Abstract
New therapeutic strategies are needed for malignant pleural mesothelioma (MPM). We conducted a single-center, open-label, nonrandomized, pilot and feasibility trial using two intrapleural doses of an adenoviral vector encoding human IFN-α (Ad.IFN-α2b). Nine subjects were enrolled at two dose levels. The first three subjects had very high pleural and systemic IFN-α concentrations resulting in severe “flu-like” symptoms necessitating dose de-escalation. The next six patients had reduced (but still significant) pleural and serum IFN-α levels, but with tolerable symptoms. Repeated vector administration appeared to prolong IFN-α expression levels. Anti-tumor humoral immune responses against mesothelioma cell lines were seen in seven of the eight subjects evaluated. No clinical responses were seen in the four subjects with advanced disease. However, evidence of disease stability or tumor regression was seen in the remaining five patients, including one dramatic example of partial tumor regression at sites not in contiguity with vector infusion. These data show that Ad.IFN-α2b has potential therapeutic benefit in MPM and that it generates anti-tumor immune responses that may induce anatomic and/or metabolic reductions in distant tumor.
Clinical trial registered with www.clinicaltrials.gov (NCT 01212367).
Keywords: clinical trials, immunotherapy, gene therapy
Malignant pleural mesothelioma (MPM) has a dismal prognosis, with current treatments only minimally affecting the disease course (1–3). Our group has previously explored the use of immuno-gene therapy using intrapleural delivery of an adenovirus (Ad) vector expressing the interferon-β gene (Ad.IFNβ) (4–6). Type 1 interferon genes (including those encoding IFN-α and IFN-β) delivered using an adenoviral vector, in contrast to using the proteins themselves, lead to very high local and prolonged IFN concentrations that induce tumor cell death in an immunogenic manner, effectively breaking tolerance and activating a strong anti-tumor immune response (7). Our initial clinical trials were helpful in showing safety, documenting pleural gene transfer, and demonstrating induction of anti-tumor humoral and cellular immune responses, as well as showing clinical responses (4–6), but the Ad.IFN-β vector supply was nonetheless withdrawn by the industry collaborator.
We were subsequently able to obtain a similar E1/E3-deleted replication-defective Ad vector expressing a homologous type 1 human interferon gene, IFN-α2b. Given that our previous trials were limited by short duration of IFN gene expression as evidenced by a rapid decline of intrapleural interferon levels after 24 hours, and that we were unable to achieve successful gene transfer after repeat vector administration with a 7-day dosing interval, we designed and conducted a new a pilot and feasiblity clinical trial involving repeated intrapleural Ad.IFN-α2b administrations in nine patients with MPM designed to answer the following questions: (1) is intrapleural Ad.IFN-α2b administration also safe and well-tolerated?; (2) does this vector give similar levels of transgene expression?; (3) does shortening the dosing interval allow for detectable transgene expression after the second dose?; (4) is there induction of equivalent humoral anti-tumor immunity as seen with Ad.IFN-β?; and (5) is there enough evidence of demonstrable clinical anti-tumor activity of the Ad.IFN-α2b vector to warrant further study?
Methods
Trial Design
This was a single-center, open-label, nonrandomized pilot and feasibility trial (see Figure E1 in the online supplement). The objectives were to determine the maximal tolerated/maximal effective dose and toxicity of Ad.IFN-α2b, to analyze gene transfer by measuring pleural fluid IFN-α, to assess systemic humoral anti-tumor immune responses, and to assess efficacy.
Adenoviral Vector
The vector used in this trial, SCH 721015 (Ad.hIFN-α2b), is a clinical-grade, serotype 5, E1/partial E3-deleted replication-incompetent adenovirus with insertion of the human IFN−α2b gene in the E1 region of the adenoviral genome (8). It was provided by the Schering-Plough Research Institute (Kenilworth, NJ). The trial was funded primarily through funds from the National Cancer Institute (P01 CA66726).
Eligibility
Patients were eligible for this study based upon: (1) a pathologically confirmed MPM diagnosis; (2) an Eastern Cooperative Oncology Group performance status of 0 or 1; and (3) an accessible pleural space for vector instillation. Exclusion criteria included pericardial effusion; recent chemotherapy or radiotherapy; inadequate pulmonary function; significant cardiac, hepatic, or renal disease; or high neutralizing anti-Ad antibody (Nabs) titers.
Protocol Summary
The protocol received full approval by the University Review boards, the Food and Drug Administration, and the NIH Recombinant DNA Advisory Committee. Written informed consent was obtained from each patient at the time of screening, and the study was registered at http://www.clinicaltrials.nih.gov.
Eligible patients with MPM underwent outpatient tunneled intrapleural catheter insertion under local anesthesia or via thoracoscopy. On Study Days 1 and 4, a dose of Ad.hIFN-α2b diluted in 25 to 50 cc of sterile normal saline was instilled into the pleural space. The initial vector dose of 1 × 1012 viral particles of Ad.hIFN-α2b was extrapolated from prior clinical trials of Ad.IFN-β, in which the maximal tolerated dose was 3 × 1012 viral particles. Since we had no experience with this new vector, for safety reasons we chose an initial dose that was one-half log lower than the maximally tolerated dose from prior studies, with the plan for dose-escalation if the initial dose was well tolerated. Subjects were assessed for anti-tumor responses approximately 60 days after initial treatment using chest computed tomography (CT) scans and 18-fluorodeoxyglucose (FDG) positron emission tomography (PET) scans as described (6). They were monitored as outpatients through Day 190 and then by phone contact. If progressive disease was documented any time after 2 months, patients proceeded with other anti-tumor therapies, as indicated and desired.
Enzyme-linked immunosorbent assays (ELISA) were used to measure pleural fluid and serum IFN-α2b levels (PBL Biomedical Labs, Piscataway, NJ) and serum mesothelin-related protein (SMRP) levels (Fujirebio, Inc., Malvern, PA). Nabs were assessed as previously described (6). To detect humoral responses against tumor antigens, immunoblotting against purified proteins and extracts from mesothelioma cell lines was performed using pre- and post-gene transfer sera as previously described (5; also see online supplement for details). Peripheral blood mononuclear cells (PBMC) from pretreatment and 2 days after gene transfer from Patient 309 were evaluated using multicolored flow cytometry with a focus on identifying natural killer (NK) cell activation markers (see Methods section in online supplement).
Results
From February 2009 until July 2010, this study enrolled nine subjects with MPM who progressed through at least one prior anti-neoplastic therapy or had refused front-line therapy (Figure E1, Table 1).
TABLE 1.
CLINICAL CHARACTERISTICS AND OUTCOMES
| ID | Age/Sex | Stage | Dose Level | Baseline Meso-thelin level (nM) | Day 64 Modified RECIST Response | Day 64 Size Change (MR) | Day 64 PET Response | Day 64 Meso-thelin Level | 6-mo Tumor Response | 6-mo Meso-thelin Level | Survival Time (mo post vector) | Reason for Death |
| 301 | 51/M | IV | 1 | 44 | SD | +19.8% | NA | NA | PD | NA | 7 | PD |
| 302 | 68/F | III/IV | 1* | 8 | SD | +14.7% | NA | 13 | PD | 14 | 14 | PD |
| 303 | 79/M | IV | 1 | 23 | NA | NA | NA | NA | NA | NA | 1 | PD |
| 304 | 56/M | I | 2 | 1.4 | SD | −15% | SD | 0.9 | PR (−46%) | NA | 22† | alive |
| 307 | 69/M | III | 2 | 11 | PR | −36% | SD | 26.4 | PD | 34.1 | 8 | PD |
| 308 | 52/M | IV | 2 | 21.9 | PD | +105% | PD | NA | NA | NA | 4 | PD |
| 309 | 71/M | III | 2 | 0.4 | SD | −10% | PR | 0.5 | SD (−20%) | 1.1 | 18† | alive |
| 312 | 80/M | III | 2 | 1.5 | NA | NA | NA | 4‡ | NA | NA | 1 | PD |
| 313 | 59/F | I | 2* | 1.0 | PR | −51 | PR | 1.0 | SD | NA | 11† | Alive |
Definition of abbreviations: Dose Level 1 = 1 × 1012 viral particles per dose, Dose Level 2 = 3 × 1011 viral particles per dose, F = Female, M = male, MR = modified RECIST measurement, NA = not available, PD = progressive disease, PR = partial response, SD = stable disease.
Table summarizes demographic data (subject identification number, age, sex, tumor stage, and dose level) and clinical response data as measured by serum mesothelin levels (in ng/ml), CT scan radiographic responses (using Modified RECIST), 18FDG PET Scan responses (based on SUVmax), survival time, and reason for death.
One dose only.
As of May 2011.
Level measured 30 d after gene transfer.
Cohort 1: Three subjects were enrolled at the first dose level of 1 × 1012 Ad.IFN-α2b viral particles (vps) on Days 1 and 4. Due to relatively severe and protracted “flu-like” symptoms in all three subjects, dose de-escalation was approved for the subsequent cohort. Six subjects were enrolled at the lower dose level of 3 × 1011 Ad.IFN-α2b vps on Days 1 and 4.
Toxicities
In general, Ad.IFN-α2b vector instillation was well tolerated. A full list of toxicities is shown in Table E1. As previously observed (5, 6), most patients developed some degree of fever and chills starting approximately 6 to 8 hours after vector instillation. Unlike our previous Ad.IFN-β trials, the high and prolonged serum IFN-α levels were sometimes associated with protracted “flu-like symptoms” lasting 7 to 10 days. These symptoms were sufficiently significant in Subjects 302 and 313 that these subjects declined the second Ad.IFN-α2b dose.
Two patients who had surgically placed catheters developed pleural catheter–related infections (one at the insertion site and one an empyema). Both were treated successfully with antibiotic therapy, and the trial was amended so that subsequent patients had their pleural catheters removed the day after the second vector instillation. No further infections were observed.
Gene Transfer Assessment
In Cohort 1, the peak concentration levels of IFN-α2b protein in pleural fluid and serum were extremely high: 144, 203, and 1,906 ng/ml. All Cohort 1 subjects had high serum IFN-α concentrations with peaks of 3.6, 4.7, and 7.7 ng/ml. At the lower vector dose, high IFN-α2b protein concentrations were still detected in pleural fluid (or pleural lavages) of all subjects with a range from 2 ng/ml to 127 ng/ml. Even at this lower Ad.IFN-α2b dose, three subjects had high serum IFN-α2b protein concentrations with values of 2.4, 1.2, and 0.5 ng/ml. There was evidence that the second Ad.IFN-α2b dose resulted in successful gene transfer (Figure E2E), unlike our findings from prior trials with Ad.IFN-β (6). For further details, see Figure E2 and Table E2.
Antiviral Immune Responses
Two potential subjects had baseline serum anti-Ad Nab titers greater than 1:1,000 and were not enrolled per protocol. Of the remaining screened patients, the baseline serum anti-Ad Nab titers ranged from less than 1:25 to 1:75, and 1 week after Ad vector instillation, all subjects tested markedly increased their anti-Ad Nab titers (to 1:3,000 or greater). However, on Day 4, at the time the second Ad.IFN-α2b dose was administered, all patient anti-Ad Nab titers were less than 1:1,000 and ranged from less than 1:25 to 1:800. For further details, see Table E3.
Anti-Tumor Immunologic Responses
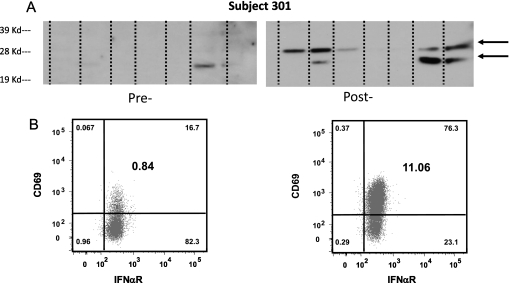
We did not see increases in humoral responses to mesothelin or SV40 virus large T-antigen (two defined mesothelioma-associated antigens) (Table 2). However, we did observe new or increased intensity bands on immunoblots containing extracts of mesothelioma cell lines in seven of eight patients. An example of induction of new bands at 25 and 30 kD on an immunoblot from Subject 301 is shown in Figure 1A. We also observed systemic NK cell activation. Flow cytometry on PBMC showed marked up-regulation of the activation marker CD69 on the NK cells in the post-treatment sample (Figure 1B).
TABLE 2.
ANTI-TUMOR ANTIGEN HUMORAL IMMUNE RESPONSES
| Patient No. | Dose (Viral Particles) | Response to Known Antigens | Response to Tumor Lysates | Response to Multiple Cell Lines |
| 301 | 1012 | None | New 30-kD band | Yes |
| New 25-kD band | ||||
| (36-d sample) | ||||
| 302* | 1012 | None | New 45-kD band | Yes |
| 303 | 1012 | strong baseline mesothelin band- no change | New 60-kD band | Yes |
| 304 | 3 × 1011 | None | New 80-kD band | Yes |
| Increase in 53- and 64-kD bands | ||||
| 307 | 3 × 1011 | None | Increase in 60- and 72-kD bands | Only to autologous cell line |
| 308 | 3 × 1011 | strong baseline mesothelin band- no change (Day 38) | None | None |
| 309 | 3 × 1011 | None | Increase in 65-kD band | Yes |
| 313* | 3 × 1011 | None | Increased in 39- and 43-kD bands | Yes |
Pretreatment serum and serum obtained 2 to4 months after gene transfer was diluted 1:1,500 and reacted with immunoblots containing known mesothelioma antigens (SV40 large T antigen, mesothelin, and Wilms Tumor-1 protein) and lysates from 7 mesothelioma tumor cell lines. The appearance of new or strongly increased bands (along with their molecular mass) are noted. We also observed whether there was a response to multiple cell lines.
Only received on dose of vector.
Figure 1.
Anti-tumor immune responses. (A) Anti-tumor humoral immune responses. Extracts from seven different mesothelioma cancer cell lines were run on an SDS-PAGE gel, transferred to nitrocellulose, and immunoblotted with diluted (1:1,500) pre- and 6-wk post-gene transfer serum from Subject 301. Note the presence of new bands at 25 and 30 kD (arrows) recognized by the post-gene transfer serum. (B) Activation of NK Cells. Peripheral blood mononuclear cells from a pretreatment sample and a sample 2 days after gene transfer were studied from Subject 309 using flow cytometry. NK cells were identified on the basis of the cell surface expression of CD56 and CD16 after gating on the CD3-/CD14-/CD19-/CD20- lymphocytes. Shown are CD3-/CD14-/CD19-/CD20-/CD56dim/CD16+ cells expressing the activation marker CD69 and IFNαR, before gene transfer (A) and 2 days after gene transfer (B). Numbers in the smaller font in the corner of each quadrant represent % of each subset in the parent gate, while numbers in larger font in the middle of the right upper quadrant represent % of activated NK cells (CD3-/CD14-/CD19-/CD20-/CD56dim/CD16+/CD69+/IFNαR+) in the lymphocyte gate. Note the marked up-regulation of the activation marker CD69 in the post-treatment sample.
Clinical Responses
At the time of first radiographic assessment (60 d), three subjects had progressive disease (two had died), four had stable disease, and two had partial responses using modified RECIST criteria (Table 1). 18FDG-PET scan responses were generally similar. As of April 2011, three subjects remain alive at 11, 18, and 22 months after vector delivery. Subjects 304 and 313 had sufficient improvement after IFN-α 2b gene transfer that they were subsequently able to undergo successful radical pleurectomy (RP), with no signs of recurrence to date (7 and 19 mo post-operatively as of May 2011). We also measured serial serum mesothelin-related peptide (SMRP) levels in the patients enrolled in the trial (Table 1). In general, changes in SMRP levels were similar to radiographic responses.
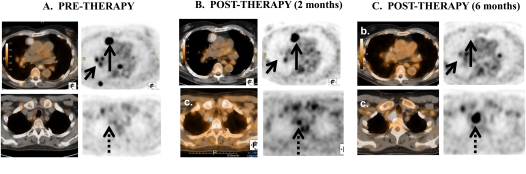
The most impressive radiographic response seen in this pilot study was a mixed response by Modified RECIST seen in Subject 309, a 71-year-old-male who had intra- and extrathoracic disease recurrence (Figure 2A) 9 months status-post RP with intraoperative photodynamic therapy followed by four cycles of adjuvant Pemetrexed/Cisplatin chemotherapy. Two months after Ad.IFN-α2b vector delivery, many of the pleural-based malignant foci had regressed on PET/CT (Figure 2B). On the 6-month follow-up PET/CT, after Ad.IFN-α2b, many lesions had completely regressed, including a dominant anteromedial right paramediastinal pleural-based mass (Figure 2C), as well as several intra- and extrathoracic nodal sites. The response was classified as “mixed,” however, as a high right paratracheal lymph node outside the pleural space demonstrated steady progression over the 6-month follow-up period.
Figure 2.
Mixed response to therapy of Subject 309. 18FDG-PET/CT and CT scans (axial cuts) at two levels from Subject 309 are shown at three time points. (A) Baseline scans showing pleural-based lesions (short arrow) and a large anteriomedial pleural mass (long arrow). There is no lesion in an area of future tumor growth (dotted arrow). (B) Two months after gene transfer, many of the pleural based lesions (short arrow) have regressed with little change in the anteriomedial pleural mass (solid arrow). A small lesion is now seen in a high right paratracheal lymph node (dotted arrow). (C) Six months after gene transfer, many lesions have now completely regressed including the dominant anteromedial right pleural mass (solid arrow). However, the high right paratracheal lymph node (dotted arrow), outside the pleural space, demonstrated steady progression over the 6-month follow-up period.
Discussion
Since the vectors used were virtually identical and the biologic activities of IFN-α and IFN-β are very similar, it was expected that many of the results in this pilot study using Ad.IFN-α2b would be similar to those seen in our previous trials using Ad.IFN-β. Accordingly, intrapleural delivery of Ad.IFN-α2b was generally well tolerated, and predictably induced a transitory innate immune response (cytokine release syndrome) characterized by 18 to 24 hours of fevers and tachycardia. Ad.IFN-α2b also resulted in consistent anti-tumor humoral immune responses (see Table 2), comparable to those detected in our previous Ad.IFN-β trials. Similar anti-tumor humoral immune responses have been demonstrated in other immunotherapy trials in which polyclonal stimulation (in contrast to a specific peptide vaccine) has been used (9). We were also able to detect evidence of significant activation of circulating NK cells in the one patient tested (Figure 1A).
However, we observed a number of interesting differences. Ad.IFN-α2b appeared to be much more potent than Ad.IFN-β, in that pleural IFN concentrations were much higher at equivalent, or even lower, vector doses. The reason for this increased potency is not entirely clear, since the vector backbones and the promoters were virtually identical, but may be related to increased intrapleural stability of IFN-α2b mRNA and/or protein. These increased pleural IFN-α2b concentrations were associated with high serum IFN-α2b levels that engendered systemic “flu-like” symptoms, similar to those commonly seen in patients receiving IFN protein for diseases such as melanoma or hepatitis (9, 10).
Despite the fact that Ad.IFN-α2b makes more interferon than Ad.IFN-β, it is an interesting but answered question whether there are actually differences in the anti-cancer activity between the two vectors in our patients. Ad.IFNα has been reported to induce bladder cancer cell killing in IFN-resistant cells through release of soluble factors (11). This has not been examined for Ad.IFNβ. Despite the fact that both type I IFNs share the same receptor, they do induce some differential responses in various cell types (primarily at low doses) that include differential gene induction (12) or differential cell killing (13). This is most likely due to the fact that IFN-β binds more tightly to the IFN receptor (14), but could also relate to recruitment of accessory proteins to the IFN subtypes receptors (15).
An important goal of this study was explore better dosing strategies. We found that successful IFN-α2b gene transfer after the administration second vector dose (without inactivation by rapidly-increasing anti-Ad Nabs) was possible by altering the dosing interval from 7 to 3 days. This allowed an increased length of expression time, which when coupled with the higher potency of the vector (see above) resulted in much higher, more prolonged levels of IFN-α exposure. Based on preclinical “dose response” data, we hypothesize this may result in improved anti-tumor cellular immune responses.
There are very few effective treatments for advanced mesothelioma refractory to surgery, radiation therapy, and standard chemotherapy agents (3). The median progression-free survival in second-line therapy is only 3 months. It is thus important that we were able to demonstrate radiographic and biochemical evidence of clinical anti-tumor activity in some of our patients. The responses seen in this pilot study, albeit anecdotal, are notable, as there are no proven second- or third-line agents for the treatment of mesothelioma.
Although it is difficult to obtain definitive clinical outcome data in a small pilot trial in a heterogenous population of heavily pretreated patients, the documentation of disease regression or stability seen in several patients is of note, given the poor prognosis of refractory MPM. We noted good congruence among CT scans, 18FDG PET scans, and serum SMRP measurements.
Given these encouraging responses in a subpopulation of our patients, we have begun to try to identify those patients most likely to respond. Our earlier trials had established that serum or pleural fluid titers of neutralizing anti-Ad antibody less than 1:1000 essentially precluded gene transfer (5, 6). By excluding potential subjects who had high baseline anti-Ad Nab titers, all of our subjects had clearly measurable gene transfer (pleural IFN concentrations > 2 ng/ml) after the first vector instillation. Age may be a factor. Subject 303 (aged 79 yr) and subject 312 (aged 80 yr) had very short survivals. It is known that immune system activity declines with age. Tumor volume also appears to be important. As with our previous trials (5, 6), and most other immunotherapy trials, we saw no benefit in our subjects with very advanced disease. Patients 303 and 308, who had short survival times, had very high SMRP levels (> 20 nM). Patient 301 also had a very high SMRP level, but had peritoneal mesothelioma with metastasis to the pleura and perhaps more indolent disease. However, in some of our younger subjects with smaller disease burden (Subjects 302, 304, 307, 309, and 313), we saw either stable disease or actual tumor regression by CT and PET (Figure 2 and Table 1). Finally, based on animal data suggesting that Ad.IFN-induced cell death is important in stimulating the anti-tumor immune response, we are generating some intriguing preliminary data that those patients whose post-treatment pleural fluid showed release of cell death markers (like HMGB1) and/or with intact IFN response pathways may be more likely to have a clinical response.
Given its safety and potential for efficacy in at least some patients, we are now working to enhance and to expand our clinical responses. Our new approaches are based on preclinical data showing that COX-2 inhibition can improve immune responses (16, 17) and that Ad.IFN gene therapy followed with either pemetrexed/cisplatin or pemetrexed/gemcitabine can markedly enhance anti-tumor efficacy (18). Accordingly, we have initiated a multi-modality trial for patients with MPM. Subjects receive two Ad.IFN-α2b doses separated by 3 days in association with oral celecoxib. Two weeks after the first vector instillation, subjects are treated with standard courses of front-line (pemetrexed/cisplatin) or second-line (carboplatin/gemcitabine) chemotherapy. Given our encouraging results in this trial with advanced-stage patients, we believe that multi-modality regimens incorporating immunogene therapy will have an important role in the treatment of patients with earlier-stage MPM.
Supplementary Material
Acknowledgments
The authors thank the following individuals at Penn for their assistance: Aaron Blouin, Kathleen Haines, and Ben Paramonte. The authors would also like to thank Drs. Beth Hutchins, David Cutler, and Thomas Haverty at Schering-Plough/Merck for their continued support and assistance. This paper is dedicated to the late Dr. Richard Carroll, a wonderful colleague, collaborator, and friend who will be greatly missed.
Footnotes
Supported primarily by a grant from the National Cancer Institute (NCI PO1 CA66726). Partially supported by the Shein Foundation for Humanity and an NIEHS funded Environmental Health Sciences Core Center grant P30-ES013508 and by U01AI065279 (to L.J.M.). ScheringPlough/Merck provided the Ad.INFα2b vector.
Author Contributions: D.H.S., A.H., C.H.J., and S.M.A. designed the study and wrote the manuscript. D.H.S., A.H., A.R., E.M., A.V., and C.G. recruited patients and conducted the trial. D.S., G.C., J.S., E.P., L.J.M., and R.C. collected and analyzed samples and helped in writing. S.I.K. evaluated all radiographs. D.F.H. did the statistical analyses. L.L. evaluated all pathology. J.F. and M.C. inserted the pleural catheters under surgical guidance.
This article has an online supplement, which is accessible form this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201103-0554CR on June 3, 2011
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Sterman DH, Albelda SM. Advances in the diagnosis, evaluation, and management of malignant pleural mesothelioma. Respirology 2005;10:266–283 [DOI] [PubMed] [Google Scholar]
- 2.Albelda S.M, Sterman D, Litzky L. Malignant mesothelioma. : Fishman AP, editor Pulmonary diseases and disorders, 4th ed New York: McGraw-Hill; 2008. pp. 1535–1552 [Google Scholar]
- 3.Fennell DA, Gaudino G, O'Byrne KJ, Mutti L, Van Meerbeeck J. Advances in the systemic therapy of malignant pleural mesothelioma. Nat Clin Pract Oncol 2008;5:136–147 [DOI] [PubMed] [Google Scholar]
- 4.Sterman DH, Gillespie CT, Carroll R, Coughlin CM, Lord EM, Sun J, Haas A, Recio A, Kaiser LR, Coukos G, et al. Interferon-beta adenoviral gene therapy in a patient with ovarian cancer. Nat Clin Oncol 2006;3:633–663 [DOI] [PubMed] [Google Scholar]
- 5.Sterman DH, Recio A, Carroll RG, Gillespie CT, Haas A, Vachani A, Kapoor V, Sun J, Hodinka R, Brown JL, et al. A phase I clinical trial of single-dose intrapleural IFN-beta gene transfer for malignant pleural mesothelioma and metastatic pleural effusions: high rate of antitumor immune responses. Clin Cancer Res 2007;13:4456–4466 [DOI] [PubMed] [Google Scholar]
- 6.Sterman DH, Recio A, Haas AR, Vachani A, Katz SI, Gillespie CT, Cheng G, Sun J, Moon E, Pereira L, et al. A phase I trial of repeated intrapleural adenoviral-mediated interferon-beta gene transfer for mesothelioma and metastatic pleural effusions. Mol Ther 2010;18:852–860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vachani A, Sterman DH, Albelda SM. Cytokine gene therapy for malignant pleural mesothelioma. J Thorac Oncol 2007;2:265–267 [DOI] [PubMed] [Google Scholar]
- 8.Demers GW, Sugarman BJ, Beltran JC, Westreich LN, Ahmed CM, Lau JY, Hong Z, Lanford RE, Maneval DC. Interferon-alpha2b secretion by adenovirus-mediated gene delivery in rat, rabbit, and chimpanzee results in similar pharmacokinetic profiles. Toxicol Appl Pharmacol 2002;180:36–42 [DOI] [PubMed] [Google Scholar]
- 9.Kijanka G, Murphy D. Protein arrays as tools for serum autoantibody marker discovery in cancer. J Proteomics 2009;72:936–944 [DOI] [PubMed] [Google Scholar]
- 10.Jonasch E, Haluska FG. Interferon in oncological practice: review of interferon biology, clinical applications, and toxicities. Oncologist 2001;6:34–55 [DOI] [PubMed] [Google Scholar]
- 11.Zhang X, Dong L, Chapman E, Benedict WF. Conditioned medium from Ad-IFN-α infected bladder cancer and normal urothelial cells is cytotoxic to cancer cells but not normal cells; further evidence for a strong bystander effect. Cancer Gene Ther 2008;15:817–822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Da Silva AJ, Brickelmaier M, Majeau GR, Lukashin AV, Peyman J, Whitty A, Hochman PS. Comparison of gene expression patterns induced by treatment of human umbilical vein endothelial cells with IFN-α2b vs. INF-β1a: understanding the functional relationship between distinct type 1 interferons that act through a common receptor. J Interferon Res 2002;22:173–188 [DOI] [PubMed] [Google Scholar]
- 13.Fleischmann CM, Fleischmann WRJ. Differential antiproliferative activities of INFs alpha, beta and gamma: kinetics of establishment of their antiproliferative effects and the rapid development of resistance to INFs alpha and beta. J Biol Regul Homeost Agents 1988;2:173–185 [PubMed] [Google Scholar]
- 14.Jaitin DA, Roisma LC, Jaks E, Gavutis M, Piehler J. Inquiring into the differential action of interferons: an IFN-α2 mutant with enhanced affinity to INFAR1 is functionally similar to INF-β. Mol Cell Biol 2006;26:1888–1897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Platanias LC, Uddin S, Domanski P, Colamonici OR. Differences in interferon alpha and beta signaling. Interferon beta selectively induces the interaction of the alpha and betaL subunits of the type 1 interferon receptor. J Biol Chem 1996;271:23630–23633 [DOI] [PubMed] [Google Scholar]
- 16.Delong P, Tanaka T, Kruklitis R, Henry A, Kapoor V, Kaiser LR, Sterman DH, Albelda SM. Use of cycloxygenase-2 inhibition to enhance the efficacy of immunotherapy. Cancer Res 2003;63:7845–7852 [PubMed] [Google Scholar]
- 17.Haas A, Sun J, Vachani A, Wallace AF, Silverberg M, Kapoor V, Albelda M. Cyclooxygenase-2 Inhibition augments efficacy of a cancer vaccine. Clin Cancer Res 2006;12:214–222 [DOI] [PubMed] [Google Scholar]
- 18.Fridlender ZG, Sun J, Singhal S, Kapoor V, Cheng G, Suzuki E, Albelda SM. Chemotherapy delivered after viral immuno-gene therapy augments anti-tumor efficacy via multiple immune-mediated mechanisms. Mol Ther 2010;18:1947–1959 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.