy and disseminated intravascular coagulation in patients with traumatic brain injury—analysis of 242 cases. Br. J. Neurosurg. 2011;25:363–368. doi: 10.3109/02688697.2011.552650. [DOI] [PubMed] [Google Scholar]
- Suzuki T. Bramlett H.M. Ruenes G. Dietrich W.D. The effects of early post-traumatic hyperthermia in female and ovariectomized rats. J. Neurotrauma. 2004;21:842–853. doi: 10.1089/0897715041526186. [DOI] [PubMed] [Google Scholar]
- Takagi K. Ginsberg M.D. Globus M.Y. Martinez E. Busto R. Effect of hyperthermia on glutamate release in ischemic penumbra after middle cerebral artery occlusion in rats. Am. J. Physiol. 1994;267:H1770–H1776. doi: 10.1152/ajpheart.1994.267.5.H1770. [DOI] [PubMed] [Google Scholar]
- Tay S.Y. Ang B.T. Lau X.Y. Meyyappan A. Collinson S.L. Chronic impairment of prospective memory after mild traumatic brain injury. J. Neurotrauma. 2010;27:77–83. doi: 10.1089/neu.2009.1074. [DOI] [PubMed] [Google Scholar]
- Terrio H. Brenner L.A. Ivins B.J. Cho J.M. Helmick K. Schwab K. Scally K. Bretthauer R. Warden D. Traumatic brain injury screening: preliminary findings in a US Army Brigade Combat Team. J. Head Trauma Rehabil. 2009;24:14–23. doi: 10.1097/HTR.0b013e31819581d8. [DOI] [PubMed] [Google Scholar]
- Thurman D.J. Branche C.M. Sniezek J.E. The epidemiology of sports-related traumatic brain injuries in the United States: recent developments. J. Head Trauma Rehabil. 1998;13:1–8. doi: 10.1097/00001199-199804000-00003. [DOI] [PubMed] [Google Scholar]
- Vasterling J.J. Verfaellie M. Sullivan K.D. Mild traumatic brain injury and posttraumatic stress disorder in returning veterans: perspectives from cognitive neuroscience. Clin. Psychol. Rev. 2009;29:674–684. doi: 10.1016/j.cpr.2009.08.004. [DOI] [PubMed] [Google Scholar]
- Vitarbo E.A. Chatzipanteli K. Kinoshita K. Truettner J.S. Alonso O.F. Dietrich W.D. Tumor necrosis factor a expression and protein levels after fluid percussion injury in rats: The effect of injury severity and brain temperature. Neurosurgery. 2004;55:416–424. doi: 10.1227/01.neu.0000130036.52521.2c. [DOI] [PubMed] [Google Scholar]
- Whalen M.J. Carlos T.M. Clark R.S. Marion D.W. DeKosky S.T. Heineman S. Schiding J.K. Memarzadeh F. Kochanek P.M. The effect of brain temperature on acute inflammation after traumatic brain injury in rats. J. Neurotrauma. 1997;14:561–572. doi: 10.1089/neu.1997.14.561. [DOI] [PubMed] [Google Scholar]