Abstract
Rationale: Idiopathic pulmonary fibrosis (IPF) is a lethal lung disease of unknown etiology with a variable and unpredictable course.
Objectives: The aim of this study was to identify and validate plasma proteins that are predictive of outcome in IPF.
Methods: Plasma samples were available for 241 patients with IPF (140 derivation and 101 validation). In the derivation cohort, concentrations of 92 proteins were analyzed using a multiplex bead-based immunoassay and concentrations of matrix metalloproteinase (MMP)-7, MMP-1, and surfactant protein D were assessed by ELISA. In the validation cohort concentrations of intercellular adhesion molecule (ICAM)-1, IL-8, and vascular cell adhesion molecule (VCAM)-1 were assessed by bead-based multiplex assay, and S100A12 and MMP-7 by ELISA. Associations of biomarkers with mortality, transplant-free survival, and disease progression were tested in the derivation and validation cohorts using nonparametric methods of survival analysis and the Cox proportional hazards model, and an integrated risk prediction score was derived and tested.
Measurements and Main Results: High concentrations of MMP-7, ICAM-1, IL-8, VCAM-1, and S100A12 predicted poor overall survival, poor transplant-free survival, and poor progression-free survival in the derivation cohort. In the independent validation cohort high concentrations of all five were predictive of poor transplant-free survival; MMP-7, ICAM-1, and IL-8 of overall survival; and ICAM-1 of poor progression-free survival. The personal clinical and molecular mortality prediction index derived in the derivation cohort was highly predictive of mortality in the validation cohort.
Conclusions: Our results suggest that plasma proteins should be evaluated as a tool for prognosis determination in prioritization of patients for lung transplantation and stratification in drug studies.
Keywords: MMP-7, adhesion molecules, biomarkers, personalized medicine, mortality prediction
At a Glance Commentary
Scientific Knowledge on the Subject
Idiopathic pulmonary fibrosis (IPF) is a chronic progressive lung disease with relatively unpredictable course. Efforts to identify molecular markers of disease have been limited. Recent evidence suggests that peripheral blood proteins are indicative of disease presence and outcome in patients with IPF.
What This Study Adds to the Field
This follow-up study identifies novel protein biomarkers of disease progression and mortality in the peripheral blood of patients with IPF, and then validates that these markers indeed indicate a high risk of death in an independent cohort. Using a combination of protein markers and patient characteristics we derived a risk score that accurately distinguishes high and low mortality risk subgroups in both cohorts. The availability of protein biomarkers and validated integrated risk scores should lead to better evaluation and stratification of patients with IPF for research, and for transplant prioritization.
Idiopathic pulmonary fibrosis (IPF) is a chronic progressive fibrotic lung disease of unknown etiology with median survival of 2.5–3 years largely unaffected by currently available medical therapies (1, 2). The prevailing hypothesis of disease pathogenesis suggests the disease begins as an alveolar epithelial injury with aberrant alveolar reepithelialization (3). What is believed to follow is a cascade of events including epithelial–mesenchymal transformation, resident fibroblast–myofibroblast transformation, and recruitment of fibrocytes to areas of injury to facilitate alveolar repair (4). Other events include macrophage activation (5), epithelial cell apoptosis (6), activation of developmental pathways (7), oxidative injury (8), and epithelial mesenchymal transition (4). Release of cytokines, chemokines, coagulation, and growth factors and angiogenic factors have been shown to play critical roles in disease pathogenesis (9, 10). Although the relative contribution of these events is unclear, the end result is fibrotic progression that is relentless but relatively unpredictable.
Traditionally, IPF has been considered a slowly progressive disease; however, recent observations suggest that some patients may experience an accelerated course, whereas others may experience acute respiratory declines (11–13). Clinical and physiologic parameters, although useful to monitor disease, have had relatively limited acceptance as predictors of disease progression or mortality in patients with IPF (14), highlighting the need for molecular predictors of disease progression and outcome, preferably in the accessible peripheral blood (15).
The use of peripheral blood proteins as potential biomarkers is supported by recent studies that demonstrate reduced survival in patients with IPF with high serum concentrations of mucin 1 (KL-6) (16), CCL-18 (17), or surfactant protein A (18). Previously, we applied a multianalyte protein assay to identify a peripheral blood protein signature that distinguished patients with IPF from control subjects and from other lung diseases (chronic obstructive pulmonary disease, sarcoidosis, and hypersensitivity pneumonitis) (19). Although we did not specifically test in that study whether the protein signature was associated with outcome, it was demonstrated that peripheral blood matrix metalloproteinase (MMP)-7 concentrations were correlated with disease severity (19).
In the present study 95 proteins in the peripheral blood were screened to identify predictors of disease progression and mortality in a derivation cohort of 140 well-characterized patients with IPF. Screening for markers in the peripheral circulation identified several that strongly predict mortality in IPF. These were then validated in an independent validation cohort of 101 patients with IPF. Some of these results were reported in an abstract and poster (20) at the 2010 International Conference of the American Thoracic Society.
Methods
See the Methods section in the online supplement for a more detailed description.
IPF Cohort
Plasma samples were available from 241 patients: 140 patients with IPF in a derivation cohort and 101 patients with IPF a validation cohort. All patients were evaluated at the University of Pittsburgh Medical Center, Pittsburgh, PA. The diagnosis of IPF was established on the basis of American Thoracic Society and European Respiratory Society criteria (21, 22), and surgical lung biopsy when clinically indicated. Clinical data were available through the Simmons Center database at the University of Pittsburgh. Smoking status was defined as previously described (23). All studies were approved by the institutional review board and all patients signed informed consent to participate in the study.
Subjects enrolled in the study were followed at intervals of 3 to 4 months according to usual care practices at the Simmons Center. Physiologic data (pulmonary function tests [PFT] and oxygen desaturation studies) and physician assessments were performed at all visits. Radiographic studies (X-rays or computed tomography scans) were performed when clinically indicated.
Analysis of Plasma Proteins
Concentrations of 92 cytokines and chemokines, MMPs, and markers of apoptosis and epithelial injury (see Table E1 in the online supplement) were analyzed using the Human DiscoveryMAP multiplex bead-based immunoassay (Rules-Based Medicine, Austin, TX) (24). Proteins were excluded from the analysis if concentrations were lower than the lowest detectable dose, defined as the mean plus 3 SDs of 20 blank readings, in at least 95% of samples in both cohorts. For the validation study, plasma concentrations of intercellular adhesion molecule (ICAM), vascular cell adhesion molecule (VCAM)-1, and IL-8 were analyzed using Luminex technology with a Bio-Plex 100 and Bio-Plex manager software 5.0 (Bio-Rad, Hercules, CA). Plasma concentrations of MMP-7, MMP-1, and surfactant protein D were measured by ELISA as recommended by the manufacturer (R&D Systems, Minneapolis, MN) as was S100A12 (MBL International, Woburn, MA).
Statistical Analysis
Time-to-event outcomes analyzed include mortality, transplant-free survival, and progression-free survival. For transplant-free survival, in addition to mortality, transplants were counted as events. For progression-free survival analysis patients were followed from the blood draw until (1) disease progression, defined as the first relative decline of 10% or more in FVC % predicted within a 1-year interval; (2) death; or (3) censoring at the last contact. Any patient receiving a lung transplant during follow-up was censored at transplant date in both mortality and progression-free survival analyses but not in transplant-free survival. Data were analyzed using the survival package (25) of the R environment (26), particularly. Survival curves were estimated using the Kaplan-Meier method. For each outcome, each protein detectable in plasma was dichotomized into high- and low-risk ranges using profile likelihood (see Methods in the online supplement) (27). Associations of biomarkers with IPF were tested using the log-rank test and Cox proportional hazards model. The proportional hazards model was used to adjust for age, sex, and baseline pulmonary function assessed by FVC or composite physiologic index (CPI) (28). For derivation of the personal clinical and molecular mortality index (PCMI), the stepAIC approach was applied (29) for variable selection in the Cox proportional hazards model and the PCMI was computed by multiplying the β coefficients by 100 and summing (see Methods in the online supplement) (30). For multiple testing in marker selection the Bonferroni method was used to control the family-wise error rate at 5%.
Results
The online supplement contains full results tables and martingale residuals plots for all outcomes and predictors for the derivation and validation cohorts.
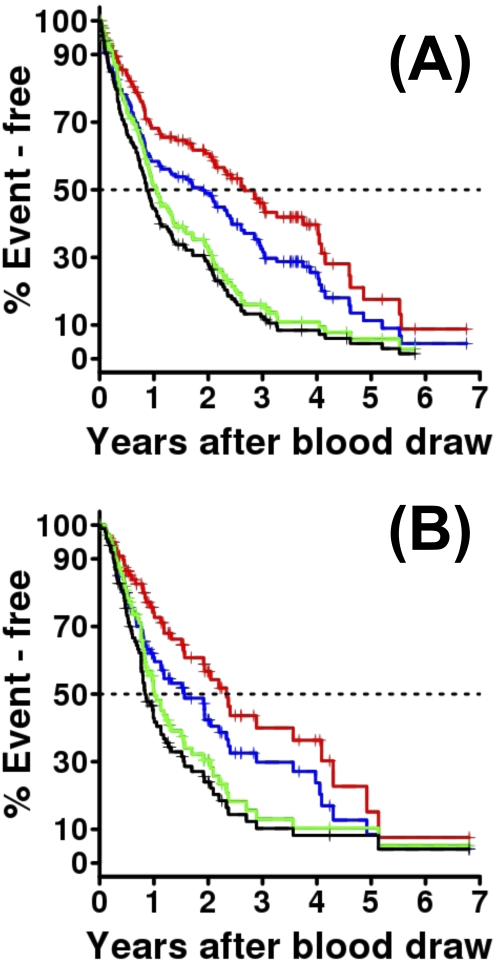
Patient Characteristics
Patient characteristics in the derivation and validation cohorts are summarized in Table 1. Nearly all (98.6%) patients in the derivation cohort were white. The average age was 67.2 ± 8.3 years, the male to female ratio was 2.6:1, and only a quarter of patients had never smoked. Overall, 60.7% of patients had a histologically confirmed diagnosis, 25.7% were listed for transplant, and 21.4% were transplanted. About one-third of patients with IPF in the derivation cohort were on immunosuppressant treatment (prednisone, azathioprine, or cyclophosphamide) at the time of the study blood draw. Although the overall demographics, PFTs, and smoking history of the validation cohort were similar to the derivation cohort, the percentages of patients diagnosed histologically and of patients treated with immunosuppressant medications was significantly lower in the validation cohort (Table 1). Importantly, the median follow-up was significantly higher in the derivation cohort (Table 1). The overall estimated median survival from blood draw for the derivation cohort was 2.66 years (Figure 1A, red line); the median transplant-free survival was 1.9 years (Figure 1A, blue line); the median progression-free survival by FVC was 1.05 years after blood draw (Figure 1A, green line); and when lung transplant was treated as an event progression-free survival was 0.86 years after blood draw (Figure 1A, black line). In the validation cohort median survival from blood draw was 2.34 years (Figure 2A, red line); the median transplant-free survival was 1.56 years (Figure 2A, blue line); the median progression-free survival by FVC was 1.01 years after blood draw (Figure 2A, green line); and transplant-free progression-free survival was 0.84 years after blood draw (Figure 2A, black line). The entire survival distribution in both cohorts was similar (P = 0.927) and to what has been reported in IPF in the general population (31–33). The similarities in demographics, PFTs, transplant rates, and outcomes between the two cohorts suggest that the differences in immunosuppressant use are potentially indicative of shifting practice patterns and not population characteristics.
TABLE 1.
PATIENT CHARACTERISTICS
| Derivation Cohort (N = 140) |
Validation Cohort (N = 101) |
|||||
| N | % | N | % | P Value | ||
| Sex | ||||||
| Male | 101 | 72.1 | 65 | 64.4 | 0.21 | |
| Female | 39 | 27.9 | 36 | 35.6 | ||
| Race | ||||||
| White | 138 | 98.6 | 97 | 96 | 0.38 | |
| African-American | 1 | 0.7 | 2 | 2 | ||
| Native American | 1 | 0.7 | 0 | 0 | ||
| Oriental | 0 | 0 | 1 | 1 | ||
| Unknown | 0 | 0 | 1 | 1 | ||
| Smoking | ||||||
| Current | 2 | 1.4 | 4 | 4 | 0.058 | |
| Former | 102 | 72.9 | 60 | 59.4 | ||
| Never | 36 | 25.7 | 37 | 36.6 | ||
| Diagnosis made | ||||||
| Clinically | 55 | 39.3 | 60 | 59.4 | 0.003 | |
| Histologically | 85 | 60.7 | 41 | 40.6 | ||
| Transplant | ||||||
| Evaluated | 68 | 48.6 | 56 | 55.4 | 0.36 | |
| Listed | 36 | 25.7 | 25 | 24.7 | 0.98 | |
| Transplanted | 30 | 21.4 | 20 | 19.8 | 0.88 | |
| Immunosuppressants* | No | 93 | 66.4 | 84 | 83.2 | 0.005 |
| Yes | 47 | 33.6 | 17 | 16.8 | ||
| Prednisone | 43 | 30.7 | 12 | 11.9 | ||
| Azathioprine | 5 | 3.6 | 3 | 3 | ||
| Cyclophosphamide | 5 | 3.6 | 0 | 0 | ||
| Mycophenolate | 2 | 1.4 | 0 | 0 | ||
| Tacrolimus | 1 | 0.7 | 0 | 0 | ||
| N-Acetylcysteine | 0 | 0 | 2 | 2 | ||
| Colchicine | 0 | 0 | 1 | 1 | ||
| Interferon | 0 | 0 | 1 | 1 | ||
| Mean | SD | |||||
| Age, yr | ||||||
| Overall | 67.2 | 8.3 | 68.2 | 9.4 | 0.4 | |
| Male | 67.5 | 8.3 | 68.3 | 8.7 | 0.5 | |
| Female | 66.4 | 8.2 | 68 | 10.5 | 0.5 | |
| Follow-up, yr | ||||||
| All patients | 1.8 | 1.6 | 1.4 | 1.3 | 0.04 | |
| Alive and not transplanted | 2.7 | 1.6 | 1.7 | 1.3 | 0.004 | |
| Baseline PFTs | ||||||
| FVC % predicted | 62 | 19.6 | 61.4 | 18 | 0.8 | |
| DLCO % predicted | 44.8 | 17.1 | 45.4 | 19 | 0.8 | |
| CPI | 53.4 | 12.7 | 53.1 | 14.1 | 0.9 | |
Definition of abbreviations: CPI = composite physiologic index; DLCO = diffusing capacity of carbon monoxide; PFT = pulmonary function tests.
Immunosuppressant therapy at time of blood sample.
Figure 1.
Patient outcomes of the derivation and validations cohorts. Red indicates the Kaplan-Meier plot of mortality. The only admissible event is death without lung transplantation. Any patient undergoing lung transplantation is censored. The dotted black line at 0.5 on the y axis intersects the survival curve at the median mortality. Blue indicates the Kaplan-Meier plot of transplant-free survival, which is similar to mortality, except that lung transplantation is treated as an event, rather than being censored. Green indicates the Kaplan-Meier plot of progression-free survival. Disease progression event is defined as a relative decline of at least 10% in FVC % predicted within any 1-year interval during follow-up. Death with no recorded disease progression is also counted as an event. A patient receiving a lung transplant during follow-up is censored at transplant date. Patients with no follow-up record of pulmonary function tests are excluded from this analysis. Black indicates the Kaplan-Meier plot of progression-free transplant-free survival: progression-free survival where lung transplant is treated as an event. (A) The derivation cohort with median time-to-event (Greenwood 95% confidence interval). (B) The validation cohort with median time-to-event (Greenwood 95% confidence interval).
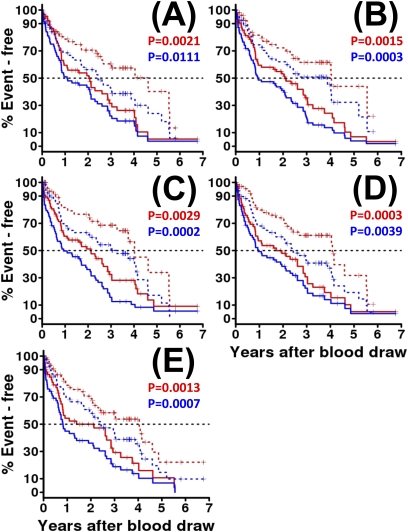
Figure 2.
Peripheral blood biomarkers strongly predict idiopathic pulmonary fibrosis outcomes in the derivation cohort. For each biomarker, red indicates the Kaplan-Meier plot of overall survival (OS) by peripheral blood concentration dichotomized at an optimal threshold as described in the text and related to OS by the log-rank test. Blue indicates the Kaplan-Meier plot of transplant-free survival, by peripheral blood concentration dichotomized at an optimal threshold and related to transplant-free survival by the log-rank test. For each outcome and biomarker, high concentrations, above the threshold, are indicated by a solid line, low concentrations, below the threshold, by a broken line. The log-rank P values comparing high with low concentrations are shown in the appropriate color for OS and transplant-free survival are shown above each plot. The markers shown are (A) matrix metalloproteinase-7, (B) intercellular adhesion molecule-1, (C) IL-8, (D) vascular cell adhesion molecule-1, and (E) S100A12.
Identification of Plasma Proteins that Predict Outcome in Derivation Cohort
Of the 95 candidate proteins tested in this study (92 Rules-Based Medicine and 3 ELISA), 75 were detectable in the plasma (see Table E1) and 5 (MMP-7, ICAM-1, IL-8, VCAM-1, and S100A12) were significantly associated with mortality or disease progression in univariate analysis after Bonferroni correction (Table 2). Concentrations higher than the calculated threshold plasma concentrations for each of those proteins were associated with significantly lower median survival times (1.4–2.1 yr) and concentrations lower were associated with significantly higher median survival times (3–4.6 yr) (Table 2 and Figure 2). The same was observed for transplant-free survival (0.8–1.1 and 2.4–3.9 yr, respectively); progression-free survival (0.8–0.9 and 1.3–1.9 yr, respectively); and transplant- and progression-free survival (0.6–0.8 and 1.1–1.3 yr, respectively) (Table 2). The shortest median survival (1.4 yr) was seen with high S100A12 concentrations, exceeding 26.5 ng/ml (Figure 2E, red lines), and the longest median survival (4.6 yr) was seen with low concentrations of MMP-7, below 4.3 ng/ml (Figure 2A, red lines). The shortest transplant-free survival (0.8 yr) was seen with high S100A12 concentrations, exceeding 26.5 ng/ml, (Figure 2E, blue lines), and the longest transplant-free survival (3.9 yr) was seen with low concentrations of ICAM-1, below 202.5 ng/ml (Figure 2B, blue lines). The mortality hazards ratios were generally similar for all five markers (unadjusted 2.1–2.4) (Table 2) meaning that high concentrations, above the threshold, of MMP-7, ICAM-1, IL-8, VCAM-1, or S100A12 are associated with at least twofold higher risk of death during follow-up. These results persisted after adjustment for age, sex, and baseline FVC or CPI, as shown in Table 2. Although for most markers hazards ratios were not changed much by adjustment for age, sex, and FVC % predicted or CPI, the mortality hazards ratio for high versus low concentrations of MMP-7 increased substantially on adjustment (from 2.1–2.9) indicating nearly threefold higher risk associated with high MMP-7 levels, above 4.3 ng/ml, when age, sex, and baseline pulmonary function were statistically controlled.
TABLE 2.
PERIPHERAL BLOOD PREDICTORS OF OUTCOMES FOR 140 PATIENTS WITH IPF, DERIVATION COHORT
| Low Marker (below threshold) |
High Marker (above threshold) |
||||||||
| Marker | Threshold (ng/ml) | Pval. | HR | Adj. Pval. | Adj. HR | Median (yr) | 95% CI | Median (yr) | 95% CI |
| Overall survival | |||||||||
| MMP-7 | 4.3 | 0.0021 | 2.1 | 0.0013 | 2.9 | 4.6 | 2.7–∞ | 2 | 0.9–2.9 |
| ICAM-1 | 202.5 | 0.0015 | 2.3 | 0.0018 | 2.6 | 4 | 2.7–∞ | 2.1 | 1–3 |
| IL-8 | 0.0072 | 0.0029 | 2.2 | 0.013 | 2.4 | 4 | 3.7–∞ | 2.1 | 1.1–2.8 |
| VCAM-1 | 418 | 0.00030 | 2.4 | 0.0015 | 2.6 | 4.1 | 2.7–∞ | 1.7 | 0.8–2.8 |
| S100A12 | 26.5 | 0.0013 | 2.1 | 0.05 | 1.8 | 4 | 2.3–∞ | 1.4 | 0.8–2.9 |
| Transplant-free survival | |||||||||
| MMP-7 | 4.2 | 0.011 | 1.7 | 0.0036 | 2 | 2.4 | 1.7–4 | 1.1 | 0.8–2.1 |
| ICAM-1 | 202.5 | 0.00029 | 2.2 | 0.00036 | 2.3 | 3.9 | 1.7–∞ | 0.9 | 0.8–2.1 |
| IL-8 | 0.0086 | 0.00025 | 2.1 | 0.038 | 1.7 | 3.3 | 2.1–4.6 | 0.9 | 0.6–1.9 |
| VCAM-1 | 418 | 0.0039 | 1.8 | 0.015 | 1.7 | 2.4 | 1.7–4.1 | 1 | 0.7–2.1 |
| S100A12 | 26.5 | 0.00073 | 1.9 | 0.027 | 1.6 | 2.4 | 1.9–4 | 0.8 | 0.6–2.1 |
| Progression-free survival: FVC 10% relative decline within 1 year(lung transplant is censored) | |||||||||
| MMP-7 | 4.4 | 0.00012 | 2.3 | 0.0087 | 2 | 1.6 | 1.1–2.5 | 0.8 | 0.6–1.1 |
| ICAM-1 | 202.5 | 0.033 | 1.5 | 0.05 | 1.6 | 1.3 | 1–2.3 | 0.9 | 0.7–1.3 |
| IL-8 | 0.0092 | 0.00014 | 2.1 | 0.006 | 2 | 1.4 | 1.1–2.2 | 0.8 | 0.6–1 |
| VCAM-1 | 399.5 | 0.0013 | 1.9 | 0.024 | 1.7 | 1.9 | 1.3–2.4 | 0.8 | 0.7–1 |
| S100A12 | 31.5 | 0.0041 | 1.8 | 0.01 | 1.9 | 1.3 | 1–2 | 0.8 | 0.4–1 |
| Progression-free survival: FVC 10% relative decline within 1 year(lung transplant counts as event) | |||||||||
| MMP-7 | 4.4 | 0.0001 | 2.2 | 0.0056 | 1.8 | 1.3 | 0.9–2.3 | 0.7 | 0.5–1 |
| ICAM-1 | 202.5 | 0.012 | 1.6 | 0.039 | 1.5 | 1.1 | 0.9–2.1 | 0.8 | 0.5–0.9 |
| IL-8 | 0.0092 | 0.0003 | 1.9 | 0.12 | 1.4 | 1.2 | 1–2 | 0.7 | 0.5–0.9 |
| VCAM-1 | 390.5 | 0.0038 | 1.8 | 0.074 | 1.4 | 1.3 | 0.9–2.3 | 0.8 | 0.6–1 |
| S100A12 | 26.5 | 0.0024 | 1.7 | 0.011 | 1.7 | 1.1 | 0.9–1.9 | 0.6 | 0.4–0.9 |
Definition of abbreviations: Adj. HR = age, sex, and baseline FVC adjusted hazards ratio; Adj. Pval. = age, sex, and baseline FVC adjusted P value; CI = confidence interval; HR = hazard ratio; ICAM = intercellular adhesion molecule; IPF = idiopathic pulmonary fibrosis; MMP = matrix metalloproteinase; Pval. = log-rank P value; VCAM-1 = vascular cell adhesion molecule-1.
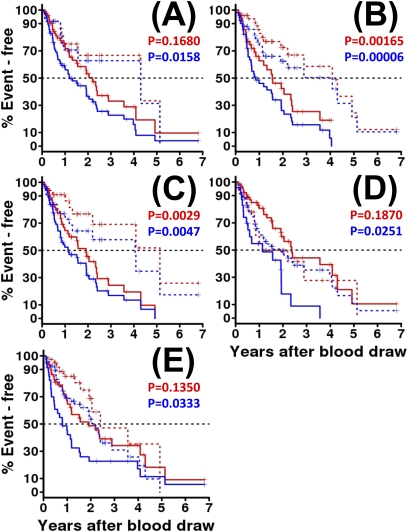
Analysis of Plasma Proteins that Predict Outcome in Validation Cohort
Increased concentrations of all five markers were significantly associated with decreased transplant-free survival (Table 3 and Figure 3). The shortest transplant-free survival (0.8 yr) was seen with high S100A12 concentrations (Figure 3E, blue lines) and the longest transplant-free survival (4.3 yr) was seen with low concentrations of MMP-7 (Figure 3A, blue line). The transplant-free survival hazards ratio ranged from 1.7–3 (Table 2). These results persisted after adjustment for age, sex, and baseline FVC or CPI, for all markers except IL-8 as shown in Table 2A. Similarly to previous observations, the hazards ratio for MMP-7 increased and the P value decreased when age, sex, and FVC were adjusted suggesting that MMP-7 may be effective for predicting outcome in patients with IPF with similar pulmonary function at presentation. When transplant was censored, increased plasma concentrations of only two markers (ICAM-1 and IL-8) were significantly predictive of shorter survival in the validation cohort (Table 3 and Figure 3). High concentrations of ICAM-1 were significantly associated with a median survival of 1.6 years, whereas lower concentrations were associated with a median survival of 4.1 years (Figure 3B, red line). High concentrations of IL-8 were significantly associated with a median survival of 1.9 years, whereas lower concentrations were associated with a median survival of 5.1 years (Figure 3C, red line). Interestingly, although MMP-7 was not significantly predictive of mortality, it became borderline predictive when age, sex, and baseline FVC were adjusted (Table 3). Only ICAM-1 was significantly associated with progression-free survival or transplant- and progression-free survival in the validation cohort (Table 3).
TABLE 3.
PERIPHERAL BLOOD PREDICTORS OF OUTCOMES FOR 101 PATIENTS WITH IPF, VALIDATION COHORT
| Low Marker (below threshold) |
High Marker (above threshold) |
||||||||
| Marker | Threshold (ng/ml) | Pval. | HR | Adj. Pval. | Adj. HR | Median (yr) | 95% CI | Median (yr) | 95% CI |
| Overall survival | |||||||||
| MMP-7 | 3.5 | 0.17 | 1.7 | 0.071 | 2.2 | 4.3 | 1.6–∞ | 2.2 | 1.5–3.6 |
| ICAM-1 | 300 | 0.0016 | 2.9 | 0.0051 | 2.8 | 4.1 | 2.9–∞ | 1.6 | 1–2.4 |
| IL-8 | 0.0097 | 0.0030 | 3.1 | 0.069 | 2.3 | 5.1 | 4.1–∞ | 1.9 | 1.2–2.9 |
| VCAM-1 | 246.8 | 0.19 | 0.7 | 0.61 | 0.8 | 1.1 | 0.8–∞ | 2.4 | 1.9–∞ |
| S100A12 | 49.1 | 0.14 | 1.7 | 0.12 | 1.8 | 2.4 | 2–∞ | 1.9 | 1.1–∞ |
| Transplant-free survival | |||||||||
| MMP-7 | 3.5 | 0.016 | 2.3 | 0.0075 | 2.8 | 4.3 | 1.6–∞ | 1.2 | 0.8–2 |
| ICAM-1 | 300 | 0.00006 | 3 | 0.00056 | 2.9 | 4.1 | 1.9–∞ | 0.9 | 0.7–1.9 |
| IL-8 | 0.0094 | 0.0048 | 2.4 | 0.34 | 2.4 | 4.1 | 1.3–∞ | 1.1 | 0.8–1.9 |
| VCAM-1 | 358.2 | 0.025 | 1.9 | 0.055 | 1.9 | 1.9 | 1.1–4 | 1.1 | 0.5–∞ |
| S100A12 | 72.1 | 0.033 | 1.7 | 0.036 | 1.8 | 2.2 | 1.9–3.6 | 0.8 | 0.5–1.6 |
| Progression-free survival: FVC 10% relative decline within 1 year (lung transplant is censored) | |||||||||
| MMP-7 | 4.7 | 0.25 | 1.3 | 0.98 | 1 | 1.1 | 0.9–2.3 | 1 | 0.8–1.6 |
| ICAM-1 | 262 | 0.0020 | 2.4 | 0.01 | 2.2 | 1.2 | 1–∞ | 0.9 | 0.7–1.5 |
| IL-8 | 0.0092 | 0.19 | 1.5 | 0.68 | 1.2 | 1 | 0.9–∞ | 1 | 0.8–1.6 |
| VCAM-1 | 246.8 | 0.26 | 0.7 | 0.81 | 1.1 | 0.8 | 0.8–2.2 | 1.2 | 0.9–1.9 |
| S100A12 | 37 | 0.067 | 0.6 | 0.065 | 0.5 | 0.9 | 0.7–1.7 | 1.1 | 0.9–2 |
| Progression-free survival: FVC 10% relative decline within 1 year (lung transplant counts as event) | |||||||||
| MMP-7 | 4.7 | 0.043 | 1.6 | 0.36 | 1.3 | 1 | 0.8–1.9 | 0.8 | 0.5–1.1 |
| ICAM-1 | 262 | 0.00075 | 2.4 | 0.0069 | 2.1 | 1.1 | 0.9–∞ | 0.7 | 0.6–1 |
| IL-8 | 0.0092 | 0.23 | 1.4 | 0.62 | 1.2 | 0.9 | 0.8–∞ | 0.8 | 0.7–1.2 |
| VCAM-1 | 246.8 | 0.16 | 0.7 | 0.94 | 1 | 0.8 | 0.7–1.1 | 1 | 0.8–1.6 |
| S100A12 | 64.8 | 0.12 | 1.4 | 0.44 | 1.2 | 1.1 | 0.8–1.7 | 0.8 | 0.5–1 |
Definition of abbreviations: Adj. HR = age, sex, and baseline FVC adjusted hazards ratio; Adj. Pval. = age, sex, and baseline FVC adjusted P value; CI = confidence interval; HR = hazard ratio; ICAM = intercellular adhesion molecule; IPF = idiopathic pulmonary fibrosis; MMP = matrix metalloproteinase; Pval. = log-rank P value; VCAM-1 = vascular cell adhesion molecule-1.
Figure 3.
Prediction of idiopathic pulmonary fibrosis. Outcome is largely validated in an independent validation set. As in Figure 2 , red indicates the Kaplan-Meier plot of overall survival by peripheral blood concentration dichotomized at an optimal threshold by profile likelihood, and blue indicates transplant-free survival. All line styles are identical to those in Figure 2 , and log-rank P values are listed at the top of each plot. As in Figure 2 , the markers shown are (A) matrix metalloproteinase-7, (B) intercellular adhesion molecule-1, (C) IL-8, (D) vascular cell adhesion molecule-1, and (E) S100A12.
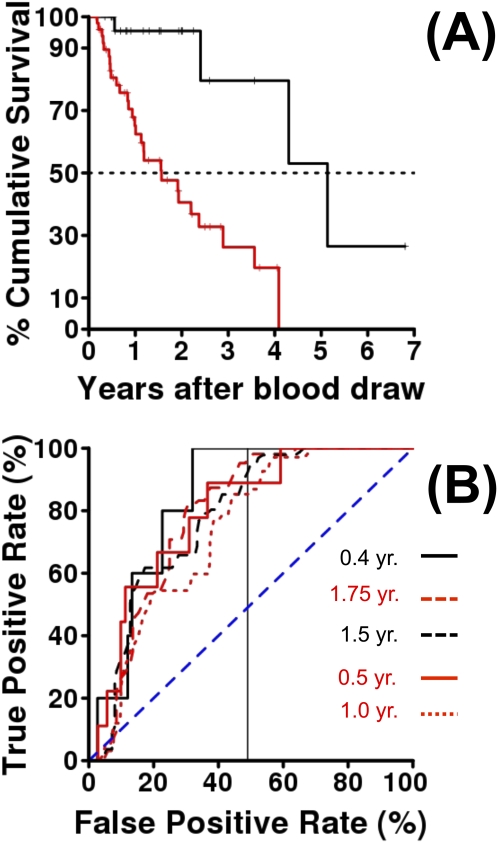
A Combined Risk Index Derived from Derivation Cohort Predicts Mortality in Validation Cohort
To demonstrate the predictive potential of our results, we used the stepAIC (29) approach to derive PCMI, using a similar approach to du Bois and coworkers (30) in our derivation set (103 patients with IPF with nonmissing biomarkers and all PFTs nonmissing), and validated the PCMI score in the validation set (n = 80 patients with nonmissing PFTs and biomarkers).
The formula derived for the PCMI from the derivation cohort is as follows:
PCMI = 114 · I(Male) + 2 · (100% - FVC % Predicted) + 3 · (100% – DlCO % Predicted) + 111 · I (MMP-7 ≥ 4.3 ng/ml), where the indicator function I() is unity (one) if and only if the condition inside the parentheses is true. For example, a male with FVC 65% of predicted and DlCO 40% of predicted and plasma MMP-7 concentration 5.2 ng/ml would have PCMI = 114 + 2 · (100–65) + 3 · (100–40) + 111, or 475; similarly, a female with FVC 75% of predicted and DlCO 60% of predicted and MMP-7 concentration 3.2 ng/ml would have PCMI = 2 · (100–75) + 3 · (100–60), or 170. Using the same statistical techniques applied to the univariate models for the biomarkers (fitting a proportional hazards model with PCMI as the single quantitative predictor, then examining loess-smoothed martingale residuals) we determined that PCMI could be fit as a dichotomous variable. Profile likelihood determined an optimal threshold of 330. Figure 4A displays the Kaplan-Meier curve for PCMI dichotomized at 330 in the validation cohort: the low-risk group (PCMI <330, black line, n = 29, four deaths) had median survival 5.13 years after blood draw (95% lower confidence limit, 4.3 yr), whereas the high-risk group (PCMI ≥330, red line, n = 51, 28 deaths) had median survival of 1.56 years (95% confidence interval, 1.01–3.56 yr). We also examined predictive performance of the PCMI to predict mortality by using receiver operating characteristic (ROC) curves and also by applying the C statistic (34). Figure 4B displays appropriate time-dependent ROC curves for a censored outcome (35), for predicting mortality at various time points using dichotomized PCMI. Following the vertical line drawn at 50% on the x axis, one can see the ROC curves relating true-positive rate to false-positive rate for all possible PCMI thresholds, for predicting mortality by, respectively from bottom to top, 1 year, 0.5 year, 1.5 year, 1.75 year, and 0.4 year. The area under the curve for these curves ranges from 0.736–0.835. Similarly, the C statistics for measuring the concordance (i.e., the probability that a patient who dies sooner will have a larger risk score [30]) ranges from 0.73–84, suggesting that the score derived from the derivation cohort is indeed predictive of mortality in the validation cohort.
Figure 4.
Peripheral blood risk index (PCMI) predicts mortality in idiopathic pulmonary fibrosis. (A) Kaplan-Meier curve for mortality of patients in validation cohort grouped by the mortality-associated PCMI derived in the derivation cohort. Black is low risk (PCMI <330) and red is high risk (PCMI ≥330). (B) Time-dependent receiver operating characteristic curves for predicting idiopathic pulmonary fibrosis mortality by PCMI. True-positive rate is plotted versus false-positive rate, for all possible thresholds in the validation set, of the PCMI derived in the derivation set. Each line is for predicting mortality within a specified time point after blood draw. The predictive performance of this combined index is high: area under the curve ranges from 0.74–0.84, C statistic from 0.73–0.84.
Discussion
In this relatively large follow-up study of 241 patients with IPF we aimed to identify and validate proteins in the plasma that predict the prognosis of patients with IPF. In a derivation cohort of 140 prospectively followed well-characterized patients with IPF we analyzed the concentrations of 95 cytokines and chemokines, MMPs, and markers of apoptosis and epithelial injury and determined that high concentrations of five (MMP-7, VCAM-1, S100A12, ICAM-1, and IL-8) were predictive of subsequent outcomes regardless of age, sex, or baseline PFTs. In the independent validation cohort of 101 patients with IPF we confirmed that high concentrations of the five markers were indeed predictive of significantly reduced survival when transplant was included as an event, ICAM-1 and IL-8 when transplant was censored, and only ICAM-1 was predictive of progression-free survival. PCMI, a risk prediction rule based on combining clinical parameters and plasma protein concentrations that distinguished high and low mortality risk subgroups in the derivation cohort, was accurately predictive of mortality in the validation cohort.
The notion that peripheral blood proteins may be informative in IPF has recently gained significant momentum (36). Multiple small cohort studies suggested that some of these proteins may indicate outcome (36); several larger studies demonstrated that high serum concentrations of mucin-1 (KL-6) (16), CCL-18 (17), or surfactant protein A (18) were associated with significantly increased mortality; and we reported that patients with IPF exhibited a unique plasma protein signature that reflected disease severity (19). Our study provides significant support to those key studies because it is significantly larger; contains a derivation and validation cohort; and started with a relatively large set of 95 proteins and concludes with five proteins (MMP-7, VCAM-1, S100A12, ICAM-1, and IL-8) that are predictive of disease outcomes. Thus, we now know that the prognosis-related protein signal is not a biologic curiosity, limited to a single protein, but indeed a general phenomenon that is potentially reflective of lung-related pathology. As an example, MMP-7 has been repeatedly implicated in IPF. MMP-7 is overexpressed and activated in alveolar epithelial cells in the IPF lung but not in normal histology lungs (37, 38). Elevated plasma concentrations of MMP-7 distinguish IPF from chronic obstructive pulmonary disease, sarcoidosis, and hypersensitivity pneumonitis (19). Most importantly, MMP-7 seems to be associated mechanistically with lung fibrosis: MMP-7 knockouts are relatively protected from fibrosis (37), it is a target gene for the WNT/β catenin pathway that has recently been shown to be aberrantly regulated in the human disease (39–42), and MMP-7 is a regulator of local lung defense mechanisms (43–46). Consistent with these observations, our results indicate that low MMP-7 concentrations define a group of patients with IPF with impressively long median survival (4.6 and 4.3 yr in the derivation and validation cohorts, respectively), whereas patients with high MMP-7 levels experience a significantly shorter median survival (2 and 2.2 yr in the derivation and validation cohorts, respectively). In contrast to all other markers, MMP-7 exhibits P values that decrease and hazard ratios that increase when age, sex, and baseline FVC are adjusted, suggesting that although increased MMP-7 concentrations may be still related to baseline pulmonary function, they may be uniquely informative for predicting outcome in patients with IPF with similar pulmonary function at presentation. This is also supported by the fact that MMP-7 emerged in our variable selection process for the PCMI as a component of the risk prediction score. Taken together, these observations suggest that MMP-7 peripheral blood concentrations may be indicative of the disease process in the lung and thus should be used to monitor disease severity and prognosis.
VCAM-1 and ICAM-1 are also probable indicators of the disease process in the lung. Increased expression of ICAM-1 and VCAM-1 is considered a sensitive marker of endothelial response to oxidative stress, a phenomenon increasingly implicated in IPF (47, 48). The two molecules are induced in radiation-induced pulmonary fibrosis and the radiation-induced increases of VCAM-1 and ICAM-1 are reversed by administration of manganese superoxide dismutase (49, 50). Treatment of bronchoalveolar macrophages obtained from patients with IPF or other interstitial lung disease with N-acetylcysteine, an agent used in the treatment of IPF (51), caused a decrease in ICAM-1 expression (52), and a reduction of neutrophil activation and IL-8 expression (53, 54), potentially suggesting that many of the changes in the peripheral blood are driven by the same mechanistic theme. The source of ICAM-1 and VCAM-1 in the blood remains unclear. Increased ICAM-1 has been found in the pulmonary capillary beds of IPF lungs (55) and in the peripheral blood of patients with IPF (56) or other fibrotic processes (57), but increased VCAM-1 has not been found (58). The predictive value of both markers and the data from the radiation-induced fibrosis suggest that the increase in the concentrations of both proteins in the peripheral blood is probably indicative of a mechanism that is active in the lung and thus should potentially be monitored when response to therapy is assessed.
Increased plasma concentrations of IL-8 and S100A12, both related to neutrophil recruitment and activation, were also associated with significantly worse outcomes in IPF. IL-8, a potent inflammatory chemokine that signals neutrophils to sites of injury (59), has been previously suggested as a regulator in IPF. Carré and coworkers (60) found elevated levels of IL-8 mRNA in alveolar macrophages and elevated levels of IL-8 protein in the bronchoalveolar lavage from patients with IPF compared with normal subjects. Xaubet and coworkers (61) found that the percentage of IL-8–positive bronchoalveolar lavage macrophages was significantly higher in areas of IPF lung with extensive fibrosis defined by high-resolution computed tomography scans compared with bronchoalveolar lavage fluid from normal smoking and nonsmoking volunteers. In contrast to IL-8 and S100A12, a calcium-binding protein secreted by neutrophils has not previously been implicated in IPF. Kikkawa and coworkers (62) recently reported that S100A12 levels in serum, bronchoalveolar lavage, and lung were significantly higher in patients developing acute lung injury after bowel surgery compared with those who do not develop acute lung injury. We found increased S100A12 mRNA in the lungs of patients with IPF compared with control subjects in a microarray dataset generated by us (GSE10667 and 59), but did not find a further increase in the lungs of patients with acute exacerbations of IPF (63), a finding consistent with a recent comparison of acute lung injury and acute exacerbations of IPF (64). The finding that increased concentration of two neutrophil-related proteins is indicative of poorer outcomes should encourage research into the role of neutrophils in IPF and potential specific therapies that target these cells considering the lack of response of patients with IPF to usual antiinflammatory therapies (65).
One potentially puzzling result of our study is that not every single observation in the derivation cohort was observed in the validation cohort. Such variability is expected given differences between cohorts and the early phase of our observations. This is especially true in the context of progression-free survival, because of the challenges to define and prove sustainable declines in lung function over a relatively short period of time and the need for a universally agreed on definition of progression, as was recently pointed out (66). Of particular interest to us is the fact that for one outcome, transplant-free survival, the results in the validation cohort indeed replicated those of the derivation cohort: increased concentrations of all of the markers were predictive of significantly shorter survival when transplant was counted as an event. This may be indicative of an overlooked phenomenon, the impact that increased accessibility of lung transplantation is having on our patient cohorts. In each of our cohorts, 50% of patients were evaluated for transplant, 25% were listed, and nearly 20% ended up being transplanted. Although it is possible that other factors, such as smaller size of cohort and significantly shorter follow-up, may account for the incomplete validation, the impressive results when transplant was included as an event suggest that similar analyses be included in the design of outcome and interventional studies.
Although the univariate results were not fully replicated, PCMI, the integrated score derived by multivariate analysis, impressively replicated the mortality predictions. PCMI, an index that integrated plasma protein concentrations and clinical parameters, identified high and low mortality risk subgroups at blood draw in the derivation cohort. Impressively, PCMI accurately predicted mortality in the validation cohort. Patients with PCMI scores below 330 in the validation cohort had a median survival of 5.13 years compared with a median survival of 1.56 years when PCMI was higher than 330 with impressive area under the ROC curve and C statistic values. An important indicator of the significant role of the proteins in PCMI is that if selection is performed with MMP-7 omitted, then a similar score is derived with VCAM-1 in its place; if VCAM-1 is further omitted, then ICAM-1 takes its place, and so on. In multivariate models derived in the derivation cohort and tested in the validation cohort, these five biomarkers jointly explain overlapping proportions of variability in IPF mortality, even though the protein concentration thresholds from the derivation cohort were not optimal for the validation cohort in the univariate analysis. Thus, our results indicate the significant value of integrating molecular information with clinical parameters in the derivation of reproducible and accurate outcome predictions rules.
Given that traditional physiologic measures are poor predictors of short- and long-term prognosis, and the critical need to identify patients with progressive and stable disease for clinical and pharmaceutical research and to prioritize for lung transplantation, the discovery of five peripheral blood proteins that predict prognosis in IPF is a key step forward in improving classification and management of these patients. Taken together with the three other previously discovered markers there is now a panel of eight proteins that should cover different aspects of disease progression in IPF: KL-6, surfactant protein A, and MMP-7 can be considered markers of alveolar epithelial cell injury; CCL-18 a marker of alveolar macrophage activation; S100A12 and IL-8 markers of neutrophil recruitment and activation; and ICAM-1 and VCAM-1 markers of oxidative stress in the lung. If this is the case, therapies that change the disease-sustaining environment in the lung should also be expected to alter the concentrations of these proteins in the bloodstream. In any case this panel of eight proteins should be incorporated in clinical research, and be critically validated for rapid implementation in the clinical setting. The impressive validated performance of PCMI suggests that integration of clinical and molecular markers for derivation of outcome prediction rules is indeed feasible in IPF. The availability of validated biomarkers and integrated risk prediction scores will address a critical unmet need in IPF research and will have the potential to transform the management of patients with IPF by allowing rational prioritization of lung transplantation.
Supplementary Material
Acknowledgments
The authors thank Lara Chensny and Mary Williams for their help in performing the research and preparation of this manuscript.
Footnotes
Supported by The Dorothy P. and Richard P. Simmons Endowed Chair for Pulmonary Research, grants P50HL0894932 and RO1HL095397; and an investigator initiated grant to N.K. from Centocor.
Author Contributions: conception and design, K.F.G., N.K., T.J.R., and F.B.; patient recruitment, diagnosis ascertainment, and quality control, M.K., K.F.G., K.O.L., T.J.R., N.K., L.J.V., and Y.Z.; plasma handling and protein assays, L.J.V., S.F., C.B., D.H., K.L., M.K., and Y.Z.; statistical analysis and intellectual contribution, K.L., T.J.R., D.H., J.C., and N.K.; and manuscript preparation, T.J.R., K.F.G., N.K., F.B., C.B., and J.C.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201101-0058OC on October 27, 2011
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Meltzer EB, Noble PW. Idiopathic pulmonary fibrosis. Orphanet J Rare Dis 2008;3:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Strieter RM. Pathogenesis and natural history of usual interstitial pneumonia: the whole story or the last chapter of a long novel. Chest 2005;128:526S–532S [DOI] [PubMed] [Google Scholar]
- 3.Selman M, King TE, Pardo A. Idiopathic pulmonary fibrosis: prevailing and evolving hypotheses about its pathogenesis and implications for therapy. Ann Intern Med 2001;134:136–151 [DOI] [PubMed] [Google Scholar]
- 4.Willis BC, Liebler JM, Luby-Phelps K, Nicholson AG, Crandall ED, du Bois RM, Borok Z. Induction of epithelial-mesenchymal transition in alveolar epithelial cells by transforming growth factor-beta1: potential role in idiopathic pulmonary fibrosis. Am J Pathol 2005;166:1321–1332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yogo Y, Fujishima S, Inoue T, Saito F, Shiomi T, Yamaguchi K, Ishizaka A. Macrophage derived chemokine (CCL22), thymus and activation-regulated chemokine (CCL17), and CCR4 in idiopathic pulmonary fibrosis. Respir Res 2009;10:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maeyama T, Kuwano K, Kawasaki M, Kunitake R, Hagimoto N, Matsuba T, Yoshimi M, Inoshima I, Yoshida K, Hara N. Upregulation of fas-signalling molecules in lung epithelial cells from patients with idiopathic pulmonary fibrosis. Eur Respir J 2001;17:180–189 [DOI] [PubMed] [Google Scholar]
- 7.Selman M, Pardo A, Kaminski N. Idiopathic pulmonary fibrosis: aberrant recapitulation of developmental programs? PLoS Med 2008;5:e62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kliment CR, Oury TD. Oxidative stress, extracellular matrix targets, and idiopathic pulmonary fibrosis. Free Radic Biol Med 2010;49:707–717 [DOI] [PubMed] [Google Scholar]
- 9.Harari S, Caminati A. IPF: new insight on pathogenesis and treatment. Allergy 2010;65:537–553 [DOI] [PubMed] [Google Scholar]
- 10.Strieter RM, Belperio JA, Keane MP. CXC chemokines in vascular remodeling related to pulmonary fibrosis. Am J Respir Cell Mol Biol 2003;29:S67–S69 [PubMed] [Google Scholar]
- 11.Martinez FJ, Safrin S, Weycker D, Starko KM, Bradford WZ, King TE, Jr, Flaherty KR, Schwartz DA, Noble PW, Raghu G, et al. The clinical course of patients with idiopathic pulmonary fibrosis. Ann Intern Med 2005;142:963–967 [DOI] [PubMed] [Google Scholar]
- 12.Selman M, Carrillo G, Estrada A, Mejia M, Becerril C, Cisneros J, Gaxiola M, Perez-Padilla R, Navarro C, Richards T, et al. Accelerated variant of idiopathic pulmonary fibrosis: clinical behavior and gene expression pattern. PLoS ONE 2007;2:e482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fernandez Perez ER, Daniels CE, Schroeder DR, St Sauver J, Hartman TE, Bartholmai BJ, Yi ES, Ryu JH. Incidence, prevalence, and clinical course of idiopathic pulmonary fibrosis: a population-based study. Chest 2010;137:129–137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ley B, Collard HR, King TE., Jr Clinical course and prediction of survival in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2010;183:431–440 [DOI] [PubMed] [Google Scholar]
- 15.Bargagli E, Olivieri C, Bennett D, Prasse A, Muller-Quernheim J, Rottoli P. Oxidative stress in the pathogenesis of diffuse lung diseases: a review. Respir Med 2009;103:1245–1256 [DOI] [PubMed] [Google Scholar]
- 16.Yokoyama A, Kondo K, Nakajima M, Matsushima T, Takahashi T, Nishimura M, Bando M, Sugiyama Y, Totani Y, Ishizaki T, et al. Prognostic value of circulating KL-6 in idiopathic pulmonary fibrosis. Respirology 2006;11:164–168 [DOI] [PubMed] [Google Scholar]
- 17.Prasse A, Probst C, Bargagli E, Zissel G, Toews GB, Flaherty KR, Olschewski M, Rottoli P, Muller-Quernheim J. Serum CC–chemokine ligand 18 concentration predicts outcome in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2009;179:717–723 [DOI] [PubMed] [Google Scholar]
- 18.Kinder BW, Brown KK, McCormack FX, Ix JH, Kervitsky A, Schwarz MI, King TE., Jr Serum surfactant protein-A is a strong predictor of early mortality in idiopathic pulmonary fibrosis. Chest 2009;135:1557–1563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rosas IO, Richards TJ, Konishi K, Zhang Y, Gibson K, Lokshin AE, Lindell KO, Cisneros J, Macdonald SD, Pardo A, et al. MMP1 and MMP7 as potential peripheral blood biomarkers in idiopathic pulmonary fibrosis. PLoS Med 2008;5:e93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Richards TJ, Lindell KO, Klesen M, Kaminski N, Zhang Y, Gibson K. Peripheral blood biomarkers predict disease progression and mortality in IPF. Am J Respir Crit Care Med 2010;181:A1120 [Google Scholar]
- 21.Demedts M, Costabel U. ATS/ERS international multidisciplinary consensus classification of the idiopathic interstitial pneumonias. Eur Respir J 2002;19:794–796 [DOI] [PubMed] [Google Scholar]
- 22.Steele MP, Speer MC, Loyd JE, Brown KK, Herron A, Slifer SH, Burch LH, Wahidi MM, Phillips JA, III, Sporn TA, et al. Clinical and pathologic features of familial interstitial pneumonia. Am J Respir Crit Care Med 2005;172:1146–1152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.King TE, Jr, Tooze JA, Schwarz MI, Brown KR, Cherniack RM. Predicting survival in idiopathic pulmonary fibrosis: scoring system and survival model. Am J Respir Crit Care Med 2001;164:1171–1181 [DOI] [PubMed] [Google Scholar]
- 24.Bouwman FG, de Roos B, Rubio-Aliaga I, Crosley LK, Duthie SJ, Mayer C, Horgan G, Polley AC, Heim C, Coort SL, et al. 2D-electrophoresis and multiplex immunoassay proteomic analysis of different body fluids and cellular components reveal known and novel markers for extended fasting. BMC Med Genomics 2011;4:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Therneau TM, Grambsch PM. Modeling survival data: extending the Cox model. New York: Springer; 2000 [Google Scholar]
- 26.Ihaka R, Gentleman RR. A language for data analysis and graphics. J Comput Graph Statist 1996;5:299–314 [Google Scholar]
- 27.Murphy SA, van der Vaart AW. On profile likelihood. J Am Stat Assoc 2000;95:449–485 [Google Scholar]
- 28.Wells AU, Desai SR, Rubens MB, Goh NS, Cramer D, Nicholson AG, Colby TV, du Bois RM, Hansell DM. Idiopathic pulmonary fibrosis: a composite physiologic index derived from disease extent observed by computed tomography. Am J Respir Crit Care Med 2003;167:962–969 [DOI] [PubMed] [Google Scholar]
- 29.Venables WN, Ripley BD. Modern applied statistics with S, 4th ed. New York: Springer; 2002 [Google Scholar]
- 30.du Bois RM, Weycker D, Albera C, Bradford WZ, Costabel U, Kartashov A, Lancaster L, Noble PW, Raghu G, Sahn SA, et al. Ascertainment of individual risk of mortality for patients with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2011;184:459–466 [DOI] [PubMed] [Google Scholar]
- 31.Flaherty KR, Toews GB, Travis WD, Colby TV, Kazerooni EA, Gross BH, Jain A, Strawderman RL, III, Paine R, Flint A, et al. Clinical significance of histological classification of idiopathic interstitial pneumonia. Eur Respir J 2002;19:275–283 [DOI] [PubMed] [Google Scholar]
- 32.King TE, Jr, Schwarz MI, Brown K, Tooze JA, Colby TV, Waldron JA, Jr, Flint A, Thurlbeck W, Cherniack RM. Idiopathic pulmonary fibrosis: relationship between histopathologic features and mortality. Am J Respir Crit Care Med 2001;164:1025–1032 [DOI] [PubMed] [Google Scholar]
- 33.Nicholson AG, Colby TV, du Bois RM, Hansell DM, Wells AU. The prognostic significance of the histologic pattern of interstitial pneumonia in patients presenting with the clinical entity of cryptogenic fibrosing alveolitis. Am J Respir Crit Care Med 2000;162:2213–2217 [DOI] [PubMed] [Google Scholar]
- 34.Uno H, Cai T, Pencina MJ, D'Agostino RB, Wei LJ. On the C-statistics for evaluating overall adequacy of risk prediction procedures with censored survival data. Harvard University Biostatistics Working Paper Series 2009:101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Heagerty PJ, Lumley T, Pepe MS. Time-dependent ROC curves for censored survival data and a diagnostic marker. Biometrics 2000;56:337–344 [DOI] [PubMed] [Google Scholar]
- 36.Prasse A, Muller-Quernheim J. Non-invasive biomarkers in pulmonary fibrosis. Respirology 2009;14:788–795 [DOI] [PubMed] [Google Scholar]
- 37.Zuo F, Kaminski N, Eugui E, Allard J, Yakhini Z, Ben-Dor A, Lollini L, Morris D, Kim Y, DeLustro B, et al. Gene expression analysis reveals matrilysin as a key regulator of pulmonary fibrosis in mice and humans. Proc Natl Acad Sci USA 2002;99:6292–6297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fujishima S, Shiomi T, Yamashita S, Yogo Y, Nakano Y, Inoue T, Nakamura M, Tasaka S, Hasegawa N, Aikawa N, et al. Production and activation of matrix metalloproteinase 7 (matrilysin 1) in the lungs of patients with idiopathic pulmonary fibrosis. Arch Pathol Lab Med 2010;134:1136–1142 [DOI] [PubMed] [Google Scholar]
- 39.Chilosi M, Poletti V, Zamo A, Lestani M, Montagna L, Piccoli P, Pedron S, Bertaso M, Scarpa A, Murer B, et al. Aberrant WNT/beta-catenin pathway activation in idiopathic pulmonary fibrosis. Am J Pathol 2003;162:1495–1502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Konigshoff M, Balsara N, Pfaff EM, Kramer M, Chrobak I, Seeger W, Eickelberg O. Functional WNT signaling is increased in idiopathic pulmonary fibrosis. PLoS ONE 2008;3:e2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Konigshoff M, Kramer M, Balsara N, Wilhelm J, Amarie OV, Jahn A, Rose F, Fink L, Seeger W, Schaefer L, et al. WNT1-inducible signaling protein-1 mediates pulmonary fibrosis in mice and is upregulated in humans with idiopathic pulmonary fibrosis. J Clin Invest 2009;119:772–787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vuga LJ, Ben-Yehudah A, Kovkarova-Naumovski E, Oriss T, Gibson KF, Feghali-Bostwick C, Kaminski N. WNT5a is a regulator of fibroblast proliferation and resistance to apoptosis. Am J Respir Cell Mol Biol 2009;41:583–589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li Q, Park PW, Wilson CL, Parks WC. Matrilysin shedding of syndecan-1 regulates chemokine mobilization and transepithelial efflux of neutrophils in acute lung injury. Cell 2002;111:635–646 [DOI] [PubMed] [Google Scholar]
- 44.McGuire JK, Li Q, Parks WC. Matrilysin (matrix metalloproteinase-7) mediates e-cadherin ectodomain shedding in injured lung epithelium. Am J Pathol 2003;162:1831–1843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Swee M, Wilson CL, Wang Y, McGuire JK, Parks WC. Matrix metalloproteinase-7 (matrilysin) controls neutrophil egress by generating chemokine gradients. J Leukoc Biol 2008;83:1404–1412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wilson CL, Schmidt AP, Pirila E, Valore EV, Ferri N, Sorsa T, Ganz T, Parks WC. Differential processing of {alpha}- and {beta}-defensin precursors by matrix metalloproteinase-7 (MMP-7). J Biol Chem 2009;284:8301–8311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Griffith B, Pendyala S, Hecker L, Lee PJ, Natarajan V, Thannickal VJ. Nox enzymes and pulmonary disease. Antioxid Redox Signal 2009;11:2505–2516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hecker L, Vittal R, Jones T, Jagirdar R, Luckhardt TR, Horowitz JC, Pennathur S, Martinez FJ, Thannickal VJ. Nadph oxidase-4 mediates myofibroblast activation and fibrogenic responses to lung injury. Nat Med 2009;15:1077–1081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Epperly MW, Sikora CA, DeFilippi SJ, Gretton JE, Bar-Sagi D, Archer H, Carlos T, Guo H, Greenberger JS. Pulmonary irradiation-induced expression of VCAM-1 and ICAM-1 is decreased by manganese superoxide dismutase-plasmid/liposome (MNSOD-PL) gene therapy. Biol Blood Marrow Transplant 2002;8:175–187 [DOI] [PubMed] [Google Scholar]
- 50.Epperly MW, Guo H, Shields D, Zhang X, Greenberger JS. Correlation of ionizing irradiation-induced late pulmonary fibrosis with long-term bone marrow culture fibroblast progenitor cell biology in mice homozygous deletion recombinant negative for endothelial cell adhesion molecules. In Vivo 2004;18:1–14 [PubMed] [Google Scholar]
- 51.Behr J, Demedts M, Buhl R, Costabel U, Dekhuijzen RP, Jansen HM, MacNee W, Thomeer M, Wallaert B, Laurent F, et al. Lung function in idiopathic pulmonary fibrosis: extended analyses of the IFIGENIA trial. Respir Res 2009;10:101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Radomska-Lesniewska DM, Skopinska-Rozewska E, Jankowska-Steifer E, Sobiecka M, Sadowska AM, Hevelke A, Malejczyk J. N-acetylcysteine inhibits IL-8 and MMP-9 release and ICAM-1 expression by bronchoalveolar cells from interstitial lung disease patients. Pharmacol Rep 2010;62:131–138 [DOI] [PubMed] [Google Scholar]
- 53.Radomska-Lesniewska DM, Sadowska AM, Van Overveld FJ, Demkow U, Zielinski J, De Backer WA. Influence of N-acetylcysteine on ICAM-1 expression and IL-8 release from endothelial and epithelial cells. J Physiol Pharmacol 2006;57:325–334 [PubMed] [Google Scholar]
- 54.Sadowska AM, Manuel-y-Keenoy B, Vertongen T, Schippers G, Radomska-Lesniewska D, Heytens E, De Backer WA. Effect of N-acetylcysteine on neutrophil activation markers in healthy volunteers: in vivo and in vitro study. Pharmacol Res 2006;53:216–225 [DOI] [PubMed] [Google Scholar]
- 55.Sato N, Suzuki Y, Nishio K, Suzuki K, Naoki K, Takeshita K, Kudo H, Miyao N, Tsumura H, Serizawa H, et al. Roles of ICAM-1 for abnormal leukocyte recruitment in the microcirculation of bleomycin-induced fibrotic lung injury. Am J Respir Crit Care Med 2000;161:1681–1688 [DOI] [PubMed] [Google Scholar]
- 56.Takehara H, Tada S, Kataoka M, Matsuo K, Ueno Y, Ozaki S, Miyake T, Fujimori Y, Yamadori I, Harada M. Intercellular adhesion molecule-1 in patients with idiopathic interstitial pneumonia. Acta Med Okayama 2001;55:205–211 [DOI] [PubMed] [Google Scholar]
- 57.Ihn H, Sato S, Fujimoto M, Kikuchi K, Kadono T, Tamaki K, Takehara K. Circulating intercellular adhesion molecule-1 in the sera of patients with systemic sclerosis: enhancement by inflammatory cytokines. Br J Rheumatol 1997;36:1270–1275 [DOI] [PubMed] [Google Scholar]
- 58.Nakao A, Hasegawa Y, Tsuchiya Y, Shimokata K. Expression of cell adhesion molecules in the lungs of patients with idiopathic pulmonary fibrosis. Chest 1995;108:233–239 [DOI] [PubMed] [Google Scholar]
- 59.Fichtner F, Koslowski R, Augstein A, Hempel U, Rohlecke C, Kasper M. Bleomycin induces IL-8 and ICAM-1 expression in microvascular pulmonary endothelial cells. Exp Toxicol Pathol 2004;55:497–503 [DOI] [PubMed] [Google Scholar]
- 60.Carre PC, Mortenson RL, King TE, Jr, Noble PW, Sable CL, Riches DW. Increased expression of the interleukin-8 gene by alveolar macrophages in idiopathic pulmonary fibrosis. A potential mechanism for the recruitment and activation of neutrophils in lung fibrosis. J Clin Invest 1991;88:1802–1810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Xaubet A, Agusti C, Luburich P, Barbera JA, Carrion M, Ayuso MC, Roca J, Rodriguez-Roisin R. Interleukin-8 expression in bronchoalveolar lavage cells in the evaluation of alveolitis in idiopathic pulmonary fibrosis. Respir Med 1998;92:338–344 [DOI] [PubMed] [Google Scholar]
- 62.Kikkawa T, Sato N, Kojika M, Takahashi G, Aoki K, Hoshikawa K, Akitomi S, Shozushima T, Suzuki K, Wakabayashi G, et al. Significance of measuring S100A12 and SRAGE in the serum of sepsis patients with postoperative acute lung injury. Dig Surg 2010;27:307–312 [DOI] [PubMed] [Google Scholar]
- 63.Konishi K, Gibson KF, Lindell KO, Richards TJ, Zhang Y, Dhir R, Bisceglia M, Gilbert S, Yousem SA, Song JW, et al. Gene expression profiles of acute exacerbations of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2009;180:167–175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Collard HR, Calfee CS, Wolters PJ, Song JW, Hong SB, Brady S, Ishizaka A, Jones KD, King TE, Jr, Matthay MA, et al. Plasma biomarker profiles in acute exacerbation of idiopathic pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol 2010;299:L3–L7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Raghu G. Idiopathic pulmonary fibrosis: treatment options in pursuit of evidence-based approaches. Eur Respir J 2006;28:463–465 [DOI] [PubMed] [Google Scholar]
- 66.Wells AU, Richards TJ, Martinez FJ. Baseline values and short serial change. A "road map" for a poor early outcome in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2011;184:395–397 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.