Abstract
In neural tissue engineering, designing materials with the right chemical cues is crucial in providing a permissive microenvironment to encourage and guide neuronal cell attachment and differentiation. Modifying synthetic hydrogels with biologically active molecules has become an increasingly important route in this field to provide a successful biomaterial and cell interaction. This study presents a strategy of using the monomer 2-methacryloxyethyl trimethylammonium chloride (MAETAC) to provide tethered neurotransmitter acetylcholine-like functionality with a complete 2-acetoxy-N,N,N-trimethylethanaminium segment, thereby modifying the properties of commonly used, non-adhesive PEG-based hydrogels. The effect of the functional monomer concentration on the physical properties of the hydrogels was systematically studied, and the resulting hydrogels were also evaluated for mice hippocampal neural cell attachment and growth. Results from this study showed that MAETAC in the hydrogels promotes neuronal cell attachment and differentiation in a concentration-dependent manner, different proportions of MAETAC monomer in the reaction mixture produce hydrogels with different porous structures, swollen states, and mechanical strengths. Growth of mice hippocampal cells cultured on the hydrogels showed differences in number, length of processes and exhibited different survival rates. Our results indicate that chemical composition of the biomaterials is a key factor in neural cell attachment and growth, and integration of the appropriate amount of tethered neurotransmitter functionalities can be a simple and effective way to optimize existing biomaterials for neuronal tissue engineering applications.
Keywords: PEG-based hydrogels, Neurotransmitters, Acetylcholine functionality, Concentration-dependent manner
1. Introduction
Injuries to the brain and spinal cord cause some of the most severe and widespread public health problems. According to the Center for Disease Control (CDC), several million people suffer from disabilities caused by brain damage each year in the U.S. alone. The causes of the damage include a wide range of conditions such as traumatic brain injury, stroke, chronic neurodegenerative disease, infections, hypoxia, and poisoning. These result in the loss of specific populations of neurons as well as connections between neurons and the development of defined psychiatric or neurological symptoms. Unfortunately, there are currently no therapies available to fully restore lost function or slow ongoing neurodegeneration in the damaged brain.
However, in recent years, knowledge of the factors influencing nerve reconstruction has increased, new surgical techniques and equipment have been developed, and experimental work in the field has made great progress. For example, neural tissue engineering [1–3] as a newly emerging field, involving the use of cells to promote nerve regeneration and to repair damage caused to nerves, provides a promising approach to repair segmental nerve defects. However, in order for cells to maintain their tissue-specific functions, a substrate material must be inserted to aid in organization of cells and the directed growth of neuronal processes [3–5]. For this application the choice of scaffolding material is crucial for success, and a number of different natural and synthetic materials have been explored that effect nerve regeneration and repair.
Compared to natural materials, synthetic materials have become increasingly important in this field, since their scaffold architectures, chemical composition, physical properties, and biochemical properties are controllable and reproducible, and each of the properties can also be tailored for specific applications. In particular, synthetic hydrogels have drawn interest as in vitro and in vivo research models for the study of neural tissue engineering applications [4], because they have many advantages over alternative scaffold materials, such as high oxygen and nutrient permeability. They also have low interfacial tensions which minimize barriers to cells migrating into the scaffold from surrounding soft tissue, or processes from cells within or out of the material crossing the scaffold-tissue boundary [6]. Furthermore, hydrogels are able to retain aqueous solutions encapsulated with drugs, growth factors and cells for desired functioning in vivo. Numerous synthetic hydrogels have been explored for drug delivery and nerve regeneration applications, and they may hold key roles in overcoming the inherent insufficiency of protection, repair and regeneration of the brain [7,8].
To date, due to availability and biocompatibility of the precursors, methacrylate based hydrogels and polyethylene glycol (PEG) based hydrogels remain the most important classes of synthetic hydrogels for CNS applications. However, a major drawback of these types of hydrogels is low protein and cell attachment: they alone cannot support cell adhesion and tissue formation due to their bio-inert nature. Cell attachment to these hydrogels is facilitated by modifying them with other molecules to create synthetic templates that can mimic some of the properties of a natural tissue matrix, such as extracellular matrix (ECM) proteins (laminin, fibronectin, or vitronectin) or short adhesive peptides (RGDS, IKVAV or YIGSR) derived from these molecules [9,10]. Modifying PEG based hydrogels with small functional biologically active molecules also provides an alternate route to give positive cues for successful biomaterial and cell interactions [11,12]. An important class of biomolecules in the nervous system is the neurotransmitters, which play important roles in cell communication, differentiation and survival [13,14]. Studies have shown that both surface-tethered and directly integrated neurotransmitters in polymers can induce specific neuronal responses [15–17]. Immobilized rat chondrocytes directly encapsulated in tyramine-substituted hyaluronan hydrogels remained metabolically active and behaved similar to cells cultured in monolayers [18]. Substantial improvement in neuronal outgrowth have been observed for neuronal cells on a bioactive polymer based on dopamine compared to tissue culture polystyrene, laminin, and poly-D-lysine [15]. Particularly, acetylcholine (ACh), as the first neurotransmitter, has been widely studied for its role in synaptic transmission [19,20], and it has been shown to regulate neuronal development and enhance neurite outgrowth [21–23]. However, studies using ACh functionality in synthetic hydrogels are limited, and the use of a structure that contains the complete acetylecholine functionality (2-acetoxy-N,N,N-trimethylethanaminium) segments is desirable to understand how immobilized acetylcholine neurotransmitter interact with neuronal cells.
In the current work, we designed and synthesized biomimetic hydrogels with a tethered acetylcholine structure to promote interaction between neuronal cells and material surfaces. 2-methacryloxyethyl trimethylammonium chloride (MAETAC) was chosen as a monomer to provide the ACh functionality. We systematically investigated a complete concentration range of MAETAC in the synthetic hydrogels by controlling the feeding volume ratio of MAETAC to PEGMA monomers in the prepolymerization solution (ranges from 90.0% to 1.0%, and the molar ratio ranges from 92.2% to 1.0%). We investigated the effect of MAETAC concentration on equilibrium swelling and mechanical properties of the hydrogels. The effect of these parameters on neuron and glial cell attachment and growth was demonstrated by seeding these hydrogels with cells derived from the mouse hippocampus.
2. Materials and methods
2.1. Synthesis of hydrogels
Random copolymer poly(MAETAC-PEGMA) hydrogels were produced by photopolymerizing monomers MAETAC and PEGMA (Sigma-Aldrich, St. Louis, MO) at various ratios (Table 1). In all experiments, the crosslinking agent ethylene glycol dimethylacrylate (EGDMA; Sigma-Aldrich, St. Louis, MO) concentration is 0.1% (v/v) to the polymerization solution, and photoinitiator 2, 2-Dimethoxy-2-Phenylacetophenone (Irgacure 651; Sigma-Aldrich, St. Louis, MO) was 53.0 mM. We synthesized the hydrogel by pipetting the mixed monomer solution between two Parafilm®-covered optical glass slides separated by a 1-mm-thick spacer. The entire assembly was exposed to UV light (365 nm, 1.8 mW/cm2) using a hand-held UV lamp (Spectroline; Westbury, NY) for 5 min. Following polymerization at room temperature, hydrogels were peeled off the Parafilm®-covered glass surface (7.6 cm diameter × 0.45cm thick, transmittance 95% at 365 nm, Esco Products, Oak Ridge, NJ). Subsequently, the gels were thoroughly washed in large quantities of distilled water for 2 days to remove unreacted monomers with periodic replacement of the water used for leaching. After washing, the hydrogel disks were cut out using a 10 mm Arch punch (10 mm diameter, McMaster-Carr, New Brunswick, NJ). Hydrogel disks were then stored in deionized water until use.
Table 1.
Feeding ratios of monomer MAETAC to monomer PEGMA in the prepolymerization solutions for the preparation of hydrogel samples and elemental analysis of these samples. The prepared hydrogel samples showed different appearance according to the feeding ratio of the two monomers.
| Hydrogel Names |
Ratio of MAETAC to PEGMA |
Elemental Analysis |
Final Gel Appearance |
||||
|---|---|---|---|---|---|---|---|
| v/v | w/w | n/n | % C | % H | % N | ||
| HS-1 | 9.00 | 6.77 | 11.74 | 40.93 | 9.10 | 4.55 | Clear |
| HS-2 | 4.00 | 3.01 | 5.22 | 42.94 | 9.09 | 4.21 | White |
| HS-3 | 2.33 | 1.76 | 3.04 | 44.15 | 8.96 | 3.74 | White |
| HS-4 | 1.50 | 1.13 | 1.96 | 46.17 | 9.06 | 3.35 | White |
| HS-5 | 1.00 | 0.75 | 1.30 | 48.65 | 9.11 | 3.00 | White |
| HS-6 | 0.67 | 0.50 | 0.87 | 49.35 | 8.93 | 2.34 | White |
| HS-7 | 0.43 | 0.32 | 0.56 | 49.64 | 8.86 | 1.74 | White |
| HS-8 | 0.25 | 0.19 | 0.33 | 50.97 | 8.77 | 1.19 | White |
| HS-9 | 0.11 | 0.08 | 0.14 | 52.79 | 8.51 | 0.63 | Clear |
| HS-10 | 0.05 | 0.04 | 0.07 | 54.30 | 8.15 | 0.29 | Clear |
| HS-11 | 0.01 | 0.01 | 0.01 | 54.39 | 8.16 | <0.05 | Clear |
Note the elemental composition of MAETAC (C9H18NO2Cl, Mw = 207.7) is as follows: C: 52.0%; H: 8.7%; and N: 6.7%. v/v, w/w, and n/n stand for the volume ratio, mass ratio and molar ratio of MAETAC monomer to PEGMA monomer, respectively.
2.2. Physical characterizations of hydrogels
Hydrogel disks were dehydrated using lyophilization for elemental analysis and scanning electron microscopy (SEM). The fully swollen hydrogel samples were put in a freeze-dryer and shock-frozen in liquid nitrogen for 10 min. The samples were then dried under vacuum at less than 1 mm Hg for 24 h. Elemental analysis for weight percentage of C, H, N of the dried hydrogels was performed by Quantitative Technologies, Inc. (QTI). For SEM experiment, the dehydrated hydrogel samples were freshly cut with a sharp blade, and then mounted on SEM stubs and sputter coated with gold for 30 s. The morphologies and pore structures of the cross-sections of the gold-coated hydrogel samples were observed under SEM (Leica 440) at 10 KV.
To measure the equilibrium swelling ratio, the fully swollen hydrogel samples were weighed after excess surface water was gently removed by blotting with Kimwipes, and then freeze-dried and weighed again. Each measurement was repeated at least three times. The equilibrium water content was calculated from the change in mass of the samples before and after freeze-drying by the following equation [24–26]: EWC% = (1− Wd/Ws) ×100, and the swelling ratio was determined according to the following equation [25]: Swelling Ratio = (Ws – Wd)/Wd, where Ws is the weight of the swollen gel and Wd is the weight of the freeze dried gel, respectively.
Mechanical properties of hydrogels were tested using a DMA 2980 (TA Instruments) in submersion compression method. The hydrogel sample disks (10 mm) were submerged in deionized (DI) water for measurements. Tests were performed in controlled force mode and the preload force was 0.01 N. The data was analyzed by calculating the compression modulus from the slope of the linear region on the plot of stress-strain curve, and each sample has been measured three times.
2.3. Hippocampal cell culture on hydrogels
Procedures used in animal experiments were processed through institutional animal care. All hydrogel samples were autoclaved for 30 min before the experiments, and all cell culture experiments were carried out in 24 well plates. One day before culture, hydrogel samples in 24-well plates were rinsed twice in neuronal culture medium consisting of Neurobasal-A (Gibco/BRL, Bethesda, MD) supplemented with B27 (2%, v/v; Gibco), and then equilibrated in the medium overnight until cell plating.
Primary hippocampal neuronal cultures were prepared through enzymatic dissociation of hippocampi removed from postnatal day 0 mouse pups as previously described [27]. Briefly, after removal of the meanings of the cerebral hemispheres, hippocampi were dissected out, chopped into small pieces and digested with 0.125% trypsin (Gibco) for 15 min at 37°C. The tissue was then dissociated into a single cell suspension by triturating. The cells were plated at a density of 40,000 cells/well onto the hydrogel samples. The cells were cultured in Neurobasal-A medium supplemented with B27 and maintained for 3 days before fixation. Each experiment was performed in triplicate and repeated 3 times.
2.4. Cell viability, immunostaining, and statistical analysis
Cell viability was measured using the standard LIVE/DEAD Viability/Cytotoxicity Assay Kit from Invitrogen (Eugene, OR). After 3 days of culture, each hydrogel sample was placed in 1 ml growth media with 0.5 µL calcein substrate and 2 µL ethidium homodimer substrate, and incubated at 37°C for 20 min. Cell viability and distribution were visualized using a Nikon Eclipse 800 fluorescence microscope. Five random fields of each sample were imaged on both green and red channels and the number of live and dead cells was counted manually for each image. The number of live cells divided by the total number of live and dead cells was defined as the fractional viability.
For immunostaining experiments, cells on hydrogel samples were fixed after three days of culture with 4% paraformaldehyde (Sigma-Aldrich) in phosphate buffered saline (PBS, pH7.4, Sigma-Aldrich) for 20 min at room temperature, and then rinsed three times with PBS buffer. The samples were then prepared for immunostaining by blocking and permeabilizing in 10% normal goat serum (NGS; Sigma-Aldrich) and 0.1% Triton-X100 (v/v, PBS-T, Sigma-Aldrich) in PBS for 1 h at room temperature. Samples were exposed to the primary antibodies, mouse monoclonal anti-β-tubulin III (1:1000, Sigma-Aldrich) and rabbit polyclonal anti-GFAP (1:1000, DAKO) diluted in PBS-T buffer containing 2% NGS and incubated overnight at 4°C. On the following day, the samples were then washed with PBS-T buffer thoroughly. The cells were then incubated for 1.5 h at room temperature with AlexaFluor488-congugated goat anti-mouse IgG (1:1000, Molecular Probes) and AlexaFluor®568 goat anti-rabbit IgG (1:1000, Molecular Probes) diluted in PBS-T containing 2% NGS, followed by incubation with DAPI (1:1000, Molecular Probes) in PBS buffer for 5 min at room temperature. After rinsing with PBS five times, and 5 min each time, images of cultures were obtained using the fluorescence microscope. The images were analyzed using ImageJ (available at http://rsbweb.nih.gov/ij/) to determine the number of neurons and astrocytes in each random field. All data are reported for triplicate samples. Statistical analyses were performed by one-way ANOVA followed by Turkey post hoc test. Results were considered statistically significant if p<0.05.
3. Results
3.1. Hydrogel preparation and characterization
Hydrogels were crosslinked by EGDMA using Irgacure 651 as a radical photoinitiator. Table 1 shows the chemical compositions of the MAETAC and PEGMA monomers used in the prepolymerization solution to make each hydrogel sample. Results from elemental analysis showed that the amount of MAETAC structure presented in the hydrogels corresponds to the feed ratio of the monomers: less MAETAC structure presented in the final gels when its concentration in the pre-polymerization solution is lower. Phase separation occurred when PEGMA was added to a MAETAC aqueous solution (75.0%, v/v) in samples from HS-2 to HS-8, and those hydrogels were white in color. When an excess amount of MAETAC or PEGMA was present in the solution, this seemed not to be a problem: samples HS-1 and HS-9 to HS-11 all were transparent and clear gels. After the polymerization process, the prepared hydrogels were purified in a large amount of deionized water to remove unreacted monomers, and the gels were also fully swollen during this procedure.
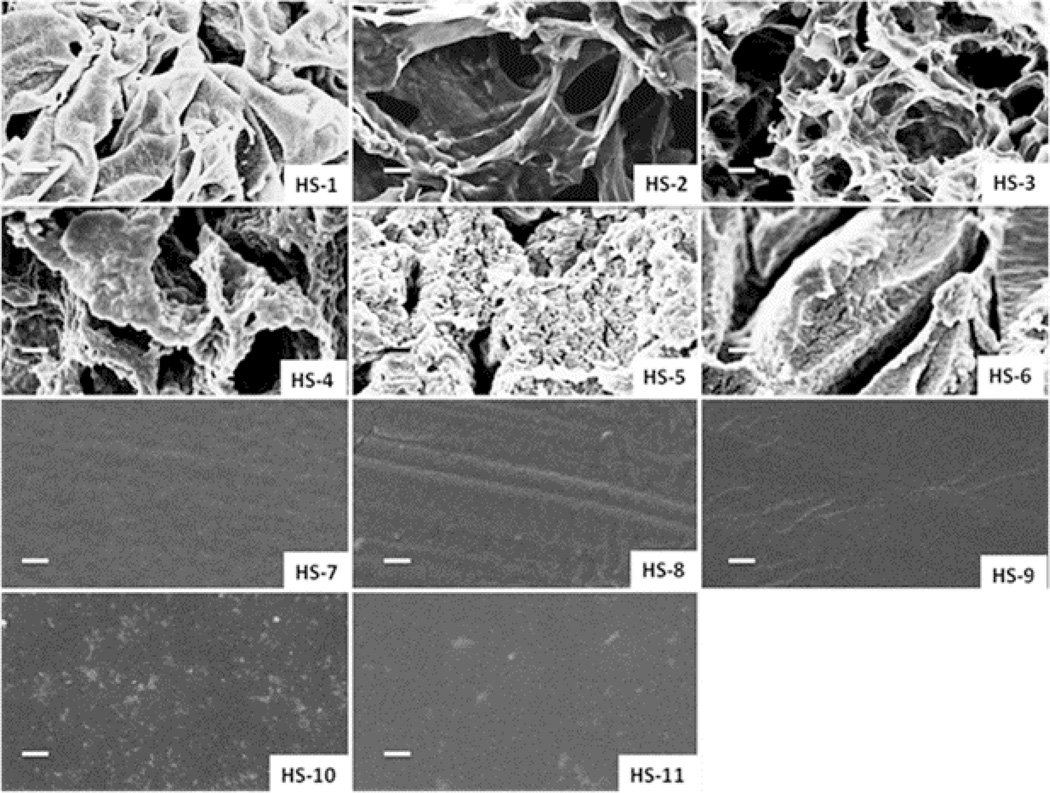
Purified hydrogels in deionized water were then freeze-dried to investigate the morphology by SEM. Fig. 2 shows sample images of a cross-sectional view of the bulk structure of the dried hydrogels. HS-1 is mechanically soft and contains a large amount of water; the structure collapsed during the freeze-drying process. HS-2 and HS-3 showed large porous structures, while due to the increased amount of PEGMA in the prepolymerized solutions; hydrogels HS-4 to HS-6 had interconnected pore morphology. From sample HS-7 to HS-11, analysis of the SEM micrographs showed no visible macrospores in the samples prepared using this method.
Fig. 2.
Cross-section structures of the hydrogels under scanning electron microscopy (SEM) after freeze-drying (scale bar is 5 µm).
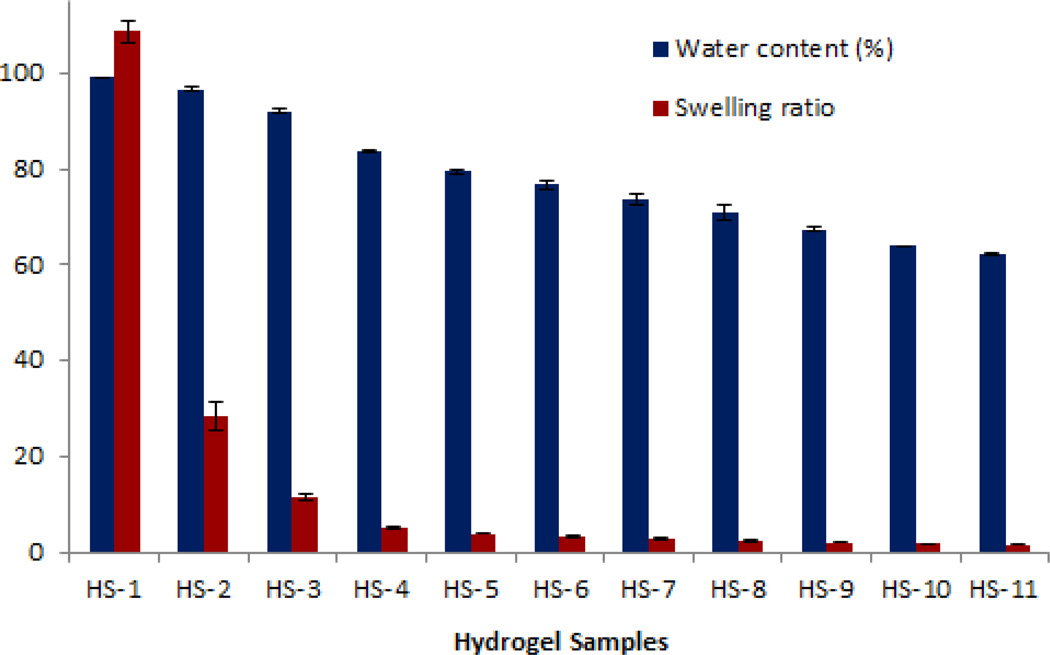
The equilibrium water content of the hydrogels decreased with the decreasing of the amount of MAETAC concentration in the monomer mixtures, which is in agreement with the SEM observation that the hydrogel porosity decreased with the increase of the PEGMA concentration of the polymerization solution (Fig. 3). This might because of the presence of positively charged groups in the matrix contribute to an osmotic force leading to water absorption, as with increasing MAETAC content, the osmotic gradient increases therefore result in higher equilibrium water content or swelling ratio. For example, the water content for HS-1 (10.0% of PEGMA) was 99.1%, while in HS-9 (90.0% of PEGMA) it was 67.4%. The dramatic change of the swelling ratio from 108.64 to 1.64 with the increased amount of PEGMA from sample HS-1 to sample HS-11 further showed that pore volume largely decreased with the PEGMA concentration increased. Note that water comprises more than 95.0% the total weight of sample HS-1 and HS-2. They can be called superabsorbent [28], and might also hold other potential applications, such as in chromatography and water purification [29,30].
Fig. 3.
Equilibriums water contents and swelling ratio of hydrogels in deionized water (Means ± SD, n=3).
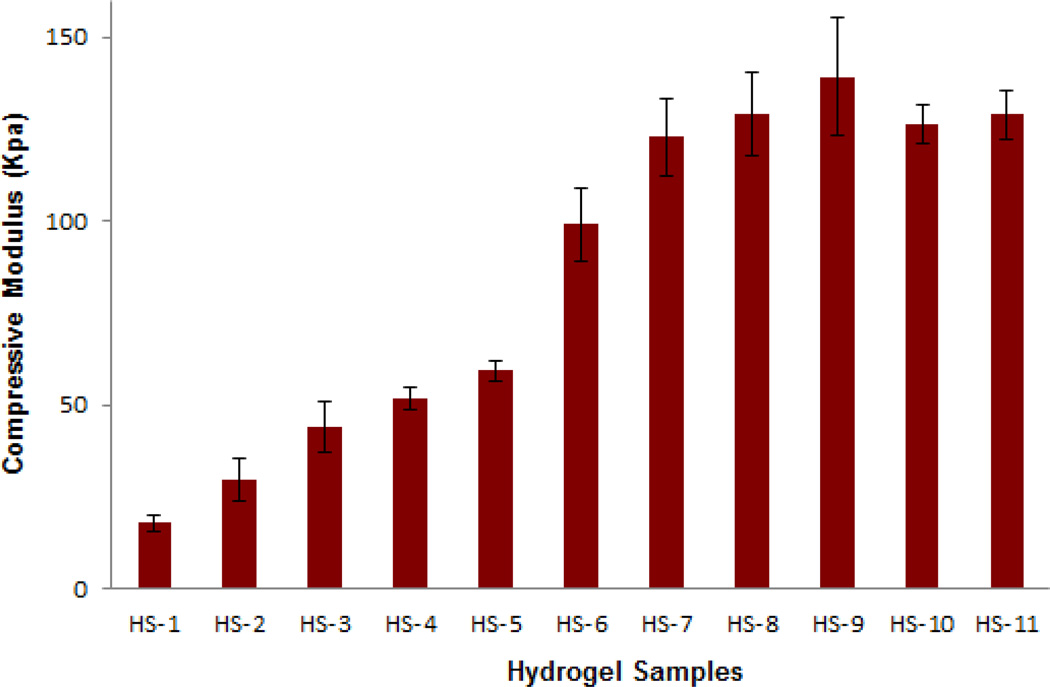
The slopes of the linear portion of the compression stress-strain curves were used to compute mean values for the compression modulus. The compression modulus is directly related through the degree of swelling and hence the water content. Higher concentrations of PEGMA were expected to result in more tightly crosslinked hydrogels and a decrease in the ability of the network to absorb water. Therefore, in most cases the compression modulus of hydrogels increased with the increase in PEGMA concentration in the polymerization solution (Fig. 4). HS-1 had a modulus of 17.7 kPa; with additional amounts of PEGMA, the compressive modulus of hydrogel HS-11 increased up to 128.9 kPa. Samples HS-10 and HS-11 were very brittle and had a lower modulus as compared to samples HS-8 and HS-9, probably because the gels were slightly cracked during the tests.
Fig. 4.
Compressive modulus of hydrogels (Means ± SD, n=3). The data was analyzed by calculating the linear region slopes of the stress-strain curves.
3.2. Attachment and viability of hippocampal neuronal cells on the hydrogels
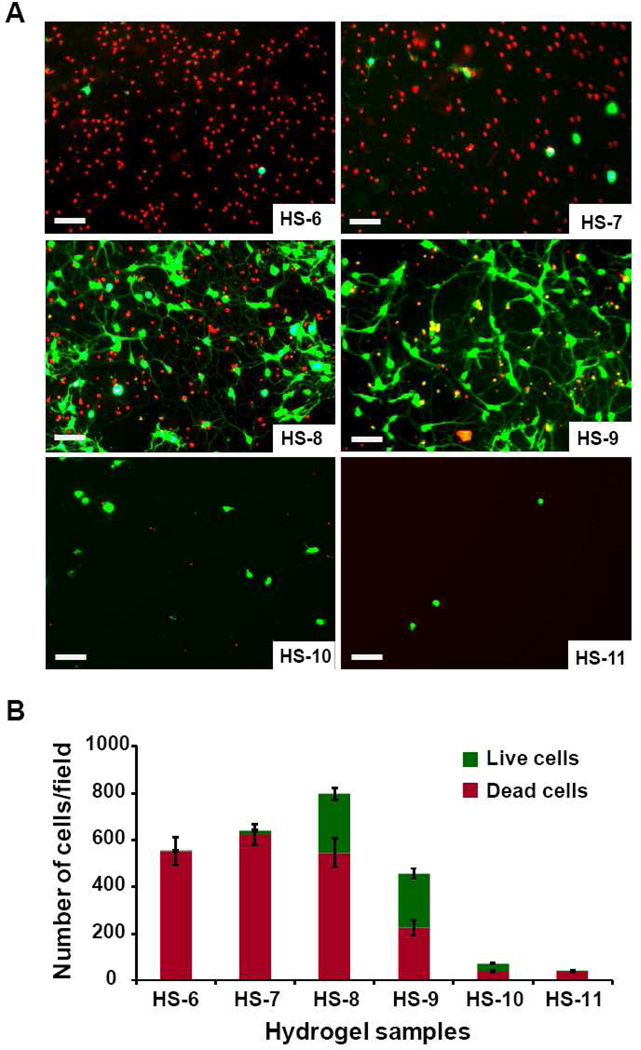
Dissociated mouse hippocampal neurons cells were plated on all hydrogel samples. After 3 days, cellular viability was assessed via the LIVE/DEAD cell viability/toxicity assay. Samples HS-1 to HS-5 showed strong cytotoxicity, with many dead cells on the surfaces, and no living cells was observed (data not shown). The other six samples, HS-6 to HS-11, had viable cells (Fig. 5). HS-6 (0.7% viability, average 3.5 live cells/field) and HS-7 (2.0% viability, average 15.5 live cells/field) also exhibited a greater number of dead cells as compared to HS-8 (33.6% viability, average 224.3 live cells/field), and HS-9 (50.9% viability, average 201.6 live cells/field). As expected, with higher concentrations of PEGMA (greater than 90.0% v/v) in the hydrogels, the samples do not favor cell attachment, as PEG is normally neutral and nonadhesive to protein and cells [31]. Samples HS-10 and HS-11 have relatively fewer cells attached to the gels as compared to sample HS-8 and HS-9 (average 35.8 and 1.7 cells/field, respectively), although the ratio of living cells are higher in these two sample (57.2% and 87.57%).
Fig. 5.
A) LIVE/DEAD viability/cytotoxicity assay of hippocampal cells on hydrogel samples (Scale bar is 50 µm). Cells on hydrogels were cultured for three days and then observed with fluorescent micrographs of live (calcein AM, green) and dead (ethidium homodimer-1, red). B) Analysis of LIVE/DEAD viability/cytotoxicity of hydrogel samples. Numbers of live (green columns) and dead (red columns) cells were counted manually in five random fields of each hydrogel sample, and each sample were repeated three times in the experiments to calculate the average number of cells on a random field.
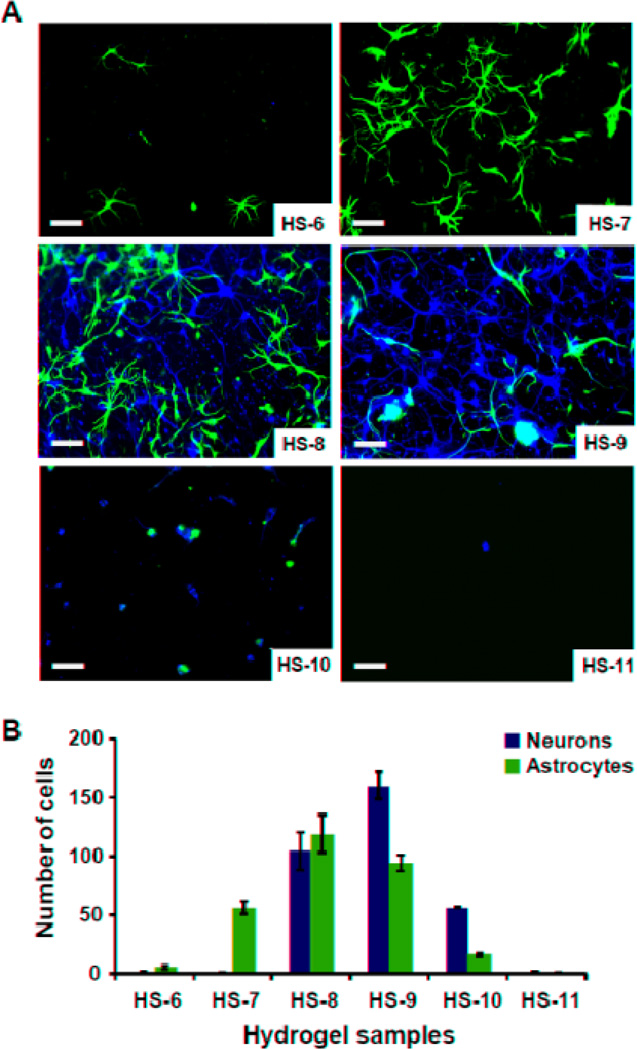
Results of double-label immunocytochemistry (Fig. 6) showed that HS-6 hardly supported either neuronal (average 0.9 neuron/field) or astrocytic (average 5.3 astrocytes/field adhesion or survival), while HS-7 greatly favored astrocytic adhesion (average 0.7 neurons/field and 56.1 astrocytes/field). Cell attachment and growth were much higher on HS-8, with about an equal number of astrocytes and neurons, with an average of 104.7 neurons/field and 118.7 astrocytes/field. HS-9 had the highest cell attachment and neurite outgrowth, with an average of 160.1 neurons and 93.6 astrocytes on a random field. At higher concentrations of MAETAC, cellular attachment and growth decreased: sample HS-10 had 56.1 neurons and 15.9 astrocytes per random field, while sample HS-11 had very few cells attached (average 1.1 neurons/field and 0.2 astrocytes/field). Cell morphologies were different on the six samples, with sample HS-9 eliciting the longest process outgrowth from neurons (Fig. 6A).
Fig. 6.
A) Immunocytochemistry performed on gel cultures demonstrates that these cells are composed of neurons (β-III-tubulin-positive cells, green) and astrocytes (GFAP-positive cells, red). Nuclei are counterstained with DAPI (blue). (Scale bar is 50 µm). B) Number of neurons (green columns) and astrocytes (red columns) grow on hydrogel samples. Cells were counted manually in five random fields of each hydrogel sample, and each sample was repeated three times in the experiments to calculate the average number of cells/field.
4. Discussion
In this study, using MAETAC monomers to emulate a tethered chemical structure of the neurotransmitter acetylcholine, we designed and synthesized a series of hydrogels by radical copolymerization of MAETAC and PEGMA monomers in the presence of the crosslinker EGDMA. The effects of the feed composition of monomers on physical properties of the hydrogels were systematically studied. In general, increasing the proportion of MAETAC monomers yielded increasing water content, swollen ratio and a corresponding decrease in compressive modulus in the gels. Modification of photo-crosslinkable PEGMA with MAETAC monomers improved mouse hippocampal neural cell attachment and growth in a concentration dependent manner, allowing identification of the critical concentration range of functional monomer MAETAC for brain neuronal cell survival and growth.
Most of the hydrogel samples prepared in this study are stiff, providing advantages over collagen gels or other naturally-derived soft gels. Nevertheless, the gels can easily be optimized to prepare softer gels by using different reaction systems (e.g., reduce the concentration of the monomers in the solution), or using different cross-linking methods (e.g., higher molecule weight of PEGDMA). Moreover, the results of this study have important implications for the recent interpretation that physical cues, such as substrate stiffness, are recognizable modifiers of cell behavior. Previous studies on polyacrylamide and polyethyleneglycol hydrogels have suggested that, within the same materials, softer gels greatly favored neurons, whereas harder gels promoted glial cultures [32]. Proliferation of encapsulated neural stem cells decreases with increase in the modulus of the alginate hydrogels [33], and primary neural stem cells differentiate into neurons on soft methacrylamide chitosan hydrogel [34]. Our results do not agree with those previously published reports which demonstrated that neurons favor soft rather than stiff substrates, both neurons and astrocytes grow better on hard gels in this particular system. Although the exact mechanism of this behavior is not yet known, it is notable that the stiffnesses varied from 17 KPa to more than 120 KPa in those hydrogels, but only hard gels (HS-7 to HS-11, with similar stiffness, 122 KPa to 128 KPa) supported cellular attachment and neurite outgrowth. Each of these gels had similar porosities, swelling behaviors and mechanical properties, but with different concentrations of functional monomers. This suggests that, in these types of materials, cellular behavior is influenced to a higher degree by the chemical components than other properties of this system. While the gels are not suitable for brain implantation at this point, the gel system may find utility in other tissue engineering applications where gelation to produce stiff materials is desired, and also in cell encapsulation where cell seeding can be performed after gelation. In addition, softer hydrogel samples based on this system can be easily prepared for the application of brain implantation or cell encapsulation, and linear, soluble polymers can be prepared with the same components for substrate coating and in vitro cell culture studies.
While PEG based materials are normally neural and non-adhesive to protein and cells, they alone cannot provide an ideal environment to neuronal cell adhesion and growth. We demonstrate that diluting the concentration of MAETAC with non-toxic PEG based material can produce materials that have reduced cytotoxicity and even promote cell attachment and outgrowth. The results from this study also showed that hydrogels with relatively low concentration (less than 60.0% v/v or 66.2% n/n) of MAETAC have statistically reduced toxicity compared to the samples containing higher concentrations of MAETAC. Neurons grew significantly better on sample HS-9 (10.0% v/v or 12.7% n/n) among the eleven hydrogel samples prepared. It is noteworthy that cell viability increased almost 77 times just by varying the concentration of MAETAC in the feed solution from 40.0% (v/v) to 10.0% (v/v), and these initial findings suggest that the presence of an optimal amount of MAETAC on the hydrogel surface is an important factor in the subsequent behavior of the cells that anchor on that surface and differentiate. This is in agreement with previous observations that cell attachment and survival are a function of MAETAC concentration [35]. Systematic changes in hydrogel monomer composition provide a valuable means for understanding the concentration-dependent pattern of cellular response on this type of materials. The results of the cellular studies suggested that this approach appeared to overcome the limited cell adhesion properties of PEG-based hydrogel systems.
Previous studies from other groups also showing that integrating acetylcholine-like functionalities (ALFs) in biocompatible polymers can induce specific neuronal responses, unfortunately, only few studies have been made on primary hippocampal neurons. Qin et al [36] prepared soluble biomimetic polymers with two initial monomers poly(ethylene glycol) monomethyl ether-glycidyl methacrylate (MePEG-GMA) and dimethylaminoethyl methacrylate (DMAEMA) at different ratios, the polymers were used to coat glass substrates and rat hippocampal neurons were plated onto the surfaces. The results showed that the ratio of the two initial monomers utilized for polymer synthesis significantly affects neuronal growth. The polymer surface prepared with 1:60 (mol/mol) of MePEG-GMA to DMAEMA induced neuronal growth response similar to that on poly-L-lysine. Christiane et al [37] have also incorporated ACh-like functionalities (ALFs) in a series of linear polymers based on diglycidylsebacate, leucine ethyl ester, and aminoethyl acetate. The polymers were also coated onto glass coverslips, and results showed that ALFs had a profound impact on sprouting and neurite extension of dorsal root ganglia (DRG) cells in a concentration-dependent manner, the polymer with 70% ALF induced regenerative responses similar to laminin. Compared to these two previous studies, our results showed that the amount of MAETAC needed to promote neuronal cell attachment and growth is very low (~10% of MAETAC). This might be because, in both previous studies, ACh-like functionalities were derived from the tertiary amine group. At neural pH, only a fraction of these amine groups will be protonated which therefore offer a close mimicry of the acetylcholine structure, while the quaternary amine groups present in the acetylcholine structure and MAETAC are permanently charged. Also, since acetylcholine is an ester of acetic acid and choline, keeping the complete original chemical structure is essential for its functions. This may cause some major differences in cell behaviors on these polyelectrolytes.
Because each sample showed different numbers and types of cells, comparing neuronal morphology by using traditional statistical analysis methods, such as measuring the lengths of neurites, cannot be correctly applied among those samples. But the fluorescence micrographs illustrate some significant differences: neurons on HS-9 seemed much healthier and exhibited longer processes as compared to all the other samples. Also, since hydrogels provided bulky 3-D scaffolds as compared to thin monolayers of polymers coated on a substrate to encourage cell adhesion, it is difficult to directly compare the results in this work to standard neuron culture substrates, such as poly-lysine or laminin-coated glass coverslips. However, soluble polymer can easily be prepared using similar methods reported in this work without adding crosslinking EGDMA. Since MAETAC is permanently charged, which enhances the electrostatic interaction with glass surfaces, the soluble polymer can be used to coat glass coverslips simply by dipping method. Comparing hippocampal neuronal cell viability and morphologies on different coated substrates will be an area of interest for future work.
5. Conclusion
Systematic changes in hydrogel monomer composition provide a simple and valuable means for discovering bioactive materials. The hydrogels containing tethered acetylcholine-like functional structures described in this study represent promising clues for the future design of candidate scaffolds for neural tissue engineering and regenerative medicine applications. The critical concentration range of functional monomer MAETAC in the system was identified for brain neuronal cell culture, and this knowledge is critical to optimize the current system. For example, softer hydrogel samples based on this system can be easily prepared for the application of brain implantation or cell encapsulation, and linear, soluble polymers can be prepared with the same components for substrate coating and in vitro cell culture studies. Since sample HS-7 favored astrocytic attachment and growth while HS-9 and HS-10 favored neuron attachment and growth specifically, selectively culturing astrocytes or neurons is also possible by properly modifying physical and chemical properties of these types of hydrogels. Furthermore, because of the rich chemistry of the free hydroxyl groups present in the PEGMA monomer, those synthetic hydrogels can be readily modified with other functionalities, such as by linking nerve growth factor, covalently bonding extracellular matrix (ECM) proteins or peptides, etc., for applications such as tissue engineering, which is also a focus of future work.
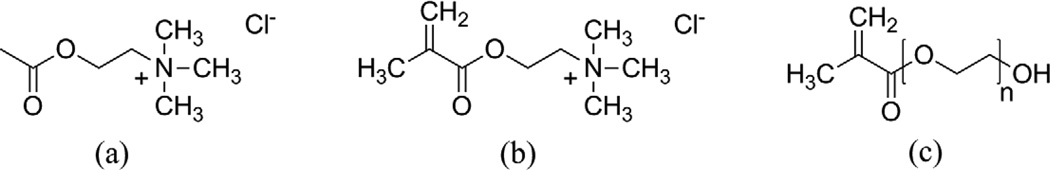
Fig. 1.
Chemical structures of (a) neurotransmitter acetylcholine chloride (2-acetoxy-N,N,N-trimethylethanaminium), monomers (b) ([2-(methacryloyloxy) ethyl]-trimethylammonium chloride (MAETAC) and (c) Polyethylene glycol methacrylate (PEGMA). (a) and (b) share common functional group except (b) contains a methyl-vinyl group which can be used for photopolymerization reaction.
Acknowledgments
We would like to acknowledge Dr. Ray Molloy and Professor Manfred Lindau for the initial cell studies. We would also like to acknowledge the use of the Microscopy and Imaging Facility at Cornell University and its facility manager, Carol Bayles, and the use of the electron microscope and the dynamic mechanical analyzer housed in the Cornell Center for Materials Research (CCMR), Shared Experimental Facilities, supported through the National Science Foundation Materials Research Science and Engineering Center Program (DMR-0520404) and the facility managers, John Hunt and Yuanming Zhang. We also acknowledge the assistance of the NHLBI DIR Light Microscopy Core Facility. This work was partially supported by NIH NSR01-044287 and by the Nanobiotechnology Centre (NBTC), an STC program of the National Science Foundation under agreement no. ECS-9876771, as well as the NHLBI Division of Intramural Research (P.Y. and H.M.G).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Nisbet DR, Crompton KE, Horne MK, Finkelstein DI, Forsythe JS. Neural tissue engineering of the CNS using hydrogels - a review. J Biomed Mater Res Part B Appl Biomater. 2008;87:251–263. doi: 10.1002/jbm.b.31000. [DOI] [PubMed] [Google Scholar]
- 2.Constans A. Neural tissue engineering. Scientist. 2004;18:40–42. [Google Scholar]
- 3.Schmidt CE, Leach JB. Neural tissue engineering: strategies for repair and regeneration. Annu Rev Biomed Eng. 2003;5:293–347. doi: 10.1146/annurev.bioeng.5.011303.120731. [DOI] [PubMed] [Google Scholar]
- 4.Subramanian A, Krishnan UM, Sethuraman S. Development of biomaterial scaffold for nerve tissue engineering: biomaterial mediated neural regeneration. J Biomed Sci. 2009;16:108–119. doi: 10.1186/1423-0127-16-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Woerly S, Plant GW, Harvey AR. Neural tissue engineering: from polymer to biohybrid organs. Biomaterials. 1996;17:301–310. doi: 10.1016/0142-9612(96)85568-2. [DOI] [PubMed] [Google Scholar]
- 6.Peppas NA, Sahlin JJ. Hydrogels as mucoadhesive and bioadhesive materials: a review. Biomaterials. 1996;17:1553–1561. doi: 10.1016/0142-9612(95)00307-x. [DOI] [PubMed] [Google Scholar]
- 7.Hejcl A, Lesny P, Pradny M, Michalek J, Jendelova P, Stulik J, et al. Biocompatible hydrogels in spinal cord injury repair. Physiol Res. 2008;57:S121–S132. doi: 10.33549/physiolres.931606. [DOI] [PubMed] [Google Scholar]
- 8.Zhong YH, Bellamkonda RV. Biomaterials for the central nervous system. J R Soc Interface. 2008;5:957–975. doi: 10.1098/rsif.2008.0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yu TT, Shoichet MS. Guided cell adhesion and outgrowth in peptide-modified channels for neural tissue engineering. Biomaterials. 2005;26:1507–1514. doi: 10.1016/j.biomaterials.2004.05.012. [DOI] [PubMed] [Google Scholar]
- 10.Geckil H, Xu F, Zhang XH, Moon S, Demirci U. Engineering hydrogels as extracellular matrix mimics. Nanomedicine. 2010;5:469–484. doi: 10.2217/nnm.10.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhu JM. Bioactive modification of poly(ethylene glycol) hydrogels for tissue engineering. Biomaterials. 2010;31:4639–4656. doi: 10.1016/j.biomaterials.2010.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yu LMY, Leipzig ND, Shoichet MS. Promoting neuron adhesion and growth. Mater Today. 2008;11:36–43. [Google Scholar]
- 13.Snyder SH. Turning off neurotransmitters. Cell. 2006;125:13–15. doi: 10.1016/j.cell.2006.03.023. [DOI] [PubMed] [Google Scholar]
- 14.Stevens CF. Neurotransmitter release at central synapses. Neuron. 2003;40:381–388. doi: 10.1016/s0896-6273(03)00643-3. [DOI] [PubMed] [Google Scholar]
- 15.Gao J, Kim YM, Coe H, Zern B, Sheppard B, Wang YD. A neuroinductive biomaterial based on dopamine. Proc Natl Acad Sci USA. 2006;103:16681–16686. doi: 10.1073/pnas.0606237103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saifuddin U, Vu TQ, Rezac M, Qian HH, Pepperberg DR, Desai TA. Assembly and characterization neurotransmitter-immobilized of biofunctional surfaces for interaction with postsynaptic membrane receptors. J Biomed Mater Res A. 2003;66A:184–191. doi: 10.1002/jbm.a.10552. [DOI] [PubMed] [Google Scholar]
- 17.Vu TQ, Chowdhury S, Muni NJ, Qian HH, Standaert RF, Pepperberg DR. Activation of membrane receptors by a neurotransmitter conjugate designed for surface attachment. Biomaterials. 2005;26:1895–1903. doi: 10.1016/j.biomaterials.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 18.Darr A, Calabro A. Synthesis and characterization of tyramine-based hyaluronan hydrogels. J Mater Sci Mater Med. 2009;20:33–44. doi: 10.1007/s10856-008-3540-0. [DOI] [PubMed] [Google Scholar]
- 19.Bernardini N, Tomassy GS, Tata AM, Augusti-Tocco G, Biagioni S. Detection of basal and potassium-evoked acetylcholine release from embryonic DRG explants. J Neurochem. 2004;88:1533–1539. doi: 10.1046/j.1471-4159.2003.02292.x. [DOI] [PubMed] [Google Scholar]
- 20.Whitteridge D. The role of acetylcholine in synaptic transmission - a critical review. J of Neurol Neurosurg Psychiatry. 1948;11:134–140. doi: 10.1136/jnnp.11.2.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lecchi M, McIntosh JM, Bertrand S, Safran AB, Bertrand D. Functional properties of neuronal nicotinic acetylcholine receptors in the chick retina during development. Eur J Neurosci. 2005;21:3182–3188. doi: 10.1111/j.1460-9568.2005.04150.x. [DOI] [PubMed] [Google Scholar]
- 22.Small DH, Reed G, Whitefield B, Nurcombe V. Cholinergic regulation of neurite outgrowth from isolated chick sympathetic neurons in culture. J Neurosci. 1995;15:144–151. doi: 10.1523/JNEUROSCI.15-01-00144.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tata AM, Cursi S, Biagioni S, Augusti-Tocco G. Cholinergic modulation of neurofilament expression and neurite outgrowth in chick sensory neurons. J Neurosci Res. 2003;73:227–234. doi: 10.1002/jnr.10650. [DOI] [PubMed] [Google Scholar]
- 24.Kim SJ, Park SJ, Kim SI. Swelling behavior of interpenetrating polymer network hydrogels composed of poly(vinyl alcohol) and chitosan. React Funct Polym. 2003;55:53–59. [Google Scholar]
- 25.Park H, Guo X, Temenoff JS, Tabata Y, Caplan AI, Kasper FK, et al. Effect of swelling ratio of injectable hydrogel composites on chondrogenic differentiation of encapsulated rabbit marrow mesenchymal stem cells in vitro. Biomacromolecules. 2009;10:541–546. doi: 10.1021/bm801197m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Woerly S, Fort S, Pignot-Paintrand I, Cottet C, Carcenac C, Savasta M. Development of a sialic acid-containing hydrogel of poly[N-(2-hydroxypropyl) methacrylamide]: Characterization and implantation study. Biomacromolecules. 2008;9:2329–2337. doi: 10.1021/bm800234r. [DOI] [PubMed] [Google Scholar]
- 27.Brewer GJ, Torricelli JR, Evege EK, Price PJ. Optimized survival of hippocampal-neurons in B27-supplemented neurobasal, a new serum-free medium combination. J Neurosci Res. 1993;35:567–576. doi: 10.1002/jnr.490350513. [DOI] [PubMed] [Google Scholar]
- 28.Zohuriaan-Mehr MJ, Kabiri K. Superabsorbent polymer materials: a review. Iran Polym J. 2008;17:451–477. [Google Scholar]
- 29.Dadhaniya PV, Patel MP, Patel RG. Swelling and dye adsorption study of novel superswelling [acrylamide/N-vinylpyrrolidone/3(2-hydroxyethyl carbamoyl) acrylic acid] hydrogels. Polym Bull. 2006;57:21–31. [Google Scholar]
- 30.Kioussis DR, Kofinas P. Characterization of anion diffusion in polymer hydrogels used for wastewater remediation. Polymer. 2005;46:9342–9347. [Google Scholar]
- 31.Guarnieri D, De Capua A, Ventre M, Borzacchiello A, Pedone C, Marasco D, et al. Covalent immobilized RGD gradient on PEG hydrogel scaffold influences cell migration parameters. Acta Biomater. 2010;6:2532–2539. doi: 10.1016/j.actbio.2009.12.050. [DOI] [PubMed] [Google Scholar]
- 32.Saha K, Keung AJ, Irwin EF, Li Y, Little L, Schaffer DV, et al. Substrate modulus directs neural stem cell behavior. Biophys J. 2008;95:4426–4438. doi: 10.1529/biophysj.108.132217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Banerjee A, Arha M, Choudhary S, Ashton RS, Bhatia SR, Schaffer DV, et al. The influence of hydrogel modulus on the proliferation and differentiation of encapsulated neural stem cells. Biomaterials. 2009;30:4695–4699. doi: 10.1016/j.biomaterials.2009.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leipzig ND, Shoichet MS. The effect of substrate stiffness on adult neural stem cell behavior. Biomaterials. 2009;30:6867–6878. doi: 10.1016/j.biomaterials.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 35.Sosnik A, Sefton MV. Poloxamine hydrogels with a quaternary ammonium modification to improve cell attachment. J Biomed Mater Res A. 2005;75A:295–307. doi: 10.1002/jbm.a.30419. [DOI] [PubMed] [Google Scholar]
- 36.Tu Q, Li L, Zhang YR, Wang JC, Liu R, Li ML, et al. The effect of acetylcholine-like biomimetic polymers on neuronal growth. Biomaterials. 2011;32:3253–3264. doi: 10.1016/j.biomaterials.2011.01.044. [DOI] [PubMed] [Google Scholar]
- 37.Gumera CB, Wang Y. Modulating neuronal responses by controlled integration of acetylcholine-like functionalities in biomimetic polymers. Adv Mater. 2007;19:4404–4409. [Google Scholar]