Abstract
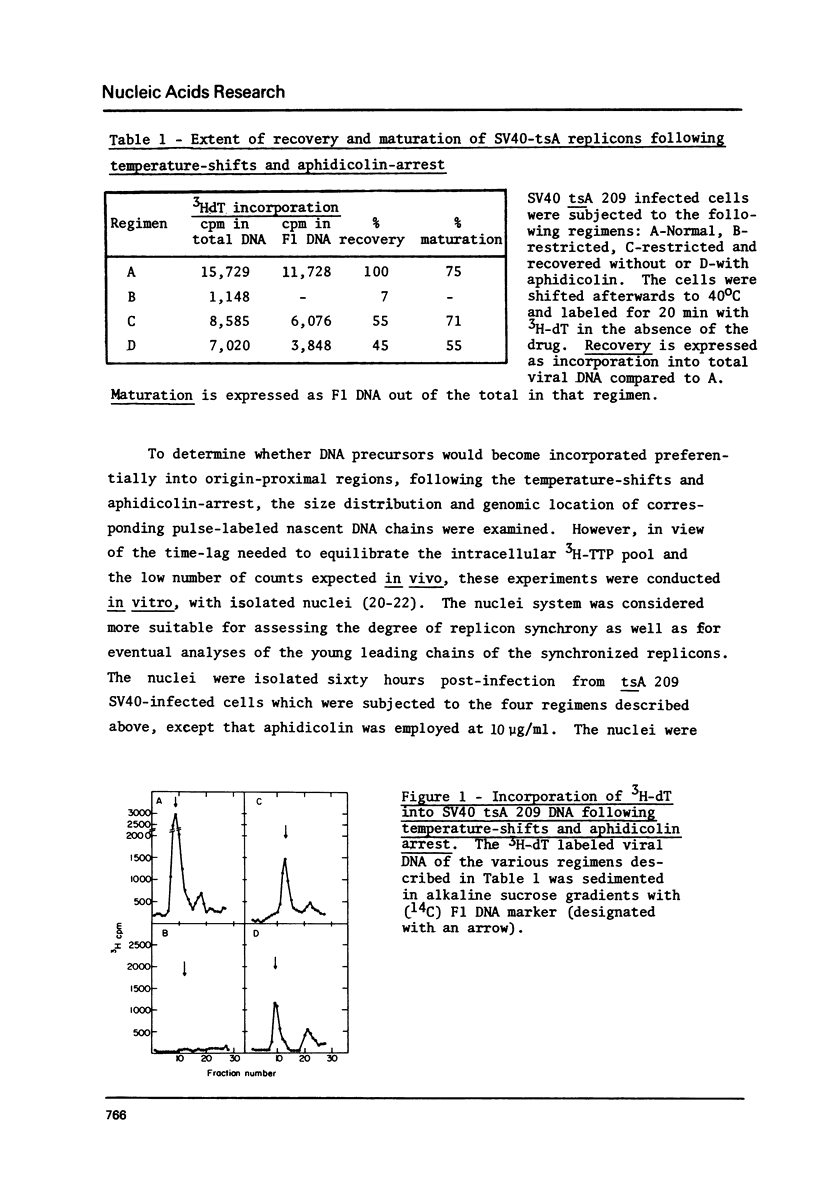
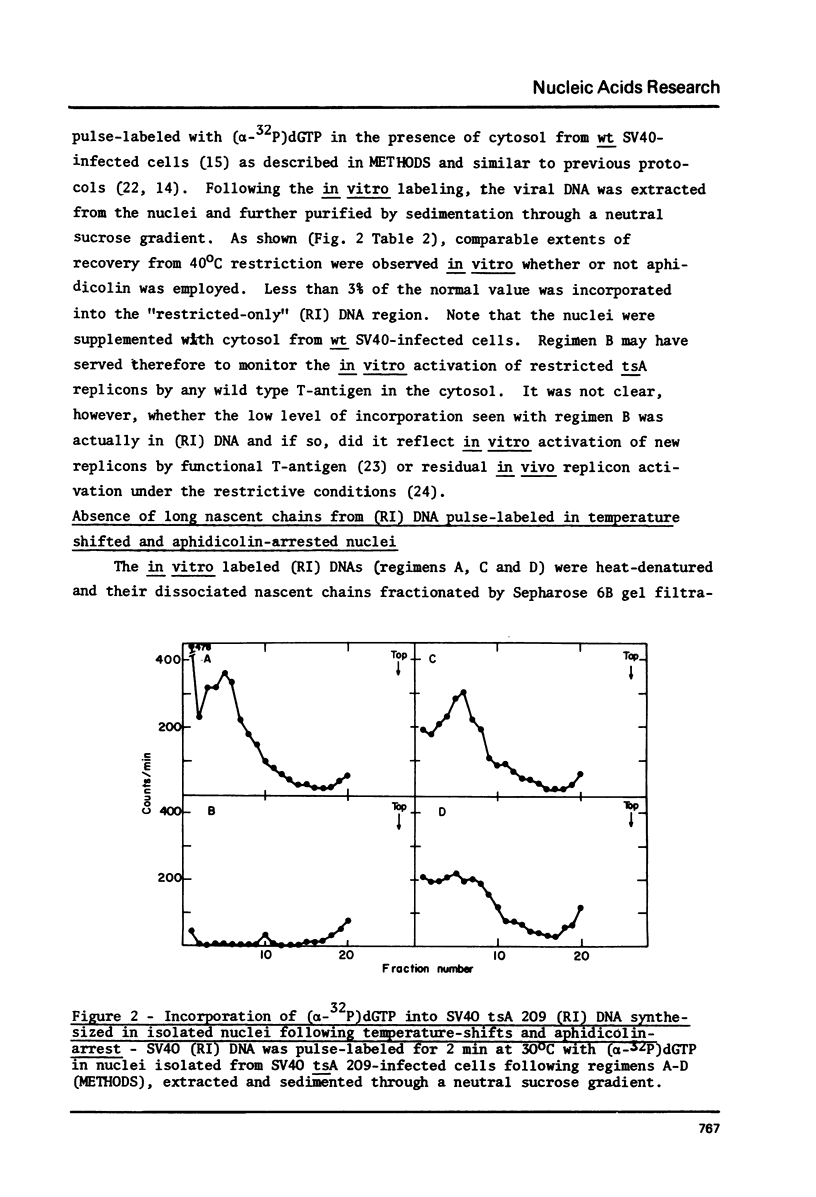
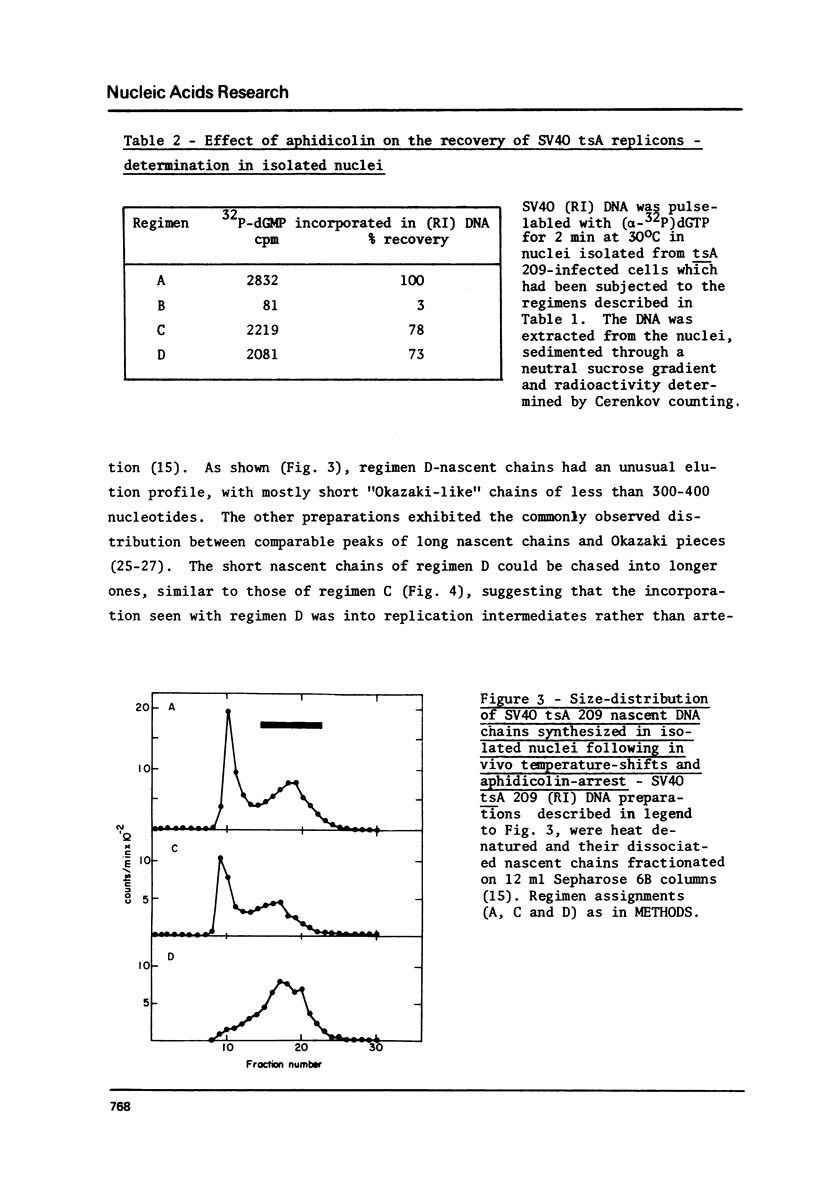
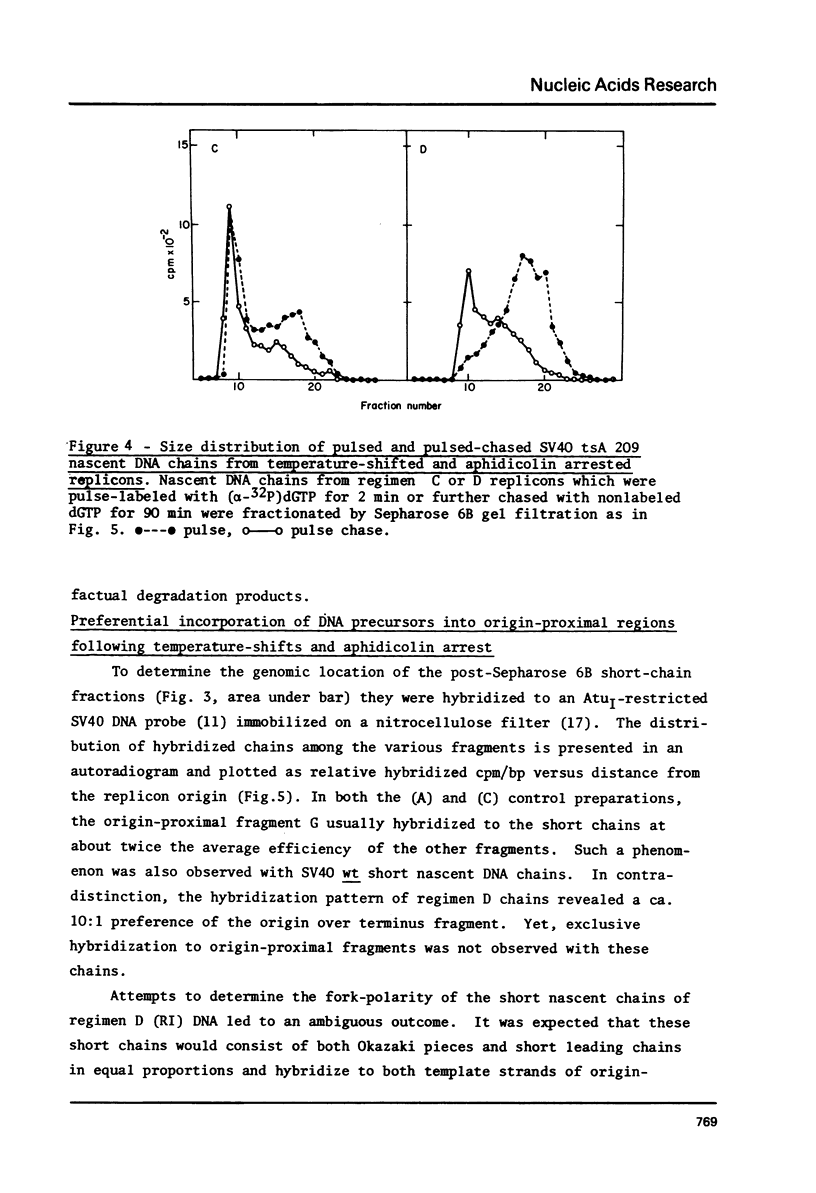
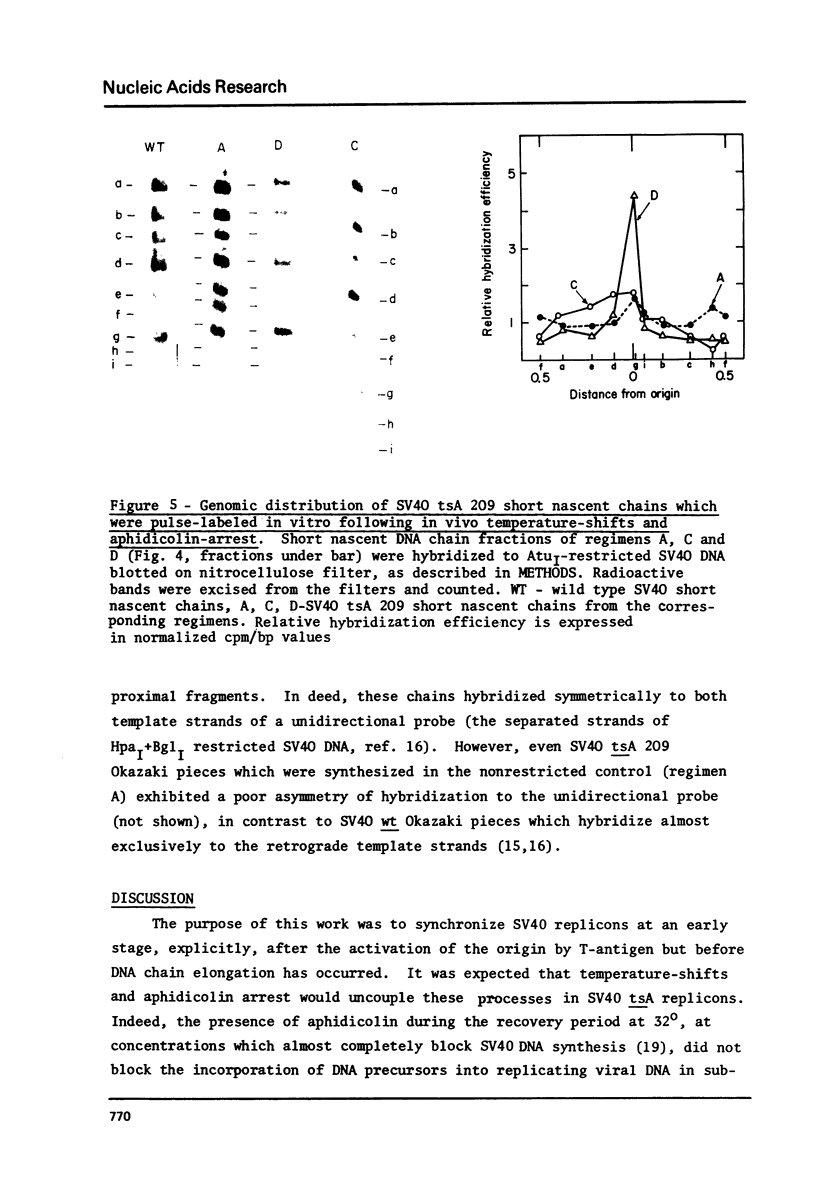
To synchronize SV40 replicons, simian cells infected with a tsA mutant were restricted at 40 degrees, to complete ongoing replication and returned to 32 degrees, to activate new replicons in the presence of the DNA chain elongation inhibitor aphidicolin. Upon further incubation at 40 degrees without the drug, 3H-dT was incorporated into SV40 FI DNA, almost to the extent seen with cells recovered in the absence of the drug. To determine whether DNA synthesis would begin from the origin, following the temperature-shifts-aphidicolin regimen, chains subsequently pulse-labeled with (alpha-32p)dGTP in isolated nuclei were analyzed for size distribution and genomic location. These chains reached up to 300-400 nucleotides in size, unlike the control which featured comparable amounts of label in long chains and Okazaki pieces. The nascent DNA of the drug-treated system could be chased into longer chains, indicating that it was a replicative intermediate; and it hybridized preferentially to an origin proximal fragment of AtuI- restricted SV40 DNA, demonstrating partial replicon synchronization. The data prove that T-antigen activates the SV40 replicon independent of DNA chain elongation and suggest means to study the mechanism of DNA chain priming at the origin.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brockman W. W., Gutai M. W., Nathans D. Evolutionary variants of simian virus 40: characterization of cloned complementing variants. Virology. 1975 Jul;66(1):36–52. doi: 10.1016/0042-6822(75)90177-4. [DOI] [PubMed] [Google Scholar]
- Bucknall R. A., Moores H., Simms R., Hesp B. Antiviral effects of aphidicolin, a new antibiotic produced by Cephalosporium aphidicola. Antimicrob Agents Chemother. 1973 Sep;4(3):294–298. doi: 10.1128/aac.4.3.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou J. Y., Avila J., Martin R. G. Viral DNA synthesis in cells infected by temperature-sensitive mutants of simian virus 40. J Virol. 1974 Jul;14(1):116–124. doi: 10.1128/jvi.14.1.116-124.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clertant P., Cuzin F. Initiation of polyoma virus DNA replication in vitro and its dependence on the viral gene A protein. Nucleic Acids Res. 1980 Oct 10;8(19):4377–4392. doi: 10.1093/nar/8.19.4377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole C. N., Landers T., Goff S. P., Manteuil-Brutlag S., Berg P. Physical and genetic characterization of deletion mutants of simian virus 40 constructed in vitro. J Virol. 1977 Oct;24(1):277–294. doi: 10.1128/jvi.24.1.277-294.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danna K. J., Nathans D. Bidirectional replication of Simian Virus 40 DNA. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3097–3100. doi: 10.1073/pnas.69.11.3097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DePamphilis M. L., Berg P. Requirement of a Cytoplasmic Fraction for Synthesis of SV40 Deoxyribonucleic Acid in Isolated Nuclei*. J Biol Chem. 1975 Jun 10;250(11):4348–4354. [PubMed] [Google Scholar]
- Edenberg H. J., Anderson S., DePamphilis M. L. Involvement of DNA polymerase alpha in simian virus 40 DNA replication. J Biol Chem. 1978 May 10;253(9):3273–3280. [PubMed] [Google Scholar]
- Fareed G. C., Salzman N. P. Intermediate in SV40 DNA chain growth. Nat New Biol. 1972 Aug 30;238(87):274–277. doi: 10.1038/newbio238274a0. [DOI] [PubMed] [Google Scholar]
- Francke B., Hunter T. In vitro polyoma DNA synthesis: discontinuous chain growth. J Mol Biol. 1974 Feb 15;83(1):99–121. doi: 10.1016/0022-2836(74)90426-4. [DOI] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Kaufmann G., Anderson S., DePamphilis M. L. RNA primers in Simian virus 40 DNA replication. II. Distribution of 5' terminal oligoribonucleotides in nascent DNA. J Mol Biol. 1977 Nov 5;116(3):549–567. doi: 10.1016/0022-2836(77)90083-3. [DOI] [PubMed] [Google Scholar]
- Kaufmann G., Bar-Shavit R., DePamphilis M. L. Okazaki pieces grow opposite to the replication fork direction during simian virus 40 DNA replication. Nucleic Acids Res. 1978 Jul;5(7):2535–2545. doi: 10.1093/nar/5.7.2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann G. Characterization of initiator RNA from replicating simian virus 40 DNA synthesized in isolated nuclei. J Mol Biol. 1981 Mar 25;147(1):25–39. doi: 10.1016/0022-2836(81)90077-2. [DOI] [PubMed] [Google Scholar]
- Krokan H., Schaffer P., DePamphilis M. L. Involvement of eucaryotic deoxyribonucleic acid polymerases alpha and gamma in the replication of cellular and viral deoxyribonucleic acid. Biochemistry. 1979 Oct 2;18(20):4431–4443. doi: 10.1021/bi00587a025. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Enhanced autoradiographic detection of 32P and 125I using intensifying screens and hypersensitized film. FEBS Lett. 1977 Oct 15;82(2):314–316. doi: 10.1016/0014-5793(77)80609-1. [DOI] [PubMed] [Google Scholar]
- LeBon J. M., Kado C. I., Rosenthal L. J., Chirikjian J. G. DNA modifying enzymes of Agrobacterium tumefaciens: effect of DNA topoisomerase, restriction endonuclease, and unique DNA endonuclease on plasmid and plant DNA. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4097–4101. doi: 10.1073/pnas.75.9.4097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee T. N., Brockman W. W., Nathans D. Evolutionary variants of simian virus 40: cloned substituted variants containing multiple initiation sites for DNA replication. Virology. 1975 Jul;66(1):53–69. doi: 10.1016/0042-6822(75)90178-6. [DOI] [PubMed] [Google Scholar]
- Magnusson G., Pigiet V., Winnacker E. L., Abrams R., Reichard P. RNA-linked short DNA fragments during polyoma replication. Proc Natl Acad Sci U S A. 1973 Feb;70(2):412–415. doi: 10.1073/pnas.70.2.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnusson G., Winnacker E. L., Eliasson R., Reichard P. Replication of polyoma DNA in isolated nuclei. II. Evidence for semi-conservative replication. J Mol Biol. 1972 Dec 30;72(3):539–552. doi: 10.1016/0022-2836(72)90173-8. [DOI] [PubMed] [Google Scholar]
- Martin R. G., Setlow V. P. The initiation of SV40 DNA synthesis is not unique to the replication origin. Cell. 1980 Jun;20(2):381–391. doi: 10.1016/0092-8674(80)90624-8. [DOI] [PubMed] [Google Scholar]
- Myers R. M., Tjian R. Construction and analysis of simian virus 40 origins defective in tumor antigen binding and DNA replication. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6491–6495. doi: 10.1073/pnas.77.11.6491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oguro M., Suzuki-Hori C., Nagano H., Mano Y., Ikegami S. The mode of inhibitory action by aphidicolin on eukaryotic DNA polymerase alpha. Eur J Biochem. 1979 Jul;97(2):603–607. doi: 10.1111/j.1432-1033.1979.tb13149.x. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Tegtmeyer P. Simian virus 40 deoxyribonucleic acid synthesis: the viral replicon. J Virol. 1972 Oct;10(4):591–598. doi: 10.1128/jvi.10.4.591-598.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjian R. The binding site on SV40 DNA for a T antigen-related protein. Cell. 1978 Jan;13(1):165–179. doi: 10.1016/0092-8674(78)90147-2. [DOI] [PubMed] [Google Scholar]
- Winnacker E. L., Magnusson G., Reichard P. Replication of polyoma DNA in isolated nuclei. I. Characterization of the system from mouse fibroblast 3T6 cells. J Mol Biol. 1972 Dec 30;72(3):523–537. doi: 10.1016/0022-2836(72)90172-6. [DOI] [PubMed] [Google Scholar]