Abstract
The current study used a rat model to investigate the underlying mechanisms of blast-induced tinnitus, hearing loss, and associated traumatic brain injury (TBI). Seven rats were used to evaluate behavioral evidence of tinnitus and hearing loss, and TBI using magnetic resonance imaging following a single 10-msec blast at 14 psi or 194 dB sound pressure level (SPL). The results demonstrated that the blast exposure induced early onset of tinnitus and central hearing impairment at a broad frequency range. The induced tinnitus and central hearing impairment tended to shift towards high frequencies over time. Hearing threshold measured with auditory brainstem responses also showed an immediate elevation followed by recovery on day 14, coinciding with behaviorally-measured results. Diffusion tensor magnetic resonance imaging results demonstrated significant damage and compensatory plastic changes to certain auditory brain regions, with the majority of changes occurring in the inferior colliculus and medial geniculate body. No significant microstructural changes found in the corpus callosum indicates that the currently adopted blast exposure mainly exerts effects through the auditory pathways rather than through direct impact onto the brain parenchyma. The results showed that this animal model is appropriate for investigation of the mechanisms underlying blast-induced tinnitus, hearing loss, and related TBI. Continued investigation along these lines will help identify pathology with injury/recovery patterns, aiding development of effective treatment strategies.
Key words: behavioral testing, blast, hearing loss, MRI, tinnitus, traumatic brain injury
Introduction
Tinnitus is a symptom characterized by the perception of sound in the absence of external stimuli. It is estimated that up to 50 million Americans have experienced tinnitus, and that as many as 16 million perceive tinnitus frequently (Adams and Marano, 1995; Shargorodsky et al., 2010). Tinnitus can inflict a wide range of distress; it has been strongly correlated with difficulty sleeping, irritability, anxiety, and depression (Andersson, 2002, 2004; Andersson and McKenna, 1998; Belli et al., 2008; Cronlein et al., 2007; Dobie, 2003; Erlandsson and Hallberg, 2000; Folmer and Griest, 2000; Folmer et al., 1999; Halford and Anderson, 1991; Hebert and Carrier, 2007; Marciano et al., 2003; McKenna, 2000; McKenna et al., 1991; Robinson et al., 2003; Tyler and Conrad-Armes, 1983; Tyler et al., 2007). Tinnitus has been shown to negatively influence cognitive functioning such as working memory and attention (Rossiter et al., 2006; Stevens et al., 2007), and at the extreme, has been linked to suicide (Johnston and Walker, 1996; Lewis et al., 1994; Turner et al., 2007). Accompanied by these clinical sequelae, the cost for seeking clinical help has been staggering. According to the American Tinnitus Association, the prevalence of tinnitus is high, with 3 to 4 million veterans suffering with this condition and up to 1 million seeking clinical services. Its economic impact becomes most apparent when military personnel transition to civilian life and seek health care services and disability benefits from the Department of Veterans Affairs. The monetary expenditures for treating this condition are extraordinary, exceeding hundreds of millions of dollars per year in disability compensation alone (Henry et al., 2009; Humes, 2006; Saunders and Griest, 2009), with costs expecting to top 2 billion dollars by 2011 (Fausti et al., 2009). This illustrates the significant economic burden to the federal government and society, prompting the need to investigate the underlying mechanisms of tinnitus so that effective treatment can be developed.
While there are many known causes of tinnitus, acoustic trauma is the most commonly reported (Axelsson and Sandh, 1985; Leske, 1981; Penner, 1995). Among the many forms of acoustic exposures, blast, a high-energy impulse noise, has received significant attention lately due to the large number of injured soldiers who were involved in recent war operations in Iraq and Afghanistan. Blast can induce a wide range of pathologies in the body, including auditory pathology such as tinnitus and hearing loss, as well as traumatic brain injury (TBI; Breeze et al., 2011; Dougherty et al., 2011; Fausti et al., 2009; Lew et al., 2009; Mrena et al., 2004a,2002; Okie, 2005). Ear injuries constitute the single most frequent injury type sustained in the Iraq War (Gondusky and Reiter, 2005). A random sample of Iraq war records revealed that 71% of soldiers experienced loud noises, and that 15.6% had tinnitus (Geckle and Lee, 2004). In addition, tinnitus is more prevalent in military personnel with TBI compared to those without TBI, with up to 38% experiencing comorbid tinnitus (Lew et al., 2007). This is significant given TBI's distinction as a signature injury from war theaters, with a prevalence rate of 15.8% among all service members (MacGregor et al., 2010). Although the impact of blast-related tinnitus, hearing loss, and TBI on soldiers involved in war theaters is substantial, there is no effective treatment for blast-induced tinnitus and its related TBI, mainly due to an unclear understanding of its underlying mechanisms.
Over the last decade, a considerable amount of research has focused on elucidating the pathophysiology of tinnitus. It is widely held that tinnitus results from damage to the auditory periphery that triggers plastic reorganization at the central level (Eggermont and Roberts, 2004; Kaltenbach, 2011; Roberts et al., 2010). The plastic changes in the brain are often manifested in an increased spontaneous firing rate, increased bursting events, and neurosynchrony. For example, noise exposure with various parameters, including pure tones and band noise of 4–15 kHz, for 20 min to 4 h at 80–127 dB sound pressure level (SPL), has been shown to cause an immediate increase in spontaneous firing rate in the primary auditory cortex (AC; Kimura and Eggermont, 1999; Komiya and Eggermont, 2000; Norena and Eggermont, 2005; Seki and Eggermont, 2003), and delayed onset of hyperactivity in the dorsal cochlear nucleus (DCN; Brozoski et al., 2002; Kaltenbach and Afman, 2000; Kaltenbach and McCaslin, 1996; Kaltenbach et al., 1998; Zhang and Kaltenbach, 1998) and inferior colliculus (IC; Bauer et al., 2008; Mulders and Robertson, 2009). Noise exposure can also elicit an increase in bursting activity in the auditory nerve, DCN, and IC (Bauer et al., 2008; Chen and Jastreboff, 1995; Finlayson and Kaltenbach, 2009; Liberman and Kiang, 1978). Bursting activity, however, has not been enduringly affected in the AC (Eggermont and Komiya, 2000; Norena and Eggermont, 2003; Seki and Eggermont, 2003). The above evidence has contributed to the understanding of the neurophysiological mechanisms underlying tinnitus.
With the advantage of being able to explore multiple structures noninvasively, neuroimaging has been utilized to investigate the neural mechanisms of tinnitus. Functional magnetic resonance imaging (fMRI) studies have found that patients with tinnitus have elevated baseline activity in the IC (Melcher et al., 2000), and differential patterns of activation in auditory centers such as the CN (Lanting et al., 2010), the IC (Lanting et al., 2009), the medial geniculate body (MGB), and the primary AC (Smits et al., 2007). Using positron emission tomography (PET), increased activity has been found in multiple brain areas, including the primary and nonprimary AC and prefrontal cortex in tinnitus patients (Arnold et al., 1996; Lockwood et al., 2001). In addition, manganese-enhanced magnetic resonance imaging uses manganese as an activity-dependent, paramagnetic contrast agent to track structural and functional information on the central nervous system (Silva et al., 2004). Ex vivo scans of rats with behavioral evidence of tinnitus depict increased activity in the ipsilateral paraflocculus, the ipsilateral posteroventral cochlear nucleus, and the contralateral IC (Brozoski et al., 2007), while in vivo scans revealed increased activity in the dorsal cortex of the IC (Holt et al., 2010). Apparently, more work needs to be done to obtain detailed and consistent information using this new imaging tool.
Over recent years, diffusion tensor imaging (DTI) has been developed to assess the microstructural integrity of white matter tissue by characterizing the movement of water molecules. DTI has been reported to be more sensitive in detecting neuropathology than conventional MRI (Kumar et al., 2009; Newcombe et al., 2010). It has been frequently utilized to investigate TBI, and has become a sensitive measurement in mild TBI, for which structural abnormalities hidden from standard imaging modalities were detected by DTI (Kumar et al., 2009). In contrast to apparently normal results from conventional MRI scanning, DTI showed significant changes in fractional anisotropy (FA), mean diffusivity value, apparent diffusion coefficient (ADC), and radial diffusivity (RD) in patients with TBI, intracerebral and subdural hemorrhages, hemispheric swelling, hematoma, and eventual encephalomalacia (Lee et al., 2006).
In addition to measuring white matter tissue, recent work has extended the use of DTI parameters to nuclei, such as the IC (Dror et al., 2010; Lin et al., 2008; Wu et al., 2009b), thalamus (Dror et al., 2010; Lutz et al., 2007), and amygdala (Kantarci et al., 2010; Lutz et al., 2008; Solano-Castiella et al., 2010). While not as dense as white matter structures, these structures still contain many passing and local nerve fibers. Indeed, decreased axial diffusivity (AD) and FA in the DCN and IC were found in subjects with sensorineural hearing loss (Wu et al., 2009a), coinciding with intense tone exposure-induced axonal degeneration (Kim et al., 1997). At the thalamic level, limited DTI studies have shown negative results in the MGB of tinnitus patients without TBI (Crippa et al., 2010; Lutz et al., 2007). However, significant neuronal loss was found in the MGB in a lateral fluid percussion-induced TBI model (Blaha et al., 2000; Hicks et al., 1996; Sanderson et al., 1999). At the cortical level, changes in grey matter volume and white matter connectivity of the AC have been seen in patients with tinnitus and hearing loss (Crippa et al., 2010; Husain et al., 2011), which is consistent with the traumatic noise exposure-induced decrease in cellular density of the AC (Basta et al., 2005; Groschel et al., 2010). However, the structural changes to the AC and its relationship to tinnitus and its related TBI remain virtually unstudied. In non-auditory structures such as the amygdala (Kovesdi et al., 2011; Lifshitz et al., 2007; Roe et al., 1998) and corpus callosum (Bendlin et al., 2008; Kraus et al., 2007; Levin et al., 2010; Xu et al., 2007), both positive and negative changes have been reported following blast- or lateral fluid percussion-induced TBI. The above information demonstrate the feasibility and advantage of using DTI to delineate the mechanisms underlying blast-induced tinnitus, hearing loss, and TBI in the current animal model.
In this study, we characterized blast-induced tinnitus using a gap detection acoustic startle reflex behavioral paradigm, which has been used as a screening tool for tinnitus (Turner and Parrish, 2008; Turner et al., 2006; Wang et al., 2009; Yang et al., 2007; Zhang et al., 2011). Acoustic startle reflex has also been used to assess sensorimotor reactivity of a rat model of TBI (Wiley et al., 1996). At the same time, we evaluated the hearing condition following blast exposure at both peripheral and central levels using auditory brainstem responses (ABRs) and a prepulse inhibition (PPI) behavioral paradigm. We used DTI to investigate the microstructural changes in both auditory and non-auditory structures of rats that demonstrated blast-induced tinnitus and hearing loss. The eventual goal is to better understand the neurological mechanisms associated with blast-related tinnitus, hearing loss, and TBI, so that prevention and treatment strategies may be developed.
Methods
Animal subjects
Ten Long-Evans rats (male, 60–70 days old) were purchased from Charles River Laboratories (Wilmington, MA). Behavioral testing and MRI scanning were conducted before and after blast exposure to induce tinnitus, hearing loss, and TBI. Three rats died over the course of the study and were not used in the behavioral and MRI data analysis. In this study, an internal control approach was taken and data from the 7 rats from after blast exposure were compared with those from before blast exposure. Since pre-blast behavioral data had stabilized, they could be reliably compared with post-exposure data in order to identify pathology. Adding further justification is our experience that repeated testing of untreated groups of rats over several months itself does not affect GAP/PPI behavioral responses. All experimental procedures were conducted in accordance with the guidelines of the Institutional Animal Care and Use Committee at Wayne State University.
Behavior testing: Before blast exposure
Before blast exposure, each rat received initial behavioral testing for the presence of tinnitus and hearing loss using gap detection (GAP) and PPI, respectively. All behavior testing was conducted with Hamilton-Kinder startle reflex hardware and software (Hamilton-Kinder Behavioral Testing Systems, Poway, CA). Each rat was placed in a polycarbonate animal holder built with openings for sound permeation. The holder reduced extraneous movement that could interfere with the acoustic startle reflex recording. Each animal holder was plugged into the inside of an SM100 StartleMonitor cabinet, and the floor of each holder was connected to a piezo transducer, which is used to measure startle force. StartleMonitor cabinets were equipped with one ceiling speaker to present background and prepulse stimuli, and a second ceiling speaker to present startle stimuli. Prior to each testing session, calibration of startle force and acoustic stimuli was completed using a Newton Impulse Calibrator (Hamilton-Kinder Behavioral Testing Systems) and a microphone (Model 4016; ACO Pacific, Belmont, CA).
The GAP procedure involved presentation of a constant 65-dB SPL background noise to the rat. The background noise was composed of 2000 Hz bandpass signals at 2–4, 6–8, 8–10, 10–12, 14–16, 18–20, 22–24, 28–30 kHz, and broadband (BBN, 6–32 kHz). The GAP procedure takes advantage of the acoustic startle reflex in rats, which can be reliably induced by a loud and intrusive sound stimulus. The introduction of a silent period or gap within the background noise prior to the startle stimulus can be detected by the rat and consequently reduces its acoustic startle reflex. It is believed that the presence of tinnitus with quality similar to the background noise will override the silent gap, and therefore the rat will not suppress its startle reflex (Turner and Parrish, 2008; Turner et al., 2006; Wang et al., 2009; Yang et al., 2007; Zhang et al., 2011). We used a 115-dB SPL, 50-msec noise burst to induce the acoustic startle reflex. The startle force of the rats (in Newtons) in response to three testing conditions was collected: (1) background noise alone, (2) startle stimulus during background noise (startle only), and (3) startle stimulus during background noise, with a silent gap in background noise preceding the startle stimulus (GAP-startle). In the last condition, a 50-msec silent gap was introduced 100 msec before the startle stimulus. Ten trials of each testing condition were administered for each background noise frequency, including BBN.
The PPI procedure and parameters were the same as for the GAP procedure, except that no background noise or silent gaps were used. For PPI, the acoustic startle reflex of rats in response to two testing conditions was measured: (1) the startle-only, and (2) a prepulse followed by the startle stimulus (PPI-startle). In the last condition, a 65-dB SPL, 50-msec prepulse was introduced 100 msec before the startle stimulus. The animal reduces its acoustic startle reflex in response to the prepulse, except when there is hearing loss at a frequency similar to the prepulse.
Both GAP and PPI procedures were run sequentially, and a 2-min acclimatization period was given at the beginning of both tests. Two trials of the startle stimulus without background noise were given after the acclimatization period to trigger and dispose of any initial, exaggerated startle reflexes. Startle stimulus, GAP-startle, or PPI-startle were pseudorandomly mixed to prevent order effects. Running time for both tests was approximately 1 h and 20 min.
Auditory brainstem responses: Before blast exposure
Prior to blast exposure, the rats underwent ABR testing. Briefly, each rat was anesthetized using a ketamine and xylazine mixture (100 mg/kg+10 mg/kg IP). The rat was placed in an acoustical enclosure (Tracoustics Inc., Austin, TX) and arranged in a prone position with its head fixed to a stereotaxic apparatus. Body temperature was regulated with a warming blanket connected to a homeothermic control unit. ABR responses were elicited by click stimuli delivered from a TDT EC1 model electrostatic speaker (Tucker Davis Technologies, Alachua, FL) through a tube inserted into the right external auditory canal. Click stimuli were generated by an RX6 multifunction processor and calibrated before experimentation with a microphone. Clicks were presented from 80 dB peak equivalent SPL down to 5 dB in 5-dB decremental steps.
Evoked potentials were recorded using platinum subdermal needle electrodes. The positive recording electrode was placed at the vertex, while the reference electrode was placed beneath the right ear pinna, and the ground electrode was placed in the contralateral temporalis muscle. ABR responses were bandpass-filtered at 300–3000 Hz, notch-filtered at 60 Hz, and averaged 300 times. The sampling rate for data acquisition was 50 kHz. Experimental operation was controlled by SigGenRP® and BioSigRP® TDT software installed in an IBM terminal connected to a System 3 TDT workstation. For analysis, ABR threshold was considered the lowest intensity at which a distinct, biological portion of the waveform remained.
MRI imaging: Before blast exposure
Rats underwent MRI scanning prior to blast exposure. Before image acquisition, each rat was anesthetized through inhalation with a mixture of oxygen flow of 0.4 L/min and isoflurane flow of 1–2.5% v/v. The rat was placed in a prone position and restrained on a cradle with a custom-built palate holder equipped with an adjustable nose cone and stereotaxic ear bars to inhibit movement during MRI scans. Its head was positioned at the isocenter of a magnet (Shen et al., 2007).
All of the MRI data acquisition was performed on a 4.7-Tesla horizontal-bore magnetic resonance spectrometer (Bruker AVANCE) with an 11.6-cm bore actively shielded gradient coil set capable of producing a magnetic field gradient of up to 250 mT/m. A whole-body birdcage radio frequency (RF) coil (72 mm inner diameter) was used as the transmitter for homogeneous RF excitation, and a surface coil (30 mm diameter) as the receiver, with active RF-decoupling to avoid signal interference.
Sequences from this set of experiments included T2- and T1-weighted imaging and DTI for the determination of water diffusion. For all sequences, the field of view was 40×40×24 mm3; thus, the whole brain was imaged. T2-weighted imaging was conducted using rapid acquisition with relaxation enhancement (RARE) with the parameters: repetition time (TR)=2500 msec, echo time (TE)=46 msec, field of view (FOV)=32×32 mm2, acquisition matrix=256×256, number of slices=24, slice thickness=1 mm, Nacq=2, TA=2 min 40 sec. T1-weighted imaging was conducted using 3D fast low angle shot (FLASH) with these parameters: TR=22 ms, TE=7 ms, flip angle (FA)=5° and 20°, FOV=32×32×24 mm3, matrix size=256×256×24, Nacq=1, TA=3 min for each.
For DTI, the standard 2D spin echo DTI sequence was used in vivo with 6 gradient encoding orientations uniformly distributed in space. Parameters were: TE=37 msec, TR=1.9 sec, b=0 and 800 sec/mm2, diffusion gradient duration/separation=8/20 msec, FOV=32×32 mm2, matrix size of 128×128, interpolated to 256×256, 19 slices of 1 mm slice thickness, Nacq=1, TA=29 min.
Blast exposure
Rats were subjected to a single blast exposure using a custom-designed shock tube located at the Wayne State University Bioengineering Center (Leonardi et al., 2010). Each rat was first anesthetized by intraperitoneal injection using a mixture of ketamine and xylazine (100 mg/kg+10 mg/kg IP). The rat was then placed on supportive netting via a metal surround and secured on a pole with a locking device 44 inches up from the open end of the tube. The rat was placed in a prone position with its head facing the oncoming shockwave. To better mimic the situation in the battlefield, no earplugs were used. The 10-msec blast exposure was estimated to be in a wide range of frequencies. The average energy under 10 kHz was measured at 14 psi, which was translated to be around 95 kPa or 194 dB SPL. After blast exposure, the rat was transferred to a polycarbonate cage. It was placed on a water circulating heating pad to prevent hypothermia, and allowed to recover from anesthesia.
Behavioral testing, ABR measures, and MRI imaging: After blast exposure
Following blast exposure, the same behavioral testing, ABR measurement, and MRI imaging were performed to examine blast-induced effects, including behavioral evidence of tinnitus, compromised hearing, and neural changes in both auditory and non-auditory centers. Both behavioral and ABR testing were performed on post-blast exposure days 1, 14, 28, and 90, while MRI imaging was performed during post-blast exposure weeks 2 and 4. MRI imaging was not performed on post-blast exposure day 1 or day 90 due to unavailability of the MRI scanner. After completion of all the above experimental procedures, each animal was euthanized by an overdose injection of mixed ketamine and xylazine.
Statistical analysis
The goal of this study was to produce blast-induced tinnitus and to examine the related neural changes in both auditory and non-auditory centers. We tracked the development of tinnitus with GAP testing and the development and recovery of hearing loss with ABR hearing thresholds and PPI data. DTI was employed to characterize the microstructural changes in the brain that accompanied blast-induced tinnitus. In all experiments, post-exposure testing data were compared with pre-blast data to identify behavioral and neuroplastic changes following blast exposure. Both behavioral and MRI imaging data were analyzed as described below.
Behavior was considered tinnitus-positive when the values of post-blast GAP ratios were greater than pre-blast ratios. Similarly, rats were considered positive for hearing impairment when the values of post-blast PPI ratios were greater than pre-blast ratios. GAP ratios were computed by taking the force of each acoustic startle reflex in response to the startle stimulus preceded-by-GAP condition (10 in total), and dividing it by the mean force of startle reflexes in response to the startle-only condition. Each of these GAP ratios is then averaged, making the final GAP ratio. Final GAP ratios were calculated for each frequency of background noise at which the GAP was tested. PPI ratios were computed in the same way, except that the startle stimulus preceded-by-PPI condition was used, and final PPI ratios were calculated for each frequency of prepulse. An elevated post-blast startle ratio at a given frequency of GAP or PPI suggests tinnitus or hearing impairment at that frequency, respectively. Construing data as tinnitus-positive, however, becomes difficult when there is positive hearing loss at the same frequency. In this scenario, GAP might be impaired simply due to an inability to hear the background noise, and thus inability to detect the silent gap, so it cannot be known if the rat exclusively has hearing loss, or if it has both hearing loss and tinnitus. Positive hearing loss data were also considered when post-blast ABR thresholds were higher than pre-blast thresholds.
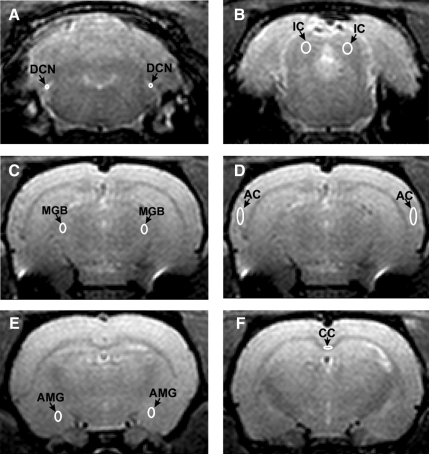
For MRI imaging analysis, we focused on both auditory and non-auditory brain structures. The auditory structures included the left and right DCN, IC, MGB, and AC, and the non-auditory structures included the amygdala and corpus callosum (CC). During analysis, a region of interest (ROI) was drawn on each of the above structures to generate corresponding mean values (comprised of the average of the left and right side structures). The locations of the structures were identified based on a rat brain atlas (Paxinos and Watston, 1998). Examples of ROIs can be seen in Figure 1. For DTI statistics, ROI drawings were performed twice by the same rater to obtain a measurement of intra-rater variability, and two rats were analyzed by a second rater for a measurement of inter-rater variability. Pearson correlation coefficient was used to compare intra-rater variability and inter-rater variability of ROI diffusion statistics.
FIG. 1.
Representative regions of interest (ROIs) for the DCN (A), IC (B), MGB (C), AC (D), AMG (E), and CC (F; DCN, dorsal cochlear nucleus; IC, inferior colliculus; MGB, medial geniculate body; AC, auditory cortex; AMG, amygdala; CC, corpus callosum).
In DTI analysis, AD, RD, FA, and ADC were evaluated to determine structural changes to the brain. Lower values in AD and FA following blast exposure are suggestive of axonal degeneration, while higher values of RD and ADC may represent myelin injury and ischemia (Kim et al., 2005; Song et al., 2003; Sun et al., 2006). Increased AD and FA are less commonly cited, although one study suggested that such findings may signify compensatory growth in one brain area in response to damage sustained by another brain area (Lutz et al., 2007). Changes, such as decreases in AD and FA in the corpus callosum, appear to be characteristic of TBI (Mac Donald et al., 2007). Controlled cortical impact that causes moderately severe TBI can reduce AD in mice (Mac Donald et al., 2007). Patients with complicated mild or moderate to severe TBI, children, and adolescents with moderate to severe TBI, and patients with severe and diffuse TBI sustained from motor vehicle accidents and falls were all found to have decreased FA in the corpus callosum (Caeyenberghs et al., 2011; Palacios et al., 2011; Wu et al., 2010). Moderate TBI has also been associated with decreased AD in the genu of the CC, while mild TBI has been linked to decreased FA in the genu of the CC (Kumar et al., 2009). In patients with mild to moderate blast-induced TBI, however, there was no significant change in FA or ADC in the CC (Levin et al., 2010).
For the above multiple comparisons of pre-blast with post-blast data, analysis of variance (ANOVA) was performed and the post-hoc Bonferroni method was used to adjust alpha values. Significance levels were set at 0.05.
Results
To induce tinnitus, hearing loss, and TBI, rats were exposed to a 10-msec, 194-dB SPL blast with both ears unprotected. Exposed rats demonstrated signs of early-onset tinnitus and hearing loss at multiple frequencies tested. While the induced tinnitus and hearing loss at a majority of frequencies had recovered by post-blast day 28, hearing loss at certain high frequencies remained through post-blast day 90 testing. Post-blast ABR thresholds were significantly elevated compared to pre-blast recordings, but recovered to baseline levels by post-blast day 14. DTI showed that blast had extensive impact in auditory brain regions, as manifested by signs of demyelination, ischemia, and compensatory axonal plastic changes. These changes may serve as the neural substrate of the induced tinnitus and hearing impairment.
Behavior data
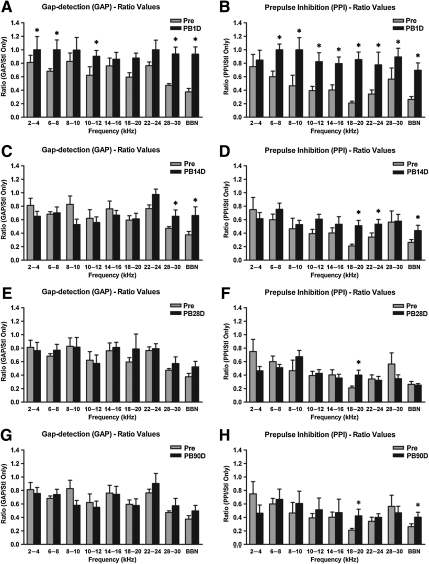
GAP and PPI were used to assess the effect of blast on tinnitus and central hearing, respectively. Ratios for GAP are represented by the average of the GAP responses divided by the mean startle-only response values, while ratios for PPI are represented by the average of the PPI responses divided by the mean startle-only values. Data for each frequency and post-blast time point, plotted next to pre-blast ratio values, are depicted in Figure 2.
FIG. 2.
Gap detection ratio values (GAP; gap detection/startle-only response) and prepulse inhibition (PPI) ratio values (PPI/startle-only response) measured at 1 day (A and B), 14 days (C and D), 28 days (E and F), and 90 days (G and H) after blast exposure. Note that the rats showed significant deficits in gap-detection and PPI inhibition at 1 day post-blast, followed by marked recovery at 14 days post-blast. Further recovery was demonstrated by the lack of deficits in GAP (E and G), and fewer deficits on PPI at post-blast days 28 and 90 (F and H). These indicate signs of transient tinnitus, as well as a complex time course change. Error bars represent standard error of the mean (*p<0.05; BBN, broadband noise; PB1D, 1 day post-blast; PB14D, 14 days post-blast; PB28D, 28 days post-blast; PB90D, 90 days post-blast; Pre, pre-blast).
The results demonstrated that blast induced significant behavioral evidence of tinnitus, especially during the early period following blast exposure. The behavioral evidence of tinnitus was manifested by significant increases in GAP ratio values, referred to here as impaired. Specifically, post-blast day 1 GAP values were significantly higher at a number of frequencies, including 2–4 kHz (F(4,345)=7.659, p=0.003), 6–8 kHz (F(4,345=6.345, p<0.001), 10–12 kHz (F(4,345)=5.347, p=0.021), 28–30 kHz (F(4,345)=17.502, p<0.001), and BBN (F(4,345)=12.843, p<0.001; ANOVA and post-hoc Bonferroni tests; Fig. 2A). GAP values showed a trend of increase at 22–24 kHz, although this was statistically insignificant (F(4,345)=2.452, p=0.068). Blast exposure also caused significant impairment in PPI responses as demonstrated by significantly increased PPI ratio values. Such PPI impairment was found to be present at all the frequency bands, including 6–8 kHz (F(4,345)=8.160, p<0.001), 8–10 kHz (F(4,345)=8.045, p<0.001), 10–12 kHz (F(4,345)=9.100, p<0.001), 14–16 kHz (F(4,345)=5.638, p=0.002), 18–20 kHz (F(4,345)=34.038, p<0.001), 22–24 kHz (F(4,345)=20.894, p<0.001), 28–30 kHz (F(4,345)=6.740, p=0.031), and BBN (F(4,345)=37.869, p<0.001; ANOVA and post-hoc Bonferroni tests; Fig. 2B).
On post-blast day 14, significant GAP impairments only occurred at 28–30 kHz (F(4,345)=7.659, p=0.032) and BBN (F(4,345)=12.843, p=0.008), indicating that the induced tinnitus persisted at higher-frequency regions (ANOVA and post-hoc Bonferroni tests; Fig. 3C). PPI impairments were maintained at 18–20 kHz (F(4,345)=34.038, p<0.001), 22–24 kHz (F(4,345)=20.894, p=0.011), and BBN (F(4,345)=37.869, p<0.001; ANOVA and post-hoc Bonferroni tests; Fig. 2D). PPI values showed a trend toward an increase at 10–12 kHz, although this was statistically insignificant (F(4,345)=9.100, p=0.083). Because PPI was not impaired at 28–30 kHz, the GAP impairment at 28–30 kHz strongly suggests the presence of high-frequency tinnitus.
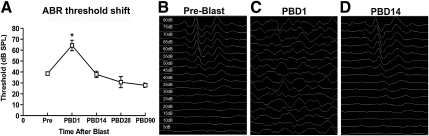
FIG. 3.
(A) Auditory brainstem response (ABR) thresholds to clicks were measured at pre-blast, post-blast day 1 (PB1D), post-blast day 14 (PB14D), post-blast day 28 (PB28D), and post-blast day 90 (PB90D). ABR thresholds were significantly elevated at post-blast day 1, and then returned to baseline level at 14, 28, and 90 days post-blast. (B–D) Representative ABR waveforms obtained from before blast, post-blast day 1, and post-blast day 14. Error bars represent standard error of the mean (*p<0.05; SPL, sound pressure level).
On post-blast days 28 and 90, GAP impairment appeared to recover to pre-blast baseline levels at all the frequency bands tested, suggesting an absence of behavioral evidence of tinnitus (Fig. 2E and 2G). However, when examining the PPI data from post-blast days 28 and 90, we found that PPI responses had also recovered to pre-blast baseline levels at all frequencies with the exception of 18–20 kHz (F(4,345)=34.038, p=0.011 for post-blast day 28, and p=0.002 for post-blast day 90), and BBN (F(4,345)=37.869, p=0.008 for post-blast day 90; ANOVA and post-hoc Bonferroni tests; Fig. 2F and 2H).
ABR data
ABR data showed that blast resulted in an immediate, temporary upward threshold shift, followed by recovery to baseline level, and a slight decrease in threshold over time (Fig. 3). The average threshold from pre-blast ABR recordings was measured at 38.57 dB SPL. Recordings 1 day after blast demonstrated a significant upward threshold shift to 61.57 dB SPL 1 day after blast (F(4,30)=16.355, p<0.001). On post-blast days 14, 28, and 90, threshold recovered to 37.86 dB SPL, 30.71 dB SPL, and 27.86 dB SPL, respectively. There was no statistically significant difference between any of these post-blast ABR thresholds and that of the pre-blast threshold (ANOVA and post-hoc Bonferroni tests; Fig. 3).
DTI data
To investigate the effects of blast and tinnitus on neural changes in auditory and non-auditory brain structures, rats underwent DTI scanning. The diffusion data across each structure and scan for AD, RD, FA, and ADC are listed in Tables 1–4.
Table 1.
Axial Diffusivity (AD) Across Scanning Time Points
| Nuclei | Pre (SD) | PBwk2 (SD) | PBwk4 (SD) |
|---|---|---|---|
| DCN | 1.434 (.134) | 1.556 (.217) (F(2,39)=2.194, p>0.05) |
1.682 (.256) (F(2,39)=2.194, p>0.05) |
| IC | 0.807 (.053) | 0.941 (.143)* (F(2,39)=6.531, p=0.003) |
0.876 (.072) (F(2,39)=6.531, p>0.05) |
| MGB | 0.809 (.061) | 0.914 (.155) (F(2,39)=4.737, p>0.05) |
0.988 (.210)* (F(2,39)=4.737, p=0.012) |
| AC | 0.755 (.061) | 0.781 (.078) (F(2,39)=0.637, p>0.05) |
0.760 (.049) (F(2,39)=0.637, p>0.05) |
| AMG | 0.879 (.104) | 0.935 (.173) (F(2,39)=0.635, p>0.05) |
0.930 (.143) (F(2,39)=0.635, p>0.05) |
| CC | 1.242 (.200) | 1.241 (.222) (F(2,100)=1.559, p>0.05) |
1.168 (.166) (F(2,100)=1.559, p>0.05) |
p<0.05.
Changes in AD across nuclei between pre-blast and post-blast weeks 2 and 4. A significant increase in AD occurred in the IC between week 2 pre- and post-blast, while a significant increase in AD occurred in the MGB between week 4 pre- and post-blast. ANOVA and post-hoc Bonferroni tests.
MGB, medial geniculate body; ANOVA, analysis of variance; IC, inferior colliculus; CC, corpus callosum; AMG, amygdala; DCN, dorsal cochlear nucleus; SD, standard deviation; Pre, pre-blast; PBwk2, post-blast week 2; PBwk4, post-blast week 4.
Table 4.
Apparent Diffusion Coefficient (ADC) Across Scanning Time Points
| Nuclei | Pre (SD) | PBwk2 (SD) | PBwk4 (SD) |
|---|---|---|---|
| DCN | 1.116 (.173) | 1.120 (.264) (F(2,39)=2.297, p>0.05) |
1.310 (.273) (F(2,39)=2.297, p>0.05) |
| IC | 0.637 (.046) | 0.678 (.067) (F(2,39)=2.364, p>0.05) |
0.679 (.056) (F(2,39)=2.364, p>0.05) |
| MGB | 0.586 (.044) | 0.651 (.078)* (F(2,39)=6.504, p=0.033) |
0.669 (.064)* (F(2,39)=6.504, p=0.004) |
| AC | 0.601 (.053) | 0.606 (.035) (F(2,39)=0.160, p>0.05) |
0.611 (.040) (F(2,39)=0.160, p>0.05) |
| AMG | 0.649 (.065) | 0.629 (.042) (F(2,39)=1.079, p>0.05) |
0.662 (.072) (F(2,39)=1.079, p>0.05) |
| CC | 0.693 (.047) | 0.704 (.078) (F(2,100)=0.731, p>0.05) |
0.686 (.060) (F(2,100)=0.731, p>0.05) |
p<0.05.
Changes in ADC across nuclei between 2 and 4 weeks pre- and post-blast. A significant increase occurred in the MGB between 2 weeks pre- and post-blast, which remained significantly elevated through post-blast 4 weeks. ANOVA and post-hoc Bonferroni tests.
MGB, medial geniculate body; ANOVA, analysis of variance; IC, inferior colliculus; CC, corpus callosum; AMG, amygdala; DCN, dorsal cochlear nucleus; SD, standard deviation; Pre, pre-blast; PBwk2, post-blast week 2; PBwk4, post-blast week 4.
Among the auditory structures studied, the IC and MGB exhibited the most differences between pre- and post-blast scans. In the IC, there was a significant increase in AD (F(2,39)=6.531, p=0.003), and FA (F(2,39)=3.934, p=0.030; ANOVA and post-hoc Bonferroni tests; Tables 1 and 3), both of which returned to baseline levels by post-blast week 4. The MGB showed a significant increase in ADC values at 2 weeks following blast (F(2,39)=6.504, p=0.033), which persisted through 4 weeks after blast (p=0.004; ANOVA and post-hoc Bonferroni tests; Table 4). There was also an increase in AD at post-blast week 4 (F(2,39)=4.737, p=0.012; ANOVA and post-hoc Bonferroni tests; Table 1). No significant changes occurred within the DCN or AC. For the non-auditory structures, neither the amygdala nor the corpus callosum demonstrated any significant changes. In addition, there were no significant changes in RD measures (Table 2).
Table 3.
Fractional Anisotropy (FA) Across Scanning Time Points
| Nuclei | Pre (SD) | PBwk2 (SD) | PBwk4 (SD) |
|---|---|---|---|
| DCN | 0.279 (.093) | 0.306 (.133) (F(2,39)=0.327, p>0.05) |
0.280 (.051) (F(2,39)=0.327, p>0.05) |
| IC | 0.262 (.056) | 0.351 (.109)* (F(2,39)=3.934, p=0.030) |
0.286 (.088) (F(2,39)=3.934, p>0.05) |
| MGB | 0.355 (.076) | 0.390 (.082) (F(2,39)=1.358, p>0.05) |
0.419 (.137) (F(2,39)=1.358, p>0.05) |
| AC | 0.255 (.034) | 0.279 (.066) (F(2,39)=1.951, p>0.05) |
0.245 (.034) (F(2,39)=1.951, p>0.05) |
| AMG | 0.341 (.082) | 0.424 (.136) (F(2,39)=2.514, p>0.05) |
0.361 (.073) (F(2,39)=2.514, p>0.05) |
| CC | 0.622 (.101) | 0.615 (.110) (F(2,100)=1.469, p>0.05) |
0.582 (.098) (F(2,100)=1.469, p>0.05) |
p<0.05.
Changes in FA values across nuclei between week 2 pre- and post-blast. A significant increase occurred in the IC between week 2 pre- and post-blast. ANOVA and post-hoc Bonferroni tests.
MGB, medial geniculate body; ANOVA, analysis of variance; IC, inferior colliculus; CC, corpus callosum; AMG, amygdala; DCN, dorsal cochlear nucleus; SD, standard deviation; Pre, pre-blast; PBwk2, post-blast week 2; PBwk4, post-blast week 4.
Table 2.
Radial Diffusivity (RD) Across Scanning Time Points
| Nuclei | Pre (SD) | PBwk2 (SD) | PBwk4 (SD) |
|---|---|---|---|
| DCN | 0.956 (.232) | 1.014 (.347) (F(2,81)=2.352, p>0.05) |
1.127 (.310) (F(2,81)=2.352, p>0.05) |
| IC | 0.553 (.098) | 0.545 (.113) (F(2,81)=0.721, p>0.05) |
0.598 (.269) (F(2,81)=0.721, p>0.05) |
| MGB | 0.476 (.113) | 0.520 (.167) (F(2,81)=0.664, p>0.05) |
0.510 (.163) (F(2,81)=0.664, p>0.05) |
| AC | 0.525 (.093) | 0.520 (.093) (F(2,81)=0.183, p>0.05) |
0.535 (.084) (F(2,81)=0.183, p>0.05) |
| AMG | 0.534 (.140) | 0.475 (.134) (F(2,81)=1.727, p>0.05) |
0.529 (.119) (F(2,81)=1.727, p>0.05) |
| CC | 0.420 (.206) | 0.439 (.237) (F(2,203)=0.296, p>0.05) |
0.448 (.210) (F(2,203)=0.296, p>0.05) |
p<0.05.
Changes in RD across nuclei between pre- and post-blast weeks 2 and 4. No significant changes in RD were found in either the auditory or non-auditory structures. ANOVA and post-hoc Bonferroni tests.
MGB, medial geniculate body; ANOVA, analysis of variance; IC, inferior colliculus; CC, corpus callosum; AMG, amygdala; DCN, dorsal cochlear nucleus; SD, standard deviation; Pre, pre-blast; PBwk2, post-blast week 2; PBwk4, post-blast week 4.
Pearson's r correlation coefficient showed a 0.97 correlation between diffusion data obtained from two sets of ROI drawings by a single rater. Correlation between data obtained from two independent raters on two rat brains showed a 0.91 correlation. This demonstrated the veracity of ROI placement during measurement.
Discussion
Summary of results
Our results revealed that the currently used blast exposure induced early-onset tinnitus and central hearing impairment at multiple frequencies. The induced tinnitus and central hearing impairment largely recovered by 28 days, with the exception of more lasting hearing impairment at 18–20 kHz and BBN. ABR hearing thresholds were significantly elevated following blast, but returned to baseline level by 14 days. Along with the blast-induced impact on tinnitus and hearing, significant microstructural changes, implying alterations to axonal and myelin integrity in auditory brain regions, were also observed on DTI scanning, with the majority of changes occurring in the IC and MGB. The DTI results suggest that damage and compensatory plastic changes were incurred in the auditory brain structures, which may be correlated with the presence of tinnitus.
Blast-induced tinnitus and hearing loss
The GAP and PPI tests revealed that blast exposure induced early-onset tinnitus and central hearing impairment at multiple frequencies 1 day after blast, followed by manifestations of tinnitus at BBN as well as a high-frequency band 14 days after blast (Fig. 2A–D). The induced tinnitus at BBN and a high-frequency band such as 28–30 kHz was reduced to an insignificant level throughout the post-blast day 90 testing period (Fig. 2E). Apparently, the blast-induced early onset of tinnitus is consistent with previous reports that tinnitus may occur immediately following acoustic insult (Loeb and Smith, 1967; Mcfeely et al., 1999; Mrena et al., 2004b). However, the fact that the induced tinnitus in our experimental paradigm recovered throughout the 90-day period measured contrasts with the results from a recent clinical study in which individuals exposed to blast reported tinnitus perception as barely improved up to 1 year after exposure (Nageris et al., 2008). The explanation for this discrepancy may be related to the parameters of blast, such as pulse duration, force, number of blasts, and time of follow-up testing.
In addition to the time course of the blast-induced tinnitus and central hearing impairment, we observed that the frequencies impacted by blast exposure tended to converge towards mid-to-high-frequency regions in addition to the more robust results at BBN (Fig. 2C–H). This observation coincides with one of the most common threads running through animal research on tinnitus, namely the association between high-frequency tinnitus and hearing loss (Roberts et al., 2010). Additional evidence supports the notion that frequency of tinnitus tends to occur at ∼ 1 octave above the region of hearing loss (Kaltenbach et al., 2004). Our findings are also in accord with the data collected from human studies following blast. For example, it has been reported that blast has typically resulted in high-frequency hearing loss (Fausti et al., 2009; Ritenour et al., 2008), characterized around 4 kHz (Fausti et al., 2009). Among 73 patients exposed to physical trauma from explosions, 78% experienced high-frequency sensorineural hearing loss, with only 7% improving over time (Nageris et al., 2008). All of the above information indicates that the currently adopted animal model of blast-induced tinnitus and hearing loss is appropriate for investigating the mechanisms underlying blast-induced tinnitus and hearing loss.
Along the same lines, the affected frequencies revealed by GAP tests (for tinnitus) did not precisely match the frequencies revealed by PPI tests (for central hearing impairment) from day 14 post-blast (compare Fig. 2C and Fig. 2D), and day 28 post-blast (compare Fig. 2E and Fig. 2F) to day 90 post-blast (compare Fig. 2G and Fig. 2H). It appears that there is a general pattern where induced tinnitus occurs at a higher frequency than the induced hearing loss. This also helps in determining that impairments to GAP and PPI did not simply result from TBI, which has been shown to reduce sensorimotor reactivity in rats with fluid percussion-induced TBI (Wiley et al., 1996), but would equally affect all frequencies. To our knowledge, there are no studies that examined the effects of blast-induced tinnitus and hearing loss in rats. Although there are no identical studies that allow us to make direct comparison, previous studies using noise exposure that have yielded different results may help shed light on the characteristics of the blast-induced tinnitus and hearing loss. For example, it has been reported that following 2-h, 12-kHz tone exposure at 120 dB SPL, rats acquired tinnitus at different frequencies, ranging from 6–24 kHz at up to 10 weeks post-exposure (Kraus et al., 2010). Other studies have found that rats acquired tinnitus at 24–32 kHz following exposure to a 17-kHz tone for 1 h (Wang et al., 2009), tinnitus at 10 kHz from exposure to a 16-kHz tone for 1 h (Turner et al., 2006), or tinnitus at 10 kHz from exposure to a 6-kHz tone for 15 min (Guitton and Dudai, 2007). Tinnitus in these studies was identified at anywhere between 1 week and several months after noise exposure. Those that implemented ABR found evidence of hearing loss early after exposure, which recovered to a certain degree at a later testing point (Turner et al., 2006; Wang et al., 2009). Although the tinnitus in most animal models of acoustic trauma developed at a higher frequency than the exposure tone (Guitton and Dudai, 2007; Kraus et al., 2010; Wang et al., 2009), there were still instances where the tinnitus developed at a lower or at various other frequencies than the exposure tone (Kraus et al., 2010; Turner et al., 2006). In this context, studies are needed to further determine the characteristics of tinnitus manifestation following blast exposure.
Finally, in the current study both ABR and PPI tests were used to evaluate the hearing condition of the rats following blast exposure. The ABR data used to measure both peripheral and central auditory neural activation and sensitivity demonstrated a temporary upward shift of 23 dB 1 day after blast, which returned to baseline level 14 days after blast exposure. The PPI data also showed significant blast-induced effects, but the residual effects at certain high frequencies lasted much longer. Although the PPI also measures both peripheral and central auditory processing, it involves much more complex brain functions. In our recent report, the underlying mechanisms of PPI were discussed (Zhang and Zhang, 2010). That is, PPI reflects fast, early-stage gating processing that can be modulated by higher-order cognitive processes in both humans and rats (Bjornsson et al., 2006). Several lines of evidence indicate that PPI involves a complex neural network extending from multiple brainstem nuclei to higher-order cortical brain regions (Campbell et al., 2007; Walton et al., 1997). It involves auditory, attentional, and emotional functions (Bjornsson et al., 2006; Bowen et al., 2003; Braff et al., 2001; Clarkson et al., 2010; Herbert et al., 1991; Miller et al., 2010; Sobin et al., 2005; Swerdlow et al., 2001; Takahashi et al., 2007). Therefore, the PPI and ABR data may be used in a complementary manner to better understand the condition of auditory processing at both the peripheral and central levels.
DTI imaging
Among the brain structures tested, only the IC and MGB showed significant increases in AD, ADC, and/or anisotropy, which returned to the pre-blast levels by post-blast week 4. First, AD is believed to reflect axonal integrity (Song et al., 2003; Sun et al., 2006). The results for AD in the IC and MGB are suggestive of alterations to their axonal integrity. The brainstem is one of the most commonly impacted structures by TBI-related axonal injury (Axelsson et al., 2000; Svetlov et al., 2009), and it is conceivable that in this type of injury structures with projections like the MGB may be affected as well. Although limited research is available that examines the effects of injury on axonal integrity of the brain within a short time post-injury, as well as the time course of injury progression, some evidence suggests that axonal changes identifiable by DTI can occur within 1 week post-injury, and can change over time (Concha et al., 2006; Sidaros et al., 2008). The increases of the AD in the IC and MGB, and the elevated anisotropy seen in the IC following blast exposure imply that axonal integrity may have been compensated in these structures. That is, at 2–4 weeks after blast-induced injury, the brain has already begun recovering, and improvements in structural integrity are indicative of neuroplastic changes occurring in response to blast injury. Such plastic changes may also account for the blast-induced tinnitus and hearing loss seen with this type of injury and the complex recovery seen over time. Second, the increases in ADC are believed to represent myelin injury and ischemia, and have been documented in TBI cases (Kim et al., 2005; Song et al., 2003; Sun et al., 2006). The observed increases in ADC in the MGB may reflect demyelination-related plasticity following blast exposure. However, it is difficult to ascertain whether tinnitus has any effects on these data, though the pathophysiology of ischemia may be similar to tinnitus (Shulman and Strashun, 2009). Third, the elevated FA values seen in the IC following blast imply that axonal integrity actually improved in these structures. This is consistent with the results from previous human studies, that the FA index of the IC was significantly higher in patient samples that had hearing loss (Chang et al., 2004; Lutz et al., 2007). This suggests that the IC may have compensated for other damaged structures (Lutz et al., 2007). Because the IC of blasted rats demonstrated a significant increase in AD, these changes may also be reflective of compensatory neuroplasticity in response to tinnitus, hearing loss, and/or TBI. This finding supports those MEMRI and PET studies that have indicated that the IC experiences plastic changes as a function of tinnitus in the form of altered baseline and induced activity (Brozoski et al., 2007; Holt et al., 2010; Melcher et al., 2000; Smits et al., 2007).
No significant changes were seen in the DCN, AC, and amygdala following blast exposure. First, it is unclear why there was no significant change in DTI measures in the DCN, although intuitively it receives more direct impact from the blast wave than other centers. One may be inclined to speculate that the blast exposure may have caused initial decreases that were followed by delayed increases in microstructural changes in the lower brainstem structures such as the DCN. Such a notion is supported by previous studies in human TBI cases (Concha et al., 2006; Sidaros et al., 2008). Future studies are needed to collect DTI data at different time points to monitor the blast-induced changes in the DCN. Second, the non-significant changes in the AC and amygdala are in contrast to previous reports about increased patency in the white matter connections between the AC and amygdala in tinnitus patients (Crippa et al., 2010), decreased integrity in white matter surrounding the AC in patients with hearing loss (Husain et al., 2010), or reduced cellular density in the primary AC (Groschel et al., 2010). Although explanations of the amygdala data remain to be explored, one may consider that certain functional changes in the AC by blast exposure may have resulted in tinnitus, but are not always identifiable in certain structures by the DTI assay. Alternatively, there might be stronger compensatory recovery at the AC level following normal acoustic input. This explanation is supported by findings that the changes in the AC may be alleviated following more direct auditory input from the ear (Eggermont and Komiya, 2000; Seki and Eggermont, 2003). Finally, our data did not demonstrate significant changes in the CC, although the CC is one of the most frequently reported sites of diffuse axonal injury, axonal degeneration, and demyelination (Kraus et al., 2007; Mac Donald et al., 2007; Singh et al., 2010; Svetlov et al., 2009; Wu et al., 2010). This further indicates that the currently adopted blast exposure induced rather mild TBI that did not lead to significant microstructural changes in many anatomical areas, as reported previously (Huang et al., 2009). This may also explain the temporary nature of the changes in behavioral measures of tinnitus and hearing impairment seen following the single blast exposure. All of the above information suggests that future studies are needed to investigate the effects of altering blasting parameters on tinnitus and related TBI pathology. In addition, MRI scanning needs to be performed at different time points following blast exposure to characterize the initiation and progression of any microstructural changes in the brain.
Conclusion
The current study demonstrated that subjecting rats to a single blast exposure was sufficient to induce early-onset behavioral manifestations of low- to high-frequency tinnitus. The induced tinnitus was accompanied by concurrent hearing impairment and very mild TBI. Results from DTI-MRI revealed certain structural changes in the IC and MGB, as well as complex evidence of injury and plastic rewiring. The fact that the CC did not show any evidence of damage indicates that the currently adopted blast-induced effects may be derived from the auditory pathways instead of through direct impact onto the brain parenchyma. Further studies adjusting blast parameters such as the number of exposures, intensity, and period of behavioral testing following blast will facilitate development of optimal blasting parameters for induction of robust tinnitus and TBI, as well as to help differentiate the effects of blast-induced tinnitus, hearing loss, and TBI. The results from this study showed that the currently adopted animal model is appropriate to investigate the mechanisms underlying blast-induced tinnitus, hearing loss, and TBI. Continued investigation along these lines will also help identify clear and definable pathology with consistent injury/recovery patterns, which in turn will aid in the construction of effective treatment strategies.
Acknowledgments
This work was supported by the Wayne State University start-up funds and the Department of Defense (grant award #W81XWH-11-2-0031).
Author Disclosure Statement
No conflicting financial interests exist.
References
- Adams P.F. Marano M.A. Current estimates from the National Health Interview Survey, 1994. Vital Health Stat. 1995:101–260. [PubMed] [Google Scholar]
- Andersson G. Carlbring P. Kaldo V. Strom L. Screening of psychiatric disorders via the Internet. A pilot study with tinnitus patients. Nord. J. Psychiatry. 2004;58:287–291. doi: 10.1080/08039480410005792. [DOI] [PubMed] [Google Scholar]
- Andersson G. McKenna L. Tinnitus masking and depression. Audiology. 1998;37:174–182. doi: 10.3109/00206099809072971. [DOI] [PubMed] [Google Scholar]
- Andersson G. Psychological aspects of tinnitus and the application of cognitive-behavioral therapy. Clin. Psychol. Rev. 2002;22:977–990. doi: 10.1016/s0272-7358(01)00124-6. [DOI] [PubMed] [Google Scholar]
- Axelsson A. Sandh A. Tinnitus in noise-induced hearing loss. Br. J. Audiol. 1985;19:271–276. doi: 10.3109/03005368509078983. [DOI] [PubMed] [Google Scholar]
- Axelsson H. Hjelmqvist H. Medin A. Persson J.K. Suneson A. Physiological changes in pigs exposed to a blast wave from a detonating high-explosive charge. Mil. Med. 2000;165:119–126. [PubMed] [Google Scholar]
- Basta D. Tzschentke B. Ernst A. Noise-induced cell death in the mouse medial geniculate body and primary auditory cortex. Neurosci. Lett. 2005;381:199–204. doi: 10.1016/j.neulet.2005.02.034. [DOI] [PubMed] [Google Scholar]
- Bauer C.A. Turner J.G. Caspary D.M. Myers K.S. Brozoski T.J. Tinnitus and inferior colliculus activity in chinchillas related to three distinct patterns of cochlear trauma. J. Neurosci. Res. 2008;86:2564–2578. doi: 10.1002/jnr.21699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belli S. Belli H. Bahcebasi T. Ozcetin A. Alpay E. Ertem U. Assessment of psychopathological aspects and psychiatric comorbidities in patients affected by tinnitus. Eur. Arch. Otorhinolaryngol. 2008;265:279–285. doi: 10.1007/s00405-007-0440-8. [DOI] [PubMed] [Google Scholar]
- Bjornsson C.S. Oh S.J. Al Kofahi Y.A. Lim Y.J. Smith K.L. Turner J.N. De S. Roysam B. Shain W. Kim S.J. Effects of insertion conditions on tissue strain and vascular damage during neuroprosthetic device insertion. J. Neural Eng. 2006;3:196–207. doi: 10.1088/1741-2560/3/3/002. [DOI] [PubMed] [Google Scholar]
- Blaha G.R. Raghupathi R. Saatman K.E. McIntosh T.K. Brain-derived neurotrophic factor administration after traumatic brain injury in the rat does not protect against behavioral or histological deficits. Neuroscience. 2000;99:483–493. doi: 10.1016/s0306-4522(00)00214-1. [DOI] [PubMed] [Google Scholar]
- Bowen G.P. Lin D. Taylor M.K. Ison J.R. Auditory cortex lesions in the rat impair both temporal acuity and noise increment thresholds, revealing a common neural substrate. Cereb. Cortex. 2003;13:815–822. doi: 10.1093/cercor/13.8.815. [DOI] [PubMed] [Google Scholar]
- Braff D.L. Geyer M.A. Swerdlow N.R. Human studies of prepulse inhibition of startle: normal subjects, patient groups, and pharmacological studies. Psychopharmacology (Berl.) 2001;156:234–258. doi: 10.1007/s002130100810. [DOI] [PubMed] [Google Scholar]
- Breeze J. Cooper H. Pearson C.R. Henney S. Reid A. Ear injuries sustained by British service personnel subjected to blast trauma. J. Laryngol. Otol. 2011;125:13–17. doi: 10.1017/S0022215110002215. [DOI] [PubMed] [Google Scholar]
- Brozoski T.J. Bauer C.A. Caspary D.M. Elevated fusiform cell activity in the dorsal cochlear nucleus of chinchillas with psychophysical evidence of tinnitus. J. Neurosci. 2002;22:2383–2390. doi: 10.1523/JNEUROSCI.22-06-02383.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brozoski T.J. Ciobanu L. Bauer C.A. Central neural activity in rats with tinnitus evaluated with manganese-enhanced magnetic resonance imaging (MEMRI) Hear. Res. 2007;228:168–179. doi: 10.1016/j.heares.2007.02.003. [DOI] [PubMed] [Google Scholar]
- Caeyenberghs K. Leemans A. Geurts M. Linden C.V. Smits-Engelsman B.C. Sunaert S. Swinnen S.P. Correlations between white matter integrity and motor function in traumatic brain injury patients. Neurorehabil. Neural Repair. 2011;25:492–502. doi: 10.1177/1545968310394870. [DOI] [PubMed] [Google Scholar]
- Campbell L.E. Hughes M. Budd T.W. Cooper G. Fulham W.R. Karayanidis F. Hanlon M.C. Stojanov W. Johnston P. Case V. Schall U. Primary and secondary neural networks of auditory prepulse inhibition: a functional magnetic resonance imaging study of sensorimotor gating of the human acoustic startle response. Eur. J. Neurosci. 2007;26:2327–2333. doi: 10.1111/j.1460-9568.2007.05858.x. [DOI] [PubMed] [Google Scholar]
- Chang Y. Lee S.H. Lee Y.J. Hwang M.J. Bae S.J. Kim M.N. Lee J. Woo S. Lee H. Kang D.S. Auditory neural pathway evaluation on sensorineural hearing loss using diffusion tensor imaging. Neuroreport. 2004;15:1699–1703. doi: 10.1097/01.wnr.0000134584.10207.1a. [DOI] [PubMed] [Google Scholar]
- Chen G.D. Jastreboff P.J. Salicylate-induced abnormal activity in the inferior colliculus of rats. Hear. Res. 1995;82:158–178. doi: 10.1016/0378-5955(94)00174-o. [DOI] [PubMed] [Google Scholar]
- Clarkson C. Lopez D.E. Merchan M.A. Long-term functional recovery in the rat auditory system after unilateral auditory cortex ablation. Acta Otolaryngol. 2010;130:326–332. doi: 10.1080/00016480903150536. [DOI] [PubMed] [Google Scholar]
- Concha L. Gross D.W. Wheatley B.M. Beaulieu C. Diffusion tensor imaging of time-dependent axonal and myelin degradation after corpus callosotomy in epilepsy patients. Neuroimage. 2006;32:1090–1099. doi: 10.1016/j.neuroimage.2006.04.187. [DOI] [PubMed] [Google Scholar]
- Crippa A. Lanting C.P. van Dijk P. Roerdink J.B. A diffusion tensor imaging study on the auditory system and tinnitus. Open Neuroimag. J. 2010;4:16–25. doi: 10.2174/1874440001004010016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronlein T. Langguth B. Geisler P. Hajak G. Tinnitus and insomnia. Prog. Brain Res. 2007;166:227–233. doi: 10.1016/S0079-6123(07)66021-X. [DOI] [PubMed] [Google Scholar]
- Dobie R.A. Depression and tinnitus. Otolaryngol. Clin. North Am. 2003;36:383–388. doi: 10.1016/s0030-6665(02)00168-8. [DOI] [PubMed] [Google Scholar]
- Dougherty A.L. MacGregor A.J. Han P.P. Heltemes K.J. Galarneau M.R. Visual dysfunction following blast-related traumatic brain injury from the battlefield. Brain Inj. 2011;25:8–13. doi: 10.3109/02699052.2010.536195. [DOI] [PubMed] [Google Scholar]
- Dror V. Eliash S. Rehavi M. Assaf Y. Biton I.E. Fattal-Valevski A. Neurodegeneration in thiamine deficient rats-A longitudinal MRI study. Brain Res. 2010;1308:176–184. doi: 10.1016/j.brainres.2009.10.032. [DOI] [PubMed] [Google Scholar]
- Eggermont J.J. Komiya H. Moderate noise trauma in juvenile cats results in profound cortical topographic map changes in adulthood. Hear. Res. 2000;142:89–101. doi: 10.1016/s0378-5955(00)00024-1. [DOI] [PubMed] [Google Scholar]
- Eggermont J.J. Roberts L.E. The neuroscience of tinnitus. Trends Neurosci. 2004;27:676–682. doi: 10.1016/j.tins.2004.08.010. [DOI] [PubMed] [Google Scholar]
- Erlandsson S.I. Hallberg L.R. Prediction of quality of life in patients with tinnitus. Br. J. Audiol. 2000;34:11–20. doi: 10.3109/03005364000000114. [DOI] [PubMed] [Google Scholar]
- Fausti S.A. Wilmington D.J. Gallun F.J. Myers P.J. Henry J.A. Auditory and vestibular dysfunction associated with blast-related traumatic brain injury. J Rehabil. Res. Dev. 2009;46:797–810. doi: 10.1682/jrrd.2008.09.0118. [DOI] [PubMed] [Google Scholar]
- Finlayson P.G. Kaltenbach J.A. Alterations in the spontaneous discharge patterns of single units in the dorsal cochlear nucleus following intense sound exposure. Hear. Res. 2009;256:104–117. doi: 10.1016/j.heares.2009.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folmer R.L. Griest S.E. Meikle M.B. Martin W.H. Tinnitus severity, loudness, and depression. Otolaryngol. Head Neck Surg. 1999;121:48–51. doi: 10.1016/S0194-5998(99)70123-3. [DOI] [PubMed] [Google Scholar]
- Folmer R.L. Griest S.E. Tinnitus and insomnia. Am. J. Otolaryngol. 2000;21:287–293. doi: 10.1053/ajot.2000.9871. [DOI] [PubMed] [Google Scholar]
- Geckle L. Lee R. Soldier perceptions of deployment environmental exposures; Force Health Protection Conference; Albuquerque, NM. 2004. [Google Scholar]
- Gondusky J.S. Reiter M.P. Protecting military convoys in Iraq: an examination of battle injuries sustained by a mechanized battalion during Operation Iraqi Freedom II. Mil. Med. 2005;170:546–549. doi: 10.7205/milmed.170.6.546. [DOI] [PubMed] [Google Scholar]
- Groschel M. Gotze R. Ernst A. Basta D. Differential impact of temporary and permanent noise-induced hearing loss on neuronal cell density in the mouse central auditory pathway. J. Neurotrauma. 2010;27:1499–1507. doi: 10.1089/neu.2009.1246. [DOI] [PubMed] [Google Scholar]
- Guitton M.J. Dudai Y. Blockade of cochlear NMDA receptors prevents long-term tinnitus during a brief consolidation window after acoustic trauma. Neural Plast. 20072007:80904. doi: 10.1155/2007/80904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halford J.B. Anderson S.D. Anxiety and depression in tinnitus sufferers. J. Psychosom. Res. 1991;35:383–390. doi: 10.1016/0022-3999(91)90033-k. [DOI] [PubMed] [Google Scholar]
- Hebert S. Carrier J. Sleep complaints in elderly tinnitus patients: a controlled study. Ear Hear. 2007;28:649–655. doi: 10.1097/AUD.0b013e31812f71cc. [DOI] [PubMed] [Google Scholar]
- Henry J.A. James K.E. Owens K. Zaugg T. Porsov E. Silaski G. Auditory test result characteristics of subjects with and without tinnitus. J. Rehabil. Res. Dev. 2009;46:619–632. doi: 10.1682/jrrd.2008.11.0157. [DOI] [PubMed] [Google Scholar]
- Herbert H. Aschoff A. Ostwald J. Topography of projections from the auditory cortex to the inferior colliculus in the rat. J. Comp. Neurol. 1991;304:103–122. doi: 10.1002/cne.903040108. [DOI] [PubMed] [Google Scholar]
- Hicks R. Soares H. Smith D. McIntosh T. Temporal and spatial characterization of neuronal injury following lateral fluid-percussion brain injury in the rat. Acta Neuropathol. 1996;91:236–246. doi: 10.1007/s004010050421. [DOI] [PubMed] [Google Scholar]
- Holt AG. Bissig D. Mirza N. Rajah G. Berkowitz B. Evidence of key tinnitus-related brain regions documented by a unique combination of manganese-enhanced MRI and acoustic startle reflex testing. PLoS ONE. 2010;5:e14260. doi: 10.1371/journal.pone.0014260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humes L.E. Noise and Military Service: Committee on Noise-Induced Hearing Loss and Tinnitus Associated with Military Service from World War II to the Present, in: Institute of Medicine of the National Academies. The National Academies Press; Washington, DC: 2006. [Google Scholar]
- Huang M.X. Theilmann R.J. Robb A. Angeles A. Nichols S. Drake A. D'Andrea J. Levy M. Holland M. Song T. Ge S. Hwang E. Yoo K. Cui L. Baker D.G. Trauner D. Coimbra R. Lee R.R. Integrated imaging approach with MEG and DTI to detect mild traumatic brain injury in military and civilian patients. J. Neurotrauma. 2009;26:1213–1226. doi: 10.1089/neu.2008.0672. [DOI] [PubMed] [Google Scholar]
- Husain F.T. Medina R.E. Davis C.W. Szymko-Bennett Y. Simonyan K. Pajor N.M. Horwitz B. Neuroanatomical changes due to hearing loss and chronic tinnitus: A combined VBM and DTI study. Brain Res. 2011;1369:74–88. doi: 10.1016/j.brainres.2010.10.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston M. Walker M. Suicide in the elderly. Recognizing the signs. Gen. Hosp. Psychiatry. 1996;18:257–260. doi: 10.1016/0163-8343(96)00039-4. [DOI] [PubMed] [Google Scholar]
- Kaltenbach J.A. Afman C.E. Hyperactivity in the dorsal cochlear nucleus after intense sound exposure and its resemblance to tone-evoked activity: a physiological model for tinnitus. Hear. Res. 2000;140:165–172. doi: 10.1016/s0378-5955(99)00197-5. [DOI] [PubMed] [Google Scholar]
- Kaltenbach J.A. Godfrey D.A. Neumann J.B. McCaslin D.L. Afman C.E. Zhang J. Changes in spontaneous neural activity in the dorsal cochlear nucleus following exposure to intense sound: relation to threshold shift. Hear. Res. 1998;124:78–84. doi: 10.1016/s0378-5955(98)00119-1. [DOI] [PubMed] [Google Scholar]
- Kaltenbach J.A. McCaslin D.L. Increases in spontaneous activity in the dorsal cochlear nucleus following exposure to high intensity sound, a possible neural correlate of tinnitus. Auditory Neurosci. 1996;3:57–78. [PMC free article] [PubMed] [Google Scholar]
- Kaltenbach J.A. Tinnitus: Models and mechanisms. Hear. Res. 2011;276:52–60. doi: 10.1016/j.heares.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaltenbach J.A. Zacharek M.A. Zhang J. Frederick S. Activity in the dorsal cochlear nucleus of hamsters previously tested for tinnitus following intense tone exposure. Neurosci. Lett. 2004;355:121–125. doi: 10.1016/j.neulet.2003.10.038. [DOI] [PubMed] [Google Scholar]
- Kantarci K. Avula R. Senjem M.L. Samikoglu A.R. Zhang B. Weigand S.D. Przybelski S.A. Edmonson H.A. Vemuri P. Knopman D.S. Ferman T.J. Boeve B.F. Petersen R.C. Jack C.R., Jr. Dementia with Lewy bodies and Alzheimer disease: neurodegenerative patterns characterized by DTI. Neurology. 2010;74:1814–1821. doi: 10.1212/WNL.0b013e3181e0f7cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H.J. Choi C.G. Lee D.H. Lee J.H. Kim S.J. Suh D.C. High-b-value diffusion-weighted MR imaging of hyperacute ischemic stroke at 1.5T. AJNR Am. J. Neuroradiol. 2005;26:208–215. [PMC free article] [PubMed] [Google Scholar]
- Kim J. Morest D.K. Bohne B.A. Degeneration of axons in the brainstem of the chinchilla after auditory overstimulation. Hear. Res. 1997;103:169–191. doi: 10.1016/s0378-5955(96)00173-6. [DOI] [PubMed] [Google Scholar]
- Kimura M. Eggermont J.J. Effects of acute pure tone induced hearing loss on response properties in three auditory cortical fields in cat. Hear. Res. 1999;135:146–162. doi: 10.1016/s0378-5955(99)00104-5. [DOI] [PubMed] [Google Scholar]
- Komiya H. Eggermont J.J. Spontaneous firing activity of cortical neurons in adult cats with reorganized tonotopic map following pure-tone trauma. Acta Otolaryngol. 2000;120:750–756. doi: 10.1080/000164800750000298. [DOI] [PubMed] [Google Scholar]
- Kovesdi E. Gyorgy A.B. Kwon S.K. Wingo D.L. Kamnaksh A. Long J.B. Kasper C.E. Agoston D.V. The effect of enriched environment on the outcome of traumatic brain injury; a behavioral, proteomics, and histological study. Front. Neurosci. 2011;5:42. doi: 10.3389/fnins.2011.00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus K.S. Mitra S. Jimenez Z. Hinduja S. Ding D. Jiang H. Gray L. Lobarinas E. Sun W. Salvi R.J. Noise trauma impairs neurogenesis in the rat hippocampus. Neuroscience. 2010;167:1216–1226. doi: 10.1016/j.neuroscience.2010.02.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus M.F. Susmaras T. Caughlin B.P. Walker C.J. Sweeney J.A. Little D.M. White matter integrity and cognition in chronic traumatic brain injury: a diffusion tensor imaging study. Brain. 2007;130:2508–2519. doi: 10.1093/brain/awm216. [DOI] [PubMed] [Google Scholar]
- Kumar R. Husain M. Gupta R.K. Hasan K.M. Haris M. Agarwal A.K. Pandey C.M. Narayana P.A. Serial changes in the white matter diffusion tensor imaging metrics in moderate traumatic brain injury and correlation with neuro-cognitive function. J. Neurotrauma. 2009;26:481–495. doi: 10.1089/neu.2008.0461. [DOI] [PubMed] [Google Scholar]
- Lanting C.P. de Kleine E. Eppinga R.N. van Dijk P. Neural correlates of human somatosensory integration in tinnitus. Hear. Res. 2010;267:78–88. doi: 10.1016/j.heares.2010.04.006. [DOI] [PubMed] [Google Scholar]
- Lanting C.P. de K.E. van D.P. Neural activity underlying tinnitus generation: results from PET and fMRI. Hear. Res. 2009;255:1–13. doi: 10.1016/j.heares.2009.06.009. [DOI] [PubMed] [Google Scholar]
- Lee J.W. Choi C.G. Chun M.H. Usefulness of diffusion tensor imaging for evaluation of motor function in patients with traumatic brain injury: three case studies. J. Head Trauma Rehabil. 2006;21:272–278. doi: 10.1097/00001199-200605000-00007. [DOI] [PubMed] [Google Scholar]
- Leske M.C. Prevalence estimates of communicative disorders in the U.S. Language, hearing and vestibular disorders. ASHA. 1981;23:229–237. [PubMed] [Google Scholar]
- Levin H.S. Wilde E. Troyanskaya M. Petersen N.J. Scheibel R. Newsome M. Radaideh M. Wu T. Yallampalli R. Chu Z. Li X. Diffusion tensor imaging of mild to moderate blast-related traumatic brain injury and its sequelae. J. Neurotrauma. 2010;27:683–694. doi: 10.1089/neu.2009.1073. [DOI] [PubMed] [Google Scholar]
- Lew H.L. Garvert D.W. Pogoda T.K. Hsu P.T. Devine J.M. White D.K. Myers P.J. Goodrich G.L. Auditory and visual impairments in patients with blast-related traumatic brain injury: Effect of dual sensory impairment on Functional Independence Measure. J. Rehabil. Res. Dev. 2009;46:819–826. doi: 10.1682/jrrd.2008.09.0129. [DOI] [PubMed] [Google Scholar]
- Lew H.L. Jerger J.F. Guillory S.B. Henry J.A. Auditory dysfunction in traumatic brain injury. J. Rehabil. Res. Dev. 2007;44:921–928. doi: 10.1682/jrrd.2007.09.0140. [DOI] [PubMed] [Google Scholar]
- Lewis J.E. Stephens S.D. McKenna L. Tinnitus and suicide. Clin. Otolaryngol. Allied Sci. 1994;19:50–54. doi: 10.1111/j.1365-2273.1994.tb01147.x. [DOI] [PubMed] [Google Scholar]
- Liberman M.C. Kiang N.Y. Acoustic trauma in cats. Cochlear pathology and auditory-nerve activity. Acta Otolaryngol. 1978;(Suppl. 358):1–63. [PubMed] [Google Scholar]
- Lin Y. Wang J. Wu C. Wai Y. Yu J. Ng S. Diffusion tensor imaging of the auditory pathway in sensorineural hearing loss: changes in radial diffusivity and diffusion anisotropy. J. Magn. Reson. Imaging. 2008;28:598–603. doi: 10.1002/jmri.21464. [DOI] [PubMed] [Google Scholar]
- Lockwood A.H. Wack D.S. Burkard R.F. Coad M.L. Reyes S.A. Arnold S.A. Salvi R.J. The functional anatomy of gaze-evoked tinnitus and sustained lateral gaze. Neurology. 2001;56:472–480. doi: 10.1212/wnl.56.4.472. [DOI] [PubMed] [Google Scholar]
- Loeb M. Smith R.P. Relation of induced tinnitus to physical characteristics of the inducing stimuli. J. Acoust. Soc. Am. 1967;42:453–455. doi: 10.1121/1.1910600. [DOI] [PubMed] [Google Scholar]
- Lutz J. Hemminger F. Stahl R. Dietrich O. Hempel M. Reiser M. Jager L. Evidence of subcortical and cortical aging of the acoustic pathway: a diffusion tensor imaging (DTI) study. Acad. Radiol. 2007;14:692–700. doi: 10.1016/j.acra.2007.02.014. [DOI] [PubMed] [Google Scholar]
- Lutz J. Jager L. de Quervain D. Krauseneck T. Padberg F. Wichnalek M. Beyer A. Stahl R. Zirngibl B. Morhard D. Reiser M. Schelling G. White and gray matter abnormalities in the brain of patients with fibromyalgia: a diffusion-tensor and volumetric imaging study. Arthritis Rheum. 2008;58:3960–3969. doi: 10.1002/art.24070. [DOI] [PubMed] [Google Scholar]
- Mac Donald C.L. Dikranian K. Song S.K. Bayly P.V. Holtzman D.M. Brody D.L. Detection of traumatic axonal injury with diffusion tensor imaging in a mouse model of traumatic brain injury. Exp. Neurol. 2007;205:116–131. doi: 10.1016/j.expneurol.2007.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacGregor A.J. Shaffer R.A. Dougherty A.L. Galarneau M.R. Raman R. Baker D.G. Lindsay S.P. Golomb B.A. Corson K.S. Prevalence and psychological correlates of traumatic brain injury in operation Iraqi freedom. J. Head Trauma Rehabil. 2010;25:1–8. doi: 10.1097/HTR.0b013e3181c2993d. [DOI] [PubMed] [Google Scholar]
- Marciano E. Carrabba L. Giannini P. Sementina C. Verde P. Bruno C. Di Pietro G. Ponsillo N.G. Psychiatric comorbidity in a population of outpatients affected by tinnitus. Int. J. Audiol. 2003;42:4–9. doi: 10.3109/14992020309056079. [DOI] [PubMed] [Google Scholar]
- Mcfeely W.J., Jr. Bojrab D.I. Davis K.G. Hegyi D.F. Otologic injuries caused by airbag deployment. Otolaryngol. Head Neck Surg. 1999;121:367–373. doi: 10.1016/S0194-5998(99)70222-6. [DOI] [PubMed] [Google Scholar]
- McKenna L. Hallam R.S. Hinchcliffe R. The prevalence of psychological disturbance in neurotology outpatients. Clin. Otolaryngol. Allied Sci. 1991;16:452–456. doi: 10.1111/j.1365-2273.1991.tb01038.x. [DOI] [PubMed] [Google Scholar]
- McKenna L. Tinnitus and insomnia. In: Tyler R.S., editor. Tinnitus Handbook. Singular; San Diego: 2000. pp. 59–84. [Google Scholar]
- Melcher J.R. Sigalovsky I.S. Guinan J.J., Jr. Levine R.A. Lateralized tinnitus studied with functional magnetic resonance imaging: abnormal inferior colliculus activation. J. Neurophysiol. 2000;83:1058–1072. doi: 10.1152/jn.2000.83.2.1058. [DOI] [PubMed] [Google Scholar]
- Miller E.J. Saint Marie L.R. Breier M.R. Swerdlow N.R. Pathways from the ventral hippocampus and caudal amygdala to forebrain regions that regulate sensorimotor gating in the rat. Neuroscience. 2010;165:601–611. doi: 10.1016/j.neuroscience.2009.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mrena R. Paakkonen R. Back L. Pirvola U. Ylikoski J. Otologic consequences of blast exposure: a Finnish case study of a shopping mall bomb explosion. Acta Otolaryngol. 2004a;124:946–952. doi: 10.1080/00016480310017045. [DOI] [PubMed] [Google Scholar]
- Mrena R. Savolainen S. Kuokkanen J.T. Ylikoski J. Characteristics of tinnitus induced by acute acoustic trauma: a long-term follow-up. Audiol. Neurootol. 2002;7:122–130. doi: 10.1159/000057660. [DOI] [PubMed] [Google Scholar]
- Mrena R. Savolainen S. Pirvola U. Ylikoski J. Characteristics of acute acoustical trauma in the Finnish Defence Forces. Int. J. Audiol. 2004b;43:177–181. doi: 10.1080/14992020400050025. [DOI] [PubMed] [Google Scholar]
- Mulders W.H. Robertson D. Hyperactivity in the auditory midbrain after acoustic trauma: dependence on cochlear activity. Neuroscience. 2009;164:733–746. doi: 10.1016/j.neuroscience.2009.08.036. [DOI] [PubMed] [Google Scholar]
- Nageris B.I. Attias J. Shemesh R. Otologic and audiologic lesions due to blast injury. J. Basic Clin. Physiol. Pharmacol. 2008;19:185–191. doi: 10.1515/jbcpp.2008.19.3-4.185. [DOI] [PubMed] [Google Scholar]
- Newcombe V.F. Williams G.B. Scoffings D. Cross J. Carpenter T.A. Pickard J.D. Menon D.K. Aetiological differences in neuroanatomy of the vegetative state: insights from diffusion tensor imaging and functional implications. J. Neurol. Neurosurg. Psychiatry. 2010;81:552–561. doi: 10.1136/jnnp.2009.196246. [DOI] [PubMed] [Google Scholar]
- Norena A.J. Eggermont J.J. Changes in spontaneous neural activity immediately after an acoustic trauma: implications for neural correlates of tinnitus. Hear. Res. 2003;183:137–153. doi: 10.1016/s0378-5955(03)00225-9. [DOI] [PubMed] [Google Scholar]
- Norena A.J. Eggermont J.J. Enriched acoustic environment after noise trauma reduces hearing loss and prevents cortical map reorganization. J. Neurosci. 2005;25:699–705. doi: 10.1523/JNEUROSCI.2226-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okie S. Traumatic brain injury in the war zone. N. Engl. J. Med. 2005;352:2043–2047. doi: 10.1056/NEJMp058102. [DOI] [PubMed] [Google Scholar]
- Palacios E.M. Fernandez-Espejo D. Junque C. Sanchez-Carrion R. Roig T. Tormos J.M. Bargallo N. Vendrell P. Diffusion tensor imaging differences relate to memory deficits in diffuse traumatic brain injury. BMC Neurol. 2011;11:24. doi: 10.1186/1471-2377-11-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G. Watston C. The Rat Brain in Stereotaxic Coordinates. Academic Press; San Diego: 1998. [Google Scholar]
- Penner M.J. Tinnitus synthesis: Fluctuant and stable matches to the pitch of tinnitus. Int. Tinnitus J. 1995;1:79–83. [PubMed] [Google Scholar]
- Ritenour A.E. Wickley A. Ritenour J.S. Kriete B.R. Blackbourne L.H. Holcomb J.B. Wade C.E. Tympanic membrane perforation and hearing loss from blast overpressure in Operation Enduring Freedom and Operation Iraqi Freedom wounded. J. Trauma. 2008;64:S174–S178. doi: 10.1097/TA.0b013e318160773e. [DOI] [PubMed] [Google Scholar]
- Roberts L.E. Eggermont J.J. Caspary D.M. Shore S.E. Melcher J.R. Kaltenbach J.A. Ringing ears: the neuroscience of tinnitus. J. Neurosci. 2010;30:14972–14979. doi: 10.1523/JNEUROSCI.4028-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson S.K. McQuaid J.R. Viirre E.S. Betzig L.L. Miller D.L. Bailey K.A. Harris J.P. Perry W. Relationship of tinnitus questionnaires to depressive symptoms, quality of well-being, and internal focus. Int. Tinnitus J. 2003;9:97–103. [PubMed] [Google Scholar]
- Rossiter S. Stevens C. Walker G. Tinnitus and its effect on working memory and attention. J. Speech Lang. Hear. Res. 2006;49:150–160. doi: 10.1044/1092-4388(2006/012). [DOI] [PubMed] [Google Scholar]
- Sanderson K.L. Raghupathi R. Saatman K.E. Martin D. Miller G. McIntosh T.K. Interleukin-1 receptor antagonist attenuates regional neuronal cell death and cognitive dysfunction after experimental brain injury. J. Cereb. Blood Flow Metab. 1999;19:1118–1125. doi: 10.1097/00004647-199910000-00008. [DOI] [PubMed] [Google Scholar]
- Saunders G.H. Griest S.E. Hearing loss in veterans and the need for hearing loss prevention programs. Noise Health. 2009;11:14–21. doi: 10.4103/1463-1741.45308. [DOI] [PubMed] [Google Scholar]
- Seki S. Eggermont J.J. Changes in spontaneous firing rate and neural synchrony in cat primary auditory cortex after localized tone-induced hearing loss. Hear. Res. 2003;180:28–38. doi: 10.1016/s0378-5955(03)00074-1. [DOI] [PubMed] [Google Scholar]
- Shargorodsky J. Curhan G.C. Farwell W.R. Prevalence and characteristics of tinnitus among US adults. Am. J. Med. 2010;123:711–718. doi: 10.1016/j.amjmed.2010.02.015. [DOI] [PubMed] [Google Scholar]
- Shen Y. Kou Z. Kreipke C.W. Petrov T. Hu J. Haacke E.M. In vivo measurement of tissue damage, oxygen saturation changes and blood flow changes after experimental traumatic brain injury in rats using susceptibility weighted imaging. Magn. Reson. Imaging. 2007;25:219–227. doi: 10.1016/j.mri.2006.09.018. [DOI] [PubMed] [Google Scholar]
- Shulman A. Strashun A.M. Fluid dynamics vascular theory of brain and inner-ear function in traumatic brain injury: a translational hypothesis for diagnosis and treatment. Int. Tinnitus J. 2009;15:119–129. [PubMed] [Google Scholar]
- Sidaros A. Engberg A.W. Sidaros K. Liptrot M.G. Herning M. Petersen P. Paulson O.B. Jernigan T.L. Rostrup E. Diffusion tensor imaging during recovery from severe traumatic brain injury and relation to clinical outcome: a longitudinal study. Brain. 2008;131:559–572. doi: 10.1093/brain/awm294. [DOI] [PubMed] [Google Scholar]
- Silva A.C. Lee J.H. Aoki I. Koretsky A.P. Manganese-enhanced magnetic resonance imaging (MEMRI): methodological and practical considerations. NMR Biomed. 2004;17:532–543. doi: 10.1002/nbm.945. [DOI] [PubMed] [Google Scholar]
- Singh M. Jeong J. Hwang D. Sungkarat W. Gruen P. Novel diffusion tensor imaging methodology to detect and quantify injured regions and affected brain pathways in traumatic brain injury. Magn. Reson. Imaging. 2010;28:22–40. doi: 10.1016/j.mri.2009.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smits M. Kovacs S. De Ridder D. Peeters R.R. van Hecke P. Sunaert S. Lateralization of functional magnetic resonance imaging (fMRI) activation in the auditory pathway of patients with lateralized tinnitus. Neuroradiology. 2007;49:669–679. doi: 10.1007/s00234-007-0231-3. [DOI] [PubMed] [Google Scholar]
- Sobin C. Kiley-Brabeck K. Karayiorgou M. Lower prepulse inhibition in children with the 22q11 deletion syndrome. Am. J. Psychiatry. 2005;162:1090–1099. doi: 10.1176/appi.ajp.162.6.1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solano-Castiella E. Anwander A. Lohmann G. Weiss M. Docherty C. Geyer S. Reimer E. Friederici A.D. Turner R. Diffusion tensor imaging segments the human amygdala in vivo. Neuroimage. 2010;49:2958–2965. doi: 10.1016/j.neuroimage.2009.11.027. [DOI] [PubMed] [Google Scholar]
- Song S.K. Sun S.W. Ju W.K. Lin S.J. Cross A.H. Neufeld A.H. Diffusion tensor imaging detects and differentiates axon and myelin degeneration in mouse optic nerve after retinal ischemia. Neuroimage. 2003;20:1714–1722. doi: 10.1016/j.neuroimage.2003.07.005. [DOI] [PubMed] [Google Scholar]
- Stevens C. Walker G. Boyer M. Gallagher M. Severe tinnitus and its effect on selective and divided attention. Int. J. Audiol. 2007;46:208–216. doi: 10.1080/14992020601102329. [DOI] [PubMed] [Google Scholar]
- Sun S.W. Liang H.F. Trinkaus K. Cross A.H. Armstrong R.C. Song S.K. Noninvasive detection of cuprizone induced axonal damage and demyelination in the mouse corpus callosum. Magn. Reson. Med. 2006;55:302–308. doi: 10.1002/mrm.20774. [DOI] [PubMed] [Google Scholar]
- Svetlov S.I. Larner S.F. Kirk D.R. Atkinson J. Hayes R.L. Wang K.K. Biomarkers of blast-induced neurotrauma: profiling molecular and cellular mechanisms of blast brain injury. J. Neurotrauma. 2009;26:913–921. doi: 10.1089/neu.2008.0609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow N.R. Geyer M.A. Braff D.L. Neural circuit regulation of prepulse inhibition of startle in the rat: current knowledge and future challenges. Psychopharmacology (Berl.) 2001;156:194–215. doi: 10.1007/s002130100799. [DOI] [PubMed] [Google Scholar]
- Takahashi K. Nagai T. Kamei H. Maeda K. Matsuya T. Arai S. Mizoguchi H. Yoneda Y. Nabeshima T. Takuma K. Yamada K. Neural circuits containing pallidotegmental GABAergic neurons are involved in the prepulse inhibition of the startle reflex in mice. Biol. Psychiatry. 2007;62:148–157. doi: 10.1016/j.biopsych.2006.06.035. [DOI] [PubMed] [Google Scholar]
- Turner J.G. Brozoski T.J. Bauer C.A. Parrish J.L. Myers K. Hughes L.F. Caspary D.M. Gap detection deficits in rats with tinnitus: a potential novel screening tool. Behav. Neurosci. 2006;120:188–195. doi: 10.1037/0735-7044.120.1.188. [DOI] [PubMed] [Google Scholar]
- Turner J.G. Parrish J. Gap detection methods for assessing salicylate-induced tinnitus and hyperacusis in rats. Am. J. Audiol. 2008;17:S185–S192. doi: 10.1044/1059-0889(2008/08-0006). [DOI] [PubMed] [Google Scholar]
- Turner O. Windfuhr K. Kapur N. Suicide in deaf populations: a literature review. Ann. Gen. Psychiatry. 2007;6:26. doi: 10.1186/1744-859X-6-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler R.S. Conrad-Armes D. The determination of tinnitus loudness considering the effects of recruitment. J. Speech Hear. Res. 1983;26:59–72. doi: 10.1044/jshr.2601.59. [DOI] [PubMed] [Google Scholar]
- Tyler R.S. Gogel S.A. Gehringer A.K. Tinnitus activities treatment. Prog. Brain Res. 2007;166:425–434. doi: 10.1016/S0079-6123(07)66041-5. [DOI] [PubMed] [Google Scholar]
- Walton J.P. Frisina R.D. Ison J.R. O'Neill W.E. Neural correlates of behavioral gap detection in the inferior colliculus of the young CBA mouse. J. Comp. Physiol. A. 1997;181:161–176. doi: 10.1007/s003590050103. [DOI] [PubMed] [Google Scholar]
- Wang H. Brozoski T.J. Turner J.G. Ling L. Parrish J.L. Hughes L.F. Caspary D.M. Plasticity at glycinergic synapses in dorsal cochlear nucleus of rats with behavioral evidence of tinnitus. Neuroscience. 2009;164:747–759. doi: 10.1016/j.neuroscience.2009.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley J.L. Compton A.D. Pike B.R. Temple M.D. McElderry J.W. Hamm R.J. Reduced sensorimotor reactivity following traumatic brain injury in rats. Brain Res. 1996;716:47–52. doi: 10.1016/0006-8993(96)00045-5. [DOI] [PubMed] [Google Scholar]
- Wu C.M. Ng S.H. Liu T.C. Diffusion tensor imaging of the subcortical auditory tract in subjects with long-term unilateral sensorineural hearing loss. Audiol. Neurootol. 2009a;14:248–253. doi: 10.1159/000191282. [DOI] [PubMed] [Google Scholar]
- Wu C.M. Ng S.H. Wang J.J. Liu T.C. Diffusion tensor imaging of the subcortical auditory tract in subjects with congenital cochlear nerve deficiency. AJNR Am. J. Neuroradiol. 2009b;30:1773–1777. doi: 10.3174/ajnr.A1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T.C. Wilde E.A. Bigler E.D. Li X. Merkley T.L. Yallampalli R. McCauley S.R. Schnelle K.P. Vasquez A.C. Chu Z. Hanten G. Hunter J.V. Levin H.S. Longitudinal changes in the corpus callosum following pediatric traumatic brain injury. Dev. Neurosci. 2010;32:361–373. doi: 10.1159/000317058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang G. Lobarinas E. Zhang L. Turner J. Stolzberg D. Salvi R. Sun W. Salicylate induced tinnitus: behavioral measures and neural activity in auditory cortex of awake rats. Hear. Res. 2007;226:244–253. doi: 10.1016/j.heares.2006.06.013. [DOI] [PubMed] [Google Scholar]
- Zhang J.S. Kaltenbach J.A. Increases in spontaneous activity in the dorsal cochlear nucleus of the rat following exposure to high-intensity sound. Neurosci. Lett. 1998;250:197–200. doi: 10.1016/s0304-3940(98)00482-0. [DOI] [PubMed] [Google Scholar]
- Zhang J.S. Zhang X.G. Electrical stimulation of the dorsal cochlear nucleus induces hearing in rats. Brain Res. 2010;1311:37–50. doi: 10.1016/j.brainres.2009.11.032. [DOI] [PubMed] [Google Scholar]
- Zhang J. Zhang Y. Zhang X. Auditory cortex electrical stimulation suppresses tinnitus in rats. J. Assoc. Res. Otolaryngol. 2011;12:185–201. doi: 10.1007/s10162-010-0246-z. [DOI] [PMC free article] [PubMed] [Google Scholar]