Abstract
Rationale: A total of 20–30% of respiratory events in obstructive sleep apnea are terminated without clear arousal. Arousals are thought to predispose to further events by promoting hyperventilation, hypocapnia, and upper-airway dilator muscle hypotonia. Therefore, events terminated without arousal may promote stable breathing.
Objectives: To compare physiologic changes at respiratory event termination with American Sleep Disorders Association (ASDA) Arousal to No Arousal, and determine whether secondary respiratory events are less common and have higher dilator muscle activity after No Arousal compared with ASDA Arousal.
Methods: Patients with obstructive sleep apnea wore sleep staging, genioglossus (EMGGG), and tensor palatini (EMGTP) electrodes plus a nasal mask and pneumotachograph. During stable sleep, continuous positive airway pressure (CPAP) was lowered for 3-minute periods to induce respiratory events. Physiologic variables were compared between events terminated with (1) ASDA Arousal, (2) No Arousal, or (3) sudden CPAP increase (CPAPinc, control).
Measurements and Main Results: Sixteen subjects had adequate data. EMGGG, EMGTP, and heart rate increased after ASDA Arousal (340 ± 57%, 215 ± 28%, and 110.7 ± 2.3%) and No Arousal (185 ± 32%, 167 ± 15%, and 108.5 ± 1.6%) but not CPAPinc (90 ± 10%, 94 ± 11%, and 102.1 ± 1%). Ventilation increased more after ASDA Arousal than No Arousal and CPAPinc, but not after accounting for the severity of respiratory event. Fewer No Arousals were followed by secondary events than ASDA Arousals. However, low dilator muscle activity did not occur after ASDA Arousal or No Arousal (EMGGG rose from 75 ± 5 to 125 ± 7%) and secondary events were less severe than initial events (ventilation rose 4 ± 0.4 to 5.5 ± 0.51 L/min).
Conclusions: Respiratory events that were terminated with ASDA Arousal were more severely flow-limited, had enhanced hyperventilation after event termination, and were more often followed by secondary events than No arousal. However, secondary events were not associated with low dilator muscle activity and airflow was improved after both No Arousal and ASDA Arousal.
Keywords: pharyngeal muscle activity, upper airway obstruction, genioglossus, tensor palatini, obstructive respiratory event
At a Glance Commentary
Scientific Knowledge on the Subject
Arousals from sleep have been suggested to predispose to further obstruction in obstructive sleep apnea (OSA) by promoting hyperventilation and upper-airway dilator muscle hypotonia, although the latter effect has not been directly tested. A proportion of respiratory events in OSA end without cortical arousal, potentially preventing subsequent dilator muscle hypotonia and leading to stable breathing.
What This Study Adds to the Field
This study demonstrates that low dilator muscle activity does not occur after termination of obstructive respiratory events either with or without cortical arousal. Rather, airflow is improved after both events. These findings raise doubt as to whether arousals predispose to further obstruction and challenge current thinking regarding the role of arousal in OSA pathogenesis.
Approximately 20–30% of respiratory events in obstructive sleep apnea (OSA) do not end with clear arousal from sleep (1–4). Whether subcriterion arousals are present or not has been debated in the literature and is not yet resolved (1, 2, 4, 5). However, the presence of cortical arousal results in enhanced hyperventilation on termination of respiratory events (4). This greater hyperventilation is expected to result in hypocapnia and dilator muscle hypotonia on the return to sleep (because dilator muscle activity, and presumably muscle tone, varies proportionately with respiratory drive), predisposing to further airway obstruction. However, whether dilator muscle activity is reduced in this setting has not been assessed.
The concept that arousal-induced hyperventilation and hypocapnia results in reduced dilator muscle tone and increased airway resistance or obstruction is based on several studies that induced hypocapnia during stable sleep with hypoxia or mechanical hyperventilation (6–9). In these studies, the individuals who had minimal increases in airway resistance during sleep had little further increase in resistance during hypocapnia. However, subjects who snored or were flow limited at baseline developed obstructive apneas and hypopneas with hypocapnia. Therefore, arousal-induced hyperventilation with subsequent hypocapnia is thought to predispose to upper airway collapse in people with upper airways susceptible to collapse.
Despite these studies conducted during stable sleep, the evidence supporting the role of arousal-induced hyperventilation leading to airway collapse is lacking. Of five separate studies that have investigated the breath-by-breath ventilatory responses to auditory-induced arousals in healthy subjects (10–14), not one found a period of significant hypoventilation (ventilation reduced >10% below baseline) after the initial hyperventilatory response, despite some subjects having flow limitation during stable sleep. In addition, airway resistance (10, 11) was reduced and dilator muscle activity increased (10, 14) for approximately 20–30 seconds after the brief arousal in these healthy individuals. In addition, three studies have induced auditory arousals in patients with OSA (while on subtherapeutic or therapeutic continuous positive airway pressure [CPAP]) and also found reduced airway resistance (15, 16) and increased dilator muscle activity of the genioglossus (GG) and tensor palatini (TP) muscles (17) during subsequent sleep. Finally, after 740 arousals in patients with OSA only seven subsequent obstructive respiratory events were observed (16). Thus, the evidence that auditory arousal-induced hyperventilation predisposes to airway collapse is somewhat lacking.
Auditory-induced arousals may differ from arousals that occur at the termination of respiratory events. Therefore, we wished to determine whether the physiologic changes (airflow, muscle activity, and airway resistance) that occur at the spontaneous termination of respiratory events differ when a cortical arousal is or is not present. In addition, we wished to determine whether respiratory events that are terminated with cortical arousal are more likely to be followed by worsened airway function (reduced dilator muscle activity and airflow) and a secondary respiratory event. We hypothesized that respiratory events terminated without cortical arousal (no American Sleep Disorders Association [ASDA] arousal) would be associated with less hyperventilation, higher residual dilator muscle activity, and fewer subsequent respiratory events than events terminated with an ASDA arousal. Some of the results of these studies have been previously reported in the form of abstracts (18, 19).
Methods
Detailed methodologic procedures are reported in the online supplement.
Subjects
Twenty CPAP-treated patients with OSA, aged 20–65 years, gave informed written consent to participate in the study. The study was approved by the Institutional Review Board of the Brigham and Women's Hospital, Boston, Massachusetts.
Measurements and Instrumentation
Subjects arrived at the laboratory 2 hours before their usual bedtime and were instrumented with EEG, electro-oculogram, submental-EMG, and ECG surface electrodes. A pressure-tipped catheter was then inserted through one decongested and anesthetized nostril until the catheter tip was located near the epiglottis. Fine wire EMG electrodes were inserted into the GG and TP muscles after surface anesthesia for bipolar recordings and a nasal mask with pneumotachograph were applied. All signals were recorded on a Spike 2 data acquisition system (1401plus; CED, Cambridge, United Kingdom). Genioglossus and TP EMGs were recorded at 1,000 Hz; EEG, electro-oculogram, and submental-EMG at 250 Hz; and all other signals at 125 Hz.
Protocol
Subjects were placed on their prescribed CPAP and were allowed to fall asleep in the supine position. Once stable non-REM sleep was achieved, the CPAP was suddenly reduced for 3 minutes unless a full awakening (>15s α) occurred, in which case the pressure was returned to the prescribed level until stable sleep resumed. The CPAP reduction was adjusted such that a range of severities of respiratory events were induced. At least 3 minutes of sleep on the prescribed CPAP level separated pressure drops. CPAP drops continued until 20–40 drops had been performed.
Data and Statistical Analysis
A trained sleep technician, blinded to the study hypotheses, staged the sleep and marked arousals according to the Rechtschaffen and Kales (20) and ASDA criteria (21). Custom written software was used to extract the physiologic information on each breath before, during, and after every CPAP drop in non-REM sleep. The specific variables extracted included breath timing, inspired minute ventilation (VI), tidal volume (VT), mean (VT/TI) and peak inspiratory flow (PIF), mean SaO2, inspiratory epiglottic pressure nadir (PEPI), plus peak and tonic EMG activity of the GG (EMGGG) and TP (EMGTP) muscles.
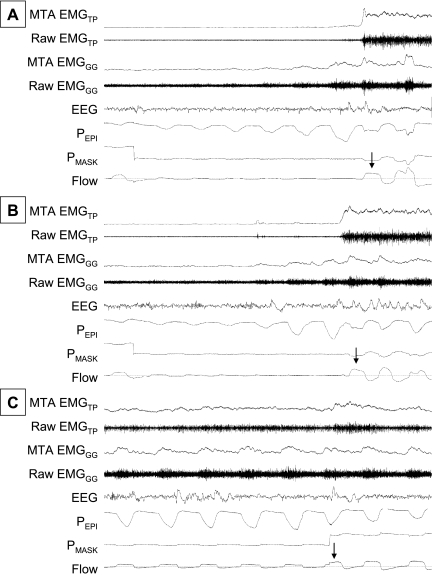
Respiratory events were defined as a period of flow limitation that ended with a sudden increase in airflow. Objective criteria were used to identify the breath on which a respiratory event was terminated. Specifically, the PIF on the breath that terminated the event was at least 50% larger than the breath immediately prior and at least 80% of the level observed on fully therapeutic CPAP (shown by arrows in Figure 1). Each event termination was then allocated to one of three types as (1) ASDA Arousal, (2) No ASDA Arousal, or (3) CPAP Increase according to the following criteria. If CPAP was constant and an ASDA arousal was scored within one respiratory cycle of the event termination, then the event was considered to be terminated with an ASDA arousal. If CPAP was constant but there was no scored arousal for at least two breaths after event termination, the event was designated a No ASDA Arousal type. Finally, if no arousal was scored for two breaths, but the increase in airflow occurred when CPAP was increased, then it was designated a CPAP Increase event. CPAP Increase events were examined as a control because of the ongoing debate regarding whether respiratory events that are terminated without clear cortical arousal actually do or do not have subtle arousals present. For this same reason, heart rate changes at the termination of events were also assessed. Examples of each of the event termination types are shown in Figure 1. Respiratory and EMG data analyses were masked because they were calculated by computer software before event termination type was determined.
Figure 1.
Examples of the three respiratory event termination types in one subject. Airflow, mask pressure (PMASK), epiglottic pressure (PEPI), central EEG, the raw and moving time averages (MTA) genioglossus (EMGGG), and tensor palatini (EMGTP) muscle activities are shown after sudden termination of respiratory events in three conditions: in association with an American Sleep Disorders Association arousal (A), without American Sleep Disorders Association arousal (B), and at the time of sudden continuous positive airway pressure increase (C). The arrows on each panel indicate the breath on which the respiratory event was considered to end.
Physiologic data were averaged for each event type for five breaths (or 20 beats for heart rate) before and after the termination of respiratory events as long as CPAP remained low (in ASDA Arousal and No Arousal events) and the subject remained asleep (for No ASDA Arousal and CPAP Increase trials). Because of the marked variability in baseline heart rate, genioglossus, and TP muscle activity, each variable was expressed as a percent of that subject's mean across all breaths and conditions, such that baseline differences in heart rate and muscle activity were not obscured. Mean tonic and phasic activities of each muscle were calculated separately. Cardiovascular and respiratory data were compared between event termination types with a three- way, repeated measures analysis of variance (ANOVA). Student t tests were used for post hoc analyses where necessary.
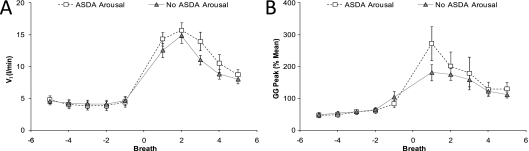
Secondary respiratory events were considered to occur when a second period of flow limitation that was terminated by a sudden increase in airflow (same criteria for initial events) occurred during the 3 minutes of reduced CPAP. The proportion of ASDA Arousals and No Arousals that were followed by a second respiratory event were compared with a paired Student t test. Furthermore, the physiologic characteristics of secondary respiratory events were compared with initial events by comparing the last two to three breaths during the initial respiratory event to the two to three most flow-limited breaths during secondary respiratory events (Figure 4) using two-way repeated measures ANOVA.
Figure 4.
Physiologic changes after respiratory event termination with and without American Sleep Disorders Association (ASDA) arousal from sleep in events matched for severity and duration of hypoventilation. Ventilatory (VI) (A) and peak inspiratory genioglossus activity (Peak GG) (B) did not differ between ASDA Arousal and No ASDA Arousal events when the severity and duration of hypopneas were matched between arousal types. See results for details regarding matching and online supplement for results of other physiologic variables.
Results
Sixteen subjects had adequate data for analysis. Reasons for incomplete data are provided in the online supplement. The anthropometric data for included subjects are shown in Table 1. In addition, ECG analyses were excluded in two subjects, one of whom had a wandering atrial pacemaker and the other who had frequent premature ventricular contractions. The intramuscular TP EMG electrodes became dislodged at some point during the night in three subjects. Because the exact time of dislodgment is unknown, TP data were discarded for the entire night in these subjects, leaving 13 subjects for TP analyses.
TABLE 1.
PATIENT CHARACTERISTICS
| Characteristic | Mean ± SEM | Range |
| Age, yr | 49 ± 3 | 20–60 |
| Sex, M:F | 11:5 | |
| Body mass index, kg/m2 | 35.1 ± 1.5 | 26.2–45.9 |
| AHI total, events/hr | 47.3 ± 6.2 | 11.1–92.4 |
| AHI non-REM, events/hr | 43.4 ± 5.4 | 11.1–87.4 |
Definition of abbreviation: AHI = apnea–hypopnea index.
Physiologic Changes at Respiratory Event Termination
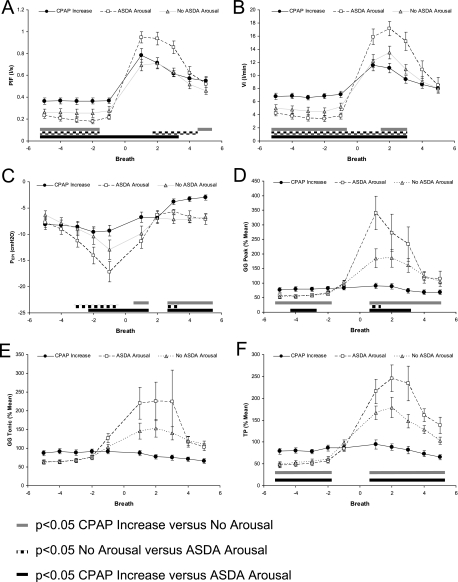
On average there were 9.6 ± 1.4 events terminated with ASDA Arousal, 6.4 ± 1.1 events terminated with No Arousal, and 5.3 ± 0.7 events terminated with CPAP Increase per subject that were suitable for analysis. The CPAP level before event termination did not differ between events terminated with CPAP increase (5.4 ± 0.8 cm H2O); events terminated with No Arousal (5.1 ± 0.8 cm H2O); or events terminated with ASDA Arousal (4.5 ± 0.8 cm H2O). The CPAP level that abolished flow limitation was 10.6 ± 0.8 cm H2O. The physiologic changes in the different event types are shown in Figure 2.
Figure 2.
(A–F) Physiologic changes after respiratory event termination with and without American Sleep Disorders Association (ASDA) arousal from sleep or by sudden continuous positive airway pressure (CPAP) increase. Peak inspiratory flow (PIF), inspired minute ventilation (VI), epiglottic pressure (PEPI), peak inspiratory and tonic genioglossus muscle (GG) activity, and tensor palatini muscle (TP) activity for five breaths before and after respiratory event termination by sudden CPAP increase, or with (ASDA Arousal) or without (No ASDA Arousal) arousal. Analysis of variance results are reported in the text, but post hoc differences are indicated for CPAP increase versus No Arousal by gray bars, differences between No Arousal versus ASDA Arousal by the checked bars, and differences between CPAP increases and ASDA Arousal in the black bars.
PIF (Figure 2A) differed between all three event types on the breaths before event termination (breaths −5 to −2). The magnitude of PIF increase at event termination was greater for ASDA Arousals than for CPAP Increases (breaths −1 to +3) and No Arousals (breaths +1 to +4). PIF did not differ between CPAP Increases and No Arousals until the fifth breath after event termination. The changes in VI (Figure 2B) are similar to that of PIF with significant differences existing among all three groups before event termination (breaths −5 to −1). VI after event termination was higher for ASDA Arousals than both CPAP Increases and No Arousals for the first three breaths (+1 to +3). In addition, VI was higher for No Arousals than CPAP Increases on breaths +2 and +3. Changes in breath timing and arterial oxygen saturation did not differ between ASDA Arousal and No Arousal event types and are presented in the online supplement (see Figure E1).
PEPI (Figure 2C) was more negative before event termination in ASDA Arousals than either No Arousals or CPAP Increases (breaths −2 and −1). After event termination PEPI became less negative with CPAP Increases compared with both ASDA Arousals and No Arousals (breaths +3 to +5). PEPI only differed between ASDA Arousals and No Arousals on the third breath after event termination where it was more negative in No Arousal events.
Before event termination peak inspiratory GG activity was higher on CPAP Increases than either ASDA Arousals or No Arousals (Figure 2D). There was a marked increase in peak inspiratory GG activity after both ASDA Arousals and No Arousals, whereas GG gradually declined after CPAP Increases. Peak inspiratory GG activity only differed between ASDA Arousal and No Arousals on the first breath after event termination (breath +1). Although tonic GG activity (Figure 2E) changes were similar to peak inspiratory GG, the ANOVA interaction effect failed to reach statistical significance. There was an overall main effect with tonic GG activity being lower for CPAP Increases than either ASDA Arousal or No Arousal events.
TP activity (Figure 2F) was higher before the event termination in CPAP increases compared with both ASDA Arousal and No Arousals. However, TP activity rose significantly at event termination in both ASDA Arousal and No Arousals. There were no significant differences in TP activity between ASDA Arousals and No Arousals at any time.
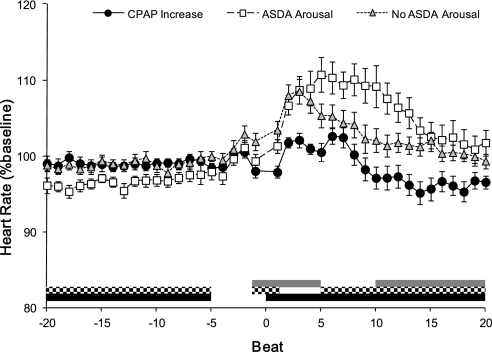
Heart rate (Figure 3) was lower before event termination in ASDA Arousals compared with both CPAP Increases and No Arousals. After event termination, heart rate was lower for CPAP Increases than both ASDA Arousals and No Arousals. Heart rate was higher for No Arousals than ASDA Arousals on the first breath after event termination. However, heart rate did not differ between ASDA Arousals and No Arousals until five heartbeats after the events were terminated. Heart rate remained elevated in ASDA Arousals for longer than No Arousals or CPAP Increases (beats +5 to +20).
Figure 3.
Heart rate changes after respiratory event termination with and without American Sleep Disorders Association (ASDA) arousal from sleep. The heart rate (expressed as a percentage of each subject's mean heart rate across the whole recording period) is shown for 20 beats before and after the termination of respiratory events by sudden continuous positive airway pressure (CPAP) increase, or with (ASDA Arousal) or without (No ASDA Arousal) arousal. Analysis of variance results are reported in the text, but post hoc differences are indicated for CPAP increase versus No Arousal by gray bars, differences between No Arousal versus ASDA Arousal by the checked bars, and differences between CPAP increases and ASDA Arousal in the black bars.
Subanalysis of Events Matched for Severity and Duration
Because the respiratory events that ended in ASDA arousal were more severe (lower ventilation and more negative epiglottic pressures) than those ending with No Arousal, we performed a subanalysis on matched events. In each subject, No Arousal and ASDA Arousal events were matched on the average ventilation during each CPAP drop (within 1 L/min) and the number of breaths during each CPAP drop (within two breaths). Fourteen subjects had data that met these criteria for subanalysis, which is presented in full in the online supplement (see Figure E2). The changes in PIF, VI (Figure 4A), PEPI, GG peak (Figure 4B), and GG Tonic were no longer significantly different between ASDA arousal and No Arousal events (no ANOVA main or interaction effects). TP activity also did not differ over time between arousal types (No ANOVA interaction effect) but was significantly higher after ASDA Arousal than No Arousal (ANOVA main effect for arousal type P = 0.03). This likely occurred as a result of the more prolonged increase in activity after termination of ASDA Arousals (Figure E2).
Secondary Respiratory Events
Twelve subjects had initial respiratory events both with and without ASDA Arousal that were followed by continued reduced CPAP such that secondary respiratory events could be observed. In these subjects, 76.1 ± 9.8% of ASDA Arousals were followed by secondary events, whereas only 50.7 ± 9.9% of events terminated with No Arousal were followed by secondary respiratory events (P = 0.01). Remaining events were followed by stable breathing until CPAP was increased at the end of the trial. Seven of the 12 subjects had secondary respiratory events after 100% of ASDA Arousals. Thus, physiologic variables could not be compared between trials ending in stable versus cyclical breathing. An additional subject had stable breathing after all respiratory events. Thus, physiologic variables were compared between ASDA Arousal and No Arousal events that were followed by secondary respiratory events in 11 subjects.
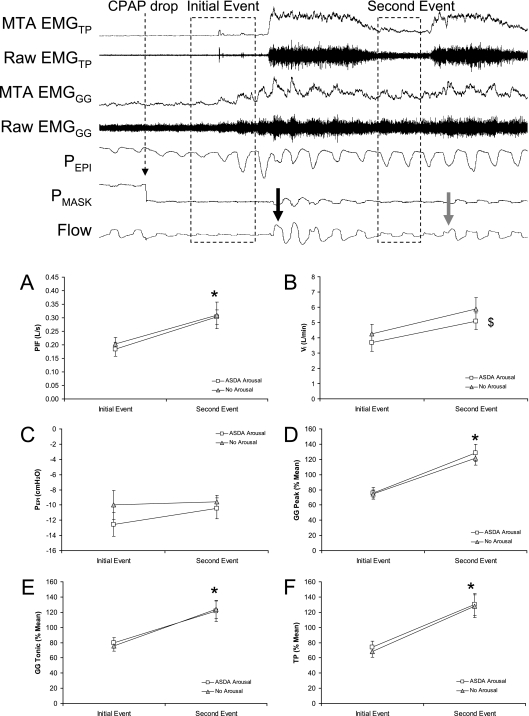
PIF was higher during secondary respiratory events than the initial event (P = 0.035) (Figure 5A). However, the PIF did not differ based on whether or not an Arousal occurred at termination of the event (no significant main or interaction effect for type of arousal on ANOVA). Results were similar for VI except that No Arousals trials had higher minute ventilation than ASDA Arousal trials both on initial and secondary events (ANOVA main effect for type of arousal P = 0.002 but no interaction effect) (Figure 5B). Epiglottic pressure was not different during secondary respiratory events compared with the initial event and no interaction effect existed (Figure 5C). Both Peak and Tonic EMGgg were higher during secondary respiratory events than initial events (Figures 5D and 5E) (ANOVA main effects for event number P = 0.001 and 0.005, respectively). TP muscle activity was also significantly higher on secondary respiratory events than initial events (ANOVA main effect P = 0.001) (Figure 5F). However, neither muscle differed between events terminated with or without arousal (no ANOVA main of interaction effects for event type).
Figure 5.
Physiologic variables during initial and secondary respiratory events that were terminated with or without American Sleep Disorders Association (ASDA) Arousal. The top panel shows an example of the breaths analyzed (dashed boxes) for initial and secondary respiratory events. Airflow (Flow), mask pressure (PMASK), epiglottic pressure (PEPI), raw and moving time averaged (MTA) genioglossus (EMGGG), and tensor palatini (EMGTP) muscle activity during the first two respiratory events after sudden continuous positive airway pressure (CPAP) drop. The large black and gray arrows indicate the breaths on which the initial and secondary respiratory events are terminated, respectively. (A–F) Peak inspiratory flow (PIF), inspired minute ventilation (VI), epiglottic pressure (PEPI), peak inspiratory and tonic genioglossus muscle (GG) activity, and tensor palatini muscle (TP) activity on initial and secondary events that were terminated either with or without ASDA arousal (white squares and gray triangles, respectively). * Significantly different to initial event. $ Indicates a significant analysis of variance main effect for arousal type.
Discussion
Consistent with the Younes paper (4), this study has demonstrated that respiratory events that are terminated with an ASDA Arousal are associated with greater hyperventilation and are more likely to be followed by a secondary respiratory event than events that are terminated without ASDA Arousal. However, we have extended the findings of Younes by assessing dilator muscle activity on both initial and secondary respiratory events. This analysis showed that, despite the greater hyperventilation with ASDA Arousal, the changes in airway dilator muscle activity did not differ compared with No Arousals. Furthermore, regardless of whether or not an ASDA Arousal was present, the secondary respiratory events were less severely flow limited and were associated with greater dilator muscle activity than initial events. This finding does not support the notion that the hyperventilation at the termination of respiratory events is excessive (reduces CO2 below baseline levels and causes reduced dilator muscle activity) and therefore that arousals predispose to further obstructive respiratory events via dilator muscle hypotonia. We propose that the reason why ASDA Arousals were associated with a higher ventilatory response and more commonly led to secondary respiratory events was because the respiratory events that preceded ASDA Arousals were more severe (lower PIF/VI and more negative PEPI) than events terminated without ASDA Arousal. This notion is strongly supported by the results of the matched subanalysis in which physiologic differences between events terminated by ASDA Arousal and No Arousal no longer existed.
Do Arousals Predispose to Further Obstructive Events?
That arousals predispose to central respiratory fluctuations is well supported by modeling (11, 22) and physiologic studies (23–25). This finding likely occurs because the hyperventilation at arousal induces subsequent hypocapnia on return to sleep. When CO2 falls below the apneic threshold a central apnea occurs, or if CO2 is just reduced below the eucapnic level a hypopnea occurs (26). However, there is very little evidence that arousals predispose to further obstructive respiratory events. Hudgel and coworkers (7) studied the activity of upper airway dilator and respiratory pump muscles during hypocapnic hypoxia in healthy subjects during stable sleep and reported that upper airway resistance only increased when the ratio of dilator muscle activity to pump muscle activity fell below a critical level, which was reproducible within a given subject (7). However, more recent data have indicated that upper airway collapse can occur at end expiration (27), when both dilator and pump muscle activities are low. Regardless, in the prior studies that have measured dilator muscle responses to arousal (10, 17), and the current study, increased genioglossus and TP activation was observed to last for 20–30 seconds. We therefore propose that on arousal there is preferential or more prolonged activation of both inspiratory and expiratory (tonic) component of upper airway dilator muscles, such that either the critical ratio of dilator to pump muscle activity is maintained despite a period of relative hypocapnia, or that expiratory airway collapse is prevented. Consistent with this idea, our own prior study of naturally occurring respiratory events in OSA patients also showed marked dilator muscle activation at the termination of respiratory events with arousal and no subsequent period of reduced activity during the next event (28). In fact, dilator muscle activity often ramped up over the course of respiratory events (see Figure 4 in Reference 28) as did the negative epiglottic pressure swings raising doubt over whether the hyperventilation at arousal was truly excessive (reduced CO2 or respiratory drive below the level present on the initial event).
Further evidence that arousals do not, on average, worsen airflow comes from the Younes study (4). In his paper, Younes calculated the severity ratio of secondary respiratory events with 0 indicating the secondary event airflow was equal to baseline flow on full CPAP (normalized breathing), or 1 indicating the second respiratory event was just as severe as the first event. On average after respiratory event termination with arousal the severity ratio was 0.76, whereas without arousal it was 0.6. Thus on average, secondary respiratory events in Younes’ study were also less severe than initial events.
We therefore propose a new model of the role of arousal in OSA, in which arousals enable sufficient ventilation to prevent serious asphyxia, while gradually allowing dilator muscle activity to build up and airflow to improve over the course of successive events. As respiratory events become less severe, they may be terminated without ASDA arousal, further reducing the associated hyperventilation and improving airflow until no further events occur. Thus, both termination events (with and without arousal) may lead to progressively greater upper airway muscle activation ultimately allowing for stable breathing during stable sleep. This series of events would account for most patients with OSA who can achieve stable breathing at some point throughout the night (28). However, there are clearly some patients with severe OSA who never achieve stable breathing. These patients may have such poor airway function (anatomy or muscle control) that the dilator muscles can never increase to a point to enable stable breathing during sleep. Alternatively, they may have particularly enhanced ventilatory responses to arousal such that hyperventilation does result in subsequent dilator muscle hypotonia in these individuals. Further investigation of this proposed model, and patients with severe OSA without any stable breathing periods, is necessary before any conclusions can be drawn.
Are “Arousal-like” Physiologic Changes Always Present at the Termination of Respiratory Events?
A secondary finding of this study was that the physiologic changes at the termination of obstructive respiratory events without ASDA arousal are very similar to those in which ASDA arousal is present. Specifically, marked heart rate acceleration and activation of the TP muscle occurred with events terminated without ASDA arousal. These changes would not be expected if no arousal of any form were present because the TP responds poorly to mechanoreceptor and chemical stimuli during sleep (29) but is strongly influenced by sleep–wake state (30, 31). Similarly, heart rate may be expected to increase slightly on the sudden removal of increased airway resistance and during the large breaths after event termination, but these changes would not be expected to differ from the changes observed after sudden CPAP Increase. We observed heart rate acceleration after events terminated without arousal that was similar to when ASDA Arousals were present for four beats. Trinder and coworkers (32) previously reported that the initial period of heart rate acceleration after arousals of different duration was near identical, but that longer arousals had more prolonged increases in heart rate consistent with our finding. Rees and coworkers (1) also demonstrated that the blood pressure changes with event termination were not different when arousals were or were not present and that the level of respiratory effort preceding event termination was also similar. Therefore we, like Rees and coworkers, suggest that most events terminated without ASDA arousal likely have near identical brainstem activation responses to when arousal is present, but lack the cortical component of arousal. If this assumption is correct, it may have implications for the cardiovascular consequences of respiratory events, particularly in individuals with low arousal indices but high apnea–hypopnea index.
Although the suggestion that subtle “subcriterion” arousals are always present at the termination of events is in agreement with several prior publications (1, 5, 33), this for the most part differs to the Younes paper (4). Younes argues that arousals were truly absent in his paper based on several points. First, because he performed EEG Fourier analyses, small less than 3-second arousals should have already been included in the arousal group. However, such analyses would not detect subcortical arousals that have no EEG changes. Second, Younes stated that bursts of delta waves, which occurred in 30% of events without clear arousal in his study, were not true arousals because the changes in airflow were the same in these events compared with events without clear arousal and without delta bursts. An alternative explanation is that subtle forms of arousal are present in both No Arousal types. Third, Younes states that the heart rate on the beat straddling the increase in airflow does not differ from the heart rate at the same point in inspiration on the preceding two respiratory efforts. These beats correspond to beats −7, −4, and +1 in Figure 3. Given that the peak in heart rate occurs at approximately three to five beats after the sudden increase in airflow, the analysis of only one beat after flow increase may be quite insensitive. Therefore, it is possible that subtle forms of arousal were present in the type 1 and 2 events in the Younes study but were not readily detectable with the methodology used. Regardless of whether or not one wants to call it an arousal, the current study has importantly shown that the physiologic changes that occur when events are terminated with or without ASDA arousal are highly conserved.
Limitations
Despite its strengths, there are limitations with the current study. First, it is possible that the moderately invasive recording equipment required for this experiment modified the responses observed. This was unavoidable and we believe it unlikely to have majorly influenced the results in a systematic direction, because they largely agree with Younes’ (4) study, which used less invasive techniques. Second, it is possible that respiratory events induced by reducing CPAP differ to naturally occurring events. However, as previously mentioned, the findings of the current study are consistent with our observations in spontaneously occurring respiratory events in OSA (28). Third, we have only assessed rapidly terminated respiratory events with a sudden increase in airflow. Theoretically, a gradual increase in dilator muscle activity throughout events may induce a gradual increase in airflow and this type of event termination would be missed with the analysis performed. However, such gradual increases in airflow have only very rarely been observed in OSA patients in several prior studies from our laboratory (28, 34). Thus, we believe that our results are representative of most respiratory events in OSA. Fourth, repetitive CPAP drops induced mild intermittent hypoxia (Figure E1) and could therefore have caused long-term facilitation across trials contaminating results. To investigate this possibility we compared genioglossus activity during the minute before the first and last CPAP drop in each subject and found that genioglossus activity did not change (4.6 ± 2.6 to 3.6 ± 1.6 %max; P = 0.2). Thus, long-term facilitation does not seem to have influenced our results. However, the presence of CPAP could have minimized any effect of long-term facilitation and we cannot exclude the possibility that some form of respiratory plasticity occurred within CPAP drops and resulted in the increased dilator muscle activity observed during secondary events. Finally, our results would have been strengthened by PaCO2 recordings to document whether hypocapnia occurred, or whether any hypercapnia (that developed during reduced CPAP) remained after hyperventilation. Such measurements using end-tidal capnometry are not possible during obstructive apneas and hypopneas because the expiratory gas sample does not represent alveolar gas.
Summary
This study indicates that reduced activity of upper airway dilator muscles does not occur after the termination of obstructive respiratory events regardless of whether or not a clear ASDA arousal is present. Furthermore, secondary respiratory events were less severe than initial events suggesting that arousal from sleep may enable gradual dilator muscle activation and improvements in airflow such that stable breathing can be attained. Further studies investigating the role of arousal in OSA, particularly focusing on such factors as the influence of arousal duration and OSA severity, are warranted.
Supplementary Material
Acknowledgments
The authors thank Magdy Younes for his thoughtful comments on the paper and acknowledge the technical assistance of Karen Stevenson, Lauren Hess, Scott Smith, and Louise Dover with the overnight data collection and analysis of the studies. The project was supported by Grant Number 1 UL1 RR025758-01, Harvard Clinical and Translational Science Center, from the National Center for Research Resources. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
Footnotes
Supported by NIH grants RO1 HL048531, P01 HL095491, K24 HL093218, and 1 UL1 RR025758–01; NHMRC (Australia) grant 510392; and the American Heart Association grants 0840159N and 10SDG3510018.
Author Contributions: Conception and design, A.S.J., A.M., and D.P.W. Data collection, A.S.J., D.J.E., and A.W. Interpretation and analysis, A.S.J., D.J.E., and J.A.T. Drafting of the manuscript and intellectual content, all authors.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201106-0975OC on August 11, 2011
Author Disclosure: A.S.J.’s institution received a modified continuous positive airway pressure device from Philips Respironics for use in the studies at no cost; she has received consultancy fees from Apnex Medical Inc. D.J.E. has received consultancy fees from Apnex Medical Inc; his institution has received grants from Sepracor. A.W.’s institution has received grants from the American Heart Association; he has received consultancy and lecture fees from Philips Respironics. J.A.T. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. A.M.’s institution has received grants from Philips, Sepracor, and Cephalon; he has received consultancy fees from Philips, Pfizer, Merck, Apnex, Apnicure, Ethicon, Medtronic, SHC, SGS, and Galleon. D.P.W. is employed by Philips Respironics; he holds stock in Philips.
References
- 1.Rees K, Spence DP, Earis JE, Calverley PM. Arousal responses from apneic events during non-rapid-eye-movement sleep. Am J Respir Crit Care Med 1995;152:1016–1021 [DOI] [PubMed] [Google Scholar]
- 2.Dingli K, Fietze I, Assimakopoulos T, Quispe-Bravo S, Witt C, Douglas NJ. Arousability in sleep apnoea/hypopnoea syndrome patients. Eur Respir J 2002;20:733–740 [DOI] [PubMed] [Google Scholar]
- 3.Berry RB, Gleeson K. Respiratory arousal from sleep: mechanisms and significance. Sleep 1997;20:654–675 [DOI] [PubMed] [Google Scholar]
- 4.Younes MK. Role of arousals in the pathogenesis of obstructive sleep apnea. Am J Respir Crit Care Med 2004;169:623–633 [DOI] [PubMed] [Google Scholar]
- 5.Thomas RJ. Arousals in sleep-disordered breathing: patterns and implications. Sleep 2003;26:1042–1047 [DOI] [PubMed] [Google Scholar]
- 6.Onal E, Burrows DL, Hart RH, Lopata M. Induction of periodic breathing during sleep causes upper airway obstruction in humans. J Appl Physiol 1986;61:1438–1443 [DOI] [PubMed] [Google Scholar]
- 7.Hudgel DW, Chapman KR, Faulks C, Hendricks C. Changes in inspiratory muscle electrical activity and upper airway resistance during periodic breathing induced by hypoxia during sleep. Am Rev Respir Dis 1987;135:899–906 [DOI] [PubMed] [Google Scholar]
- 8.Warner G, Skatrud JB, Dempsey JA. Effect of hypoxia-induced periodic breathing on upper airway obstruction during sleep. J Appl Physiol 1987;62:2201–2211 [DOI] [PubMed] [Google Scholar]
- 9.Badr MS, Kawak A, Skatrud JB, Morrell MJ, Zahn BR, Babcock MA. Effect of induced hypocapnic hypopnea on upper airway patency in humans during NREM sleep. Respir Physiol 1997;110:33–45 [DOI] [PubMed] [Google Scholar]
- 10.Jordan AS, Eckert DJ, Catcheside PG, McEvoy RD. Ventilatory response to brief arousal from non-rapid eye movement sleep is greater in men than in women. Am J Respir Crit Care Med 2003;168:1512–1519 [DOI] [PubMed] [Google Scholar]
- 11.Khoo MC, Berry RB. Modeling the interaction between arousal and chemical drive in sleep-disordered breathing. Sleep 1996;19:S167–S169 [DOI] [PubMed] [Google Scholar]
- 12.Carley DW, Applebaum R, Basner RC, Onal E, Lopata M. Respiratory and electrocortical responses to acoustic stimulation. Sleep 1996;19:S189–S192 [DOI] [PubMed] [Google Scholar]
- 13.Badr MS, Morgan BJ, Finn L, Toiber FS, Crabtree DC, Puleo DS, Skatrud JB. Ventilatory response to induced auditory arousals during NREM sleep. Sleep 1997;20:707–714 [DOI] [PubMed] [Google Scholar]
- 14.Carlson DM, Carley DW, Onal E, Lopata M, Basner RC. Acoustically induced cortical arousal increases phasic pharyngeal muscle and diaphragmatic EMG in NREM sleep. J Appl Physiol 1994;76:1553–1559 [DOI] [PubMed] [Google Scholar]
- 15.Khoo MC, Shin JJ, Asyali MH, Kim TS, Berry RB. Ventilatory dynamics of transient arousal in patients with obstructive sleep apnea. Respir Physiol 1998;112:291–303 [DOI] [PubMed] [Google Scholar]
- 16.Jordan AS, McEvoy RD, Edwards JK, Schory K, Yang CK, Catcheside PG, Fogel RB, Malhotra A, White DP. The influence of gender and upper airway resistance on the ventilatory response to arousal in obstructive sleep apnoea in humans. J Physiol 2004;558:993–1004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yeo A, Catcheside PG, George K, Thomson K, McEvoy RD. Prolonged post-arousal upper airway dilator muscle activity in men with obstructive sleep apnoea on continuous positive airway pressure [abstract]. Sleep Biol Rhythms 2007;5:A133 [Google Scholar]
- 18.Jordan AS, Eckert DJ, Wellman A, Stevenson KE, Hess L, Malhotra A, White DP. Termination of respiratory events without cortical arousal from sleep [abstract]. Sleep 2009;32:A173 [Google Scholar]
- 19.Jordan AS, Eckert DJ, Wellman A, Smith SA, Stevenson KE, Malhotra A, White DP. A comparison of the respiratory changes at the termination of flow limitation with and without cortical arousal from sleep [abstract]. Am J Respir Crit Care Med 2008;177:A594 [Google Scholar]
- 20.Rechtschaffen A, Kales AE. A manual of standardised terminology, techniques and scoring systems for sleep stages of human subjects. UCLA Los Angeles: National Institute of Health; 1968 [Google Scholar]
- 21.American Sleep Disorders Association Task Force EEG arousals: scoring rules and examples. Sleep 1992;15:173–184 [PubMed] [Google Scholar]
- 22.Longobardo GS, Evangelisti CJ, Cherniack NS. Influence of arousal threshold and depth of sleep on respiratory stability in man: analysis using a mathematical model. Exp Physiol 2009;94:1185–1199 [DOI] [PubMed] [Google Scholar]
- 23.Xie A, Wong B, Phillipson EA, Slutsky AS, Bradley TD. Interaction of hyperventilation and arousal in the pathogenesis of idiopathic central sleep apnea. Am J Respir Crit Care Med 1994;150:489–495 [DOI] [PubMed] [Google Scholar]
- 24.Bonnet MH, Dexter JR, Arand DL. The effect of triazolam on arousal and respiration in central sleep apnea patients. Sleep 1990;13:31–41 [DOI] [PubMed] [Google Scholar]
- 25.Quadri S, Drake C, Hudgel DW. Improvement of idiopathic central sleep apnea with zolpidem. J Clin Sleep Med 2009;5:122–129 [PMC free article] [PubMed] [Google Scholar]
- 26.Badr MS, Kawak A. Post-hyperventilation hypopnea in humans during NREM sleep. Respir Physiol 1996;103:137–145 [DOI] [PubMed] [Google Scholar]
- 27.Morrell MJ, Arabi Y, Zahn B, Badr MS. Progressive retropalatal narrowing preceding obstructive apnea. Am J Respir Crit Care Med 1998;158:1974–1981 [DOI] [PubMed] [Google Scholar]
- 28.Jordan AS, White DP, Lo YL, Wellman A, Eckert DJ, Yim-Yeh S, Eikermann M, Smith SA, Stevenson KE, Malhotra A. Airway dilator muscle activity and lung volume during stable breathing in obstructive sleep apnea. Sleep 2009;32:361–368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Malhotra A, Pillar G, Fogel RB, Beauregard J, Edwards JK, Slamowitz DI, Shea SA, White DP. Genioglossal but not palatal muscle activity relates closely to pharyngeal pressure. Am J Respir Crit Care Med 2000;162:1058–1062 [DOI] [PubMed] [Google Scholar]
- 30.Lo YL, Jordan AS, Malhotra A, Wellman A, Heinzer RA, Eikermann M, Schory K, Dover L, White DP. Influence of wakefulness on pharyngeal airway muscle activity. Thorax 2007;62:799–805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fogel RB, Trinder J, White DP, Malhotra A, Raneri J, Schory K, Kleverlaan D, Pierce RJ. The effect of sleep onset on upper airway muscle activity in patients with sleep apnoea versus controls. J Physiol 2005;564:549–562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Trinder J, Allen N, Kleiman J, Kralevski V, Kleverlaan D, Anson K, Kim Y. On the nature of cardiovascular activation at an arousal from sleep. Sleep 2003;26:543–551 [PubMed] [Google Scholar]
- 33.Dingli K, Assimakopoulos T, Fietze I, Witt C, Wraith PK, Douglas NJ. Electroencephalographic spectral analysis: detection of cortical activity changes in sleep apnoea patients. Eur Respir J 2002;20:1246–1253 [DOI] [PubMed] [Google Scholar]
- 34.Jordan AS, Wellman A, Heinzer RC, Lo YL, Schory K, Dover L, Gautam S, Malhotra A, White DP. Mechanisms used to restore ventilation after partial upper airway collapse during sleep in humans. Thorax 2007;62:861–867 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.