Abstract
Resveratrol (3,5,4’-trihydroxystilbene) has been ascribed multiple beneficial biological effects but the influence of resveratrol on glucocorticoid-induced muscle atrophy is not known. We examined the effects of resveratrol on dexamethasone-induced atrogin-1 and MuRF1 expression, FOXO1 acetylation, protein degradation and atrophy in cultured L6 myotubes. In addition, the role of the deacetylase SIRT1 in the effects of resveratrol was determined by transfecting myotubes with SIRT1 siRNA. The catabolic effects of dexamethasone were prevented by resveratrol and the protective effects of resveratrol on dexamethasone-induced atrogin-1 and MuRF1 expression were abolished in myotubes transfected with SIRT1 siRNA. Results suggest that resveratrol can prevent glucocorticoid-induced muscle wasting and that this effect is at least in part SIRT1-dependent.
Keywords: Muscle wasting, glucocorticoids, acetylation, deacetylation, resveratrol
INTRODUCTION
Resveratrol (3,5,4’-trihydroxystilbene) is a natural polyphenol present in peanuts, pines, the skin of grapes, and red wine [1,2]. It has been ascribed multiple beneficial biological effects, including cardioprotection [3] and amelioration of some of the metabolic consequences of diabetes [4]. Different mechanisms by which resveratrol may exert beneficial effects have been described and include antioxidant effects [5,6], inhibition of NF-kB [7], and activation of AMP-activated protein kinase (AMPK) [8]. In other reports, resveratrol stimulated the activity of the histone deacetylase SIRT1 and recent studies suggest that this may be the most important mechanism of the metabolic effects of the drug [9–12].
Previous studies suggest that resveratrol may protect skeletal muscle from the influence of certain catabolic conditions, including diabetes [4], mechanical unloading [13], muscular dystrophy [14], and cancer [15]. Conflicting results have been reported, however, and in recent experiments, muscle wasting was not prevented, or was even worsened, by resveratrol [16]. In addition, the mechanisms by which resveratrol protects skeletal muscle from muscle wasting are unclear. In particular, the role of SIRT1 activation in resveratrol-induced protection from muscle wasting is not well understood. This is important, because recent studies from our and other laboratories suggest that muscle wasting is associated with reduced expression and activity of histone deacetylases, including SIRT1 [17,18].
High levels of glucocorticoids result in increased expression of the muscle atrophy-related ubiquitin ligases atrogin-1 and MuRF1, increased ubiquitin-proteasome-dependent muscle proteolysis, and loss of muscle mass [19,20]. In addition, the catabolic effects of certain conditions, such as sepsis and severe injury, are at least in part mediated by glucocorticoids [19–22]. The effects of resveratrol on glucocorticoid-induced atrogin-1 and MuRF1 expression and muscle atrophy and the role of SIRT1 activation have not been reported. Here, we tested the hypothesis that resveratrol prevents dexamethasone-induced expression of atrogin-1 and MuRF1, protein degradation and atrophy in cultured myotubes and that the protective effects of resveratrol are SIRT1-dependent.
Previous studies suggest that atrogin-1 and MuRF1 expression is at least in part regulated by the transcription factor FOXO1 [23,24]. Other reports provided evidence that FOXO1 activity is increased by acetylation and can be inhibited by SIRT1 [25,26] although apparently contradictory results have also been reported [27], possibly reflecting differential regulation of FOXO1 activity by acetylation in different cell types. The regulation by glucocorticoids of FOXO1 acetylation in skeletal muscle and the effects of resveratrol are not known. In the present study, we therefore also tested the influence of dexamethasone and resveratrol on FOXO1 acetylation in cultured myotubes.
MATERIALS AND METHODS
Cell culture
L6 muscle cells, a rat skeletal muscle cell line (American Type Culture Collection, Manassas, VA), were maintained and cultured as described in detail recently [28]. Differentiated myotubes were treated for 24 h with 1 μM dexamethasone (Sigma Aldrich, St. Louis, MO), 100 μM resveratrol (Sigma Aldrich), or both drugs in combination. The concentrations of dexamethasone and resveratrol used here were based on previous studies [28–30]. Control myotubes were treated with solvent (0.1% ethanol).
Preparation of total cell lysates and nuclear extracts
Total cell lysates were prepared by harvesting the myotubes directly in RIPA buffer (50 mM Tris-HCl, 150 mM NaCl, 0.5% sodium deoxycholate, 0.1% SDS, and 1% Nonidet P-40) containing Protease Inhibitor Cocktail Tablets (Roche Applied Science, Indianapolis, IN). After scraping the lysates into eppendorf tubes, the samples were briefly sonicated using a Sonic Dismembrator (Fisher Scientific, Model 100) followed by centrifugation at 14,000 x g for 10 minutes at 4°C. Nuclear extracts were prepared using the NE-PER® Nuclear and Cytoplasmic Extraction Reagents (Thermo Fisher Scientific, Asheville, NC) according to the manufacturer’s instructions. Concentrations of soluble proteins in the supernatants of the nuclear extracts and total cell lysates were determined by using the Bradford Protein Assay Kit (Theromo Fisher Scientific) with bovine serum albumin as standard. Nuclear extracts and cell lysates were stored at 80°C until analyzed.
Real-Time PCR
Messenger RNA levels for atrogin-1, MuRF1, and SIRT1 were determined by real-time PCR performed as described in detail recently [17,28,29,31]. The sequences of the forward, reverse, and double-labeled oligonucleotides for rat atrogin-1 and MuRF1 used here were reported recently [17,31]. SIRT1 mRNA levels were determined using the ABI Taqman Gene Expression Assay (Assay ID: RN01428093_m1) from Applied Biosystems, Foster City, CA.
Western blotting
Western blotting was performed as described in detail recently [17,28] using the following antibodies: a rabbit polyclonal anti-mouse atrogin-1 antibody (1:1,000; kindly supplied by Dr. Stewart Lecker, Harvard Medical School); a mouse polyclonal anti-rat MuRF1 antibody (1:1,000; kindly supplied by Regeneron Pharmaceuticals, NY); a rabbit monoclonal anti-rat α-tubulin antibody (1:5,000; Sigma Aldrich); a goat anti-rabbit IgG horseradish peroxidase-conjugated secondary antibody (1:5,000; Santa Cruz Biotechnology, Santa Cruz, CA). Levels of acetylated FOXO1 and PGC-1α were determined by co-immunoprecipitation. Nuclear proteins (300 μg) were pulled down with an anti-acetyl lysine antibody (Cell Signaling Technology, Danver, MA) followed by Western blotting using a rabbit polyclonal anti-rat FOXO1 antibody (Cell Signaling Technology) or a rabbit polyclonal anti-rat PGC-1α antibody (Cell Signaling Technology). Immunoreactive protein bands were visualized by chemiluminescence using the Western Lighting Kit (Perkin Elmer Life Sciences Inc., Boston, MA) followed by exposure to X-Ray blue film (Cole-Parmer, Vernon Hills, IL). Molecular weights of protein bands were determined by Dual Precision molecular weight standards (BioRad, Hercules, CA). The protein bands were quantified by densitometry using ImageJ software (NIH, Frederick, MD).
Measurement of protein degradation and myotube diameter
Protein degradation rates were determined by measuring the release of trichloroacetic acid (TCA)-soluble radioactivity during 24 h from proteins prelabeled with [3H]-tyrosine as described [28,29]. For the measurement of myotube diameter, myotube cultures were photographed under a phase contrast microscope at 100X magnification. The diameters were measured in a total of 60 myotubes from at least 10 random fields using ImageJ software (NIH) as described in detail [28,29]. The measurements were conducted in a “blinded” fashion on coded pictures with the investigator being unaware of the group (control, dexamethasone-, resveratrol-, or dexamethasone + resveratrol-treated myotubes) from which the cultures originated. Results were expressed as per cent of control.
Cell transfections
L6 myotubes were transfected with SIRT1 siRNA or non-targeting (scrambled) siRNA (Santa Cruz Biotechnology) utilizing the transfection reagent Lipofectamine RNAiMAX (Invitrogen, Grand Island, NY). The siRNA constructs were added to the culture medium at a concentration of 165 nM in combination with Lipofectamine according to the manufacturer’s instructions. After 5 h, the medium was changed to fresh DMEM containing 2% FBS. After 48 h, myotubes were exposed for 24 h to1 μM dexamethasone, 100 μM resveratrol, a combination of the drugs, or solvent alone (0.1% ethanol).
Statistics
Results are reported as means ± SEM. Statistical analysis was performed by Student's t-test or ANOVA followed by Tukey’s post hoc test as appropriate. p<0.05 was considered statistically significant.
RESULTS
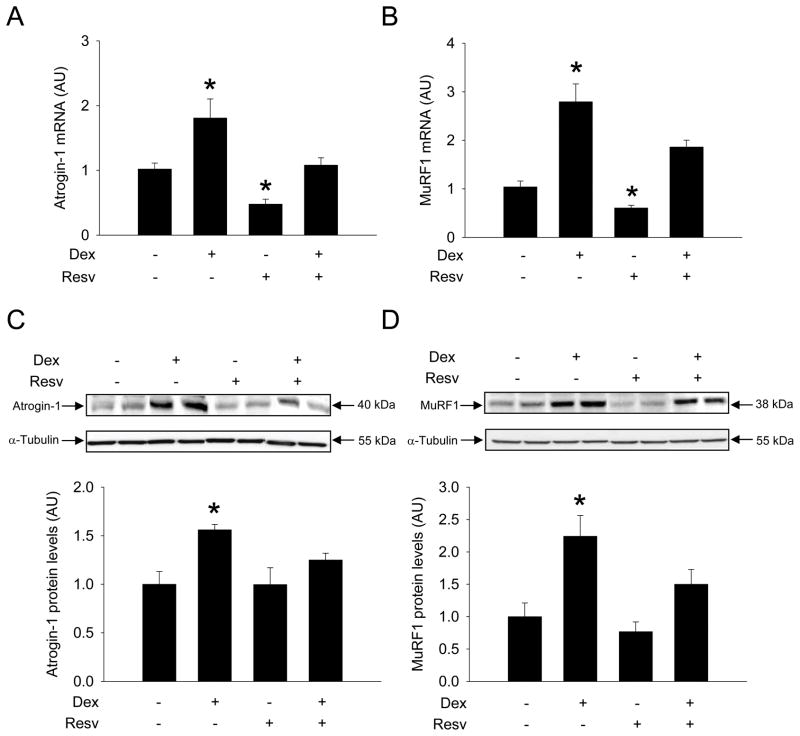
Treatment of cultured L6 myotubes with 1 μM dexamethasone for 24 h resulted in an approximately 2- to 3-fold increase in atrogin-1 and MuRF1 mRNA levels (Fig 1A and B) and a 1.5- to 2-fold increase in corresponding protein levels (Fig 1C and D). These effects of dexamethasone are similar to previous reports [28,29]. When dexamethasone-treated myotubes were exposed to 100 μM resveratrol, atrogin-1 and MuRF1 mRNA and protein levels were not different from those in untreated control myotubes. Of note, resveratrol by itself reduced atrogin-1 and MuRF1 mRNA levels but did not influence the corresponding protein levels. Taken together, the results suggest that resveratrol inhibits dexamethasone-induced expression of atrogin-1 and MuRF1 in cultured myotubes.
Figure 1.
Resveratrol inhibits dexamethasone-induced expression of atrogin-1 and MuRF1 in cultured L6 myotubes. Myotubes were treated for 24 h with 1 μM dexamethasone, 100 μM resveratrol, both drugs in combination or solvent (0.1% ethanol) followed by measurement of mRNA levels for (A) atrogin-1 and (B) MuRF1 and protein levels for (C) atrogin-1 and (D) MuRF1. Results are means ± SEM with n=8 per group. *p<0.05 vs all other groups by ANOVA.
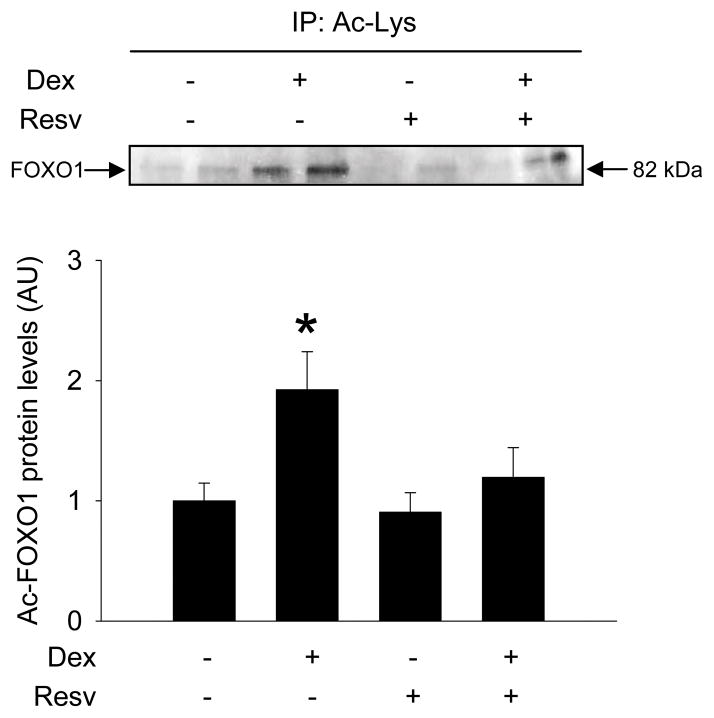
Because previous studies suggest that atrogin-1 and MuRF1 expression is at least in part regulated by FOXO1 [23,24] and that FOXO1 activity may be stimulated by acetylation [25], we next tested whether FOXO1 acetylation is increased in dexamethasone-treated myotubes and can be reduced by resveratrol. When L6 myotubes were treated with dexamethasone, nuclear levels of acetylated FOXO1 increased, consistent with increased FOXO1 activity [25], and this effect of dexamethasone was blocked by resveratrol (Fig 2).
Figure 2.
Resveratrol blocks dexamethasone-induced acetylation of FOXO1 in cultured L6 myotubes. Myotubes were treated with dexamethasone and resveratrol as described in Fig 1. Nuclear levels of acetylated FOXO1 were determined by co-immunopecipitation as described in Materials and Methods. Results are means ± SEM with n=6 per group. *p<0.05 vs all other groups by ANOVA.
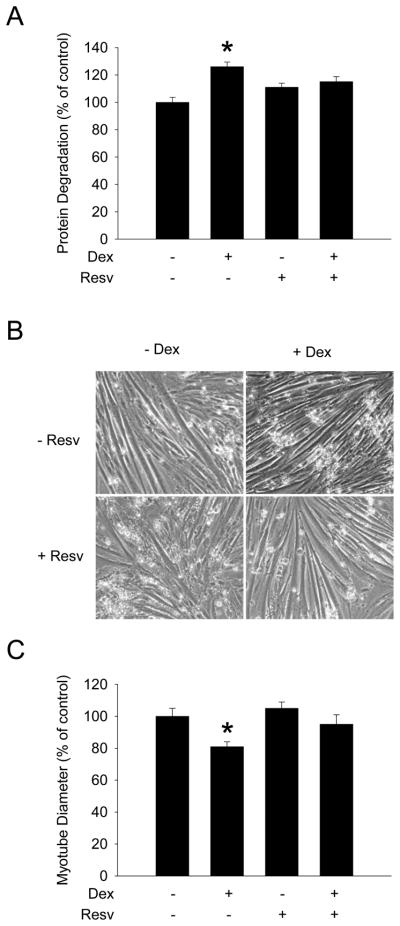
Since glucocorticoid-induced loss of muscle mass is at least in part regulated by FOXO1-mediated upregulation of atrogin-1 and MuRF1 expression [23,24], we next examined whether resveratrol may prevent dexamethasone-induced increase in muscle protein degradation and myotube atrophy. Treatment of L6 myotubes with dexamethasone stimulated protein degradation by approximately 20% (Fig 3A) and reduced myotube diameter by 20-30% (Fig 3B and C), similar to previous reports [28,29]. Importantly, both the dexamethasone-induced increase in protein degradation and myotube atrophy were prevented by resveratrol.
Figure 3.
Resveratrol inhibits dexamethasone-induced protein degradation and atrophy in cultured L6 myotubes. Myotubes were treated with dexamethasone and resveratrol as described in Fig 1. (A) Protein degradation, (B) myotube morphology, and (C) myotube diameter were determined as described in Materials and Methods. Results in (A) and (B) are means ± SEM with n=8 per group. *p<0.05 vs all other groups by ANOVA.
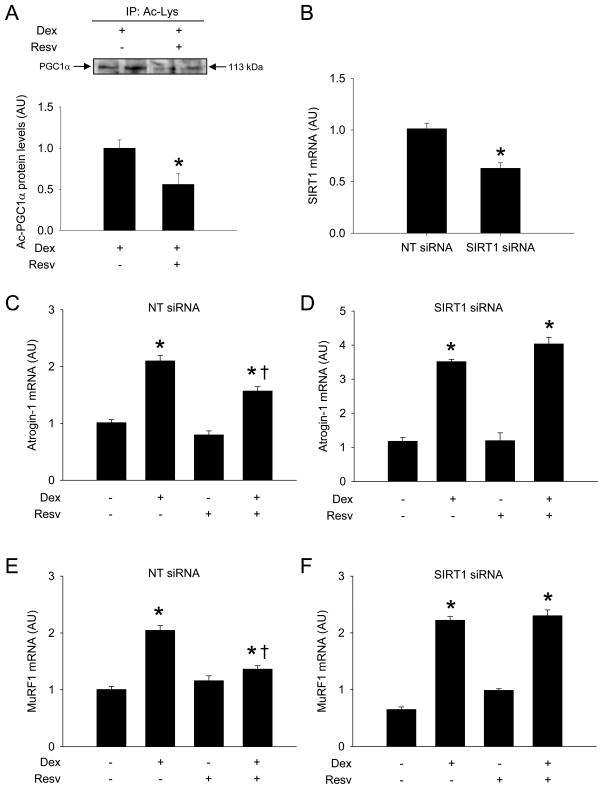
Because studies suggest that activation of SIRT1 may be the most important mechanism of the metabolic effects of resveratrol [9–12], we next tested whether SIRT1 activation may be involved in the effects of resveratrol observed here. This was done by using two experimental approaches. First, we examined whether resveratrol activated SIRT1 in dexamethasone-treated myotubes by determining the acetylation status of the nuclear cofactor PGC-1α, a well established marker of SIRT1 activity [8,32]. Treatment of the myotubes with resveratrol resulted in an approximately 50% decrease in acetylated PGC-1α (Fig 4A) suggesting that resveratrol activated SIRT1 under the present experimental conditions. Next, we transfected cultured myotubes with SIRT1 siRNA or non-targeting (scrambled) siRNA to test whether the effects of resveratrol on dexamethasone-induced atrogin-1 and MuRF1 expression would be reduced after knockdown of SIRT1 expression. Transfection of myotubes with SIRT1 siRNA reduced SIRT1 expression by approximately 40% (Fig 4B). The protective effects of resveratrol on dexamethasone-induced atrogin-1 and MuRF1 expression were abolished in myotubes with downregulated expression of SIRT1 (Fig 4C-F), suggesting that SIRT1 is involved in the effects of resveratrol in dexamethasone-treated myotubes. Of note, resveratrol alone did not influence atrogin-1 and MuRF1 mRNA levels in this experiment (see Fig 4C-F) which differs from the results in resveratrol-treated, non-transfected myotubes (compare with Fig 1A and B). Although we can not offer an explanation for these apparently contradictory results, taken together, the results in Fig 1 and 4 support the concept that dexamethasone-induced increase in atrogin-1 and MuRF1 expression is inhibited by resveratrol.
Figure 4.
SIRT1 is involved in the protective effects of resveratrol in dexamethasone-treated L6 myotubes. (A) Nuclear levels of acetylated PGC-1α were determined by co-immunoprecipitation in dexamethasone-treated myotubes in the absence or presence of resveratrol. (B) SIRT1 mRNA levels were determined in myotubes after transfection with non-targeting (NT) or SIRT1 siRNA. In other experiments, myotubes were treated with dexamethasone and resveratrol as described in Fig 1 following transfection with non-targeting (NT) or SIRT1 siRNA and atrogin-1 (C and D) and MuRF1 (E and F) mRNA levels were determined by real-time PCR. Results are means ± SEM with n=6 (A) or 8 per group. *p<0.05 by Student’s t-test (A and B); *p<0.05 vs all other groups; +p<0.05 vs Dex by ANOVA (C-F).
DISCUSSION
The present results suggest that resveratrol may inhibit glucocorticoid-induced muscle wasting and upregulation of atrogin-1 and MuRF1 and that these effects of resveratrol are at least in part regulated by SIRT1. The results have important clinical implications because muscle wasting is commonly seen as a significant side-effect in patients treated with corticosteroids [33,34] and in patients with Cushing’s syndrome [35]. In addition, muscle wasting in several catabolic conditions, including sepsis and burn injury, is in part regulated by glucocorticoids [19–22]. Therefore, treatment that reduces glucocorticoid-mediated muscle wasting may benefit a large number of patients with different muscle wasting conditions. Other studies provided evidence of muscle sparing effects of resveratrol in additional conditions associated with muscle wasting, including diabetes [4], muscular dystrophy [14], muscle disuse [13], and cancer cachexia [15], further supporting the potential role of resveratrol in the prevention and treatment of muscle wasting. Of note, an apparently contradictory study in which resveratrol did not prevent muscle wasting in experimental cancer cachexia [16] may reflect insufficient doses of resveratrol used in that study (up to 25 mg/kg/day). Recent experiments suggest that a resveratrol dose of at least 200 mg/kg/day may be necessary to prevent cancer-induced cachexia [15].
Although the effects of resveratrol on glucocorticoid-induced muscle atrophy have not been reported previously, the influence of resveratrol on glucocorticoid-induced upregulation of uncoupling protein-3 (UCP3) in skeletal muscle was reported recently [30]. In that study, resveratrol prevented dexamethasone-induced increase in UCP3 expression in cultured muscle cells and evidence was found that this effect of resveratrol was SIRT1-dependent. Although the precise role of UCP3 in muscle wasting is not known at present, sepsis [36], endotoxemia [37], and cancer cachexia [38] are associated with increased UCP3 expression in skeletal muscle, suggesting that inhibited UCP3 expression may play a role in the muscle sparing effects of resveratrol.
The involvement of the histone deacetylase SIRT1 in the anti-catabolic effects of resveratrol is important because we and others found recently that hyperacetylation may be an important factor in muscle wasting [17,18,39–41]. Resveratrol-induced deacetylation of FOXO1, consistent with reduced FOXO1 activity [25,26], and deacetylation of PGC-1α, consistent with increased PGC-1α activity [8,32,42], as observed in the present study, are particularly important because atrogin-1 and MuRF1 expression is increased by FOXO1 activity [23,24] and reduced by PGC-1α [43,44]. Thus, it is possible that the prevention by resveratrol of dexamethasone-induced atrogin-1 and MuRF1 expression observed here at least in part reflected reduced FOXO1 and increased PGC-1α activities.
Although the present study and several previous reports suggest that activation of SIRT1 is involved in the muscle sparing effects of resveratrol [9–12], the results reported here do not rule out the possibility that some of the effects of resveratrol were SIRT1-independent. It should be noted that other potential mechanisms that may be involved in the anti-catabolic effects of resveratrol have been described. These mechanisms include antioxidant effects [5,6], inhibition of NF-kB [7] and iNOS activities [8], prevention of insulin resistance [4,45], and increased microvascular recruitment in skeletal muscle [46]. It is of course possible, and perhaps even likely, that several of these mechanisms are interconnected. For example, inhibition of NF-kB by resveratrol may reflect SIRT1-dependent deacetylation of NF-kB/p65 [47] and antioxidant effects may reflect improved PGC-1α-regulated mitochondrial biogenesis [11,42].
Interestingly, a recent study by Centeno-Baez et al [8] suggests that mechanism(s) regulating the anti-catabolic effects of resveratrol may depend on the mediators involved in muscle wasting and that different effects of resveratrol may be regulated by different mechanisms. Thus, in that study [8], treatment of cultured L6 muscle cells with a mixture of TNFα, IFNγ, and LPS resulted in increased iNOS expression and NO levels. These effects of the cytokine/LPS mixture were inhibited by resveratrol in a dose-dependent manner and because the effects of resveratrol were not blocked by different SIRT1 inhibitors, the authors concluded that the beneficial effects of resveratrol did not reflect SIRT1 activation. This conclusion was further supported by the inability of resveratrol to deacetylase PGC-1α in untreated control cells (although the influence of resveratrol on the status of PGC-1α acetylation in cytokine/LPS-treated muscle cells was not reported). In contrast, downregulation of AMPK with siRNA or treatment of the muscle cells with the AMPK inhibitor Compound C blunted the ability of resveratrol to prevent the cytokine/LPS-induced iNOS expression and NO production and the authors concluded that resveratrol inhibition of iNOS in skeletal muscle involves AMPK but not SIRT1 [8]. Although these results suggest that resveratrol targets a different mechanism in muscle cells treated with cytokines and LPS [8] than in muscle cells treated with dexamethasone [present study and ref # 30], the apparently contradictory results may also suggest that resveratrol inhibits the expression of atrogin-1, MuRF1, and UCP3 and the expression of iNOS through different mechanisms.
The present study is important because it provides novel information about the influence of resveratrol on glucocorticoid-induced muscle wasting. In addition, the results provide molecular evidence that the muscle-sparing effects of resveratrol are at least in part regulated by SIRT1 and reflect inhibited upregulation of the ubiquitin ligases atrogin-1 and MuRF1.
High levels of glucocorticoids result in loss of muscle mass
We tested if resveratrol prevents glucocorticoid-induced muscle atrophy
Myotubes were treated with dexamethasone with or without resveratrol
Resveratrol blocked dexamethasone-induced expression of atrogin-1, MuRF1 and atrophy
The effects of resveratrol were abolished by SIRT1 siRNA
Acknowledgments
The study was supported in part by NIH grant R01 DK37908. ZA was supported in part by Department of Clinical Medicine, Sapienza University of Rome, Rome, Italy. EC was supported in part by Gobierno Vasco, Spain (BFI2010-240).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Soleas GJ, Diamandis EP, Goldberg DM. The world of resveratrol. Adv Exp Med Biol. 2001;492:159–182. doi: 10.1007/978-1-4615-1283-7_13. [DOI] [PubMed] [Google Scholar]
- 2.Burns J, Yokota T, Ashikara H, Lean ME, Crozier A. Plant foods and herbal sources of resveratrol. J Agric Food Chem. 2002;50:3337–3340. doi: 10.1021/jf0112973. [DOI] [PubMed] [Google Scholar]
- 3.Chen CJ, Yu W, Fu YC, Wang X, Li JL, Wang W. Resveratrol protects cardiomyocytes from hypoxia-induced apoptosis through the SIRT1-FoxO1 pathway. Biochem Biophys Res Commun. 2009;378:389–393. doi: 10.1016/j.bbrc.2008.11.110. [DOI] [PubMed] [Google Scholar]
- 4.Chen KH, Cheng ML, Jung YH, Chiu DTY, Shiao MS, Chen JK. Resveratrol ameliorates metabolic disorders and muscle wasting in streptozotocin-induced diabetic rats. Am J Physiol Endocrinol Metab. 2011;301:E853–E863. doi: 10.1152/ajpendo.00048.2011. [DOI] [PubMed] [Google Scholar]
- 5.Das S, Khan N, Mukherjee S, Bagchi D, Gurusamy N, Swartz H, Das DK. Redox regulation of resveratrol-mediated switching of death signal into survival signal. Free Rad Biol Med. 2008;44:82–90. doi: 10.1016/j.freeradbiomed.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 6.Jackson JR, Ryan MJ, Hao Y, Alway SE. Mediation of endogenous antioxidant enzymes and apoptotic signaling by resveratrol following muscle disuse in the gastrocnemius muscles of young and old rats. Am J Physiol Regul Integr Comp Physiol. 2010;299:R1572–R1581. doi: 10.1152/ajpregu.00489.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Holmes-McNary M, Baldwin AS. Chemopreventive properties of trans-resveratrol are associated with inhibition of activation of the IkappaB kinase. Cancer Res. 2000;60:3477–3483. [PubMed] [Google Scholar]
- 8.Centeno-Baez C, Dallaire P, Marette A. Resveratrol inhibition of inducible nitric oxide synthase in skeletal muscle involves AMPK but not SIRT1. Am J Physiol Endocrinol Metab. 2011;301:E922–E930. doi: 10.1152/ajpendo.00530.2010. [DOI] [PubMed] [Google Scholar]
- 9.Howitz KT, Bitterman KJ, Cohen HY, Lamming DW, Lavu S, Wood JG, Zipkin RE, Chung P, Kisielewski A, Zhang LL, Scherer B, Sinclair DA. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature. 2003;425:191–196. doi: 10.1038/nature01960. [DOI] [PubMed] [Google Scholar]
- 10.Borra MT, Smith BC, Denn JM. Mechanism of human SIRT1 activation by resveratrol. J Biol Chem. 2005;280:17187–17195. doi: 10.1074/jbc.M501250200. [DOI] [PubMed] [Google Scholar]
- 11.Lagouge M, Argmann C, Gerhart-Hines Z, Meziane H, Lerin C, Daussin F, Messadeq N, Millne J, Lambert P, Elliott P, Geny B, Laakso M, Puigserver P, Auwerx J. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1α. Cell. 2006;127:1109–1122. doi: 10.1016/j.cell.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 12.Shakibaei M, Buhrmann C, Mobaskeri A. Resveratrol-mediated SIRT-1 interactions with p300 modulate receptor activator of NF-kB ligand (RANKL) activation of NF-kB signaling and inhibits osteoclastogenesis in bone-derived cells. J Biol Chem. 2011;286:11492–11505. doi: 10.1074/jbc.M110.198713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Momken I, Stevens L, Bergonignau A, Desplanches D, Rudwill F, Chery I, Zahariev A, Zahn S, Stein TP, Sebedio JL, Pujos-Guillot E, Falempin M, Simon C, Coxam V, Andrianjafinony T, Gauquelin-Koch G, Picquet F, Blanc S. Resveratrol prevents the wasting disorders of mechanical unloading by acting as a physical exercise mimetic in the rat. FASEB J. 2011;25:3646–3660. doi: 10.1096/fj.10-177295. [DOI] [PubMed] [Google Scholar]
- 14.Hori YS, Kuno A, Hosoda R, Tanno M, Miura T, Shimamoto K, Horio Y. Resveratrol ameliorates muscular pathology in the dystrophic mdx mouse, a model for Duchenne muscular dystrophy. J Pharm Exp Ther. 2011;338:784–794. doi: 10.1124/jpet.111.183210. [DOI] [PubMed] [Google Scholar]
- 15.Shadfar S, Couch ME, McKinney KA, Weinstein LJ, Yin X, Rodriguez JE, Guttridge DC, Willis M. Oral resveratrol therapy inhibits cancer-induced skeletal muscle and cardiac atrophy in vivo. Nutr Canc. 2011;63:749–762. doi: 10.1080/01635581.2011.563032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Busquets S, Fuster G, Ametller E, Olivan M, Figueras M, Costelli P, Carbo N, Argiles JM, Lopez-Soriano FJ. Resveratrol does not ameliorate muscle wasting in different types of cancer cachexia models. Clin Nutr. 2007;26:239–244. doi: 10.1016/j.clnu.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 17.Alamdari N, Smith IJ, Aversa Z, Hasselgren PO. Sepsis and glucocorticoids upregulate p300 and downregulate HDAC6 expression and activity in skeletal muscle. Am J Physiol Regul Integr Comp Physiol. 2010;299:R509–R520. doi: 10.1152/ajpregu.00858.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sadoul K, Boyault C, Pabion M, Khochbin S. Regulation of protein turnover by acetyltransferases and deacetylases. Biochimie. 2008;90:306–312. doi: 10.1016/j.biochi.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 19.Hasselgren PO, Alamdari N, Aversa Z, Gonnella P, Smith IJ, Tizio S. Corticosteroids and muscle wasting – role of transcription factors, nuclear cofactors, and hyperacetylation. Curr Opin Clin Nutr Metabol Care. 2010;13:423–428. doi: 10.1097/MCO.0b013e32833a5107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tiao G, Fagan J, Roegner V, Lieberman M, Wang JJ, Fischer JE, Hasselgren PO. Energy-ubiquitin-dependent muscle proteolysis during sepsis in rats is regulated by glucocorticoids. J Clin Invest. 1996;97:339–348. doi: 10.1172/JCI118421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fang CH, James JH, Ogle C, Fischer JE, Hasselgren PO. Influence of burn injury on protein metabolism in different types of skeletal muscle and the role of glucocorticoids. J Am Coll Surg. 1995;180:33–42. [PubMed] [Google Scholar]
- 22.Schakman O, Gilson H, Thiessen JP. Mechanisms of glucocorticoid-induced myopathy. J Endocrinol. 2008;197:1–10. doi: 10.1677/JOE-07-0606. [DOI] [PubMed] [Google Scholar]
- 23.Waddell DS, Baehr LM, van den Brandt J, Johnsen SA, Reichardt HM, Furlow JD, Bodine SC. The glucocorticoid receptor and FOXO1 synergistically activate the skeletal muscle atrophy-associated MuRF1 gene. Am J Physiol Endocrinol Metab. 2008;295:E785–E797. doi: 10.1152/ajpendo.00646.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smith I, Alamdari N, O’Neal P, Gonnella P, Aversa Z, Hasselgren PO. Sepsis increases the expression and activity of the transcription factor Forkhead Box O 1 (FOXO1) in skeletal muscle by a glucocorticoid-dependent mechanism. Int J Biochem Cell Biol. 2010;42:701–711. doi: 10.1016/j.biocel.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Perrot V, Rechler MM. The coactivator p300 directly acetylates the forkhead transcription factor Foxo1 and stimulates Foxo1-induced transcription. Mol Endocr. 2005;19:2283–2298. doi: 10.1210/me.2004-0292. [DOI] [PubMed] [Google Scholar]
- 26.Motta MC, Divecha N, Lemieux M, Kamel C, Chen D, Gu W, Bultsma Y, McBurney M, Guarente L. Mammalian SIRT1 represses forkhead transcription factors. Cell. 2004;116:551–563. doi: 10.1016/s0092-8674(04)00126-6. [DOI] [PubMed] [Google Scholar]
- 27.Park JM, Kim TH, Bae JS, Kim MY, Kim KS, Ahn YH. Role of resveratrol in FOXO1-mediated gluconeogenic gene expression in the liver. Biochem Biophys Res Commun. 2010;403:329–334. doi: 10.1016/j.bbrc.2010.11.028. [DOI] [PubMed] [Google Scholar]
- 28.Menconi M, Gonnella P, Petkova V, Lecker S, Hasselgren PO. Dexamethasone and corticosterone induce similar, but not identical muscle wasting responses in cultured L6 and C2C12 myotubes. J Cell Biochem. 2008;105:353–364. doi: 10.1002/jcb.21833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gonnella P, Alamdari N, Tizio S, Aversa Z, Petkova V, Hasselgren PO. C/EBPβ regulates dexamethasone-induced muscle cell atrophy and expression of atrogin-1 and MuRF1. J Cell Biochem. 2011;112:1737–1748. doi: 10.1002/jcb.23093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Amat R, Solanes G, Giralt M, Villarroya F. SIRT1 is involved in glucocorticoid-mediated control of uncoupling protein-3 gene transcription. J Biol Chem. 2007;282:34066–34076. doi: 10.1074/jbc.M707114200. [DOI] [PubMed] [Google Scholar]
- 31.Fareed MU, Evenson AR, Wei W, Menconi M, Poylin V, Petkova V, Pignol B, Hasselgren PO. Treatment of rats with calpain inhibitors prevents sepsis-induced muscle proteolysis independent of atrogin-1/MAFbx and MuRF1 expression. Am J Physiol Regual Integr Comp Physiol. 2006;290:R1589–R1597. doi: 10.1152/ajpregu.00668.2005. [DOI] [PubMed] [Google Scholar]
- 32.Rodgers JT, Lerin C, Haas W, Gygi SP, Spiegelman BM, Puigserver P. Nutrient control of glucose homeostasis through a complex of PGC-1α and SIRT1. Nature. 2005;434:113–118. doi: 10.1038/nature03354. [DOI] [PubMed] [Google Scholar]
- 33.Dekhuijzen PNR, Decramer M. Steroid-induced myopathy and its significance to respiratory disease: a known disease rediscovered. Eur Respir J. 1992;5:997–1003. [PubMed] [Google Scholar]
- 34.Pereira RM, Frere de Carvalho J. Glucocorticoid-induced myopathy. Joint Bone Spine. 2011;78:41–44. doi: 10.1016/j.jbspin.2010.02.025. [DOI] [PubMed] [Google Scholar]
- 35.Orth DN, Kovacs WJ, DeBold CR. The adrenal cortex. In: Wilson JD, Foster DW, editors. Williams Textbook of Endocrinology. 8. W.B. Saunders Co; 1992. pp. 489–619. [Google Scholar]
- 36.Sun X, Wray CJ, Tian X, Hasselgren PO, Lu J. The expression of uncoupling protein 3 is upregulated in skeletal muscle during sepsis. Am J Physiol Endocrinol Metab. 2003;285:E512–E520. doi: 10.1152/ajpendo.00446.2002. [DOI] [PubMed] [Google Scholar]
- 37.Yu XX, Barger JL, Boyer BB, Brand MD, Pan G, Adams SH. Impact of endotoxin on UCP homolog mRNA abundance, thermoregulation, and mitochondrial proton leak kinetics. Am J Physiol Endocrinol Metab. 2000;279:E433–E446. doi: 10.1152/ajpendo.2000.279.2.E433. [DOI] [PubMed] [Google Scholar]
- 38.Busquets S, Almendro V, Barreiro E, Figueras M, Argiles JM, Lopez-Soriano FJ. Activation of UCPs gene expression in skeletal muscle can be independent on both circulating fatty acids and food intake. Involvement of ROS in a model of mouse cancer cachexia. FEBS Lett. 2005;579:717–722. doi: 10.1016/j.febslet.2004.12.050. [DOI] [PubMed] [Google Scholar]
- 39.Yang H, Menconi M, Wei W, Petkova V, Hasselgren PO. Dexamathasone upregulates the expression and activity of the nuclear cofactor p300 and its interaction with C/EBP in cultured myotubes. J Cell Biochem. 2005;94:1058–1067. doi: 10.1002/jcb.20371. [DOI] [PubMed] [Google Scholar]
- 40.Yang H, Wei W, Menconi M, Hasselgren PO. Dexamethasone-induced protein degradation in cultured myotubes is p300/HAT-dependent. Am J Physiol Regul Integr Comp Physiol. 2007;292:R337–R344. doi: 10.1152/ajpregu.00230.2006. [DOI] [PubMed] [Google Scholar]
- 41.Tobimatsu K, Noguchi T, Hosooka T, Sakai M, Inagaki K, Matsuki Y, Hiramatsu R, Kasuga M. Overexpression of the transcriptional coregulator Cited2 protects against glucocorticoid-induced atrophy of C2C12 myotubes. Biochem Biophys Res Commun. 2009;378:399–403. doi: 10.1016/j.bbrc.2008.11.062. [DOI] [PubMed] [Google Scholar]
- 42.Gerhart-Hines Z, Rodgers JT, Bare O, Lerin C, Kim SH, Mostoslavsky R, Alt FW, Wu Z, Puigserver P. Metabolic control of muscle mitochondrial function and fatty acid oxidation through SIRT1/PGC-1α. EMBO J. 2007;26:1913–1923. doi: 10.1038/sj.emboj.7601633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Menconi MJ, Arany ZP, Alamdari N, Aversa Z, Gonnella P, O’Neal P, Smith I, Tizio S, Hasselgren PO. Sepsis and glucocorticoids downregulate the expression of the nuclear cofactor PGC-1beta in skeletal muscle. Am J Physiol Endocrinol Metab. 2010;299:E533–E543. doi: 10.1152/ajpendo.00596.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lin J, Handschin C, Spiegleman BM. Metabolic control through the PGC-1 family of transcription coactivators. Cell Metab. 2005;1:361–370. doi: 10.1016/j.cmet.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 45.Kang W, Hong HJ, Guan J, Kim DG, Yang EJ, Koh G, Park D, Han CH, Lee YJ, Lee DH. Resveratrol improves insulin signaling in a tissue-specific manner under insulin-resistant conditions only: in vitro and in vivo experiments in rodents. Metabolism. 2011 Sep 23; doi: 10.1016/j.metabol.2011.08.003. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 46.Wang N, Ko SH, Chai W, Li G, Barrett EJ, Tao L, Cao W, Liu Z. Resveratrol recruits rat muscle microcirculature via a nitric oxide-dependent mechanism that is blocked by TNFα. Am J Physiol Endocrinol Metab. 2011;300:E195–E201. doi: 10.1152/ajpendo.00414.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rothgiesser KM, Fey M, Hottiger MO. Acetylation of p65 at lysine 314 is important for late NF-kB-dependent gene expression. BMC Genomics. 2010;11:22. doi: 10.1186/1471-2164-11-22. [DOI] [PMC free article] [PubMed] [Google Scholar]