Abstract
Background: Bisphenol A (BPA) is a synthetic estrogen commonly used in polycarbonate plastic and resin-lined food and beverage containers. Exposure of animal and cell models to doses of BPA below the recommended tolerable daily intake (TDI) of 50 μg/kg/day have been shown to alter specific estrogen-responsive gene expression, but this has not previously been shown in humans.
Objective: We investigated associations between BPA exposure and in vivo estrogenic gene expression in humans.
Methods: We studied 96 adult men from the InCHIANTI population study and examined in vivo expression of six estrogen receptor, estrogen-related receptor, and androgen receptor genes in peripheral blood leukocytes.
Results: The geometric mean urinary BPA concentration was 3.65 ng/mL [95% confidence interval (CI): 3.13, 4.28], giving an estimated mean excretion of 5.84 μg/day (95% CI: 5.00, 6.85), significantly below the current TDI. In age-adjusted models, there were positive associations between higher BPA concentrations and higher ESR2 [estrogen receptor 2 (ER beta)] expression (unstandardized linear regression coefficient = 0.1804; 95% CI: 0.0388, 0.3221; p = 0.013) and ESRRA (estrogen related receptor alpha) expression (coefficient = 0.1718; 95% CI: 0.0213, 0.3223; p = 0.026): These associations were little changed after adjusting for potential confounders, including obesity, serum lipid concentrations, and white cell subtype percentages. Upper-tertile BPA excretors (urinary BPA > 4.6 ng/mL) had 65% higher mean ESR2 expression than did lower-tertile BPA excretors (0–2.4 ng/mL).
Conclusions: Because activation of nuclear-receptor–mediated pathways by BPA is consistently found in laboratory studies, such activation in humans provides evidence that BPA is likely to function as a xenoestrogen in this sample of adults.
Keywords: bisphenol A, endocrine disruption, estrogen receptor-β, estrogen-related receptor-α, human biomonitoring, InCHIANTI, toxicogenomics
Bisphenol A (BPA) is a synthetic compound that is suspected to act as an endocrine disruptor (i.e., a compound capable of causing dysfunction to hormonally regulated body systems) (Talsness et al. 2009). It was originally synthesized as a synthetic estrogen (Dodds and Lawson 1936). It is used extensively as a monomer in polycarbonate plastics and in the epoxy resins that are used to line food and beverage containers and is one of the world’s highest-production-volume chemicals (Ritter 2011). Ubiquitous exposure to BPA is believed to occur mainly through the diet, with additional contributions from dental sealants, dermal exposure, and inhalation of household dusts. BPA metabolites have been reported in the urine of > 90% of people in representative population samples in the United States and Europe (Calafat et al. 2008; Galloway et al. 2010).
Whether BPA can cause human health effects is a matter of some debate. There has been concern about the potential for a relationship between BPA and negative health effects, including increases in abnormal penile/urethra development in males, early sexual maturation in females, an increase in neurobehavioral problems such as attention deficit–hyperactivity disorder (ADHD) and autism, an increase in childhood and adult obesity and type 2 diabetes, and an increase in hormonally mediated cancers, such as prostate and breast cancers (reviewed by Hengstler et al. 2011; vom Saal et al. 2007). Cross-sectional epidemiological studies have shown higher BPA exposure to be associated with adverse health effects in the general adult population. In a study of 1,455 respondents in the 2003–2004 U.S. population-representative National Health and Nutrition Examination Survey (NHANES), higher urinary BPA concentrations were associated with cardiovascular disease diagnoses and with diagnosed diabetes but not with other common diseases, suggesting specificity of the reported findings (Lang et al. 2008). In a further study of data from NHANES 2005/2006, higher BPA concentrations were again associated with coronary heart disease, providing independent replication of the findings (Melzer et al. 2010). Higher exposure to BPA has also been associated with reproductive and developmental abnormalities. In a study of 249 mothers and their children, prenatal urinary BPA concentrations in mothers were prospectively associated with externalizing behavior scores among their children when measured at 2 years of age (Braun et al. 2009). A positive association was also shown between BPA exposure and recurrent miscarriage in a prospective study of 67 women (Sugiura-Ogasawara et al. 2005). The mechanisms underlying these potential health effects remain to be determined.
Most studies of the health effects of BPA have focused on its estrogenic activity because it is widely documented to function as an agonist of certain estrogen receptors (ERs) (Lee et al. 2003) and as an androgen antagonist and to suppress aromatase activity (Bonefeld-Jørgensen et al. 2007). Additional receptor-mediated biological activities, including binding to the orphan estrogen-related receptor ERRγ (Okada et al. 2008), thyroid hormone disruption (Moriyama et al. 2002), altered pancreatic β-cell function (Ropero et al. 2008), and obesity-promoting effects (Newbold et al. 2008), have been reported in different model systems. Many of these effects are already detectable in the nanomolar range, prompting calls for a revision to the current tolerable daily intake (TDI) of 50 μg/kg/day. However, until now, there has been no evidence that BPA at these low levels exerts significant biological effects in humans, and hence the TDI has remained unaltered (European Food Safety Authority 2010).
A recent cross-sectional examination of circulating sex hormone concentrations in 307 men showed higher BPA levels to be associated with changes in total testosterone concentrations [β = 0.046; 95% confidence interval (CI): 0.015, 0.076; p = 0.004 in fully adjusted models] (Galloway et al. 2010). There was no significant trend in 17β-estradiol levels with higher BPA in men, although an earlier study of 167 men recruited through an infertility clinic used multiple adjusted regression models to show BPA concentrations in urine to be inversely associated with the estradiol:testosterone ratio (Meeker et al. 2010). Plausible explanations for these endocrine changes include altered expression of hormone-responsive genes. To date there is no in vivo evidence for changes in sex-hormone–responsive gene expression associated with human exposure to BPA.
Here, we aimed to test the hypothesis that exposure to BPA would be associated with changes in the in vivo expression of estrogen- and androgen-responsive genes. To do this, we conducted a cross-sectional study to characterize six candidate estrogen- or androgen-related transcripts for differential in vivo expression in response to BPA exposure. The study population was selected from the InCHIANTI study, a large European population representative sample based in Chianti, Italy.
Materials and Methods
Study population. The InCHIANTI study, a prospective population-based study of Italian adults (InCHIANTI 2011), was designed to identify risk factors for mid- and late-life morbidity in urban and rural populations and has been described extensively elsewhere (Ferrucci et al. 2000). InCHIANTI is performed in two sites: Greve in Chianti (11,709 inhabitants) and Bagno a Ripoli (Village of Antella, 4,704 inhabitants). The final study population included 1,453 persons (age range 20–102 years) stratified across age ranges using a multistage sampling process, with a response rate of 91.6% from the baseline interview. Subjects and specimens selected for the present study were those with the most adequate RNA and urine specimens in the 2008/2009 follow-up, and ≤ 76 years of age, in line with previous work. Women were excluded from this analysis because of cyclic hormonal variations in premenopausal subjects. The Instituto Nazionale Riposo e Cura Anziani Institutional Review Board (Florence, Italy) provided ethical approval. All participants gave informed (or surrogate) consent.
Sample collection. Participants who consented to give a blood sample were also asked to provide a spot morning urine sample, which was stored at –20°C until further analysis. First thing in the morning on the day of the study visit, after participants had been sedentary for 15 min, fasting blood samples were collected for routine blood examination, and peripheral blood specimens preserving in vivo RNA expression were collected using PAXgene technology (Debey-Pascher et al. 2009).
Analysis of urinary BPA concentrations. Samples were analyzed at the Brixham Environmental Laboratory Division of Analytical Chemistry (a division of AstraZeneca PLC; Brixham, UK) in compliance with Good Laboratory Practice, EU Directive 88/32/EEC (United Kingdom 2004). BPA ingested in humans is almost completely metabolized and rapidly excreted, so urine is considered the most appropriate matrix for assessment of exposure (Calafat et al. 2005). As part of our extensive Good Laboratory Practice–compliant quality control, we included reagent blanks and confirmed that samples stored for up to 10 years contained predominantly metabolized compound, confirming minimal leaching of BPA from collection or storage vessels during this time. BPA concentrations were measured in spot urine samples by liquid chromatography–mass spectrometry. Total (free and conjugated) urinary concentrations of BPA were obtained using online, solid-phase extraction coupled with high-performance liquid chromatography–isotope dilution tandem mass spectrometry with peak focusing, as described previously (Galloway et al. 2010). Calibration was linear from 0.50 to 100 μg/L (R2 > 0.996), limit of detection was < 0.50 ng/mL BPA, the limit of quantification was 0.50 ng/mL BPA, and the lowest calibration standard gave a signal height:noise ratio > 10 (relative standard deviations < 20%, all other standards < 15%).
Gene expression by real-time reverse-transcriptase polymerase chain reaction (RT-PCR). Blood leukocytes were used for transcript analysis because they are convenient and available and because they provide sufficient power in large cohorts where access to other tissues is lacking. Because BPA is metabolized in the intestines and liver to form predominantly BPA-monoglucuronide, which is passed through the bloodstream to the kidney, exposure of leukocytes to BPA and/or its metabolites is inevitable. To test the hypothesis that exposure to BPA would be associated with changes in the expression of estrogen- and androgen-responsive genes, we correlated BPA levels as a continuous trait with the expression of ER, androgen receptor (AR), and estrogen-related receptor (ERR) genes by quantitative real-time PCR in a subset of 100 male subjects. These genes were chosen because the nuclear hormone receptors they encode are transcription factors that control essential developmental and physiological pathways and because activation of these nuclear-receptor–mediated pathways by BPA is consistently found in laboratory studies.
Total RNA (100 ng) was reversed transcribed in 20 μL reactions using the Superscript III VILO kit (Invitrogen, Paisley, UK), according to the manufacturer’s instructions.
The expression levels of ESR1 (estrogen receptor 1; ERα), ESR2 [estrogen receptor 2 (ER beta); ERβ], ESRRA (estrogen related receptor alpha; ERRα), ESRRB (estrogen related receptor beta; ERRβ), ESRRG (estrogen related receptor gamma), and AR (androgen receptor) genes were then assessed relative to the endogenous control genes GUSB (glucuronidase, beta) and ACTB (actin, beta; β-actin) on the TaqMan Low Density Array (TLDA) platform (Applied Biosystems, Foster City, CA, USA). Probes were inventoried with Applied Biosystems assays Hs01046812_m1, Hs01100358_m1, Hs01584024_m1, Hs00155006_m1, Hs00907244_m1, Hs99999908_m1, and Hs03023943_g1 for ESR1, ESR2, ESRRB, ESRRG, AR, GUSB, and ACTB genes, respectively. These probes were chosen because they are documented to pick up all isoforms and splice variants for the genes of interest.
The expression of the ESRRA gene was assessed by the use of a custom assay (probe and primer sequences available on request). Reaction mixes included 50 μL 2× TaqMan universal master mix (no AMPerase; Applied Biosystems), 40 μL distilled H2O, and 10 μL cDNA template per TLDA loading port. PCR amplifications were performed on the ABI 7900HT platform (Applied Biosystems). Cycling conditions were 50°C for 2 min, 94.5°C for 10 min followed by 40 cycles of 97°C for 30 sec and 57.9°C for 1 min. The expression of each gene was measured in triplicate for each sample. Gene expression relative changes were quantified using the 2–ΔΔCt method (Livak and Schmittgen 2001) relative to the geometric mean of the endogenous controls listed above using the StatMiner relative quantification software for high-throughput integrated analysis of TLDA data (Integromics, Grenada, Spain).
Statistical analysis. We assessed the association of candidate gene expression levels with urinary BPA concentration by multivariable linear regression. Data were adjusted for potential confounding factors that could influence BPA exposure or candidate gene expression: age (reported in years at the last birthday and used as a continuous variable); body mass index (BMI) calculated as weight in kilograms divided by height in meters squared; waist circumference (as a continuous trait); highest level of education attained (in four categories: none/elementary, secondary, high school, and university/professional); low-density lipoprotein (LDL) cholesterol (milligrams per deciliter); triglycerides (milligrams per deciliter); and study site [individuals were drawn from a rural village (Greve) and an urban population (Bagno a Ripoli)]. Models were also adjusted for the percentage of neutrophils (neutrophil%), lymphocytes (lymphocyte%), monocytes (monocyte%), and eosinophils (eosinophil%) [the percentage of basophils (basophil%) was not added because the cell subtype percentages would have equaled 100%].
The expression value of each of the target genes was not normally distributed, and we used natural log transformation when gene expression was considered as a dependent variable. In all analyses, an upper age cutoff was 76 years to minimize the problem of comorbidity. Data analysis was performed using STATA (version 10 SE; StataCorp LP, College Station, TX, USA); p < 0.05 was considered significant.
We used generalized additive models with penalized cubic regression splines (Wood 2006) to explore the functional form of the relationship between candidate gene expression levels and urinary BPA concentration. Linearity of the relationship between log-transformed expression level and log-transformed BPA concentration was assessed by visual inspection of the estimated spline functions and by examining the estimated degrees of freedom (edf) for the smoothed BPA term. Values of the edf close to 1 were taken as evidence of linearity. Adjustment was made for the same potential confounding factors that were included in the multivariable linear regression models. The prediction error criterion for smoothness selection was generalized cross-validation. Robustness of the smoothness selection was assessed by making comparisons with the use of maximum likelihood estimation. The spline models were fitted using R statistical software using the mgcv package for generalized additive modeling (version 2.12.1; R Project for Statistical Computing 2010).
Results
The sample (n = 96; Table 1) had a mean age of 58.3 years (range, 32–76 years) and a mean (± SD) BMI of 27.8 ± 4.1 kg/m2. The geometric mean urinary BPA concentration was 3.65 ng/mL (95% CI: 3.13, 4.28) ranging from 0.73 to 56.94 ng/mL (limit of detection < 0.5 ng/mL). The distribution was skewed, with a 10th percentile of 1.3 ng/mL and a 90th percentile of 10.4 ng/mL. The estimated mean excretion was 5.84 μg/day (95% CI: 5.00, 6.85).
Table 1.
Characteristics of the sample (n = 96).
| Characteristic | Mean ± SD (range)a |
|---|---|
| Age (years) | 58.3 ± 15.2 (32–76) |
| Site (%) | |
| Greve | 38.4 |
| Bagno a Ripoli | 61.5 |
| Education (%) | |
| None/elementary | 22.9 |
| Secondary school | 26.0 |
| High school | 35.4 |
| Professional/university | 16.6 |
| BMI (kg/m2) | 27.8 ± 4.1 (18.38–42.99) |
| LDL cholesterol (mg/dL) | 125.4 ± 29.8 (60–220) |
| Triglycerides (mg/dL) | 137.3 ± 75.3 (45–469) |
| Neutrophils% | 55.2 ± 9.7 (26.2–79.1) |
| Lymphocytes% | 32.8 ± 9.2 (9.1–59.9) |
| Monocytes% | 8.4 ± 2 (4.3–21.3) |
| Eosinophils% | 3 ± 1.7 (0.1–10.3) |
| Basophils% | 0.5 ± 0.2 (0.1–1.4) |
| aValues shown are mean ± SD (range) except where indicated. | |
The expression of transcripts associated with sex-hormone–related signaling was quantified by real-time RT-PCR (Table 2). Expression of ESRRG was not detected in our samples. There was only one significant correlation of expression intensities between probes: between ESR1 and ESR2 (pairwise correlation = 0.24; p = 0.02). We obtained valid expression intensity measures for 96 men for the ESR2 gene and 83 men for the ESRRA gene (Table 2). BPA concentrations in the 96 respondents with successful ESR1 expression measures were no different from the remaining 55 respondents < 76 years of age (age-adjusted regression with log-transformed BPA concentration: unstandardized linear regression coefficient = 0.012; 95% CI: –0.114, 0.138; p = 0.848) for which measured BPA values were available.
Table 2.
Expression characteristics of the tested estrogen and androgen target genes.
| Target gene | Assay IDa | Accession numberb | n | Mean ± SD (range) | ||||
|---|---|---|---|---|---|---|---|---|
| ESR1 | Hs01046812_m1 | NM_000125 | 96 | 1.21 ± 0.535 (0.365–3.165) | ||||
| ESR2 | Hs01100358_m1 | NM_001040275 | 96 | 1.294 ± 0.899 (0.167–5.585) | ||||
| ESRRA | Hs01067166_g1 | NM_004451 | 83 | 0.882 ± 0.33 (0.105–1.991) | ||||
| ESRRB | Hs01584024_m1 | NM_004452 | 96 | 2.974 ± 2.434 (0.000–10.363) | ||||
| ESRRG | Hs00155006_m1 | NM_206595 | Not expressed | |||||
| AR | Hs00907244_m1 | NM_000044.2 | 96 | 1.232 ± 0.673 (0.188–3.295) | ||||
| aTaqMan Gene Expression assay identification number. bAccession numbers from the National Center for Biotechnology Information (2011). | ||||||||
Using urinary BPA concentrations as a continuous variable, we tested linear associations between BPA and gene expression. In age-adjusted regression models of log-transformed BPA concentrations against log-transformed expression levels (Table 3), we observed positive associations with ESR2 (ERβ; coefficient = 0.1804; 95% CI: 0.0388, 0.3221; p = 0.013) and ESRRA (ERRα, coefficient = 0.1718, 95% CI: 0.0213, 0.3223, p = 0.026) but not with ESR1(ERα), ESRRB(ERRβ), or AR.
Table 3.
Estimates for the associations between natural log of urinary BPA concentrations and gene expression intensity (log transformed), in age-adjusted and fully adjusteda regression models.
| Age-adjusted model | Fully adjusted model | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gene | Coefficient (95% CI) | p-Value | Std β | Coefficient (95% CI) | p-Value | Std β | ||||||
| ESR1 | –0.0657 (–0.1815, 0.0500) | 0.262 | –0.117 | –0.1071 (–0.2205, 0.0063) | 0.064 | –0.1909 | ||||||
| ESR2 | 0.1804 (0.0388, 0.3221) | 0.013 | 0.231 | 0.1387 (0.001, 0.2764) | 0.048 | 0.1775 | ||||||
| ESRRA | 0.1718 (0.0213, 0.3223) | 0.026 | 0.250 | 0.1886 (0.0324, 0.3448) | 0.019 | 0.2699 | ||||||
| ESRRB | –0.2816 (–1.3969, 0.8337) | 0.617 | –0.054 | –0.4857 (–1.6669, 0.6955) | 0.416 | –0.0925 | ||||||
| ESRRG | ND | ND | ||||||||||
| AR | 0.0115 (–0.1404, 0.1634) | 0.881 | 0.016 | 0.0925 (–0.0646, 0.2495) | 0.245 | 0.1285 | ||||||
| Abbreviations: ND, not detected; Std, standardized. aFull adjustment included age, BMI, study site, educational attainment, and LDL cholesterol and triglyceride concentrations, plus percentages of neutrophils, lymphocytes, monocytes, and eosinophils. | ||||||||||||
In models additionally adjusted for previously suggested confounders (Sharpe 2010) (BMI, LDL cholesterol and triglyceride concentrations, study site, and educational attainment—a proxy for social position) and white cell subtype percentages, the results were little changed: for ESR2, coefficient = 0.1387; 95% CI: 0.001, 0.2764; p = 0.048; for ESRRA coefficient = 0.1886; 95% CI: 0.0324, 0.3448; p = 0.019) (Table 4).
Table 4.
Multiple regression model estimates for the associations between explanatory variables and natural logs of ESR2and ESRRA gene expression.
| ESR2 | ESRRA | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | Coefficient (95% CI) | p-Value | Std β | Coefficient (95% CI) | p-Value | Std β | ||||||
| BPA concentration (log transformed) | 0.1387 (0.001, 0.2764) | 0.048 | 0.1775 | 0.1886 (0.0324, 0.3448) | 0.019 | 0.2699 | ||||||
| Age | –0.0169 (–0.0261, –0.0078) | < 0.001 | –0.4256 | 0.0018 (–0.0086, 0.0122) | 0.733 | 0.0470 | ||||||
| BMI | 0.0206 (–0.0057, 0.0468) | 0.122 | 0.1399 | –0.0023 (–0.0312, 0.0265) | 0.873 | –0.0194 | ||||||
| Study site | 0.2016 (–0.0098, 0.413) | 0.061 | 0.1629 | –0.218 (–0.4489, 0.0129) | 0.064 | –0.2087 | ||||||
| Educational attainment | ||||||||||||
| None/elementary | 1 (Reference) | 1 (Reference) | ||||||||||
| Secondary | 0.1146 (–0.2084, 0.4375) | 0.482 | 0.0834 | –0.4742 (–0.8521, –0.0962) | 0.015 | –0.4176 | ||||||
| High school | –0.0347 (–0.3704, 0.3011) | 0.838 | –0.0275 | –0.099 (–0.4865, 0.2885) | 0.612 | –0.0929 | ||||||
| Professional/university | 0.0899 (–0.2604, 0.4403) | 0.611 | 0.0542 | –0.0379 (–0.4521, 0.3764) | 0.856 | –0.0268 | ||||||
| LDL cholesterol (mg/dL) | –0.0024 (–0.006, 0.0012) | 0.195 | –0.117 | 0.0016 (–0.0022, 0.0055) | 0.407 | 0.094 | ||||||
| Triglycerides (mg/dL) | 0.0012 (–0.0002, 0.0025) | 0.095 | 0.1444 | –0.0009 (–0.0023, 0.0006) | 0.255 | –0.1339 | ||||||
| Neutrophil% | –0.3194 (–0.8618, 0.2229) | 0.245 | –5.1245 | 0.3622 (–0.2803, 1.0047) | 0.265 | 6.5463 | ||||||
| Lymphocyte% | –0.302 (–0.845, 0.2411) | 0.272 | –4.606 | 0.3611 (–0.2828, 1.0049) | 0.267 | 6.1452 | ||||||
| Monocyte% | –0.2982 (–0.8349, 0.2385) | 0.272 | –1.0051 | 0.3678 (–0.265, 1.0007) | 0.250 | 1.5245 | ||||||
| Eosinophil% | –0.3075 (–0.8726, 0.2575) | 0.282 | –0.8726 | 0.3624 (–0.2947, 1.0196) | 0.275 | 1.2623 | ||||||
| Constant | 31.078 (–23.088, 85.244) | 0.257 | –36.1216 | 0.264 | 0 | |||||||
| Std, standardized. “Constant” refers to the intercept term in the multiple regression model; it gives the expected log-transformed gene expression level when all other variables in the model are set to zero. | ||||||||||||
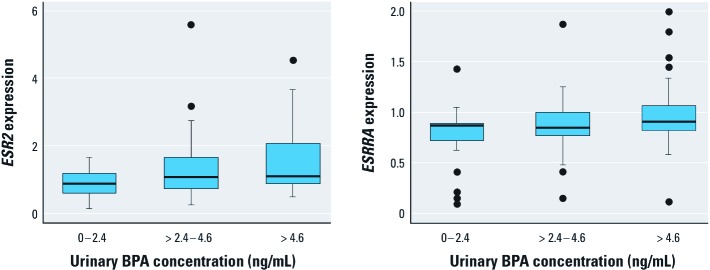
When using an alternative exposure metric of dividing BPA concentrations into tertiles in the fully adjusted models (Figure 1), participants in the lowest BPA exposure tertile had a geometric mean expression of ESR2 of 0.80 IU (95% CI: 0.65, 0.99), rising to 1.32 IU (95% CI: 1.08, 1.60) in the highest tertile, a 65% increase in mean expression. For ESRRA, the same measures were 0.66 IU (95% CI: 0.49, 0.89) and 0.91 IU (95% CI: 0.78, 1.06), a 38% increase in mean expression of the gene.
Figure 1.
Box plot of ESR2 and ESRRA probe intensity by urinary BPA concentration. Boxes extend from the 25th to the 75th percentile, horizontal bars represent the median, whiskers indicate the 10th and 90th percentiles, and outliers are represented as circles.
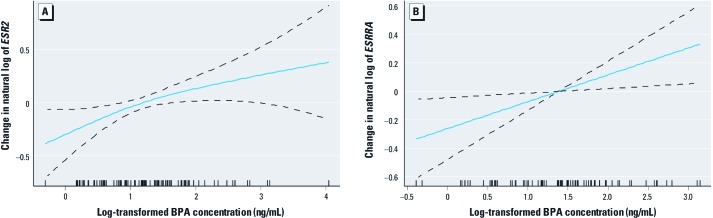
Figure 2A shows a spline plot for the change in natural log of ESR2 expression as a function of log-transformed urinary BPA concentration. This suggests that the positive association between ESR2 and BPA concentration is curvilinear (edf = 1.45; p-value for smoothed term = 0.027), with evidence of a diminishing effect as BPA concentration increases. A similar spline plot for ESRRA expression is shown in Figure 2B. This suggests that the relationship with BPA concentration is linear for this ERR (edf = 1.00; p-value for smoothed term = 0.017).
Figure 2.
Cubic regression spline models illustrating the functional form of the relationship between log-transformed urinary BPA concentration and ESR2 (A) and ESSRA (B) gene expression.
Discussion
In this study, we aimed to assess whether increased urinary BPA concentrations were associated with changes in gene expression in vivo in the general adult population. We made use of a large-scale and high-quality population-representative data set for which specimens preserving in vivo RNA expression were available. We were able to measure in vivo expression of five ER, ERR, and AR genes in peripheral blood leukocytes in 96 adult men. Using urinary BPA excretion as a marker of exposure, we found that those with higher BPA exposures had higher expression of two estrogen-responsive genes, ESR2 (ERβ) and ESRRA (ERRα).
These findings are important because they suggest that BPA is bioactive in the human body and that associations with hormone signaling and related disorders are biologically plausible. ERβ, which showed the strongest association with BPA exposure, is one of two ER subtypes that, along with ERα, mediates the physiological actions of estrogens (Swedenborg et al. 2009). ERβ and ERα have distinct tissue distribution, ligand specificities, and functions; ERα is predominant in the regulation of female reproduction, whereas ERβ is important in maintaining the structure and function of nonclassic target tissues, including prostate, colon, and cardiovascular and central nervous systems (Imamov et al. 2005). BPA displays estrogenic agonist activities against both ERα and ERβ subtypes in vitro, with relatively high ERβ selectivity (Matthews et al. 2001), consistent with our findings. The modulation by BPA of ER gene expression has previously been shown in animal models, at environmentally relevant concentrations. For example, exposure of rat prostate mesenchyme cells to 1 nM BPA led to altered ER gene expression, accompanied by modest stimulation of cell growth, with a threshold of induction around 30-fold less potent than 17β-estradiol (Richter et al. 2007).
ERRα belongs to the NR3B orphan nuclear receptor subgroup, which consists of ERRα, ERRβ, and ERRγ (Hong et al. 1999). All three ERRs show close sequence identity to the ERα DNA binding domain and also feature a conserved C-terminal domain with a putative ligand binding domain and interaction surfaces for coregulators, and a less conserved N-terminal domain (Giguère 2002). Despite this close structural homology to the ERs, estradiol does not bind to ERRα, and X-ray crystallography of the putative ligand-binding domain pocket of ERRα shows it to be almost completely occupied by side chains. This supports the suggestion that ERRα shows ligand-independent transcriptional activation and is largely dependent on its functional interaction with coregulators, including peroxisome proliferator-activated receptor-γ (PPARγ) coactivator 1α (PGC-1A) and PGC-1B for optimal gene regulation (Ranhotra 2010). In adults, ERRα is constitutively expressed in tissues that preferentially use fatty acids as energy sources, including adipose tissue, heart, and skeletal muscle, where it plays a significant role in regulating energy homeostasis and adaptive oxidative capacity (Dufour et al. 2007). These functions are thought to involve close cooperation with PGC-1A and ERRγ (Villena and Kralli 2008). Crucially, BPA binds to ERRγ with high affinity (Okada et al. 2008), and ERβ has been identified as an important regulator of PPARγ (Foryst-Ludwig et al. 2008).
Given the structural homology between ERs and ERRs, particularly in the DNA-binding domain, involvement of ERRs in estrogenic signaling pathways is not unexpected (Giguère 2002). ERRα has been proposed as a regulator of aromatase activity (Yang et al. 1998), and in turn, estradiol induces up-regulation of ERRα in some tissues (Shigeta et al. 1997). ERRα stimulation of androgen-responsive element–containing promoters illustrates the potential for cross-talk with signaling driven by other steroid hormones (Teyssier et al. 2008).
The functional relevance of changes in ERβ and ERRα expression in blood leukocytes has not been determined. Because estrogens and androgens can exert differential effects in function depending on the cell type and its stage of development, the consequences of BPA exposure on a wider range of adult reproductive and somatic tissues merits further attention (Goodman et al. 2008). However, up to 50% of expression changes in leukocytes for highly heritable cis-acting traits are also mirrored in other tissues such as adipose tissue, making them viable surrogates for exposure of other tissues (Emilsson et al. 2008). Human adipocytes express both ERβ and ERRα (Hugo et al. 2008), and adipocyte explants respond to both BPA and 17β-estradiol exposure in the nanomolar range by accumulating lipid. Taken together, these results are strongly suggestive of specific and targeted bioactivity of BPA in vivo, even if the clinical relevance, if any, of these findings is not yet clear.
One limitation of this analysis is its cross-sectional nature. Virtually all individuals are exposed, and because clinical trials to administer BPA in human subjects are ethically unacceptable, collecting longitudinal data demonstrating that BPA exposure induces gene expression changes in vivo is not currently achievable.
It is feasible that the increases in gene expression that we measured are associated with confounding variables that have not been accounted for in our models. For example, there are time-dependent changes in ERβ expression, both on a long-term scale, such as in fetal and postnatal development, and in short-term oscillations during the circadian cycle (Swedenborg et al. 2009). Although it is not possible to completely account for circadian cycles, all samples were taken at a similar time of day, and we restricted our analysis to men rather than women to minimize the influence of cyclic variation in endogenous hormones. Confounding variables that could affect BPA exposure include higher food intakes or obesity, which could be accompanied by incidentally higher intakes of BPA (Sharpe 2010). Our secondary analyses included adjustment for BMI and LDL cholesterol and triglyceride concentrations, and these made minimal difference to the overall results, arguing against obesity as an explanation for our findings. We found no associations between serum lipids and expression intensities of our candidate genes.
Another consideration is that we quantified BPA metabolites in urine, while gene expression was measured in blood leukocytes. BPA ingested in humans is rapidly excreted, so urine is considered the most viable biomonitoring approach for BPA, as detailed by Calafat et al. (2005). Single spot samples are limited measures of longer-term exposure, but a in study of temporal variability in urinary BPA metabolites, Mahalingaiah et al. (2008) found that a single spot sample had moderate sensitivity for predicting an individual’s tertiary categorization. Nepomnaschy et al. (2009) measured stability of BPA over 2-week intervals in first voided urine samples from 60 women and found a Spearman correlation of 0.5, indicating that within-individual BPA exposures were generally stable over periods of weeks. They also showed that the stability of BPA in long-term frozen samples is good. The stability of free BPA in urine was also confirmed by Ye et al. (2011).
The mean BPA concentration in our study was 3.65 ng/mL, and assuming an average 24-hr urine volume of 1,600 mL in adult men (Galloway et al. 2010), a 100% excretion rate, and a total blood volume of 6 L, the estimated concentration of BPA in the blood was in the low nanogram per milliliter range. The in vitro IC50 (half-maximal inhibitory concentration) for human ERβ receptor binding of BPA is in the micromolar range (Matthews et al. 2001), which would imply low ER occupancy rates. Given that functional effects of BPA on nuclear receptor expression have also been reported in both animal and human cells at this concentration, in vitro measurement may not be indicative of the in vivo situation where differential binding to carrier proteins and receptors may occur. There are no in vivo data on the rate at which BPA is converted to BPA-monoglucuronide and excreted from the body, only estimates, and because BPA is lipophilic with a log octanol–water partition coefficient (Kow) of 2.2–3.82, distribution to lipid-rich tissues is a possibility. This suggestion is supported by population-based half-lives for BPA calculated by Stahlhut et al. (2009) to be significantly longer than previous predictions of 6 hr.
The major metabolite of BPA, BPA-monoglucuronide, has no estrogenic activity, but oxidative cleavage of BPA to form the estrogenically active metabolite 4-methyl-2,4-bis(4-hydroxyphenyl)pent-1-ene (MBP) has been observed in rat liver. Okuda et al. (2010) reported that MBP was 500-fold more potent than BPA itself in inducing dose-dependent changes in expression of ERα and ERβ mRNA. The significance of this metabolite in humans is not yet known. However, a comparison of the phase 1 metabolism of BPA in rat and human liver microsomes identified the oxidation product BPA-catechol to be a minor (~ 10%) metabolite in both species. BPA-catechol is considered to be a weak estrogen (Nakagawa and Suzuki 2001), suggesting that further investigation of the phase 1 metabolism of BPA in humans and the estrogenic potency of all metabolites is merited (Ye et al. 2011).
Conclusion
We provide the first report of associations between BPA exposure and in vivo estrogenic gene expression in humans. We examined in vivo expression of six ER, ERR, and AR genes in peripheral blood leukocytes from 96 adult men from the InCHIANTI population study. We observed positive associations between higher urinary BPA concentrations and higher expression of two estrogen-responsive genes, encoding ERβ and ERRα. The associations remained statistically significant when adjusted for potential confounders, including obesity and serum lipid concentrations. The individuals in the upper tertile of BPA exposure showed 65% higher mean expression of the ESR2 (ERβ) gene in peripheral blood leukocytes than did those in the lower tertile. Although the clinical significance of these results is not yet known, such activation in humans provides evidence that BPA is likely to function as a xenoestrogen in this population-representative sample of adults. This prompts a need for replication and scientific follow-up, for example, in examining the relationship between gene expression changes and protein expression and effects of BPA exposure in a wider range of estrogen-regulated target tissues.
Acknowledgments
We thank all who contributed to the InCHIANTI study, including the anonymous participants.
Footnotes
R.C. was supported by University of Exeter internal funding. This project was supported in part by the (U.K. government–funded) Peninsula NIHR (National Institute for Health Research) Clinical Research Facility; the Intramural Research Program, of the National Institute on Aging, U.S. National Institutes of Health; and the European Centre for Environment and Human Health, University of Exeter.
C.M., P.M., and A.Y. are employed by Brixham Environmental Laboratory, AstraZeneca UK Ltd. Their input was limited to conducting and documenting the bisphenol A (BPA) assays, and they were blind to the other data examined. The analysis of BPA samples on contract was funded from independent Peninsula College of Medicine and Dentistry sources. The authors declare that they have no other actual or potential competing financial interests.
References
- Bonefeld-Jørgensen EC, Long M, Hofmeister MV, Vinggaard AM. Endocrine-disrupting potential of bisphenol A, bisphenol A dimethacrylate, 4-n-nonylphenol, and 4-n-octylphenol in vitro: new data and a brief review. Environ Health Perspect. 2007;115(suppl 1):69–76. doi: 10.1289/ehp.9368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun JM, Yolton K, Dietrich KN, Hornung R, Ye X, Calafat AM, et al. Prenatal bisphenol A exposure and early childhood behavior. Environ Health Perspect. 2009;117:1945–1952. doi: 10.1289/ehp.0900979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calafat AM, Kuklenyik Z, Reidy JA, Caudill SP, Ekong J, Needham LL. Urinary concentrations of bisphenol A and 4-nonylphenol in a human reference population. Environ Health Perspect. 2005;113:391–395. doi: 10.1289/ehp.7534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calafat AM, Ye X, Wong LY, Reidy JA, Needham LL. Exposure of the U.S. population to bisphenol A and 4-tertiary-octylphenol: 2003–2004. Environ Health Perspect. 2008;116:39–44. doi: 10.1289/ehp.10753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debey-Pascher S, Eggle D, Schultze JL. RNA stabilization of peripheral blood and profiling by bead chip analysis. Methods Mol Biol. 2009;496:175–210. doi: 10.1007/978-1-59745-553-4_13. [DOI] [PubMed] [Google Scholar]
- Dodds E, Lawson W. Synthetic estrogenic agents without the phenanthrene nucleus. Nature. 1936;137:996. [Letter] [Google Scholar]
- Dufour CR, Wilson BJ, Huss JM, Kelly DP, Alaynick WA, Downes M, et al. Genome-wide orchestration of cardiac functions by the orphan nuclear receptors ERRα and γ. Cell Metab. 2007;5:345–356. doi: 10.1016/j.cmet.2007.03.007. [DOI] [PubMed] [Google Scholar]
- Emilsson V, Thorleifsson G, Zhang B, Leonardson AS, Zink F, Zhu J, et al. Genetics of gene expression and its effect on disease. Nature. 2008;452(7186):423–428. doi: 10.1038/nature06758. [DOI] [PubMed] [Google Scholar]
- European Food Safety Authority. 2010. Scientific opinion on bisphenol A: evaluation of a study investigating its neurodevelopmental toxicity, review of recent scientific literature on its toxicity and advice on the Danish risk assessment of bisphenol A. EFSA J 8(9):1829–1945.
- Ferrucci L, Bandinelli S, Benvenuti E, Di Iorio A, Macchi C, Harris TB, et al. Subsystems contributing to the decline in ability to walk: bridging the gap between epidemiology and geriatric practice in the InCHIANTI study. J Am Geriatr Soc. 2000;48:1618–1625. doi: 10.1111/j.1532-5415.2000.tb03873.x. [DOI] [PubMed] [Google Scholar]
- Foryst-Ludwig A, Clemenz M, Hohmann S, Hartge M, Sprang C, Frost N, et al. 2008Metabolic actions of estrogen receptor beta (ERβ) are mediated by a negative cross-talk with PPARγ. PLoS Genet 4e1000108; doi: 10.1371/journal.pgen.1000108[Online 27 June 2008] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galloway T, Cipelli R, Guralnick J, Ferrucci L, Bandinelli S, Corsi AM, et al. Daily bisphenol A excretion and associations with sex hormone concentrations: results from the InCHIANTI adult population study. Environ Health Perspect. 2010;118:1603–1608. doi: 10.1289/ehp.1002367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giguère V. To ERR in the estrogen pathway. Trends Endocrinol Metab. 2002;13:220–225. doi: 10.1016/s1043-2760(02)00592-1. [DOI] [PubMed] [Google Scholar]
- Goodman JE, Witorsch RJ, McConnell EE, Sipes IG, Slayton TM, Yu CJ, et al. Weight-of-evidence evaluation of reproductive and developmental effects of low doses of bisphenol A. Crit Rev Toxicol. 2008;20:1–75. doi: 10.3109/10408440903279946. [DOI] [PubMed] [Google Scholar]
- Hengstler JG, Foth H, Gebel T, Kramer P-J, Lilienblum W, Schweinfurth H, et al. Critical evaluation of key evidence on the human health hazards of exposure to bisphenol A. Crit Rev Toxicol. 2011;41:263–291. doi: 10.3109/10408444.2011.558487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong H, Yang L, Stallcup MR. Hormone-independent transcriptional activation and coactivator binding by novel orphan nuclear receptor ERR3. J Biol Chem. 1999;274:22618–22626. doi: 10.1074/jbc.274.32.22618. [DOI] [PubMed] [Google Scholar]
- Hugo ER, Brandebourg TD, Woo JG, Loftus J, Alexander JW, Ben-Jonathan N. Bisphenol A at environmentally relevant doses inhibits adiponectin release from human adipose tissue explants and adipocytes. Environ Health Perspect. 2008;116:1642–1647. doi: 10.1289/ehp.11537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imamov O, Shim G, Warner M, Gustafsson JA. Estrogen receptor beta in health and disease. Biol Reprod. 2005;73:866–871. doi: 10.1095/biolreprod.105.043497. [DOI] [PubMed] [Google Scholar]
- InCHIANTI. The Study Design. 2011. Available: http://www.inchiantistudy.net/study.html [accessed 2 August 2011]
- Lang IA, Galloway TS, Scarlett A, Henley WE, Depledge M, Wallace RB, et al. Association of urinary bisphenol A concentration with medical disorders and laboratory abnormalities in adults. JAMA. 2008;300:1303–1310. doi: 10.1001/jama.300.11.1303. [DOI] [PubMed] [Google Scholar]
- Lee HJ, Chattopadhyay S, Gong EY, Ahn RS, Lee K. Antiandrogenic effects of bisphenol A and nonylphenol on the function of androgen receptor. Toxicol Sci. 2003;75:40–46. doi: 10.1093/toxsci/kfg150. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Mahalingaiah S, Meeker JD, Pearson KK, Calafat AM, Ye X, Petrozza J, et al. Temporal variability and predictors of urinary bisphenol A concentrations in men and women. Environ Health Perspect. 2008;116:173–178. doi: 10.1289/ehp.10605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews JB, Twomey K, Zacharewski TR. In vitro and in vivo interactions of bisphenol A and its metabolite, bisphenol A glucuronide, with estrogen receptors α and β. Chem Res Toxicol. 2001;14:149–157. doi: 10.1021/tx0001833. [DOI] [PubMed] [Google Scholar]
- Meeker JD, Calafat AM, Hauser R. Urinary bisphenol A concentration in relation to serum thyroid and reproductive hormones in men from an infertility clinic. Environ Sci Technol. 2010;44:1458–1463. doi: 10.1021/es9028292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melzer D, Rice NE, Lewis C, Henley WE, Galloway TS.2010Association of urinary bisphenol A concentration with heart disease: evidence from NHANES 2003/06. PLoS One 51e8673; doi: 10.1371/journal.pone.0008673[Online 13 January 2010] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriyama K, Tagami T, Akamizu T, Usui T, Saijo M, Kanamoto N, et al. Thyroid hormone action is disrupted by bisphenol A as an antagonist. J Clin Endocrinol Metab. 2002;87:5185–5190. doi: 10.1210/jc.2002-020209. [DOI] [PubMed] [Google Scholar]
- Nakagawa Y, Suzuki T. Metabolism and cytotoxicity of bisphenol A and other bisphenols in isolated rat hepatocytes. Xenobiotica. 2001;31:113–123. [Google Scholar]
- National Center for Biotechnology Information. PubMed Nucleotide. 2011. Available: http://www.ncbi.nlm.nih.gov/nuccore [accessed 26 October 2011]
- Nepomnaschy PA, Baird DD, Weinberg CR, Hoppin JA, Longnecker MP, Wilcox AJ. Within-person variability in urinary bisphenol A concentrations: measurements from specimens after long term storage. Environ Res. 2009;109(6):734–737. doi: 10.1016/j.envres.2009.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newbold RR, Padilla Banks E, Jefferson WN, Heindel JJ. Effects of endocrine disruptors on obesity. Int J Androl. 2008;31:201–208. doi: 10.1111/j.1365-2605.2007.00858.x. [DOI] [PubMed] [Google Scholar]
- Okada H, Tokunaga T, Liu X, Takayanagi S, Matsushima A, Shimohigashi Y. Direct evidence revealing structural elements essential for the high binding ability of bisphenol A to human estrogen-related receptor. Environ Health Perspect. 2008;116:32–38. doi: 10.1289/ehp.10587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuda K, Takiguchi M, Yoshihara S. In vivo estrogenic potential of 4-methyl-2,4-bis(4-hydroxyphenyl)pent-1-ene, an active metabolite of bisphenol A, in uterus of ovariectomized rat. Toxicol Lett. 2010;197:7–11. doi: 10.1016/j.toxlet.2010.04.017. [DOI] [PubMed] [Google Scholar]
- Ranhotra HS. The estrogen-related receptor alpha: the oldest, yet an energetic orphan with robust biological functions. J Recept Signal Transduct. 2010;2010:1–13. doi: 10.3109/10799893.2010.487493. [DOI] [PubMed] [Google Scholar]
- R Development Core Team. R Project for Statistical Computing. 2010. Available: http://www.r-project.org/ [acceessed 20 January 2011]
- Richter CA, Taylor JA, Ruhlen RL, Welshons WV, vom Saal FS. Estradiol and bisphenol a stimulate androgen receptor and estrogen receptor gene expression in fetal mouse prostate mesenchyme cells. Environ Health Perspect. 2007;115:902–908. doi: 10.1289/ehp.9804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritter S. Debating BPA’s toxicity. Chem Eng News. 2011;89:14–19. [Google Scholar]
- Ropero A, Alonso Magdalena P, García-García E, Ripoll C, Fuentes E, Nadal A. Bisphenol A disruption of the endocrine pancreas and blood glucose homeostasis. Int J Androl. 2008;31:194–200. doi: 10.1111/j.1365-2605.2007.00832.x. [DOI] [PubMed] [Google Scholar]
- Sharpe R. Bisphenol A and sexual dysfunction in men. Hum Reprod. 2010;25(2):292–294. doi: 10.1093/humrep/dep385. [DOI] [PubMed] [Google Scholar]
- Shigeta H, Zuo W, Yang N, Diaugustine R, Teng C. The mouse estrogen receptor-related orphan receptor alpha 1: molecular cloning and estrogen responsiveness. J Mol Endocrinol. 1997;19:299–309. doi: 10.1677/jme.0.0190299. [DOI] [PubMed] [Google Scholar]
- Stahlhut RW, Welshons WV, Swan SH. Bisphenol A data in NHANES suggest longer than expected half life, substantial nonfood exposure, or both. Environ Health Perspect. 2009;117:784–789. doi: 10.1289/ehp.0800376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiura-Ogasawara M, Ozaki Y, Sonta S, Makino T, Suzumori K. Exposure to bisphenol A is associated with recurrent miscarriage. Human Reprod. 2005;20:2325–2329. doi: 10.1093/humrep/deh888. [DOI] [PubMed] [Google Scholar]
- Swedenborg E, Power KA, Cai W, Pongratz I, Rüegg J. Regulation of estrogen receptor beta activity and implications in health and disease. Cell Mol Life Sci. 2009;66(24):3873–3894. doi: 10.1007/s00018-009-0118-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talsness CE, Andrade AJ, Kuriyama SN, Taylor JA, vom Saal FS. Components of plastic: experimental studies in animals and relevance for human health. Philos Trans R Soc Lond B Biol Sci. 2009;364(1526):2079–2096. doi: 10.1098/rstb.2008.0281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teyssier C, Bianco S, Lanvin O, Vanacker JM. The orphan receptor ERRα interferes with steroid signaling. Nucleic Acids Res. 2008;36:5350–5361. doi: 10.1093/nar/gkn520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- United Kingdom (GLP Monitoring Authority, Medicines and Healthcare Products Regulatory Agency) 2004. Good Laboratory Practice Regulations 1999 [Statutory Instrument 1999 No. 3106], as amended by the Good Laboratory Practice (Codification Amendments Etc.) Regulations 2004.
- Villena JA, Kralli A. ERRα: a metabolic function for the oldest orphan. Trends Endocrinol Metab. 2008;19:269–276. doi: 10.1016/j.tem.2008.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- vom Saal F, Akingbemi BT, Belcher SM, Birnbaum LS, Carin DA, Eriksen M, et al. Chapel Hill bisphenol A expert panel consensus statement: integration of mechanisms, effects in animals and potential to impact human health at current levels of exposure. Reprod Toxicol. 2007;24:131–138. doi: 10.1016/j.reprotox.2007.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood SN. 2006. Generalized Additive Models: An Introduction with R. Boca Raton, FL:Chapman & Hall/CRC. [Google Scholar]
- Yang C, Zhou D, Chen S. Modulation of aromatase expression in the breast tissue by ERR-1 orphan receptor. Cancer Res. 1998;58:5695–5699. [PubMed] [Google Scholar]
- Ye X, Zhou X, Needham LL, Calafat AM. In-vitro oxidation of bisohenol A: is bisphenol A catechol a suitable biomarker for human exposure to bisphenol A? Anal Bioanal Chem. 2011;399:1071–1079. doi: 10.1007/s00216-010-4344-x. [DOI] [PubMed] [Google Scholar]