Abstract
The insula and the amygdala have been implicated as components of central networks subserving evaluative and affective processes, although their precise contributions have not been fully elucidated. The present study examined evaluative valence and arousal ratings to picture stimuli in patients with lesions of the insula or the amydala. Ratings of positivity, negativity and arousal to picture stimuli (extending from very negative to very positive) were obtained. Lesions of the amygdala did not alter either positive or negative valence ratings, but were associated with attenuated arousal ratings for negative stimuli. In contrast, patients with insular lesions displayed reduced arousal to both negative and positive stimuli, as well as marked attenuation of corresponding valence ratings. Results support the view that the insular cortex may play a broader role in integrating both affective and cognitive processes, whereas the amygdala may have a more selective role in affective arousal, especially for negative stimuli.
Keywords: insula, amygdala, lesions, evaluative processes, valence, arousal, affect
The amygdala has been a major focus of research and theory on affective processes, since Kluver and Bucy’s (1939) early report on affective blunting following amygdala/anterior temporal lobe lesions. The amygdala appears to play an important role in fear conditioning, preattentive processing of threat-related stimuli, emotional memories and decision making based on environmental punishment/reward contingencies (Bechara, Damasio & Damasio, 2003; Labar, 2007; McGaugh, 2004; Ohman et al., 2007; Phelps, 2006; Roozendaal, McEwen & Chattarji, 2009).
Emotional stimuli or contexts have been shown to induce amygdala activation as measured by functional brain imaging methods (Critchley, 2009; Norris et al., 2004; Sabatinelli et al., 2005). The magnitude of this activation is related to affective intensity and is generally greater for negative than for positive emotional stimuli (Critchley, 2009; Norris et al., 2004; Sabatinelli et al., 2005). Lesions of the amygdala, on the other hand, disrupt fear conditioning and the perception of potential danger (Bauman et al, 2004; Bechara et al., 1995; LeDoux, 2003; LaBar, 2007; Phelps, 2006). These and other findings clearly implicate the amygdala in negative affect, although the precise role of this structure remains to be fully elucidated. While the amygdala appears to have a predominant role in negative emotions, it may also play a role in appetitive conditioning and positive affect (Hamann et al., 2002; Mather et al., 2004) and may code emotional intensity as well as emotional valence (Adolphs et al., 1999; Winston et al., 2005).
Human patients with amygdala lesions can provide important insights into the functional contributions of the amygdala. Such patients have been reported to display less intense negative emotions (Tranel et al., 2006); to be impaired at recognizing facial expressions of negative emotions (Adolphs et al., 2001); to show deficits in episodic or autobiographical emotion-related memories (LaBar, 2007; Phelps; 2006) and to display reduced emotional potentiation of memory (McGaugh, 2004). Based on these and other findings, t has been hypothesized that amygdala lesions may be associated more with a deficiency in emotional arousal rather than a cognitive/perceptual deficit in processing emotional valence (Glascher and Adolphs, 2003), especially for negative stimuli (Adolphs et al., 1999; Bauman et al., 2004). A recent study supported this hypotheses. Patients with amygdala lesions were found to show reduced emotional arousal to negative picture stimuli, while still accurately rating the valence (positivity/negativity) of the stimuli (Berntson et al., 2007). This rather selective deficit in the arousal component of evaluative processing, with sparing of the cognitive aspects of identification and labeling of the valence content of the stimuli, may relate to the relative level of the amygdala in the neural hierarchy of evaluative processes.
The amygdala represents a single nodal point in a broader network underlying affective processes. The amygdala is heavily interconnected with structures, such as the hypothalamus, the prefrontal and cingulate cortices and the insular cortex, which have been implicated in affective and autonomic regulation (Swanson, 2003; Pressoa, 2008; Price, 2003). The insular cortex is another nodal point which has received increasing attention for its role in affective processes. The insula occupies a relatively unique position in the neural hierarchy subserving evaluative processes. The posterior insular cortex represent an important integrative site for interoceptive representations and autonomic control (Craig, 2009; Critchley, 2009) and its segue through the anterior insula represents a ‘fronto-insular’ junction and more broadly a limbic-insular link to many structures implicated in affective processes, including the prefrontal cortex and the amygdala (Augustine, 1996; Craig, 2009; Critchley, 2009). In particular, the insula has been suggested to be the critical substrate linking visceral function and interoception to awareness and consciousness, serving as a critical bridge between affective and cognitive processes (see Craig, 2009; Damasio, 1999).
Over a century ago, William James proposed that emotions were the perceptual consequences of somatovisceral feedback from bodily responses (James, 1884). Although the strong form of this model—that emotions are nothing more than these perceptual consequences— may no longer be tenable, it is increasingly recognized that visceral feedback can have powerful modulatory effects on affective as well as cognitive processes. The perception of bodily states, for example, has been shown to correlate with insular activity and with the intensity of reactions to emotional stimuli (Critchley, 2009; Pollatos et al., 2007). In addition, visceral afference has been shown to enhance memory and cortical reactivity to evocative stimuli via ascending noradrenergic relays through the amygdala (Roozendaal et al., 2009). According to the “somatic marker” model (see Bechara & Damasio, 2005), visceral afferent signals serve as critical markers of bodily states that serve to guide emotion, cognition, and behavior. Consistent with this suggestion, anterior insula activation has been reported prior to risk-averse decisions (Kuhnen & Knutson, 2005) and insular lesions impair sensitivity to aversive outcomes and the ability to adjust bets based on the odds (Clark et al., 2008). The role of the insula in evaluative processing may be somewhat broader than that of the amygdala, as interoceptive awareness was associated with increased arousal ratings to both positive and negative stimuli in the study of Pollatos et al. (2007). Moreover, activation of the insula has been reported to be associated with the expected magnitude of reward (Smith et al., 2009), with decisions about pleasantness and expected value of odors (Rolls et al., 2009) and with anticipation of both risky gains as well as risky losses (Knudson & Geer, 2008).
In order to clarify the potential differential contributions of the of the insula and the amygdala to evaluative processing, the present study examined the valence and arousal dimensions of evaluative judgments of lesion patients, in the context of a comprehensive, bivariate model of evaluative space (Cacioppo and Berntson, 1994; Larsen et al., 2004). Separate valence (positivity and negativity) ratings as well as arousal ratings were obtained to standard pictures (very positive to very negative) in a group of patients with amygdala lesions, a group with insula lesions, and a clinical contrast group.
METHOD
Participants
Seven patients with lesions of the insula (1female and 6 males; age 46 – 69 years, mean = 53.56), constituted the primary focus of the study. Comparison groups included 12 patients with lesions of the amygdala (6 males and 6 females; age 33–65, mean = 47.24), and 10 lesion contrast patients (5 males and 4 females; age 43 – 81, mean= 56.56) with damage that spared the insula, the amygdala/temporal lobe area and other areas implicated in affective processing. All patients had undergone neuroanatomical characterization according to the standard protocols of the University of Iowa Laboratory of Neuroimaging and Human Neuroanatomy (Damasio, 2005). The patient selection criteria were: (i) a stable and chronic lesion at least three months after onset; and (ii) involvement of a brain region that either included the insula, the amygdala or (for the clinical contrast group) excluded these structure or other areas thought to be critical for emotional processing, including the ventromedial prefrontal cortex and the orbitofrontal cortex. The distribution of the Insula lesions is illustrated in Figure 1. The amygdala patients all had anterior temporal lobectomies for the control of seizure disorders. Although this confounds the locus of the lesion with etiology, the primary focus of the study was on the insular goup, with the amygdala group as an experimental contrast group. We also have prior published data on the effects of amygdala lesions (not associated with seizure disorders) on the evaluative task a (Berntson et al., 2007), which can be compared to the present results.
Figure 1.
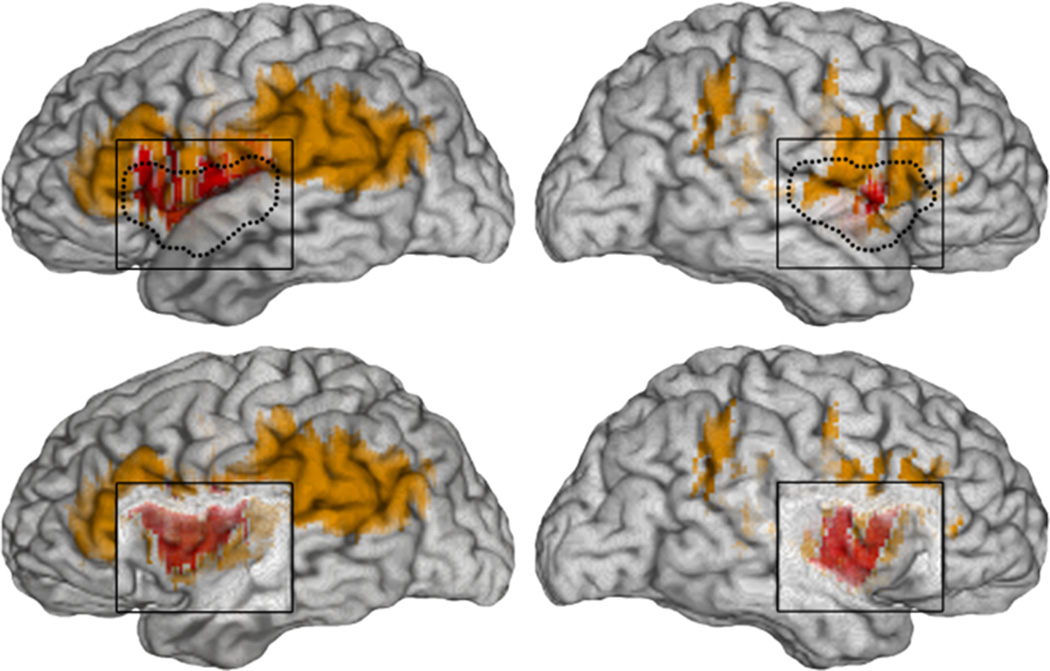
Lesion overlap in the insular cortex lesion group, in views of the right and left lateral surfaces, and with overlaying cortex (insula outlined in dotted lines) in top rows, and with cortex removed exposing the insular cortex underneath in bottom rows. All cases had unilateral lesions in the insular cortex, and the area of damage in the right- or left-sided cases was fairly symmetrical. There is maximal lesion overlap (reflected by red color) across the group in the insular cortex (anterior and posterior) and somatosensory SII region. The lesions in a few patients (reflected by lighter color) were broader and extended posteriorly into the inferior parietal cortex in some subjects, and anteriorly into the inferior frontal gyrus in other subjects.
Patient groups did not appreciably differ on most demographic or neuropsychological characteristics (see Table 1). Amygdala patients were somewhat younger than the other groups, but no significant differences were apparent in pairwise comparisons. The only neuropsychological test to show a difference was the CESD, with a higher depression score for the Amygdala group. This was largely attributable to one subject who had a CESD score 3 SDs above the mean of the group. In view of this result, however, all significant statistical results from the behavioral measures in the study were also evaluated with a covariate on CESD. In no case did this change the pattern of significance, and so this was omitted from the results.
Table 1.
Demographic and psychosocial characteristics of lesion groups.
| Contrast | Amygdala | Insula | |
|---|---|---|---|
| Age* | 51.38 ± 4.69 | 41.25 ± 4.35 | 56.56 ± 4.27 |
| Education | 13.58 ± .69 | 12.75 ± .85 | 13.77 ± .80 |
| Handedness | 98.33 ± 2.02 | 96.85 ± 2.47 | 97.78 ± 2.33 |
| Chronicity | 7.83 ± 1.46 | 10.75 ± 1.79 | 5.77 ± 1.69 |
| IQ (WAIS-III) | 100.63 ± 3.32 | 97.13 ± 4.17 | 104.65 ± 3.90 |
| Memory (WMS) | 104.44 ± 4.24 | 97.26 ± 4.80 | 104.49 ± 2.80 |
| Boston Naming | 45.02 ± 3.59 | 49.87 ± 4.39 | 56.11 ± 4.14 |
| Token Task | 40.41 ± 2.24 | 39.87 ± 2.50 | 42.77 ± 2.36 |
| CESD* | 7.56 ± 1.94 | 13.3 ± 3.4* | 8.14 ± 2.93 |
| UCLA | 40.34 ± 3.03 | 46.76 ± 2.52 | 43.01 ± 3.43 |
Data are represented as mean ± S.E.M.
denotes significant differences between groups (p<.05). Including age and CESD ratings as covariates do not alter the significance of the data discussed above.
Apparatus
Experimental control and response recording was implemented using E-prime (Psychology Software Tools, Pittsburgh). A mouse served as the response device whereby the subjects indicated valence and arousal ratings to the stimuli. For positivity/negativity ratings, participants positioned a cursor on a bivariate display (5×5 grid) with the horizontal dimension indicating positivity and the vertical, negativity. The arousal rating entailed a similar cursor placement on a single dimension (9 point) scale.
Stimuli
Stimuli were positive, negative, and neutral pictures from the IAPS (Lang et al., 1999). Pictures were matched on normative arousal ratings and on evaluative extremity from neutral (12 very positive, 6 moderately positive, 12 neutral, 6 moderately negative and 12 very negative). The number of pictures were limited to 48, to minimize potential fatigue and attentional confounds, and the stimuli were selected to sample the range of affective ratings, distinct emotions, and social vs nonsocial contexts.
Procedure
Participants rated the picture stimuli on positivity, negativity and arousal dimensions. Pictures were presented in random order on a computer monitor for 6 s. Participants were instructed to focus on the emotional content of the pictures. After viewing each picture, participants were instructed to rate it on a 5-point bivariate scale of positivity and negativity and a univalent scale of how aroused it made them feel. The response grid was presented on the screen immediately after termination of the stimulus picture (Larsen et al., 2004). After responding, a second screen displayed a single response continuum and the subject was instructed to rate how aroused they felt to the stimulus. Three seconds after completing the ratings, the next slide was presented. In addition to the separate ratings of positivity, negativity and arousal for each of the picture stimuli, a net valence rating for each picture was calculated as the positivity rating minus the negativity rating.
Neuropsychological testing
In addition to the experimental task, patients were evaluated on a range of neuropsychological tests. These included the Wechsler Adult Intelligence Scale (3rd edition), the Wechsler Memory Scale (3rd edition), the Wisconsin Card Sorting Task, and the Beck Depression Inventory.
Data analysis
Primary statistical evaluation of ratings was by between-within (repeated measures on stimulus categories) analysis of variance (ANOVA) with trends, followed up by simple ANOVAs for pairwise contrasts. Derived measures entailing two-sample comparisons were tested by Student’s t tests.
RESULTS
Evaluative Valence Ratings
As illustrated in Figure 2, valence ratings of the picture stimuli were comparable for all groups and slide categories, with the exception of the insula group. Patients with lesions of the insula were similar to other groups for neutral stimuli, but showed a smaller increment in valence ratings with either more positive or more negative stimuli.
Figure 2.
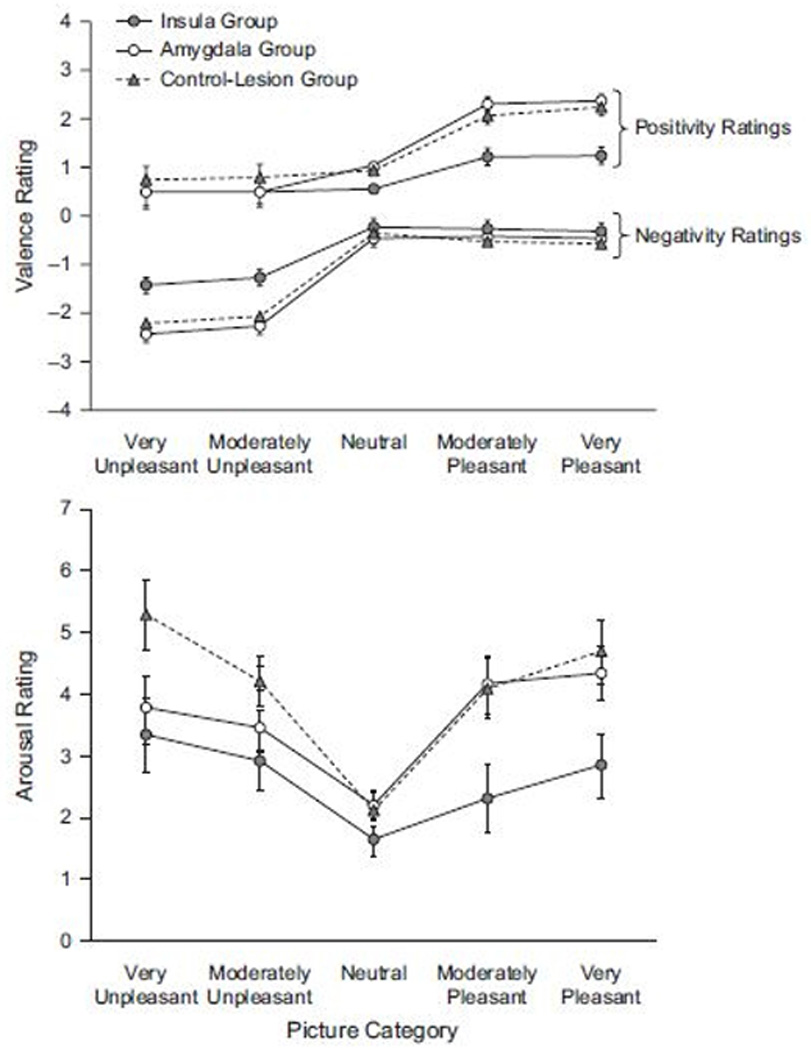
Valence (top) and arousal ratings (bottom) to different categories of picture stimuli. Ins = insula group; Amy = amygdala group; cnt = contrast group; norm = normative group. Data represented as mean ± S.E.M
Omnibus ANOVAs revealed the expected effects of picture category on both positivity (F4,108) = 62.24, p = .000) and negativity (F(4,108) = 87.97, p = .000) ratings, characterized by significant linear, quadratic and cubic trends over picture categories (for positivity ratings, Fs(1,27) > 34.64, ps = .000; for negativity ratings, Fs(1,27) > 70.48, ps = .000). Significant lesion group × picture category interactions also emerged (for positivity, F(8,108) = 3.98, p = .001; f = .15, for negativity, F(8,108) = 2.55, p = .014; f = .11). These interactions reflect the selective reduction in ratings of the insula group to positive and negative, relative to neutral, stimuli. In addition, three way interactions between picture category, lesion group and sociality also emerged for both positivity ratings (F(8,108) = 2.59, p = .013) and negativity ratings (F(8,108) = 3.42, p = .002). To clarify the source of these interactions, further pairwise ANOVAs were pursued.
These analyses showed that the insula group differed from the amygdala group in both positivity and negativity ratings. For positivity ratings, this was reflected by a main effect of lesion group (F(1,19) = 9.61, p = .006) and a lesion group × picture category interaction (F(4,76) = 12.11, p = .000, f = .16). The latter was characterized by differences in the linear and cubic trends in ratings across the picture categories (F(1, 19)s > 12.45, Ps < .003). Trend differences again reflect the low (and comparable) positivity ratings for neutral and negative stimuli, but an attenuated increment for the insula group in positivity ratings for more positive stimuli. Similar results were obtained for negativity, as revealed by a main effect of lesion group (F(1,19) =7.12, p = .015) and a lesion group × picture category interaction (F(4,76) = 5.98, p = .000; f = .12), again characterized by differences in the linear and cubic trends across picture categories (F(1, 19)s > 6.2, Ps < .022). Paralleling the results for positivity ratings, the two groups gave comparable low negativity ratings to neutral and positive stimuli, but the insula group showed a smaller increment in negativity ratings for more negative stimuli.
The insula group, but not the amygdala group, also differed significantly from the lesion contrast group. This was true for positivity ratings as evidenced by a significant main effect of lesion group (F(1,15) = 12.59, p = .003), and for negativity ratings, as revealed by a significant lesion group × picture category × sociality interaction (F(4,60) = 9.13, p = .000; f = .14). The latter was associated with significant differences in both the linear and cubic trends across picture categories (F(1,15)s > 11.28, ps< .005). Like the contrast group, the insula group displayed low positive ratings to negative and neutral stimuli and low negative ratings to positive and neutral stimuli. Again, however, they displayed a smaller increment in positivity ratings to positive stimuli and negativity ratings to negative stimuli.
The interactions with sociality outlined above reflect the somewhat greater effects of lesions on social compared to non-social stimuli. Although significant, these differences were small, and the general pattern of reduced valence ratings depicted in Figure 1 applies to both social and nonsocial pictures.
Arousal
An omnibus ANOVA revealed significant effects of picture category on arousal ratings (F(4,108) = 20.36; p < .001); f = .18. As illustrated in Figure 2, this was characterized by a significant quadratic trend (F(1,52) = 155.78, p < .001) reflecting the overall increased arousal to positive or negative, relative to neutral pictures. The ANOVA also revealed a significant lesion group by picture category by sociality interaction, reflecting differences in arousal ratings among the lesion groups (F(8,108) = 2.29, p = .026). As illustrated in figure 2, this interaction is attributable to attenuated arousal ratings of the insula group to both positive and negative picture categories, and the reduction in arousal ratings of the amygdala group selectively to negative pictures. The latter effect (amygdala group) is consistent with our previous report (Berntson et al., 2007), and differs from the more generalized attenuation of arousal ratings of the insula patients.
Results were followed up by pairwise ANOVAs to identify specific effects. These analyses revealed that the amygdala group, relative to the lesion contrast group, showed reduced arousal ratings selectively to the negative picture categories. This was indicated by a lesion group × picture category interaction on the quadratic trend in ratings across picture categories (F(1,20) = 7.31, p < .014). Whereas the contrast group showed a progressive increase in arousal for both positive and negative pictures, the amygdala group showed a typical increment for positive, but an attenuated increase for negative pictures.
The insula group also showed significant attenuation of arousal ratings. In contrast to the amygdala group, however, the arousal ratings of the insula group were attenuated for both positive and negative picture categories. This was indicated by a significant main effect of lesion group on arousal (F(1,16) = 9.27, p =.008; f = .12) and a significant difference in the quadratic trend of arousal across picture categories. A pairwise ANOVA further revealed that the insula group and the amygdala group differed significantly from each other. This was indicated by a significant main effect of lesion group (insula vs. amygdala) on arousal (F(1,18) = 4.02, p < .05) and a significant difference in the cubic trend on arousal across picture categories (F(1,18) = 4.34, p = .05). As illustrated in Figure 2, the insula group had generally lower arousal ratings than the amygdala group, but this difference was most apparent for the positive pictures.
Differences among lesion groups on arousal ratings were similar for social and nonsocial stimuli, although effects were slightly greater for social stimuli. This was revealed by an overall three-way interaction between lesion groups, picture categories, and sociality in the omnibus ANOVA (F(8,108) = 2.29, p = .026), and by significant interactions on sociality in the pairwise comparisons between the insula and contrast groups (F(4,64) = 4.61, p = .002 f = .12) and between the amygdala and contrast groups (on the cubic trend across categories; F(1,20) =4.65, p =.04). This was most apparent for the extreme positive picture category, where arousal ratings of the insula group for positive social stimuli were lower than for nonsocial stimuli, whereas for other groups the opposite was the case. This is generally consistent with the somewhat lower valence ratings of the insula group for social stimuli as described above.
Laterality and gender effects
Laterality differences have been reported in the literature for both the amygdala and the insula. The present study included both males and females, and lesions were mostly unilateral. Unfortunately, the small Ns for right (4) and left (3) insula lesions and for amygdala lesions (1 right, 11 left) precluded meaningful statistical comparisons. Similarly, although gender differences may have been present, the small Ns again precluded meaningful analyses.
Although bilateral lesions of a given structure generally have much larger effects than unilateral injuries, unilateral lesions in the present study yielded notable effects on evaluative processes. In fact, results from the unilateral amygdala group in the present study closely replicated our previous preliminary report which included participants with bilateral amygdala injuries.
DISCUSSION
The present results reveal distinct differences in the evaluative processes of patients with lesions of the insula and the amygdala. In accord with our previous preliminary finding (Berntson et al., 2007), amygdala lesions did not affect either positivity or negativity valence ratings of the picture stimuli, but were associated with significantly reduced arousal ratings, selectively for negative pictures. This is consistent with the report that patients with amygdala lesions may accurately judge the valence and extremity of both positive and negative facial expressions, but show lower arousal judgments specifically for negative facial displays (Adolphs et al., 1999).
In contrast, patients with lesions of the insula displayed similarly reduced arousal ratings for negative stimuli, but also showed attenuated arousal to positive stimuli. This did not appear to reflect an overall bias in ratings, as ratings of neutral stimuli were similar to other groups. Rather, insula patients failed to report the typical arousal increments to affective picture content. In further contrast with the amygdala patients, the reduced arousal ratings of the insula group were also associated with reductions in positive and negative valence ratings of the affective stimuli.
Results for arousal ratings are in general accord with the literature, which suggests that the amygdala may play a particularly important role in negative affect (Berntson et al., 2007; Critchley, 2009; Norris et al., 2004; Phelps, 2006; Sabatinelli et al., 2005), whereas the insula may be involved more equivalently in both positive and negative contexts (Knudson & Geer, 2008; Pollatos et al., 2007; Rolls et al., 2009; Smith et al., 2009). Activation of the insula, for example, has been shown to be associated with the expected magnitude of reward (Smith et al., 2009) and with decisions about pleasantness and expected value of odors (Rolls et al., 2009). Insula activation has also been reported in anticipation of gains as well as losses, and insula lesions disrupt performance for both risky gains and risky losses (Knudson & Geer, 2008).
The present results suggest that amygdala lesions may not necessarily disrupt the basic perception, categorization and labeling of affective picture content, as evidenced by typical valence ratings of the picture stimuli. Rather, these lesions appear to preferentially attenuate arousal effects to negative stimuli, even when the negative picture content is recognized and accurately categorized. This is not likely attributable to the fact that the present group of amygdala patients had only unilateral lesions, as the results are highly consistent with a prior study which included bilateral lesions (Berntson et al., 2007). In addition, the present results did not appear to be uniquely related to the eitiology (seizure disorders) of the present amygdala patients, as non-seizure patients with amygdala lesions yielded comparable results in the prior study. Overall, results are consistent with reports that amygdala lesions may disrupt the development of conditioned autonomic arousal responses, despite the fact that patients may acquire explicit cognitive knowledge about the stimulus and outcome contingencies (Bechara et al., 1995; LaBar et al., 1995). Although patients with amygdala lesions have been reported to show deficits in the recognition of emotion in facial displays, these deficits may be attributable in large part to inadequate visual search and fixation rather than a fundamental inability to discriminate and identify negative facial features (Adolphs et al., 2005).
In contrast to the effects of amygdala lesions, damage to the insular cortex appears to more broadly impact evaluative processes, including both arousal and valence judgments for both positive and negative as well as social and nonsocial stimuli. The insula is a major relay and integrative site for interoceptive information and appears to represent an important link between autonomic, affective and cognitive processes (Craig, 2009; Critchley, 2009). Its interconnections with the prefrontal cortex and anterior cingulate may represent an important route by which insular activity could impact cognitive processes (Augustine, 1996; Craig, 2009; Critchley, 2009). Craig (2009) has suggested that the insula may be a critical nodal point in systems underlying awareness and consciousness. This functional linkage may have emerged through evolutionary co-option of networks that link inherently hedonic sensory qualities (such as odors/tastes) to adaptive behavioral approach/withdrawal dispositions, and ultimately to goal-oriented decision-making (Rolls et al., 2009). Insula activation to odors, for example, was greater when subjects were required to make a decision as to the relative pleasantness and intensity of two odorants, rather than simply rating them (Rolls et al., 2009).
According to the somatic-marker model, somatic and visceral representations are maintained in the insula and somatosensory cortex (Bechara & Damasio, 2005). In decision-making contexts, the representations associated with previous choices and actions are suggested to be related to prior outcomes through prefrontal circuits (Bechara & Damasio, 2005). Together, these theoretical models and empirical findings emphasize the potential role of the insula not only in affective states, but at the interface between affective processes and awareness, judgments and cognition. This is in general accord with the rather pervasive effects of insula damage not only in arousal reactions in the present study, but in the perception, recognition, and labeling of affective stimuli.
In summary, the present results point to distinct roles for the amygdala and the insular cortex in evaluative processers. The amygdala may not be necessary to determine whether and to what extent a stimulus is appetitive or aversive, hostile or hospitable. Rather, it may play a more important role in registering the arousal or emotional impact especially of aversive stimuli. In contrast, the insular cortex appears to be more broadly involved in the recognition, processing, and assignment of evaluative valence, as well as contributing to affective arousal. The latter may represent a direct effect of the lesion or may derive secondarily from effects on valence judgments.
Supplementary Material
Acknowledgments
This research was supported by a program project grant from the National Institute of Neurological Disorders and Stroke (NS19632; D.T. & A.B.), by the National Institute on Drug Abuse (DA022549, D.T.; R01DA023051, A.B.), and grants from the National Institute of Mental Health and the Templeton Foundation to J.T.C.
REFERENCES
- Adolphs R, Gosselin F, Buchanan TW, Tranel D, Schyns P, Damasio AR. A mechanism for impaired fear recognition after amygdala damage. Nature. 2005;433(7021):68–72. doi: 10.1038/nature03086. [DOI] [PubMed] [Google Scholar]
- Adolphs R, Russell JA, Tranel D. A role for the human amygdala in recognizing emotional arousal from unpleasant stimuli. Psychological Science. 1999;10:167–171. [Google Scholar]
- Adolphs R, Tranel D, Damasio H. Emotion recognition from faces and prosody following temporal lobectomy. Neuropsychology. 2001;15(3):396–404. doi: 10.1037//0894-4105.15.3.396. [DOI] [PubMed] [Google Scholar]
- Augustine JR. Circuitry and functional aspects of the insular lobe in primates including humans. Brain Research Review. 1996;22(3):229–244. doi: 10.1016/s0165-0173(96)00011-2. [DOI] [PubMed] [Google Scholar]
- Bauman MD, Lavenex P, Mason WA, Capitanio JP, Amaral DG. The development of mother-infant interactions after neonatal amygdala lesions in rhesus monkeys. Journal of Neuroscience. 2004;24(3):711–721. doi: 10.1523/JNEUROSCI.3263-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara A, Damasio A. The somatic marker hypothesis: a neural theory of economic decision. Games and Economic Behavior. 2005;52:336–372. [Google Scholar]
- Bechara A, Damasio H, Damasio AR. Role of the amygdala in decision-making. Annals of the New York Academy of Sciences. 2003;985:356–369. doi: 10.1111/j.1749-6632.2003.tb07094.x. [DOI] [PubMed] [Google Scholar]
- Bechara A, Tranel D, Damasio H, Adolphs R, Rockland C, Damasio AR. Double dissociation of conditioning and declarative knowledge relative to the amygdala and hippocampus in humans. Science. 1995;269(5227):1115–1118. doi: 10.1126/science.7652558. [DOI] [PubMed] [Google Scholar]
- Berntson GG, Bechara A, Damasio H, Tranel D, Cacioppo JT. Amygdala contribution to selective dimensions of emotion. Social Cognitive and Affective Neuroscience. 2007;2(2):123–129. doi: 10.1093/scan/nsm008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacioppo JT, Berntson GG. Relationship between attitudes and evaluative space: A critical review, with emphasis on the separability of positive and negative substrates. Psychological Bulletin. 1994;115:401–423. [Google Scholar]
- Clark L, Bechara A, Damasio H, Aitken MR, Sahakian BJ, Robbins TW. Differential effects of insular and ventromedial prefrontal cortex lesions on risky decision-making. Brain. 2008;131(Pt 5):1311–1322. doi: 10.1093/brain/awn066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig AD. How do you feel--now? The anterior insula and human awareness. Nature Reviews Neuroscience. 2009;10(1):59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- Critchley HD. Psychophysiology of neural, cognitive and affective integration: fMRI and autonomic indicants. International Journal of Psychophysiology. 2009;73(2):88–94. doi: 10.1016/j.ijpsycho.2009.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damasio AR. The Feeling of What Happens: Body and Emotion in the Making of Consciousness. New York: Harcourt Brace; 1999. [Google Scholar]
- Damasio H. Human brain anatomy in computerized images. 2nd edn. Oxford New York: Oxford University Press; 2005. [Google Scholar]
- Glascher J, Adolphs R. Processing of the arousal of subliminal and supraliminal emotional stimuli by the human amygdala. Journal of Neuroscience. 2003;23(32):10274–10282. doi: 10.1523/JNEUROSCI.23-32-10274.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamann SB, Ely TD, Hoffman JM, Kilts CD. Ecstasy and agony: activation of the human amygdala in positive and negative emotion. Psychological Science. 2002;13(2):135–141. doi: 10.1111/1467-9280.00425. [DOI] [PubMed] [Google Scholar]
- James W. What is an emotion? Mind. 1884;(9):188–205. [Google Scholar]
- Kluver H, Bucy PC. Preliminary analysis of functions of the temporal lobes in monkeys. Archives of Neurology and Psychiatry. 1939;4:979–1000. doi: 10.1176/jnp.9.4.606. [DOI] [PubMed] [Google Scholar]
- Kuhnen CM, Knutson B. The neural basis of financial risk taking. Neuron. 2005;47(5):763–770. doi: 10.1016/j.neuron.2005.08.008. [DOI] [PubMed] [Google Scholar]
- Labar KS. Beyond Fear Emotional Memory Mechanisms in the Human Brain. Current Directions in Psychological Science. 2007;16(4):173–177. doi: 10.1111/j.1467-8721.2007.00498.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaBar KS, LeDoux JE, Spencer DD, Phelps EA. Impaired fear conditioning following unilateral temporal lobectomy in humans. Journal of Neuroscience. 1995;15(10):6846–6855. doi: 10.1523/JNEUROSCI.15-10-06846.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. International Affective Picture System (IAPS): Instruction manual and affective ratings (Technical Report No. A-4). Paper presented at the Center for Research in Psychophysiology; Gainesville, FL. 1999. [Google Scholar]
- Larsen JT, McGraw AP, Mellers BA, Cacioppo JT. The agony of victory and thrill of defeat: Mixed emotional reactions to disappointing wins and relieving losses. Psychological Science. 2004;15(5):325–330. doi: 10.1111/j.0956-7976.2004.00677.x. [DOI] [PubMed] [Google Scholar]
- LeDoux J. The emotional brain, fear, and the amygdala. Cellular and Molecular Neurobiology. 2003;23(4–5):727–738. doi: 10.1023/a:1025048802629. [DOI] [PubMed] [Google Scholar]
- Mather M, Canli T, English T, Whitfield S, Wais P, Ochsner K, et al. Amygdala responses to emotionally valenced stimuli in older and younger adults. Psychological Science. 2004;15(4):259–263. doi: 10.1111/j.0956-7976.2004.00662.x. [DOI] [PubMed] [Google Scholar]
- McGaugh JL. The amygdala modulates the consolidation of memories of emotionally arousing experiences. Annual Review of Neuroscience. 2004;27:1–28. doi: 10.1146/annurev.neuro.27.070203.144157. [DOI] [PubMed] [Google Scholar]
- Norris CJ, Chen EE, Zhu DC, Small SL, Cacioppo JT. The interaction of social and emotional processes in the brain. Journal of Cognitive Neuroscience. 2004;16(10):1818–1829. doi: 10.1162/0898929042947847. [DOI] [PubMed] [Google Scholar]
- Ohman A, Carlsson K, Lundqvist D, Ingvar M. On the unconscious subcortical origin of human fear. Physiology and Behavior. 2007;92(1–2):180–185. doi: 10.1016/j.physbeh.2007.05.057. [DOI] [PubMed] [Google Scholar]
- Pessoa L. On the relationship between emotion and cognition. Nature Reviews Neuroscience. 2008;9(2):148–158. doi: 10.1038/nrn2317. [DOI] [PubMed] [Google Scholar]
- Phelps EA. Emotion and cognition: insights from studies of the human amygdala. Annual Review of Psychology. 2006;57:27–53. doi: 10.1146/annurev.psych.56.091103.070234. [DOI] [PubMed] [Google Scholar]
- Pollatos O, Gramann K, Schandry R. Neural systems connecting interoceptive awareness and feelings. Human Brain Mapping. 2007;28(1):9–18. doi: 10.1002/hbm.20258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price JL. Comparative aspects of amygdala connectivity. Annals of the New York Academy of Sciences. 2003;985:50–58. doi: 10.1111/j.1749-6632.2003.tb07070.x. [DOI] [PubMed] [Google Scholar]
- Rolls ET, Grabenhorst F, Parris BA. Neural systems underlying decisions about affective odors. Journal of Cognitive Neuroscience. 2009 doi: 10.1162/jocn.2009.21231. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- Roozendaal B, McEwen BS, Chattarji S. Stress, memory and the amygdala. Nature Reviews Neuroscience. 2009;10:423–433. doi: 10.1038/nrn2651. [DOI] [PubMed] [Google Scholar]
- Sabatinelli D, Bradley MM, Fitzsimmons JR, Lang PJ. Parallel amygdala and inferotemporal activation reflect emotional intensity and fear relevance. Neuroimage. 2005;24:1265–1270. doi: 10.1016/j.neuroimage.2004.12.015. [DOI] [PubMed] [Google Scholar]
- Smith BW, Mitchell DG, Hardin MG, Jazbec S, Fridberg D, Blair RJ, et al. Neural substrates of reward magnitude, probability, and risk during a wheel of fortune decision-making task. Neuroimage. 2009;44(2):600–609. doi: 10.1016/j.neuroimage.2008.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson LW. The amygdala and its place in the cerebral hemisphere. Annals of the New York Academy of Sciences. 2003;985:174–184. doi: 10.1111/j.1749-6632.2003.tb07081.x. [DOI] [PubMed] [Google Scholar]
- Tranel D, Gullickson G, Koch M, Adolphs R. Altered experience of emotion following bilateral amygdala damage. Cognitive Neuropsychiatry. 2006;11(3):219–232. doi: 10.1080/13546800444000281. [DOI] [PubMed] [Google Scholar]
- Winston JS, Gottfried JA, Kilner JM, Dolan RJ. Integrated neural representations of odor intensity and affective valence in human amygdala. Journal of Neuroscience. 2005;25(39):8903–8907. doi: 10.1523/JNEUROSCI.1569-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


