Obstructive sleep apnea is a disorder characterized by recurrent episodes of pharyngeal obstruction during sleep (1), leading to repeated oxyhemoglobin desaturations and arousals from sleep. These acute physiologic perturbations can be characterized by polysomnographic metrics that quantify the degree of oxyhemoglobin desaturation and sleep fragmentation including the oxygen desaturation index, time spent with an SaO2 below 90%, respiratory disturbance indices (apnea and hypopnea indices) in non–rapid eye movement (non-REM) and REM sleep, and alterations in sleep architecture (arousal frequencies, sleep stage distribution, and transition indices). Increasing degrees of sleep fragmentation and oxyhemoglobin desaturation likely increase the patient's risk of developing clinical sequelae including neurocognitive dysfunction, metabolic dysregulation, and cardiovascular morbidity and mortality (2–5). Nevertheless, these metrics of sleep apnea severity provide little information about the underlying pathogenic mechanisms of this disorder.
Obstructive sleep apnea is due to increases in pharyngeal collapsibility during sleep (6). These increases can result from upper airway anatomic defects (7) and/or disturbances in neuromuscular control (6, 8). Structural defects have been linked to boney and soft tissue abnormalities, which load the pharynx and predispose to airflow obstruction during sleep. These defects are best summarized by measurements of pharyngeal critical closing pressures under conditions of absent or markedly reduced neuromotor tone (passive Pcrit) (9). Elevations in passive Pcrit have been associated with sleep apnea risk factors including obesity, central adiposity, male sex, and age (10, 11), suggesting its role as a physiologic mediator of sleep apnea susceptibility. As passive Pcrit increases, dynamic collapse of the pharynx limits inspiratory airflow to a maximal level (Vimax) as airway pressure downstream to the pharynx falls below Pcrit. Mechanical loads on the pharynx predispose to inspiratory airflow limitation, during which Vimax characterizes the severity of airflow obstruction during sleep (Figure 1) (12).
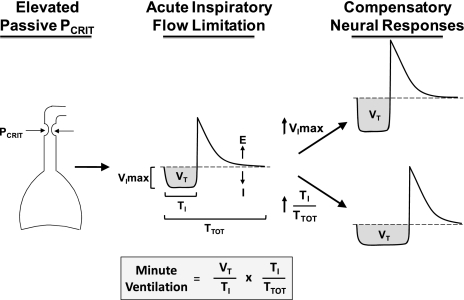
Figure 1.
Schematic illustrating intermediate physiologic phenotypes for sleep apnea pathogenesis. Pharyngeal mechanical loads elevate passive Pcrit (left panel). Dynamic collapse of the airway leads to the development of inspiratory airflow limitation (middle panel), as characterized by a plateauing of the inspiratory airflow waveform (I = inspiration, E = expiration) at a maximal level (Vimax) as effort increases (not shown). Compensatory neural responses (right panel) mitigate the fall in ventilation by increasing Vimax (upper right panel) or inspiratory duty cycle (lower right panel). Compensatory increases in mean inspiratory airflow (Vt/Ti) and inspiratory duty cycle (Ti/Ttot) restore minute ventilation, as described in the equation, where Vt is tidal volume, Ti is inspiratory time, and Ttot is the respiratory cycle period. E, expiration; I, inspiration.
Inspiratory airflow limitation can elicit neural responses that preserve ventilation during sleep (Figure 1) (8, 13, 14). Activation of pharyngeal muscles can decrease airway collapsibility (active Pcrit), which decreases the severity of airflow obstruction (see increased Vimax, Figure 1, top right) (6, 8, 15). Alternatively, individuals can compensate for inspiratory flow limitation by increasing the inspiratory duty cycle, as defined by the inspiratory time (Ti) over total respiratory period (Ttot) (see increased Ti/Ttot, Figure 1, lower right) (8, 16). At any given level of Vimax, lengthening the inspiratory duty cycle is the only means available to preserve ventilation when the pharynx flow-limits during sleep (17). As the inspiratory duty cycle increases, minute ventilation will increase accordingly, irrespective of respiratory rate and tidal volume (Figure 1, lower right) (17). Thus, neural responses can either mitigate or compensate for pharyngeal obstruction (flow limitation), helping to maintain ventilation during sleep.
Severe airflow obstruction can overwhelm these neural responses. What happens when these responses fail to protect the individual from developing obstructive sleep apnea? In the current issue of the Journal, Jordan and coworkers (pp. 1183) address this question by inducing airflow obstruction and monitoring the response (18). Airflow obstruction triggered an overshoot in ventilation with and without frank arousals from sleep. These investigators examined whether arousals further destabilized ventilation during sleep. Compared with trials that did not precipitate arousals, those with arousals were associated with greater degrees of ventilatory overshoot and a greater frequency of obstructive hypopneas. Of note, ventilatory instability ensued after arousals, regardless of the severity of airflow obstruction or associated decreases in pharyngeal muscle activity. These findings suggest that when neuromuscular responses fail to compensate for upper airway obstruction during sleep, arousals contribute to the development of respiratory instability and help to propagate recurrent obstructive apneas and hypopneas.
Jordan's study suggests that ventilatory responses to arousals constitute an independent physiologic phenotype of sleep apnea susceptibility. If so, we should extend our ability to characterize physiologic phenotypes in every sleep recording. If suitable technology were adopted to quantify airflow accurately, ventilatory metrics including Vimax, timing indices, and minute ventilation could be determined during sleep, respiratory-triggered arousals, and sleep-wake transitions. Moreover, robust physiologic markers of sleep-wake stability could be used to quantify effects of arousals on ventilatory stability. Current evidence suggests that distinct neural mechanisms govern sleep-wake stability (19). Sleep-disordered breathing episodes and sleep-wake propensities can interact, triggering alterations in sleep architecture, arousals, sleep-wake transitions, and α intrusion during sleep (20). Much work remains to discern which metrics predict ventilatory and sleep-wake instability once compensatory neural responses fail to stabilize ventilation during sleep.
Footnotes
Supported by NIH HL50381 and HL37379.
Author Disclosure: J.P.K.'s institution has received grants from ResMed for duties not related to editorial tasks; he has also received consultancy fees from Respicardia for duties not related to editorial tasks; his institution has received lecture fees on airflow phenotypes of OSA from Case Western University and University of Pittsburgh Medical Center. B.M.M. has received consultancy fees from Sleep Sciences. S.P. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. H.S. has received scientific advisory consultancy fees from Apnex Medical, inSleep, and Fisher and Paykel. A.R.S. has received scientific advisory consultancy fees from Apnex Medical, inSleep, and Respicardia; he and his institution have a patent pending for a disposable sleep and breathing monitor; he has stock options in Sova Pharmaceuticals. P.L.S. has received scientific advisory consultancy fees from Apnex Medical and inSleep; he and his institution have a patent pending for a disposable sleep and breathing monitor.
References
- 1.Remmers JE, deGroot WJ, Sauerland EK, Anch AM. Pathogenesis of upper airway occlusion during sleep. J Appl Physiol 1978;44:931–938 [DOI] [PubMed] [Google Scholar]
- 2.Punjabi NM, Caffo BS, Goodwin JL, Gottlieb DJ, Newman AB, O'Connor GT, Rapoport DM, Redline S, Resnick HE, Robbins JA, et al. Sleep-disordered breathing and mortality: a prospective cohort study. PLoS Med 2009;6:e1000132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: An observational study. Lancet 2005;365:1046–1053 [DOI] [PubMed] [Google Scholar]
- 4.Young T, Finn L, Peppard PE, Szklo-Coxe M, Austin D, Nieto FJ, Stubbs R, Hla KM. Sleep disordered breathing and mortality: eighteen-year follow-up of the Wisconsin sleep cohort. Sleep 2008;31:1071–1078 [PMC free article] [PubMed] [Google Scholar]
- 5.Marshall NS, Wong KK, Liu PY, Cullen SR, Knuiman MW, Grunstein RR. Sleep apnea as an independent risk factor for all-cause mortality: the Busselton Health Study. Sleep 2008;31:1079–1085 [PMC free article] [PubMed] [Google Scholar]
- 6.Patil SP, Schneider H, Marx JJ, Gladmon E, Schwartz AR, Smith PL. Neuromechanical control of upper airway patency during sleep. J Appl Physiol 2007;102:547–556 [DOI] [PubMed] [Google Scholar]
- 7.Isono S, Remmers JE, Tanaka A, Sho Y, Sato J, Nishino T. Anatomy of the pharynx in patients with obstructive sleep apnea and in normal subjects. J Appl Physiol 1997;82:1319–1326 [DOI] [PubMed] [Google Scholar]
- 8.McGinley BM, Schwartz AR, Schneider H, Kirkness JP, Smith PL, Patil SP. Upper airway neuromuscular compensation during sleep is defective in obstructive sleep apnea. J Appl Physiol 2008;105:197–205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Patil SP, Punjabi NM, Schneider H, O'Donnell CP, Smith PL, Schwartz AR. A simplified method for measuring critical pressures during sleep in the clinical setting. Am J Respir Crit Care Med 2004;170:86–93 [DOI] [PubMed] [Google Scholar]
- 10.Kirkness JP, Schwartz AR, Schneider H, Punjabi NM, Maly JJ, Laffan AM, McGinley BM, Magnuson T, Schweitzer M, Smith PL, et al. Contribution of male sex, age, and obesity to mechanical instability of the upper airway during sleep. J Appl Physiol 2008;104:1618–1624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kirkness JP, McGinley BM, Sgambati FP, Patil SP, Schwartz AR, Smith PL. Developing quantitative physiological phenotypes of sleep apnea for epidemiological studies. Conf Proc IEEE Eng Med Biol Soc 2011;1:8319–8322 [DOI] [PubMed] [Google Scholar]
- 12.Kirkness JP, Verma M, McGinley BM, Erlacher M, Schwartz AR, Smith PL, Wheatley JR, Patil SP, Amis TC, Schneider H. Pitot-tube flowmeter for quantification of airflow during sleep. Physiol Meas 2011;32:223–237 [DOI] [PubMed] [Google Scholar]
- 13.Kirkness JP, Schwartz AR, Patil SP, Pichard LE, Marx JJ, Smith PL, Schneider H. Dynamic modulation of upper airway function during sleep: a novel single breath method. J Appl Physiol 2006;101:1489–1494 [DOI] [PubMed] [Google Scholar]
- 14.Schneider H, Boudewyns A, Smith PL, O'Donnell CP, Canisius S, Stammnitz A, Allan L, Schwartz AR. Modulation of upper airway collapsibility during sleep: influence of respiratory phase and flow regimen. J Appl Physiol 2002;93:1365–1376 [DOI] [PubMed] [Google Scholar]
- 15.Jordan AS, Wellman A, Heinzer RC, Lo YL, Schory K, Dover L, Gautam S, Malhotra A, White DP. Mechanisms used to restore ventilation after partial upper airway collapse during sleep in humans. Thorax 2007;62:861–867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schneider H, Krishnan V, Pichard LE, Patil SP, Smith PL, Schwartz AR. Inspiratory duty cycle responses to flow limitation predict nocturnal hypoventilation. Eur Respir J 2009;33:1068–1076 [DOI] [PubMed] [Google Scholar]
- 17.Tagaito Y, Schneider H, O'Donnell CP, Smith PL, Schwartz AR. Ventilating with tracheal gas insufflation and periodic tracheal occlusion during sleep and wakefulness. Chest 2002;122:1742–1750 [DOI] [PubMed] [Google Scholar]
- 18.Jordan A, Eckert DJ, Wellman A, Trinder J, Malhotra A, White DP. Termination of respiratory events with and without cortical arousal in obstructive sleep apnea. Am J Respir Crit Care Med 2011;184:1183–1191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lo CC, Chou T, Penzel T, Scammell TE, Strecker RE, Stanley HE, Ivanov PC. Common scale-invariant patterns of sleep-wake transitions across mammalian species. Proc Natl Acad Sci USA 2004;101:17545–17548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Laffan A, Caffo B, Swihart BJ, Punjabi NM. Utility of sleep stage transitions in assessing sleep continuity. Sleep 2010;33:1681–1686 [DOI] [PMC free article] [PubMed] [Google Scholar]