Abstract
Rationale: Adults with cystic fibrosis (CF) possess multiple potential risk factors for chronic kidney disease, including CF-related diabetes (CFRD) and lifetime nephrotoxic drug exposure.
Objectives: To determine whether cumulative intravenous (IV) aminoglycoside exposure and CFRD increase the risk of chronic kidney disease in adults with CF.
Methods: This was a cohort study using adults (≥ 18 yr) in the CF Foundation registry from 2001–2008. Chronic kidney disease (stage 3 or greater) was defined by an estimated glomerular filtration rate of less than 60 ml/min/1.73 m2. Time-dependent multivariable Cox proportional hazards models were used to determine whether cumulative number of acute pulmonary exacerbations (surrogate for IV aminoglycoside exposure) and CFRD requiring insulin increase the risk of chronic kidney disease, adjusting for confounders.
Measurements and Main Results: The study cohort included 11,912 adults with a median follow-up of 4 years. During the study period, 204 subjects had chronic kidney disease, with an annual disease prevalence of 2.3%. Disease prevalence doubled with every 10-year increase in age. CFRD requiring insulin therapy substantially increased the risk of chronic kidney disease (1–4 yr of CFRD requiring insulin vs. no CFRD, hazard ratio [HR] = 2.40, 95% confidence interval [CI] 1.74–3.32; ≥ 5 yr, HR = 4.56, 95% CI 2.84–7.31). Pulmonary exacerbations did not significantly increase the risk of chronic kidney disease (one to five exacerbations vs. none, HR = 0.79, 95% CI 0.56–1.11; six to nine exacerbations, HR = 0.92, 95% CI 0.58–1.46; ≥ 10 exacerbations, HR = 1.16, 95% CI 0.75–1.81).
Conclusions: CF-related diabetes is a significant risk factor for chronic kidney disease in adults with CF, but additional studies examining IV aminoglycoside exposure directly are required.
Keywords: kidney, renal insufficiency, aminoglycosides, diabetes
At a Glance Commentary
Scientific Knowledge on the Subject
Adults with cystic fibrosis (CF) possess multiple potential risk factors for the development of chronic kidney disease, including CF-related diabetes and lifetime nephrotoxic drug exposure. The prevalence and risk factors for chronic kidney disease in adults with CF are unknown.
What This Study Adds to the Field
This study demonstrates a dramatic increase in the prevalence of stage 3 chronic kidney disease with advancing age. This study also suggests that CF-related diabetes is a strong risk factor for chronic kidney disease in adults with CF.
Survival among individuals with cystic fibrosis (CF) in the United States continues to increase. The number of adults living with CF is expected to surpass the number of children living with CF in the next few years (1). As a consequence of survivorship, complications resulting from lifetime drug exposures and chronic CF-related comorbidities are destined to emerge.
Dysfunction in the CF transmembrane regulator (CFTR) protein is not thought to impair renal function despite its abundant expression in the renal cortex and outer medulla (2). However, adults with CF possess multiple potential risk factors for the development of chronic kidney disease (CKD). More than 70% of adults with CF are infected with Pseudomonas aeruginosa (1) and thus receive repeated courses of intravenous (IV) aminoglycoside antibiotics for exacerbations over their lifetime (3). In a prospective study of 80 adults and adolescents with CF chronically infected with P. aeruginosa, 30% had abnormal renal function in follow-up and this was highly correlated with lifetime use of aminoglycosides (3). Although aminoglycosides are a recognized cause of acute renal failure (ARF) (4–6), this small study suggests that they also contribute importantly to the pathogenesis of CKD.
Twenty percent of individuals with CF develop CF-related diabetes (CFRD) by the age of 20 and the prevalence increases to over 30% by the age of 40 (1). Although diabetes mellitus is one of the most important risk factors for renal disease in the general population (7), the CFRD Consensus Committee recently reported that the microvascular consequences of CFRD seem to be less frequent and less severe than in other diabetic populations (8, 9). Contrary to this report, a small study comparing CFRD with type 1 diabetes mellitus showed a similar rate of overall microvascular complications (29%) but a significantly higher prevalence of microalbuminuria (21% vs. 4%) among those with CFRD (10).
To our knowledge, this is the first population registry–based cohort study examining the prevalence and predictors of incident CKD cases in the adult population with CF. We speculate that adult patients with CF are at increased risk for developing CKD and we hypothesize that cumulative IV aminoglycoside exposure and CFRD are important risk factors.
Methods
Study Population and Data Sources
Data from the United States Cystic Fibrosis Foundation Registry (CF Registry) were used for this study, which contains longitudinal data on 32,653 patients from more than 110 care centers spanning the years 2001–2008 (1). A detailed description of the database has been previously published (11). We studied adults with CF (≥ 18 yr old), who had at least two estimated glomerular filtration rate (eGFR) measurements, not separated by more than 2 years, from January 1, 2001 to December 31, 2008. Individuals entered the study cohort after their first eGFR measurement and were followed to the earliest of CKD diagnosis, solid organ transplant, or last eGFR measurement.
Outcome Definition
Renal function was estimated using the Cockcroft-Gault formula standardized for body surface area (12, 13). Data on serum creatinine, age, weight, height, and sex were required for this calculation (see online supplement). CKD was defined by eGFR measured less than 60 ml/min/1.73 m2 in two consecutive registry years. Based on National Kidney Foundation KDOQI guidelines, this corresponds to stage 3 CKD severity and is the earliest stage that can be diagnosed using serum creatinine alone (14). Because our definition of CKD required 2 consecutive years with eGFR less than 60 ml/min/1.73 m2, incident CKD cases could not be ascertained in 2008, our last year of follow-up. More advanced stages of CKD were defined as follows: stage 4, eGFR less than 30 ml/min/1.73 m2; and stage 5, eGFR less than 15 ml/min/1.73 m2 or need for hemodialysis.
Classification of Exposures
Predictors of interest and other covariates were characterized using annual-level registry data. A priori we had two main predictors of interest for incident CKD cases: cumulative courses of IV aminoglycoside antibiotics and cumulative years of CFRD. The class of antibiotic used to treat acute pulmonary exacerbations was not specified in the CF Registry and the number of annual IV antibiotic courses was only available starting in 2003. In preliminary analyses, we found a very strong correlation between numbers of IV antibiotic courses and numbers of acute exacerbations since 2003, and therefore number of acute pulmonary exacerbations was chosen as a surrogate for IV aminoglycoside exposure. To ensure a consistent case definition of CFRD, it was defined as CFRD requiring chronic insulin therapy. Other covariates included age, sex, CF genotype, body mass index (BMI), percent-predicted FEV1, P. aeruginosa and Burkholderia cepacia culture status, previous episodes of ARF defined by eGFR of less than 60 ml/min/1.73 m2 in a single registry year, and cumulative years of exposure to inhaled tobramycin and high-dose ibuprofen.
Statistical Analyses
Descriptive analyses were used to summarize baseline characteristics in the year before cohort entry stratified by subsequent CKD status. Differences and 95% confidence intervals (CI) between groups were reported.
Age-adjusted Cox proportional hazards models were used to identify significant independent predictors of CKD. A multivariable Cox proportional hazards model was then developed including our primary predictors of interest, after adjustment for age, other independent predictors of CKD, and potential confounders (see online supplement).
Sensitivity analyses were defined a priori to assess the robustness of our results (see online supplement). A two-sided P value of less than 0.05 was considered statistically significant for all analyses. Assuming 200 CKD events in the cohort and a one-sided 0.025 level of significance, there was 80% power to detect a hazard ratio (HR) of 1.48 or greater. Analyses were performed using STATA 10.0 (StataCorp, College Station, TX). The Human Subjects Review Board at the University of Washington approved this study.
Results
Cohort Characteristics
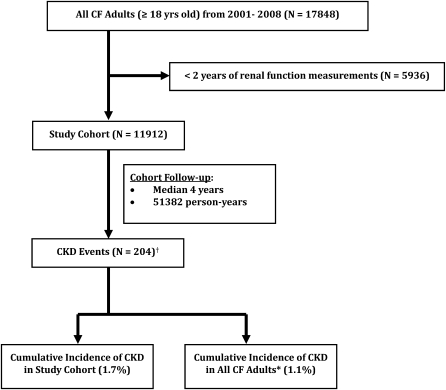
Among the greater than 110 CF care centers in the United States, there were 17,848 adults (aged ≥18 yr) with CF entered into the CF Registry during the study period of January 1, 2001 to December 31, 2008. After the exclusion of 5,936 patients because of insufficient renal function data, the total number entering the study cohort was 11,912, resulting in a total follow-up of 51,382 person-years (Figure 1).
Figure 1.
Diagram of study population with number excluded, cohort follow-up time, and CKD cumulative incidence. CF = cystic fibrosis; CKD = chronic kidney disease. *Assumes adult patients excluded from study cohort do not have CKD; †A total of 81 cases were diagnosed in the year of cohort entry.
The baseline characteristics of the study cohort comparing those who developed CKD with those who did not are shown in Table 1. Adults with CF diagnosed with CKD were older (39 vs. 25.8 yr; mean difference 13.2 yr; 95% CI for difference, 11.3–15.1 yr); more likely to be female (53.2% vs. 45.6%; difference 7.6%; 95% CI for difference, 0.7–14.6%); with a lower BMI (20.9 vs. 21.4 kg/m2; mean difference 0.5 kg/m2; 95% CI for difference, 0.0–0.9 kg/m2); and more likely to be diagnosed with CFRD requiring insulin (33.3% vs. 13.3%; difference 20%; 95% CI difference, 13.4–26.6%) in the year before cohort entry. Although those eventually diagnosed with CKD had a lower percent-predicted FEV1 (56.2% vs. 65.4%; difference 9.2%; 95% CI for difference, 6.1–12.3%), they were less likely to have experienced an acute pulmonary exacerbation (33.3% vs. 40.6%; 7.2% difference; 95% CI for difference, 0.7–13.8%) or have required inhaled tobramycin therapy (33.3% vs. 50.7%; difference 17.4%; 95% CI for difference, 10.8–24%) in the year before cohort entry. Other baseline characteristics were similar between CKD cases and noncases including homozygosity for the delta F508 mutation.
TABLE 1.
BASELINE CHARACTERISTICS OF CHRONIC KIDNEY DISEASE CASES AND NONCASES
| Characteristic* | CKD (−) (n = 11,708) | CKD (+) (n = 204) | Difference† (95% CI) |
| Mean age, yr | 25.8 | 39.0 | 13.2 (11.3 to 15.1) |
| Male sex, yr | 53.2 | 45.6 | 7.6 (0.7 to 14.6) |
| Homozygous ΔF508, % | 48.7 | 48.2 | 0.5 (−7.2 to 8.3) |
| Mean BMI, kg/m2 | 21.4 | 20.9 | 0.5 (0 to 0.9) |
| Mean percent-predicted FEV1, % | 65.4 | 56.2 | 9.2 (6.1 to 12.3) |
| Greater than or equal to one acute pulmonary exacerbation, % | 40.6 | 33.3 | 7.2 (0.7 to 13.8) |
| CFRD requiring insulin, % | 13.3 | 33.3 | 20.0 (13.4 to 26.6) |
| Inhaled tobramycin use, % | 50.7 | 33.3 | 17.4 (10.8 to 24) |
| HD ibuprofen use, % | 3.7 | 2 | 1.7 (0.2 to 3.7) |
| Pseudomonas aeruginosa culture, % positive | 74.6 | 75.4 | 0.8 (−5.4 to 7) |
| Burkholderia cepacia culture, % positive | 4.4 | 3.1 | 1.2 (−1.3 to 3.7) |
Definition of abbreviations: BMI = body mass index; CFRD = cystic fibrosis–related diabetes; CI = confidence interval; CKD = chronic kidney disease; HD = high dose.
In the year before cohort entry.
Absolute difference with 95% CI.
Adults with CF diagnosed with CKD were more likely to have been previously diagnosed with ARF (6.9% vs. 3.2%; difference 3.7%; 95% CI for difference, 0.2–7.2%), and were more likely to require hemodialysis after CKD diagnosis (22.5% vs. 0.9%; difference 21.6%; 95% CI for difference, 15.9–27.5%) compared with those without CKD.
CKD Incidence and Prevalence
There were 204 CKD cases detected over the observation period of 2001–2008. Eighty-one cases were diagnosed in the year of cohort entry, which was the first year of renal function measurement. The mean annual prevalence of stage 3 or greater CKD over this time period was 2.3%. Just over half (53%) progressed to stage 4 or greater CKD with a mean annual prevalence of 0.7%, and 44% progressed to stage 5 CKD with a mean annual prevalence of 0.6%. The cumulative incidence of stage 3 or greater CKD was 1.7% of those adults included in the cohort and 1.1% of all adults enrolled in the CF Registry (Figure 1). The overall incidence rate of stage 3 or greater CKD was 4 events per 1,000 person-years of follow-up.
Both the prevalence and incidence rate of CKD increased substantially with age (Figure 2; see online supplement). Stage 3 or greater CKD prevalence increased from 0.6% for the 18- to 25-year-old age group to 19.2% for those greater than 55 years of age.
Figure 2.
Prevalence of chronic kidney disease (CKD) by age category and severity. Stage 3 CKD severity: estimated glomerular filtration rate (eGFR) less than 60 ml/min/1.73 m2. Stage 4 CKD severity: eGFR less than 30 ml/min/1.73 m2. Stage 5 CKD severity: eGFR less than 15 ml/min/1.73 m2 (or need for hemodialysis).
Twenty-five percent of individuals with stage 3 or greater CKD, 58% of individuals with stage 4 or greater CKD, and 78% of individuals with stage 5 or greater CKD required hemodialysis in follow-up.
Independent Associations between Each Predictor of Interest and Risk of CKD
Based on age-adjusted Cox proportional hazards analysis, the HR of CKD did not significantly increase with an increase in the cumulative number of acute pulmonary exacerbations (Table 2). The risk of CKD increased significantly for patients with CFRD requiring insulin and the age-adjusted HR increased from 2.52 (95% CI, 1.82–3.49) for those with 1 to 4 years of disease versus no CFRD to 4.71 (95% CI, 2.96–7.50) for those with at least 5 years of disease versus no CFRD. Homozygosity for the delta F508 mutation (HR 1.48; 95% CI, 1.12–1.96) and at least one previous episode of ARF (HR 1.91; 95% CI, 1.05–3.46) also increased the age-adjusted risk of CKD. Higher BMI decreased the age-adjusted risk of CKD (HR 0.85; 95% CI, 0.81–0.89). Male sex, percent-predicted FEV1, duration of inhaled tobramycin therapy, duration of high-dose ibuprofen therapy, and B. cepacia or P. aeruginosa culture status were not found to modify the risk of CKD after adjustment for age.
TABLE 2.
HAZARD RATIOS AND 95% CONFIDENCE INTERVALS FROM AGE-ADJUSTED COX PROPORTIONAL HAZARD MODELS USED TO ASSESS THE INDEPENDENT ASSOCIATION BETWEEN EACH COVARIATE AND RISK OF CKD
| Exposure | CKD Hazard Ratio (95% CI) |
| Time-varying | |
| Acute pulmonary exacerbations* | |
| No exacerbations | REF |
| One to five exacerbations | 0.86 (0.62–1.19) |
| Six to nine exacerbations | 1.08 (0.69–1.69) |
| ≥ 10 exacerbations | 1.48 (0.99–2.22) |
| CFRD (insulin-requiring)* | |
| 0 yr | REF |
| 1–4 yr | 2.52 (1.82–3.49) |
| ≥ 5 yr | 4.71 (2.96–7.50) |
| Per additional year of inhaled tobramycin use | 0.94 (0.86–1.02) |
| Per additional year of HD ibuprofen use | 1.03 (0.85–1.24) |
| Previous episode of acute renal failure | 1.91 (1.05–3.46) |
| Time-fixed† | |
| Male sex | 0.78 (0.59–1.02) |
| Homozygous ΔF508‡ | 1.48 (1.12–1.96) |
| BMI, kg/m2 | 0.85 (0.81–0.89) |
| Percent-predicted FEV1 | 0.99 (0.99–1.00) |
| Pseudomonas aeruginosa culture (+) | 0.99 (0.71–1.37) |
| Burkholderia cepacia culture (+) | 0.86 (0.38–1.95) |
Definition of abbreviations: BMI = body mass index; CFRD = cystic fibrosis–related diabetes; CI = confidence interval; CKD = chronic kidney disease; HD = high dose.
Includes exposure up to 3 years before cohort entry.
Data from the year before cohort entry.
Reference group = no ΔF508 gene mutations.
Multivariable Model for Risk of CKD
Based on multivariable Cox proportional hazards regression, cumulative number of acute pulmonary exacerbations did not significantly increase the risk of CKD after adjustment for age, years of CFRD requiring insulin, BMI, and years of inhaled tobramycin use (Table 3). Increasing duration of CFRD requiring insulin therapy substantially increased the risk of CKD with a HR of 2.40 (95% CI, 1.74–3.32) for 1 to 4 years of disease versus no CFRD and a HR of 4.56 (95% CI, 2.85–7.28) for at least 5 years of disease, after adjustment for age, cumulative number of acute pulmonary exacerbations, BMI, and years of inhaled tobramycin use. A 1-year increase in age also significantly increased the risk of CKD (HR 1.11; 95% CI, 1.10–1.12) (Table 3). Each additional year of inhaled tobramycin use (HR 0.89; 95% CI, 0.81–0.97) and each unit increase in BMI (HR 0.86; 95% CI, 0.82–0.90) slightly decreased the risk of CKD. Homozygosity for the delta F508 mutation was no longer a significant predictor of CKD in the multivariate model.
TABLE 3.
FINAL MULTIVARIABLE COX PROPORTIONAL HAZARD MODEL
| Exposure | CKD Hazard Ratio (95% CI) |
| Acute pulmonary exacerbations* | |
| No exacerbations | REF |
| One to five exacerbations | 0.79 (0.56–1.11) |
| Six to nine exacerbations | 0.92 (0.58–1.46) |
| ≥ 10 exacerbations | 1.16 (0.75–1.81) |
| CFRD (insulin-requiring)* | |
| 0 yr | REF |
| 1–4 yr | 2.40 (1.74–3.32) |
| ≥ 5 yr | 4.56 (2.85–7.28) |
| Per additional year of age | 1.11 (1.10–1.12) |
| Per additional year of inhaled tobramycin use | 0.89 (0.81–0.97) |
| Per unit increase in BMI, kg/m2 | 0.86 (0.82–0.90) |
Definition of abbreviations: BMI = body mass index; CFRD = cystic fibrosis–related diabetes; CI = confidence interval; CKD = chronic kidney disease.
Includes exposure up to 3 years before cohort entry.
In a post hoc analysis, we explored the relationship between CFRD complicated by microalbuminuria and risk of CKD. After multivariable adjustment, CFRD complicated by microalbuminuria further increased the risk of CKD with a HR of 4.19 (95% CI, 1.57–11.21) for 1–3 years of microalbuminuria versus none and a HR of 11.44 (95% CI, 3.46–37.81) for greater than or equal to 4 years of microalbuminuria.
Sensitivity Analyses
To examine the potential influence of case ascertainment bias, choice of GFR estimating equation, and short cohort follow-up time on our results, we performed multiple sensitivity analyses. Our estimates of CKD (stage 3 or greater) prevalence and incidence were similar to our primary analysis, and CFRD requiring insulin therapy remained a strong risk factor for CKD (see online supplement). Furthermore, restricting our cohort to individuals with P. aeruginosa infection did not change the lack of association between number of acute pulmonary exacerbations and CKD found in our primary analysis.
Discussion
Our cohort study is the first to comprehensively examine the prevalence of CKD (stage 3 or greater) in the adult population with CF and to identify predictors of incident disease. Previous observational studies have looked at ARF (6) or have been small and used non–guidelines-based definitions of CKD (3, 15).
Based on our results, moderate severity CKD is not uncommon in patients with CF with an annual disease prevalence of about 2% and prevalence that doubles with every 10-year increase in age. Previous studies in the general population have shown that advanced age is an independent risk factor for CKD (7, 16). Our age-adjusted prevalence estimates for CKD (stage 3 or greater) are about twofold higher than estimates from the United States general population using 1999–2004 National Health and Nutrition Examination Survey data (17). Earlier stages of CKD often progress to end-stage renal disease (18). This seemed to be the case in our cohort with up to one-quarter of cases with moderate-severity CKD requiring hemodialysis in short-term follow-up.
An important finding of this study is that CFRD requiring insulin is an important risk factor for CKD in the adult population with CF. The increase in risk with duration of disease is compatible with the natural history of diabetic microvascular complications, because diabetic nephropathy tends to arise 10–15 years after diabetes diagnosis (19). Although it seems that we see an increase in risk much earlier, our exposure times reflect minimum values, because we were unable to capture lifetime exposure. This key finding is interesting in light of a recent statement on behalf of the American Diabetes Association and CF-related Diabetes Consensus Report Committee that the “kidneys may be somewhat protected in CFRD” because of residual insulin secretion, and the absence of other known risk factors for microvascular disease, including hypertension and hyperlipidemia (8). Our findings support a recent study by van den Berg and coworkers (10) comparing the microvascular complications of CFRD with type 1 diabetes. They found a similar rate of overall microvascular complications between the two types of diabetes but a much higher rate of microalbuminuria (21% vs. 4%; P = 0.003) in CFRD. Our registry-based definition of CFRD was rigorous, because it required that the subject be on chronic insulin therapy. Although this may result in selection bias for those patients with more severe CFRD, this definition is generalizable because about 65% of patients with CFRD are chronic users of insulin as coded in the CF registry.
Cumulative number of acute pulmonary exacerbations (surrogate for cumulative IV aminoglycoside exposure) did not independently increase the risk of CKD in this study. Previous studies have found an increased risk of renal dysfunction after IV aminoglycoside exposure (3, 5, 20). IV aminoglycosides are directly toxic to the kidneys but the precise mechanism leading to nephrotoxicity remains uncertain (21). In addition to tubular damage, renal vasoconstriction and mesangial contraction are suspected to play important roles at the glomerular level (22). There are a number of potential reasons why our findings are inconsistent with previous studies. First, we used a very crude proxy measure for IV aminoglycoside exposure because of a lack of detailed information available on this exposure in the CF Registry. Although cumulative number of IV aminoglycoside courses likely correlates with cumulative number of acute pulmonary exacerbations, we are uncertain as to what proportion of acute pulmonary exacerbations actually required IV aminoglycoside antibiotics. If this proportion was small, the impact of nondifferential misclassification of exposure may have been significant, thus leading to an underestimation of risk. We restricted our analysis to individuals with P. aeruginosa infection because they were more likely to require IV aminoglycosides during acute pulmonary exacerbations but we still failed to demonstrate a relationship between number of acute pulmonary exacerbations and CKD risk. A more likely reason for our negative findings is that we had a relatively short exposure period and therefore we may have failed to detect an increase in risk because of limited cumulative drug exposure. In previous studies, renal function was inversely associated with lifetime IV aminoglycoside exposure (3). We conducted a sensitivity analysis restricting to CKD cases with normal renal function on cohort entry because they had a slightly longer exposure period before CKD diagnosis but we still failed to detect an association between number of acute pulmonary exacerbations and CKD risk.
Although homozygosity for the delta F508 CFTR mutation seemed to increase the risk of CKD in age-adjusted analysis, it was not an independent predictor after adjustment in multivariate analysis. Most of the age-adjusted increased risk was likely caused by confounding by CFRD, because CFRD is a strong predictor of CKD and is also more prevalent in individuals homozygous for the delta F508 CFTR mutation. This supports previous speculation that CFTR mutations do not predispose to major renal dysfunction despite abundant expression of CFTR in the renal cortex and outer medulla (2), and evidence of reduced renal excretion of NaCl and decreased capacity to dilute and concentrate urine (23, 24).
We found that an increase in the number of years of inhaled tobramycin use had a slightly protective effect on the risk of developing CKD. This finding is reassuring, because previous case reports have suggested nephrotoxicity related to inhaled tobramycin use (25, 26), which was not observed in randomized clinical trials using this drug (27–31). Although speculative, a possible explanation for this observed protective effect is that individuals on maintenance inhaled tobramycin therapy are less likely to develop acute pulmonary exacerbations resulting in reduced exposure to potentially nephrotoxic IV antibiotics.
Although our study demonstrates a strong association between CFRD requiring insulin and CKD, epidemiologic studies are susceptible to biases that may influence the results and therefore we undertook multiple sensitivity analyses to ensure our results were robust. We were concerned about possible case ascertainment bias because those patients with risk factors for CKD might be more likely to have their serum creatinine measured. As an example, serum creatinine is routinely measured around the time of IV aminoglycoside use because of concerns about nephrotoxicity. Because measurement of serum creatinine is required to make a diagnosis of CKD, presence of a potential risk factor can lead to higher rates of diagnosis, thus inducing a spurious association. We thus limited our analysis to centers that monitored serum creatinine in greater than 70% of their enrolled patients to minimize this possible selection bias. Our relative risk estimates actually increased after this sensitivity analysis and therefore we do not believe case ascertainment bias was a significant issue in our analysis. The strong relative risk estimates, positive dose–response relationship, and a post hoc analysis demonstrating an even higher risk for those with CFRD complicated by microalbuminuria all support the biological plausibility of this association.
Another limitation of our study is that we relied on serum creatinine to estimate renal function. Diagnosis of earlier stages of CKD (stages 1 and 2) require evidence of kidney damage on urine or nuclear testing but this information was not available through the CF Registry (14). Diagnosis of renal dysfunction using serum creatinine alone is subject to interpretation error because baseline serum creatinine decreases with low muscle mass and malnutrition, which are prevalent in the CF population. Therefore, we used the Cockcroft-Gault formula to estimate glomerular filtration rate as it adjusts for age, body mass, and sex. We also performed a sensitivity analysis using the abbreviated modified diet in renal disease (aMDRD) equation, which adjusts for similar factors plus ethnicity. Our CKD prevalence estimates were similar for the two estimating equations with a slightly higher rate of CKD diagnosis using the aMDRD equation. Without a gold standard available for comparison, it is difficult to know which prediction equation performs better. Unfortunately, there is no consensus on the best creatinine-based estimating equation for glomerular filtration rate in patients with CF, although one study found Cockcroft-Gault to be superior to aMDRD (32). Choice of estimating equation is unlikely to influence the diagnosis of more advanced disease (as demonstrated in our study) but may play an important role in identifying borderline cases of renal dysfunction. Urinary biomarkers, such as N-acetyl-β-D-glucosaminidase, may play a role in detecting earlier kidney injury (21, 33).
Another limitation of this study is the relatively short follow-up period of our cohort, with a median follow-up of just 4 years. The short follow-up period is potentially a significant limitation in the assessment of the association between our cumulative exposure covariates and CKD. Ideally, we would have looked at lifetime exposure but this was not possible with the data available in the CF Registry. For a cumulative exposure variable, such as CFRD, cumulative exposure during the short follow-up period of our cohort likely correlates with lifelong exposure because most individuals with CFRD were likely diagnosed many years previously. This is probably why we were able to establish such a strong association between CFRD requiring insulin and CKD with an exposure period of just 1–4 years. The suspected correlation between short-term and lifetime exposure observed with CFRD may not be true for acute pulmonary exacerbations, and thus remains a potential limitation.
Finally, the overall prevalence of exposure to ibuprofen was low at just 2.5%. The highest observed use of ibuprofen was in the youngest age groups also at the lowest risk for developing CKD. We thus had limited statistical power to detect an association between this exposure and CKD, leading to a possible Type II error. Therefore, we cannot exclude the possibility of increased risk of CKD after ibuprofen use.
Given the dramatic increase in disease prevalence observed with advancing age, we anticipate that the prevalence of CKD will rise with improvements in survival of the CF population. CKD diagnosis can pose a significant challenge to physicians caring for patients with CF. Doses of nephrotoxic antibiotics, such as IV tobramycin and colistin, that are relied on heavily to treat resistant strains of Pseudomonas must be altered or sometimes avoided altogether. Earlier stages of CKD can progress to end-stage disease with its attendant complications, including the need for dialysis. More importantly, a diagnosis of CKD can result in preclusion from lung transplant consideration given the significant risk of progressive renal dysfunction in the posttransplant setting (34), or result in worse outcomes in those transplanted with milder degrees of renal dysfunction (35). With all these potential implications in mind, diagnosing and aggressively managing CFRD is of paramount importance to decrease the risk of CKD.
In conclusion, to assess the prevalence and predictors of incident cases of CKD in adults with CF we have longitudinally analyzed nearly 12,000 individuals with CF for a median follow-up period of 4 years from 2001–2008. During that period, 204 individuals were diagnosed with stage 3 CKD, with a disease prevalence of 2.3% and incidence rate of 4 events per 1,000 person-years. After controlling for other independent predictors and potential confounders, the results suggest that CFRD is a strong risk factor for CKD but that IV aminoglycoside exposure requires further study.
Supplementary Material
Footnotes
Supported by NIH/NIDDK (P30 DK089507–01). B.S.Q. was supported by a British Columbia Lung Association Fellowship Award.
Author contributions: Conception and design, B.S.Q., C.H.G., and N.M.-H. Analysis and interpretation, B.S.Q., C.H.G., and N.M.-H. Drafting the manuscript for important intellectual content, all authors.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201105-0932OC on July 28, 2011
Author Disclosure: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Cystic Fibrosis Foundation Patient Registry 2009 Annual Data Report. Bethesda, Maryland: Cystic Fibrosis Foundation; 2011 [Google Scholar]
- 2.Morales MM, Carroll TP, Morita T, Schwiebert EM, Devuyst O, Wilson PD, Lopes AG, Stanton BA, Dietz HC, Cutting GR, et al. Both the wild type and a functional isoform of CFTR are expressed in kidney. Am J Physiol 1996;270:F1038–F1048 [DOI] [PubMed] [Google Scholar]
- 3.Al-Aloul M, Miller H, Alapati S, Stockton PA, Ledson MJ, Walshaw MJ. Renal impairment in cystic fibrosis patients due to repeated intravenous aminoglycoside use. Pediatr Pulmonol 2005;39:15–20 [DOI] [PubMed] [Google Scholar]
- 4.Shusterman N, Strom BL, Murray TG, Morrison G, West SL, Maislin G. Risk factors and outcome of hospital-acquired acute renal failure: clinical epidemiologic study. Am J Med 1987;83:65–71 [DOI] [PubMed] [Google Scholar]
- 5.Bertenshaw C, Watson AR, Lewis S, Smyth A. Survey of acute renal failure in patients with cystic fibrosis in the UK. Thorax 2007;62:541–545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smyth A, Lewis S, Bertenshaw C, Choonara I, McGaw J, Watson A. Case-control study of acute renal failure in patients with cystic fibrosis in the UK. Thorax 2008;63:532–535 [DOI] [PubMed] [Google Scholar]
- 7.Fox CS, Larson MG, Leip EP, Culleton B, Wilson PW, Levy D. Predictors of new-onset kidney disease in a community-based population. JAMA 2004;291:844–850 [DOI] [PubMed] [Google Scholar]
- 8.Moran A, Brunzell C, Cohen RC, Katz M, Marshall BC, Onady G, Robinson KA, Sabadosa KA, Stecenko A, Slovis B. Clinical care guidelines for cystic fibrosis-related diabetes: A position statement of the American Diabetes Association and a clinical practice guideline of the Cystic Fibrosis Foundation, endorsed by the Pediatric Endocrine Society. Diabetes Care 2010;33:2697–2708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schwarzenberg SJ, Thomas W, Olsen TW, Grover T, Walk D, Milla C, Moran A. Microvascular complications in cystic fibrosis-related diabetes. Diabetes Care 2007;30:1056–1061 [DOI] [PubMed] [Google Scholar]
- 10.van den Berg JM, Morton AM, Kok SW, Pijl H, Conway SP, Heijerman HG. Microvascular complications in patients with cystic fibrosis-related diabetes (CFRD). J Cyst Fibros 2008;7:515–519 [DOI] [PubMed] [Google Scholar]
- 11.FitzSimmons SC. The changing epidemiology of cystic fibrosis. J Pediatr 1993;122:1–9 [DOI] [PubMed] [Google Scholar]
- 12.Mosteller RD. Simplified calculation of body-surface area. N Engl J Med 1987;317:1098. [DOI] [PubMed] [Google Scholar]
- 13.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron 1976;16:31–41 [DOI] [PubMed] [Google Scholar]
- 14.National Kidney Foundation K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification and stratification. Am J Kidney Dis 2002;39(Suppl 1):S1–S266 [PubMed] [Google Scholar]
- 15.Mukherjee R, Whitehouse J, Honeybourne D. Renal impairment in cystic fibrosis. Thorax 2008;63:473. [DOI] [PubMed] [Google Scholar]
- 16.Culleton BF, Larson MG, Evans JC, Wilson PW, Barrett BJ, Parfrey PS, Levy D. Prevalence and correlates of elevated serum creatinine levels: the Framingham Heart Study. Arch Intern Med 1999;159:1785–1790 [DOI] [PubMed] [Google Scholar]
- 17.Coresh J, Selvin E, Stevens LA, Manzi J, Kusek JW, Eggers P, Van Lente F, Levey AS. Prevalence of chronic kidney disease in the United States. JAMA 2007;298:2038–2047 [DOI] [PubMed] [Google Scholar]
- 18.Iseki K, Ikemiya Y, Fukiyama K. Risk factors of end-stage renal disease and serum creatinine in a community-based mass screening. Kidney Int 1997;51:850–854 [DOI] [PubMed] [Google Scholar]
- 19.Brennan AL, Geddes DM, Gyi KM, Baker EH. Clinical importance of cystic fibrosis-related diabetes. J Cyst Fibros 2004;3:209–222 [DOI] [PubMed] [Google Scholar]
- 20.Pedersen SS, Jensen T, Osterhammel D, Osterhammel P. Cumulative and acute toxicity of repeated high-dose tobramycin treatment in cystic fibrosis. Antimicrob Agents Chemother (Bethesda) 1987;31:594–599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Prayle A, Smyth AR. Aminoglycoside use in cystic fibrosis: therapeutic strategies and toxicity. Curr Opin Pulm Med 2010;16:604–610 [DOI] [PubMed] [Google Scholar]
- 22.Lopez-Novoa JM, Quiros Y, Vicente L, Morales AI, Lopez-Hernandez FJ. New insights into the mechanism of aminoglycoside nephrotoxicity: an integrative point of view. Kidney Int 2011;79:33–45 [DOI] [PubMed] [Google Scholar]
- 23.Donckerwolcke RA, van Diemen-Steenvoorde R, van der Laag J, Koomans HA, Boer WH. Impaired diluting segment chloride reabsorption in patients with cystic fibrosis. Child Nephrol Urol 1992;12:186–191 [PubMed] [Google Scholar]
- 24.Stenvinkel P, Hjelte L, Alvan G, Hedman A, Hultman E, Strandvik B. Decreased renal clearance of sodium in cystic fibrosis. Acta Paediatr Scand 1991;80:194–198 [DOI] [PubMed] [Google Scholar]
- 25.Ahya VN, Doyle AM, Mendez JD, Lipson DA, Christie JD, Blumberg EA, Pochettino A, Nelson L, Bloom RD, Kotloff RM. Renal and vestibular toxicity due to inhaled tobramycin in a lung transplant recipient. J Heart Lung Transplant 2005;24:932–935 [DOI] [PubMed] [Google Scholar]
- 26.Hoffmann IM, Rubin BK, Iskandar SS, Schechter MS, Nagaraj SK, Bitzan MM. Acute renal failure in cystic fibrosis: association with inhaled tobramycin therapy. Pediatr Pulmonol 2002;34:375–377 [DOI] [PubMed] [Google Scholar]
- 27.Ryan G, Singh M, Dwan K. Inhaled antibiotics for long-term therapy in cystic fibrosis. Cochrane Database Syst Rev 2011;CD001021. [DOI] [PubMed] [Google Scholar]
- 28.Konstan MW, Geller DE, Minic P, Brockhaus F, Zhang J, Angyalosi G. Tobramycin inhalation powder for P. aeruginosa infection in cystic fibrosis: the EVOLVE trial. Pediatr Pulmonol 2010;46:230–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ramsey BW, Pepe MS, Quan JM, Otto KL, Montgomery AB, Williams-Warren J, Vasiljev KM, Borowitz D, Bowman CM, Marshall BC, et al. Intermittent administration of inhaled tobramycin in patients with cystic fibrosis. Cystic Fibrosis Inhaled Tobramycin Study Group. N Engl J Med 1999;340:23–30 [DOI] [PubMed] [Google Scholar]
- 30.Ramsey BW, Dorkin HL, Eisenberg JD, Gibson RL, Harwood IR, Kravitz RM, Schidlow DV, Wilmott RW, Astley SJ, McBurnie MA, et al. Efficacy of aerosolized tobramycin in patients with cystic fibrosis. N Engl J Med 1993;328:1740–1746 [DOI] [PubMed] [Google Scholar]
- 31.MacLusky IB, Gold R, Corey M, Levison H. Long-term effects of inhaled tobramycin in patients with cystic fibrosis colonized with Pseudomonas aeruginosa. Pediatr Pulmonol 1989;7:42–48 [DOI] [PubMed] [Google Scholar]
- 32.Soulsby N, Greville H, Coulthard K, Doecke C. What is the best method for measuring renal function in adults and children with cystic fibrosis? J Cyst Fibros 2010;9:124–129 [DOI] [PubMed] [Google Scholar]
- 33.Glass S, Plant ND, Spencer DA. The effects of intravenous tobramycin on renal tubular function in children with cystic fibrosis. J Cyst Fibros 2005;4:221–225 [DOI] [PubMed] [Google Scholar]
- 34.Orens JB, Estenne M, Arcasoy S, Conte JV, Corris P, Egan JJ, Egan T, Keshavjee S, Knoop C, Kotloff R, et al. International guidelines for the selection of lung transplant candidates: 2006 update–a consensus report from the Pulmonary Scientific Council of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant 2006;25:745–755 [DOI] [PubMed] [Google Scholar]
- 35.Hmiel SP, Beck AM, de la Morena MT, Sweet S. Progressive chronic kidney disease after pediatric lung transplantation. Am J Transplant 2005;5:1739–1747 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.