Abstract
Rationale: Sleep fragmentation (SF) is one of the major characteristics of sleep apnea, and has been implicated in its morbid consequences, which encompass excessive daytime sleepiness and neurocognitive impairments. We hypothesized that absence of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase activity is neuroprotective in SF-induced cognitive impairments.
Objectives: To examine whether increased NADPH oxidase activity may play a role in SF-induced central nervous system dysfunction.
Methods: The effect of chronic SF during the sleep-predominant period on sleep architecture, sleep latency, spatial memory, and oxidative stress parameters was assessed in mice lacking NADPH oxidase activity (gp91phox-/Y) and wild-type littermates.
Measurements and Main Results: SF for 15 days was not associated with differences in sleep duration, sleep state distribution, or sleep latency in both gp91phox-/Y and control mice. However, on a standard place training task, gp91phox-/Y mice displayed normal learning and were protected from the spatial learning deficits observed in wild-type littermates exposed to SF. Moreover, anxiety levels were increased in wild-type mice exposed to SF, whereas no changes emerged in gp91phox-/Y mice. Additionally, wild-type mice, but not gp91phox-/Y mice, had significantly elevated NADPH oxidase gene expression and activity, and in malondialdehyde and 8-oxo-2′-deoxyguanosine levels in cortical and hippocampal lysates after SF exposures.
Conclusions: This work substantiates an important role for NADPH oxidase in hippocampal memory impairments induced by SF, modeling sleep apnea. Targeting NADPH oxidase, therefore, is expected to minimize hippocampal impairments from both intermittent hypoxia and SF associated with the disease.
Keywords: NADPH oxidase, sleep fragmentation, neurocognitive impairments
At a Glance Commentary
Scientific Knowledge on the Subject
Sleep apnea induces behavioral impairments in rodents partially because of increase in NADP reduced (NADPH) oxidase activity leading to oxidative stress. However, the contribution of sleep fragmentation to cognitive dysfunction and oxidative stress remains unknown.
What This Study Adds to the Field
NADPH oxidase activity underlies components of sleep fragmentation–induced oxidative stress and cognitive impairments. These findings indicate that targeted therapy focused on NADPH oxidase activity may be useful in the treatment of patients with sleep disorders.
The manifestations of obstructive sleep apnea (OSA) reflect the interactions of intermittent hypoxia (IH), intermittent hypercapnia, increased intrathoracic pressure swings, and sleep fragmentation (SF) as elicited by the episodic changes in upper airway resistance during sleep. SF is a common phenomenon among several clinical disorders, and can lead to impaired cognitive function via mechanisms that remain poorly understood (1). Indeed, uninterrupted sleep for a minimum length of time is required for optimal daytime vigilance and neurocognitive function (1–3). Preliminary studies in rodents using short-term SF paradigms have also confirmed the adverse effects of SF on learning and seem to be independent of adenosine-mediated synaptic inhibition (4–7).
In clinical populations with severe SF (e.g., in OSA) total sleep time typically diminishes only slightly (8). The effects of experimentally induced SF on sleep patterns have not been critically characterized in rodents. It is likely that OSA-induced sleep perturbations are accompanied by obvious cognitive deficits because of increased levels of systemic markers of oxidative stress and inflammation, the latter leading to gray matter loss in neural sites contributing to cognitive function. Thus, neural deficits could be mediated by IH, SF, or the interactions between these two disturbances (9–14). Several theories have suggested that sleep deprivation elicits oxidative stress cellular damage (i.e., the overall result of an imbalance between the reactive oxygen species [ROS] generated and clearance by the endogenous antioxidant defense system) (15) in discrete areas of the brain (16, 17), and that sleep decreases oxidative stress (18) by removing oxidants produced during waking time (19). Overall, it is generally accepted that sleep induces repair, restoration, and detoxification (20, 21). Conversely, sleep deprivation has been reported to cause oxidative stress resulting in the formation of ROS, and eventually could lead to neuronal and cellular damage (16). However, it remains unclear whether similar to sleep deprivation SF paradigms induce increased oxidative stress.
The primary catalytic function nicotinamide adenine dinucleotide phosphate (NADPH) oxidase was primarily studied in the context of its role in phagocyte oxidative burst (22). More recently, this enzyme has emerged as a major source of ROS generation in most mammalian cells, including neurons and synapses (23), either as a by-product of normal catalytic activity or as the result of aberrant function in disease (24–28). The authors have recently shown that the oxidative stress responses and neurobehavioral impairments induced by IH during sleep were partially mediated by excessive NADPH oxidase activity (14). However, it remains unknown whether NADPH oxidase activity is activated during SF, and whether such oxidative stress underlies putative SF-induced neurobehavioral dysfunctions. To explore this issue, NADPH oxidase mutant mice (gp91phox-/Y) and wild-type littermates were exposed to SF and changes in sleep patterns, NADPH oxidase activity and expression, markers of oxidative stress, and hippocampus-dependent learning and memory tasks were all assessed. In addition, other behavioral paradigms for anxiety and depression were also tested, because such problems are frequently encountered in patients with sleep apnea (14, 29–34).
Methods
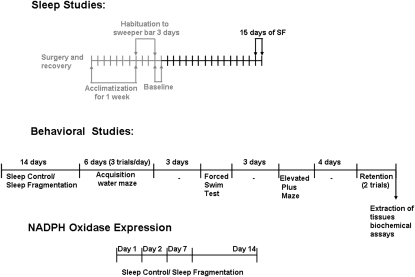
Figure 1 summarizes the experimental design for sleep, behavior, and biochemical studies. After each behavioral paradigm the mice were immediately returned to their respective exposures.
Figure 1.
Schematic diagram illustrating sleep, behavior, and biochemical experiments with exposures to either sleep fragmentation (SF) or sleep control in both wild-type and nicotinamide adenine dinucleotide phosphate (NADPH) oxidase null mice.
SF Exposures
Male transgenic mice and control littermates were maintained in custom-made cages operated under a 12-hour light–dark cycle (7:00 am to 7:00 pm) for 14 days before behavioral testing, and then for the duration of behavioral testing. The custom fabricated device used to induce SF in mice has been previously described (catalog #Model 80390; Lafayette Instruments, Lafayette, IN) (35).
Surgical Procedure and Implantation of Telemetric Transmitter and Electrodes
All surgical procedures were performed under sterile conditions and general anesthesia. A telemetric transmitter weighing 3.5 g (F20-EET; DSI, St. Paul, MN), which allows simultaneous monitoring of two biopotential channels (temperature and locomotor activity), was chronically implanted to record the EEG from the frontal area and EMG from superior nuchal muscle (35). Sleep recordings, SF, and sleep scoring were done as previously described (35, 36).
Behavioral Testing
In the Morris water maze the maze protocol is similar to that described by Morris and coworkers (37) with modifications for mice. Briefly, a standard place-training reference memory task was conducted on mice in the water maze after exposure to 14 days of SF or sleep control (SC). Place learning was assessed over 6 consecutive days with three trials per day with an intertrial interval of 10 minutes (i.e., one block) using a spaced training regimen that has been demonstrated to produce optimal learning in mice (38). The performance in the water maze was assessed using mean escape latencies and swim distance. Retention tests were performed 14 days after acquisition of the task as previously described (14). For the elevated plus maze mice were placed in the center of the maze facing a closed arm, and allowed to explore for 10 minutes in isolation. The percent time spent in open and closed arms, number of entries to closed arms, and time spent in the center were analyzed. In the forced swimming test mice were individually forced to swim for 6 minutes on 2 consecutive days in an open cylindrical container (diameter 14 cm; temperature 25 ± 1°C). Mice were marked as immobile if they performed minimal amount of work required to float at least 1 second as previously described (14, 39, 40).
Measurement of ATP Levels and Assessment of Adenosine Monophosphate Kinase α Activation
For more in-depth information see the online supplement.
NADPH oxidase expression.
The mRNA expression of p47phox and p67phox was determined by quantitative reverse transcriptase polymerase chain reaction using ABI PRISM 7500 System (Applied Biosystems, Foster City, CA) and commercially available specific primers (Applied Biosystems assays number Mm00447921_m1 and Mm00726636_m1, respectively).
NADPH oxidase activity.
As previously described (41), cortical tissue homogenate incubated in assay buffer (200 μl) containing acetylated cytochrome-c (100 μM) in a 96-well plate at 30°C. NADPH (200 μM) was then added in the absence or presence of superoxide dismutase (3 U/μl) and the reduction of cytochrome-c was monitored at 550 nm for 10 minutes. NOX activity was calculated as the superoxide dismutase–inhibitable reduction of cytochrome-c. All chemicals were from Sigma (St. Louis, MO).
Lipid peroxidation and 8-OHDG assay.
For the assays, the frontal cortex and the hippocampus was dissected from mice and samples were processed for the lipid peroxidation malondialdehyde (MDA)-586 kits (OxisResearch, Portland, OR) and 8-oxo-2′-deoxyguanosine (8-OHDG) assay (Cell Biolabs, San Diego, CA). The assay was performed as described (14).
Data Analysis
For sleep and behavioral tests, two-way repeated measures analysis of variance (ANOVA) followed by post hoc tests was used. For biochemical assays of MDA and 8-OHDG and NADPH expression levels were analyzed using one-way ANOVA. Statistics were performed using SPSS version 17.0 (IBM, Chicago, IL).
Results
Sleep Measures
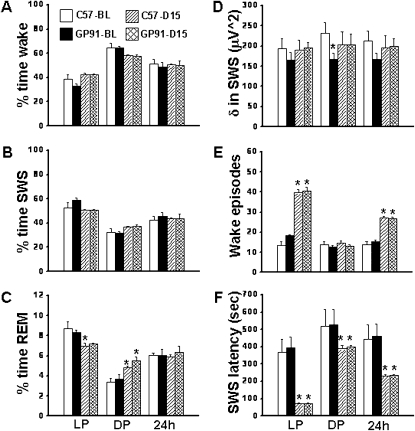
Overall analysis of the polysomnographic data for a period of 24 hours revealed significant changes between baseline (BL) and SF for wake (F = 22.57; P < 0.001), slow wave sleep (SWS) (F = 22.57; P < 0.001), and REM sleep (F = 22.57; P < 0.001), and delta power during SWS (F = 22.57; P < 0.001) indicating there was a significant effect on light period versus dark period. SF increased wake episodes (F = 52.563; P < 0.001) and reduced SWS latency (F = 7.643; P < 0.002), indicating that SF continued to elicit the desired frequency of awakenings and increased sleep propensity.
During the light period, C57BL6/J mice were awake for 38.52 ± 4.11% and gp91-/Y mice were awake for 33.19 ± 1.92% of the time (Figure 2A). During the dark period, C57BL6/J mice were awake for 64.59 ± 3.67% and gp91-/Y mice were awake for 64.69 ± 1.25% of the time. Thus, no differences emerged between C57BL6/J and gp91-/Y mice (Figure 2). Similar findings occurred for SWS (Figure 2B) and REM sleep (Figure 2C). However, there were significant, albeit minor, differences in REM sleep between C57BL6/J at BL and C57BL6/J after SF (q = 4.22; P < 0.04) during the light period, and minor differences in REM sleep during the dark period (C57BL6/J BL vs. C57BL6/J SF [q = 4.10; P < 0.04]; C57BL6/J BL vs. gp91-/Y SF [q = 6.06; P < 0.003]; and gp91-/Y BL vs. gp91-/Y SF [q = 5.30; P < 0.009]).
Figure 2.
Sleep patterns in mice exposed to sleep fragmentation (SF). There were no changes in total wake (A), slow wave sleep (SWS) (B), REM sleep (C), and delta power (D). An increase in the number of wake episodes (E) and decreases in SWS latency (F) after 15 days of SF are apparent. n = 6 per experimental group. * P < 0.05; see text for more details.
Delta Power during SWS
No changes in delta power between C57BL6/J mice and gp91-/Y mice emerged in SF (Figure 2D).
Wake Episodes
During the light period there were significant changes in wake episodes between C57BL6/J BL and C57BL6/J SF (q = 17.70; P < 0.001) and between gp91-/Y BL and gp91-/Y SF (q = 15.09; P < 0.001), as targeted by the SF paradigm (Figure 2E). There were no changes in wake episodes during the dark period. As a result, there were significant increases in wake episodes for the 24-hour period in SF for C57BL6/J and gp91-/Y mice (Figure 2E).
SWS Latency
Significant decreases in SWS latency emerged in all SF-exposed mice during the light period (C57BL6/J BL vs. C57BL6/J SF, q = 7.307, P < 0.001; gp91-/Y BL vs. gp91-/Y SF, q = 7.97, P < 0.001), but no differences between mouse strains were apparent (Figure 2F). Reductions in SWS latency were also identified during the dark period and the total 24-hour period for both mouse strains after SF (Figure 2F).
Spatial Learning Performance
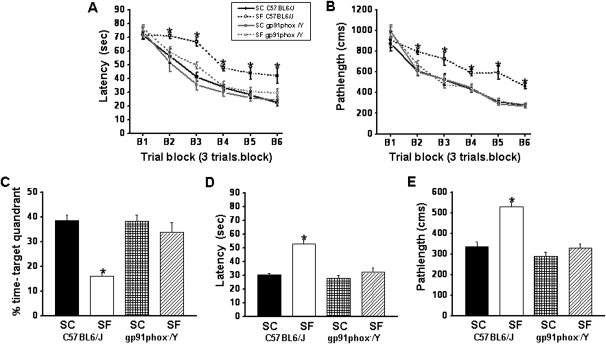
On the standard place discrimination task, wild-type mice exposed to 14 days of SF (SF-C57BL6/J) exhibited longer latencies and pathlengths to locate the hidden platform when compared with either control animals (SC-C57BL6/J, SC-gp91phox-/Y) or with gp91phox_/Y mice exposed to 14 days SF (SF-gp91phox-/Y; n = 12 per experimental condition) (Figures 3A and 3B). Overall latency analysis for the entire trial blocks revealed significant changes between the different treatment groups (F = 26.128; P < 0.001) and pathlength (F = 28.605; P < 0.001) indicating that SF adversely affected task performance. Significant differences in latencies were observed during blocks 2 (F = 2.90; P < 0.049); 3 (F = 18.136; P < 0.001); 4 (F = 6.811; P < 0.001); 5 (F = 7.279; P < 0.001); and 6 (F = 5.421; P < 0.004). There were no significant differences in Block 1. Repeated measures ANOVA revealed significant differences in path lengths during blocks 2 (F = 6.875; P < 0.001); 3 (F = 4.524; P < 0.009); 4 (F = 7.832; P < 0.001); 5 (F = 17.465; P < 0.001); and 6 (F = 20.417; P < 0.001), with no significant differences in Blocks 1 and 2. There were no significant differences in swim speed in these mice (data not shown). In the probe-trial test, one-way ANOVA revealed a significant effect of treatment (SF vs. SC: F = 23.219; P < 0.001). The magnitude of impairment was present only in SF-C57BL6/J mice (Figure 3C). In the reference memory tests, SF-C57BL6/J mice exhibited significant deficits in memory retention in both latency (F = 23.454; P < 0.001) and pathlength (F = 20.189; P < 0.001). However, the SF- gp91phox-/Y mice performed similar to control animals (Figure 3D and 3E).
Figure 3.
gp91phox_/Y mice exposed to sleep fragmentation (SF) do not exhibit any deficits in acquisition and retention. (A and B) Mean latencies (s) and pathlengths (cm) to locate the target platform during place training in C57BL6/J and gp91phox-/Y either exposed to SF or sleep control (SC) (n = 12 per group). (C) Mean percentage time in the target quadrant during probe trial after completion of water maze testing in either C57BL6/J and gp91phox-/Y exposed to SF or SC (n = 12 per group). (D) Mean latencies (s) and (E) pathlengths (cm) to locate the target platform during retention in C57BL6/J and gp91phox-/Y either exposed to SF or SC during assessment of retention of the Morris water maze task. n = 12 per group. * P < 0.05.
Repeated measures multivariate ANOVA with latency, groups, and conditions (F[5,39] = 44.13; P < 0.0001) revealed that SC C57BL6/J mice required significantly less time than their littermates exposed to SF to find the hidden platform in a Morris water maze (Figure 3A), whereas the SF-gp91phox-/Y mice were not affected. Repeated measures multivariate ANOVA with pathlength, groups, and conditions (F[5,39] = 15.304; P < 0.0001) indicated that as the training progressed the SC gp91phox-/Y and SC C57BL6/J mice could reach the hidden platform and covered the shortest distance compared with the distance covered by C57BL6/J mice exposed to SF (Figure 3B). In addition, repeated measures multivariate ANOVA with swim speed, groups, and conditions on the swim speed showed no significant differences between the groups and treatments (data not shown).
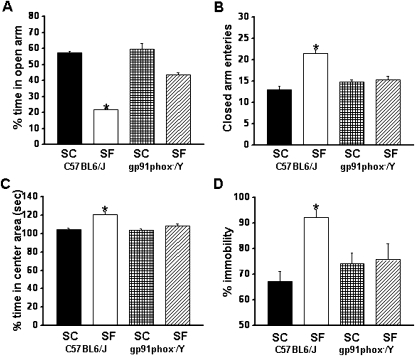
Elevated Plus Maze
SF-C57BL6/J mice showed significant differences in the percentage of time spent in the open arm (F = 74.625; P < 0.001) and in the number of entries into the closed arm (F = 13.018; P < 0.001) (Figures 4A and 4B). The results of the elevated plus maze showed that SF-C57BL6J spent significantly less time in the open arms (Figure 4A) (group effect, F = 29.903; P < 0.0001) and significantly more time in the center area (Figure 4C) (group effect, F = 9.277; P < 0.0001). The number of entries into the closed arms was significantly increased (Figure 4B) (condition effect, F = 9.899; P < 0.0001). Although the percentage of time spent in the open arm is commonly used as a measure of anxiety, the time spent on the center platform of the maze and the closed arm entries all reflect anxiety-like behaviors in mice.
Figure 4.
Exposure to sleep fragmentation (SF) induces anxiety and depression in mice, which is abrogated in NADP reduced oxidase null mice. (A) C57BL6/J mice exposed to SF spent significantly less time in the open arm of the elevated plus maze compared with sleep control (SC) C57BL6/J, or with gp91phox-/Y mice exposed to SF or maintained under SC. (B) An increased number of closed-arm entries emerged in wild-type mice exposed to SF. (C) Time spent in the Center Area was increased in wild-type mice exposed to SF. n = 12 per group;* P < 0.05. (D) gp91phox-/Y exposed to SF show less immobility compared with C57BL6/J mice exposed to SF. n = 12 per experimental group. * P < 0.05; see text for more details.
Forced Swim Test
SF-C57BL6/J mice had significantly higher immobility durations during the last 4 minutes of the forced swim test (F = 5.614; P < 0.004) compared with all other treatment groups, including SF-gp91phox-/Y (Figure 4D), suggesting the presence of depressive-like behaviors.
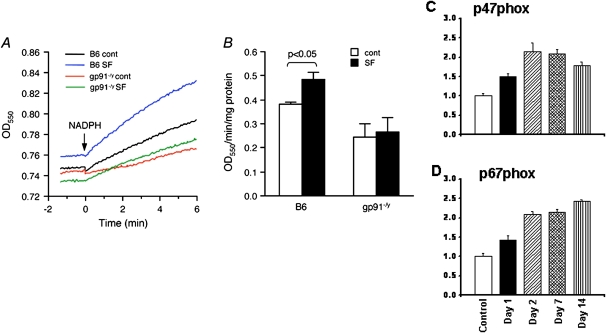
NADPH Oxidase Expression and Activity
SF induced increased expression of the P47phox and P67phox subunits of the NADPH oxidase gene (Figures 5C and 5D). Furthermore, SF-exposed wild-type mice showed NADPH oxidase activity increases, which were absent in gp91phox-/Y mice (Figure 5B).
Figure 5.
Nicotinamide adenine dinucleotide phosphate (NADPH) oxidase activity and expression. NADPH oxidase activity in the cerebral cortex. (A) Kinetic NADPH oxidase activity measured as NADPH-dependent cytochrome-c reduction. Shown are representative tracings from the four experimental groups. (B) Summary of NADPH oxidase activities. Sleep fragmentation (SF) resulted in a significant increase in NADPH oxidase activities in wild-type mice. Such SF-induced increases in NADPH oxidase activity were abolished in gp91phox-/Y mice. n = 4 for each group. Data are mean ± SE. (C and D) Expression of p47phox and p67phox subunits of NADPH oxidase was significantly increased during the course of SF in C57BL6/J mice.
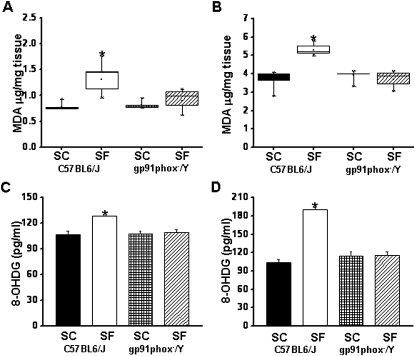
Lipid Peroxidation and 8-OHDG Levels
After the behavioral experiments, cortical tissues and hippocampus were harvested and processed for assessment of lipid peroxidation as indicated by MDA levels. Figures 6A and 6B show MDA concentrations in homogenates of cerebral cortex from all treatment groups. A significant increase in MDA levels was observed in SF-C57BL6/J mice in the cortex (F = 14.051; P < 0.001) and in the hippocampus (F = 34.22; P < 0.001) compared with all three other experimental groups.
Figure 6.
Lipid peroxidation and oxidative DNA damage after sleep fragmentation (SF). Lipid peroxidation is reduced in the frontal cortex (A) and hippocampus (B) of gp91phox_/Y exposed to SF. Malondialdehyde tissue levels of gp91phox-/Y and C57BL6/J mice exposed to SF or control sleep conditions (SC) for 14 days. n = 6 per experimental group; * P < 0.05. Oxidative DNA damage is absent in the cortex (C) and hippocampus (D) of gp91phox_/Y exposed to SF. 8-oxo-2′-deoxyguanosine levels in cortex and hippocampus of gp91phox-/Y and C57BL6/J mice exposed to SF or SC for 14 days. n = 6 per experimental group; * P < 0.05. MDA = ; OHDG = .
The levels of 8-OHDG in homogenates of cerebral cortex and the hippocampus were significantly higher in SF-C57BL6/J mice (F = 68.588, P < 0.001; and F = 13.392, P < 0.001), respectively, compared with all other groups (Figures 6C and 6D). However, there were no significant differences in the levels of 8-OHDG in cortex of SF-gp91phox-/Y compared with control animals SF-gp91phox-/Y and SF-C57BL6/J.
Discussion
The present study demonstrates that 12 hours of SF during the daylight period for 14 days, which aims to mimic sleep apnea, interferes with spatial learning and retention memory tests in a well-defined hippocampus-dependent learning and memory task (14, 42). The SF-induced performance deficits in the water maze could be mediated by a disturbance in hippocampal-dependent memory consolidation. In addition, chronic SF is not accompanied by prominent differences in sleep state distribution and duration, and yet the recurrent arousals induced during daylight hours are manifest as marked decreases in sleep latency (i.e., increased sleep propensity). Current findings also point out for the first time that chronic SF induces increased expression and activation of NADPH oxidase, but that the SF-induced oxidative stress that is abrogated in NADPH oxidase null mice does not seem to play a role in the emergence of increased sleep propensity, because both SF- gp91phox-/Y and SF-C57BL6/J showed similar reductions in SWS latencies. However, excessive NADPH oxidase activity likely mediated the cognitive and behavioral deficits elicited by chronic SF.
During sleep, patients with sleep apnea are subjected to recurrent and chronic SF and IH, both of which induce substantial increases in NADPH oxidase expression and activity, and ROS production and consequent cellular dysfunction (43). In the context of sleep perturbations and learning, most studies have reported on the presence of cognitive impairments with sleep deprivation by exploring a large array of learning and memory tests including the radial arm maze (44, 45), Morris water maze (6, 46), contextual fear conditioning (47), and eight-box task (48). However, there is only a paucity of cognitive studies exploring the effects of SF, and those were restricted to short-term SF, the latter using methodologies that were associated with substantial alterations in sleep architecture (4–6). In addition, we postulate that because the SF-exposed mice exhibited overall preserved sleep architecture, the fact that SF-gp91phox-/Y mice were protected against SF-induced cognitive deficits is highly unlikely to be explained by any underlying disruption of sleep architecture, or by reduced brain energy source bioavailability. This is particularly pertinent when considering the preserved ATP levels and absence of any phosphorylation of AMPK-α (see online supplement), whereas both of these reporters of cellular bioenergetics are markedly altered as a consequence of sleep deprivation (49). Consequently, it is assumed that NADPH-mediated ROS were the major contributor to the disrupted neurobehavioral and cognitive manifestations of SF. ROS can be generated from various subcellular compartments, including mitochondria, the cellular membrane, lysosomes, peroxisomes, and the endoplasmic reticulum (27, 50, 51). Certain levels of ROS are extremely important for a variety of normal biologic functions including growth factor regulation, calcium signaling, and phagocytosis/inflammation (52–54). For example, NADPH oxidase (27, 55, 56), xanthine oxidase (57), phospholipase A2 (57), lipoxygenases and cyclooxygenase (50), and cytochrome P-450 (58) have all been identified as sources of ROS in various subcellular compartments under both physiologic and pathologic conditions. Indeed, physiologic concentrations of hydrogen peroxide seem to be essential to the integrity of hippocampal long-term potentiation (59–61), whereas increased concentrations of the hydrogen peroxide are markedly detrimental to hippocampal function (62). Similar inferences regarding other ROS sources including NADPH oxidase biologic functions have been reported (63–65). Thus, excessive ROS bioavailability, such as that generated by SF-induced increased expression and activation of the various subunits of NADPH oxidase that are present in cortical and hippocampal neurons (25, 26, 66, 67), results in deleterious effects on the performance in a spatial task acquisition and retention, and also underlies the changes in behavior, as measured in the forced swim test (depression) or the elevated plus maze (anxiety). Although it cannot be inferred from the current findings on the cellular source of NADPH oxidase contribution to SF-induced cognitive and behavioral deficits, the near complete abrogation of such deficits in the gp91phox-/Y mice clearly and conclusively assigns a critical role for NADPH oxidase in this context. Of note, activation of NADPH oxidase and consequent ROS production and oxidative stress can also occur in sleep apnea, particularly in the context of IH, and may mediate components pulmonary vasoconstriction (68, 69), chemoreceptor hypersensitivity (70), and mobilization of progenitor cells (71).
Depressive and anxiety symptoms are frequent in OSA patients (34, 72, 73), and in the present study some of these frequent clinical complaints are reproduced in the model of chronic SF, suggesting that the recurrent arousals and increased oxidative stress play a role in the depressive and anxiety symptoms of the disease. Indeed, depression may account for the fatigue seen in OSA patients, even after adjusting for OSA severity (31). Some studies have reported higher responsiveness of hippocampus and hypothalamus and reduced susceptibility to oxidative stress in cortex and brainstem in response to sleep deprivation. Conversely, others have reported on the presence of increased oxidative stress in the hippocampus and cortex in sleep-deprived rats (74–76). The divergent reports are perhaps caused by the inherently different models of sleep deprivation used and also by the different brain regions being assessed. Notwithstanding such considerations, ROS have been implicated in the pathophysiology of depression and anxiety (77). The elevated plus-maze is the most frequently used animal model for assessing anxiety-like behaviors (78, 79) because it enables researchers to observe the conflict between two innate rodent behaviors: the avoidance of open space exposure as countering the tendency to explore novel environments (79). The results further imply that SF modified anxiety-like behaviors in wild-type mice. In contrast, gp91phox-/Y exposed to SF showed preserved performances in this test, suggesting that regions underlying these behavioral patterns are susceptible to SF, most likely via the oxidant stress mediated by activation of NADPH oxidase.
In summary, the current findings on chronic SF-induced oxidative stress (Figure 6) pave the way for a viable rationale supporting the use of antioxidant therapies aiming to reduce the excessive ROS generation, accumulation, and propagation, and thus palliate the neurocognitive and mood and behavioral dysfunction associated with sleep apnea. Indeed, transgenic mice null for NADPH oxidase activity displayed significantly reduced lipid peroxidation and DNA damage after SF exposures, and preserved cognition and behavior (Figures 3 and 6). The present study used a combination of sleep recordings, and behavioral, molecular, and biochemical studies, and demonstrated that oxidative stress induced by excessive NADPH oxidase-mediated superoxide release is a critically important mediator of the neuronal dysfunction induced by SF, but is not involved in the mechanisms underlying SF-induced excessive sleepiness.
Supplementary Material
Footnotes
Author Contributions: Conception and design, D.G. and Y.W. Analysis and interpretation, D.N., S.X.L.Z., V.R., F.H., Y.W., and D.G. Drafting the manuscript for important intellectual content, D.N., S.X.L.Z., and D.G. Experimental procedures, D.N., S.X.L.Z., V.R., N.K., and F.H.
Supported by National Institutes of Health grant HL-086662.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Author disclosures are available with the text of this article at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.201107-1173OC on August 25, 2011
References
- 1.Bonnet MH. Sleep restoration as a function of periodic awakening, movement, or electroencephalographic change. Sleep 1987;10:364–373 [DOI] [PubMed] [Google Scholar]
- 2.Franken P. Long-term vs. short-term processes regulating REM sleep. J Sleep Res 2002;11:17–28 [DOI] [PubMed] [Google Scholar]
- 3.Stepanski EJ. The effect of sleep fragmentation on daytime function. Sleep 2002;25:268–276 [DOI] [PubMed] [Google Scholar]
- 4.Tartar JL, Ward CP, McKenna JT, Thakkar M, Arrigoni E, McCarley RW, Brown RE, Strecker RE. Hippocampal synaptic plasticity and spatial learning are impaired in a rat model of sleep fragmentation. Eur J Neurosci 2006;23:2739–2748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ward CP, McCoy JG, McKenna JT, Connolly NP, McCarley RW, Strecker RE. Spatial learning and memory deficits following exposure to 24 h of sleep fragmentation or intermittent hypoxia in a rat model of obstructive sleep apnea. Brain Res 2009;1294:128–137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ward CP, McCarley RW, Strecker RE. Experimental sleep fragmentation impairs spatial reference but not working memory in Fischer/brown Norway rats. J Sleep Res 2009;18:238–244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tartar JL, McKenna JT, Ward CP, McCarley RW, Strecker RE, Brown RE. Sleep fragmentation reduces hippocampal Ca1 pyramidal cell excitability and response to adenosine. Neurosci Lett 2010;469:1–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coleman RM, Roffwarg HP, Kennedy SJ, Guilleminault C, Cinque J, Cohn MA, Karacan I, Kupfer DJ, Lemmi H, Miles LE, et al. Sleep-wake disorders based on a polysomnographic diagnosis. A national cooperative study. JAMA 1982;247:997–1003 [PubMed] [Google Scholar]
- 9.Montplaisir J, Bedard MA, Richer F, Rouleau I. Neurobehavioral manifestations in obstructive sleep apnea syndrome before and after treatment with continuous positive airway pressure. Sleep 1992;15:S17–S19 [DOI] [PubMed] [Google Scholar]
- 10.Beebe DW, Gozal D. Obstructive sleep apnea and the prefrontal cortex: towards a comprehensive model linking nocturnal upper airway obstruction to daytime cognitive and behavioral deficits. J Sleep Res 2002;11:1–16 [DOI] [PubMed] [Google Scholar]
- 11.Carpagnano GE, Kharitonov SA, Resta O, Foschino-Barbaro MP, Gramiccioni E, Barnes PJ. Increased 8-isoprostane and interleukin-6 in breath condensate of obstructive sleep apnea patients. Chest 2002;122:1162–1167 [DOI] [PubMed] [Google Scholar]
- 12.Macey PM, Henderson LA, Macey KE, Alger JR, Frysinger RC, Woo MA, Harper RK, Yan-Go FL, Harper RM. Brain morphology associated with obstructive sleep apnea. Am J Respir Crit Care Med 2002;166:1382–1387 [DOI] [PubMed] [Google Scholar]
- 13.Gozal D, Crabtree VM, Sans Capdevila O, Witcher LA, Kheirandish-Gozal L. C-reactive protein, obstructive sleep apnea, and cognitive dysfunction in school-aged children. Am J Respir Crit Care Med 2007;176:188–193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nair D, Dayyat EA, Zhang SX, Wang Y, Gozal D. Intermittent hypoxia-induced cognitive deficits are mediated by NADPH oxidase activity in a murine model of sleep apnea. PLoS ONE 2011;6:e19847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Silva RH, Abilio VC, Takatsu AL, Kameda SR, Grassl C, Chehin AB, Medrano WA, Calzavara MB, Registro S, Andersen ML, et al. Role of hippocampal oxidative stress in memory deficits induced by sleep deprivation in mice. Neuropharmacology 2004;46:895–903 [DOI] [PubMed] [Google Scholar]
- 16.Gopalakrishnan A, Ji LL, Cirelli C. Sleep deprivation and cellular responses to oxidative stress. Sleep 2004;27:27–35 [DOI] [PubMed] [Google Scholar]
- 17.Ramanathan L, Gulyani S, Nienhuis R, Siegel JM. Sleep deprivation decreases superoxide dismutase activity in rat hippocampus and brainstem. Neuroreport 2002;13:1387–1390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kumar A, Kalonia H. Protective effect of withania somnifera dunal on the behavioral and biochemical alterations in sleep-disturbed mice (grid over water suspended method). Indian J Exp Biol 2007;45:524–528 [PubMed] [Google Scholar]
- 19.Ikeda M, Ikeda-Sagara M, Okada T, Clement P, Urade Y, Nagai T, Sugiyama T, Yoshioka T, Honda K, Inoue S. Brain oxidation is an initial process in sleep induction. Neuroscience 2005;130:1029–1040 [DOI] [PubMed] [Google Scholar]
- 20.Ikeda-Sagara M, Ikeda M. Oxidative stress and sleep homeostasis. Nippon Yakurigaku Zasshi 2007;129:404–407 [DOI] [PubMed] [Google Scholar]
- 21.Honda K, Komoda Y, Inoue S. Oxidized glutathione regulates physiological sleep in unrestrained rats. Brain Res 1994;636:253–258 [DOI] [PubMed] [Google Scholar]
- 22.Quinn MT, Gauss KA. Structure and regulation of the neutrophil respiratory burst oxidase: comparison with nonphagocyte oxidases. J Leukoc Biol 2004;76:760–781 [DOI] [PubMed] [Google Scholar]
- 23.Tejada-Simon MV, Serrano F, Villasana LE, Kanterewicz BI, Wu GY, Quinn MT, Klann E. Synaptic localization of a functional NADPH oxidase in the mouse hippocampus. Mol Cell Neurosci 2005;29:97–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dringen R. Oxidative and antioxidative potential of brain microglial cells. Antioxid Redox Signal 2005;7:1223–1233 [DOI] [PubMed] [Google Scholar]
- 25.Serrano F, Kolluri NS, Wientjes FB, Card JP, Klann E. NADPH oxidase immunoreactivity in the mouse brain. Brain Res 2003;988:193–198 [DOI] [PubMed] [Google Scholar]
- 26.Vallet P, Charnay Y, Steger K, Ogier-Denis E, Kovari E, Herrmann F, Michel JP, Szanto I. Neuronal expression of the NADPH oxidase NOX4, and its regulation in mouse experimental brain ischemia. Neuroscience 2005;132:233–238 [DOI] [PubMed] [Google Scholar]
- 27.Bedard K, Krause KH. The NIX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev 2007;87:245–313 [DOI] [PubMed] [Google Scholar]
- 28.Abramov AY, Jacobson J, Wientjes F, Hothersall J, Canevari L, Duchen MR. Expression and modulation of an NADPH oxidase in mammalian astrocytes. J Neurosci 2005;25:9176–9184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kaplan R. Obstructive sleep apnoea and depression: diagnostic and treatment implications. Aust N Z J Psychiatry 1992;26:586–591 [DOI] [PubMed] [Google Scholar]
- 30.Borak J, Cieslicki JK, Koziej M, Matuszewski A, Zielinski J. Effects of CPAP treatment on psychological status in patients with severe obstructive sleep apnoea. J Sleep Res 1996;5:123–127 [DOI] [PubMed] [Google Scholar]
- 31.Bardwell WA, Ancoli-Israel S, Dimsdale JE. Types of coping strategies are associated with increased depressive symptoms in patients with obstructive sleep apnea. Sleep 2001;24:905–909 [DOI] [PubMed] [Google Scholar]
- 32.Sanchez AI, Buela-Casal G, Bermudez MP, Casas-Maldonado F. The effects of continuous positive air pressure treatment on anxiety and depression levels in apnea patients. Psychiatry Clin Neurosci 2001;55:641–646 [DOI] [PubMed] [Google Scholar]
- 33.Akashiba T, Kawahara S, Akahoshi T, Omori C, Saito O, Majima T, Horie T. Relationship between quality of life and mood or depression in patients with severe obstructive sleep apnea syndrome. Chest 2002;122:861–865 [DOI] [PubMed] [Google Scholar]
- 34.El-Sheikh M, Kelly RJ, Buckhalt JA, Benjamin Hinnant J. Children's sleep and adjustment over time: the role of socioeconomic context. Child Dev 2010;81:870–883 [DOI] [PubMed] [Google Scholar]
- 35.Ramesh V, Kaushal N, Gozal D. Sleep fragmentation modifies EEG delta power during slow wave sleep in socially isolated and paired mice. Sleep Science 2009;2:64–75 [Google Scholar]
- 36.Espana RA, McCormack SL, Mochizuki T, Scammell TE. Running promotes wakefulness and increases cataplexy in orexin knockout mice. Sleep 2007;30:1417–1425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Morris RG, Garrud P, Rawlins JN, O'Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature 1982;297:681–683 [DOI] [PubMed] [Google Scholar]
- 38.Gerlai R, Clayton NS. Analysing hippocampal function in transgenic mice: an ethological perspective. Trends Neurosci 1999;22:47–51 [DOI] [PubMed] [Google Scholar]
- 39.Eckeli AL, Dach F, Rodrigues AL. Acute treatments with GMP produce antidepressant-like effects in mice. Neuroreport 2000;11:1839–1843 [DOI] [PubMed] [Google Scholar]
- 40.Dias Elpo Zomkowski A, Oscar Rosa A, Lin J, Santos AR, Calixto JB, Lucia Severo Rodrigues A. Evidence for serotonin receptor subtypes involvement in agmatine antidepressant like-effect in the mouse forced swimming test. Brain Res 2004;1023:253–263 [DOI] [PubMed] [Google Scholar]
- 41.Miller FJ, Jr, Griendling KK. Functional evaluation of nonphagocytic NAD(P)H oxidases. Methods Enzymol 2002;353:220–233 [DOI] [PubMed] [Google Scholar]
- 42.Gozal D, Nair D, Goldbart AD. Physical activity attenuates intermittent hypoxia-induced spatial learning deficits and oxidative stress. Am J Respir Crit Care Med 2010;182:104–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhan G, Serrano F, Fenik P, Hsu R, Kong L, Pratico D, Klann E. NADPH oxidase mediates hypersomnolence and brain oxidative injury in a murine model of sleep apnea. Am J Respir Crit Care Med 2005;172:921–929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Smith CT, Conway JM, Rose GM. Brief paradoxical sleep deprivation impairs reference, but not working, memory in the radial arm maze task. Neurobiol Learn Mem 1998;69:211–217 [DOI] [PubMed] [Google Scholar]
- 45.Alhaider IA, Aleisa AM, Tran TT, Alzoubi KH, Alkadhi KA. Chronic caffeine treatment prevents sleep deprivation-induced impairment of cognitive function and synaptic plasticity. Sleep 2010;33:437–444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Youngblood BD, Smagin GN, Elkins PD, Ryan DH, Harris RB. The effects of paradoxical sleep deprivation and valine on spatial learning and brain 5-HT metabolism. Physiol Behav 1999;67:643–649 [DOI] [PubMed] [Google Scholar]
- 47.Graves LA, Heller EA, Pack AI, Abel T. Sleep deprivation selectively impairs memory consolidation for contextual fear conditioning. Learn Mem 2003;10:168–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bjorness TE, Riley BT, Tysor MK, Poe GR. REM restriction persistently alters strategy used to solve a spatial task. Learn Mem 2005;12:352–359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dworak M, McCarley RW, Kim T, Kalinchuk AV, Basheer R. Sleep and brain energy levels: ATP changes during sleep. J Neurosci 2010;30:9007–9016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Droge W. The plasma redox state and ageing. Ageing Res Rev 2002;1:257–278 [DOI] [PubMed] [Google Scholar]
- 51.Akki A, Zhang M, Murdoch C, Brewer A, Shah AM. NADPH oxidase signaling and cardiac myocyte function. J Mol Cell Cardiol 2009;47:15–22 [DOI] [PubMed] [Google Scholar]
- 52.Lambeth JD. NOX enzymes and the biology of reactive oxygen. Nat Rev Immunol 2004;4:181–189 [DOI] [PubMed] [Google Scholar]
- 53.Lambeth JD. NOX enzymes, ROS, and chronic disease: an example of antagonistic pleiotropy. Free Radic Biol Med 2007;43:332–347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol 2007;39:44–84 [DOI] [PubMed] [Google Scholar]
- 55.Berry CE, Hare JM. Xanthine oxidoreductase and cardiovascular disease: molecular mechanisms and pathophysiological implications. J Physiol 2004;555:589–606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Muralikrishna Adibhatla R, Hatcher JF. Phospholipase A2, reactive oxygen species, and lipid peroxidation in cerebral ischemia. Free Radic Biol Med 2006;40:376–387 [DOI] [PubMed] [Google Scholar]
- 57.Yasui H, Hayashi S, Sakurai H. Possible involvement of singlet oxygen species as multiple oxidants in P450 catalytic reactions. Drug Metab Pharmacokinet 2005;20:1–13 [DOI] [PubMed] [Google Scholar]
- 58.Fukui T, Lassegue B, Kai H, Alexander RW, Griendling KK. Cytochrome B-558 alpha-subunit cloning and expression in rat aortic smooth muscle cells. Biochim Biophys Acta 1995;1231:215–219 [DOI] [PubMed] [Google Scholar]
- 59.Colton CA, Fagni L, Gilbert D. The action of hydrogen peroxide on paired pulse and long-term potentiation in the hippocampus. Free Radic Biol Med 1989;7:3–8 [DOI] [PubMed] [Google Scholar]
- 60.Auerbach JM, Segal M. Peroxide modulation of slow onset potentiation in rat hippocampus. J Neurosci 1997;17:8695–8701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kamsler A, Segal M. Hydrogen peroxide modulation of synaptic plasticity. J Neurosci 2003;23:269–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kamsler A, Segal M. Paradoxical actions of hydrogen peroxide on long-term potentiation in transgenic superoxide dismutase-1 mice. J Neurosci 2003;23:10359–10367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kishida KT, Klann E. Sources and targets of reactive oxygen species in synaptic plasticity and memory. Antioxid Redox Signal 2007;9:233–244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hidalgo C, Carrasco MA, Munoz P, Nunez MT. A role for reactive oxygen/nitrogen species and iron on neuronal synaptic plasticity. Antioxid Redox Signal 2007;9:245–255 [DOI] [PubMed] [Google Scholar]
- 65.Benoit CE, Bastianetto S, Brouillette J, Tse Y, Boutin JA, Delagrange P, Wong T, Sarret P, Quirion R. Loss of quinone reductase 2 function selectively facilitates learning behaviors. J Neurosci 2010;30:12690–12700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Noh KM, Koh JY. Induction and activation by zinc of NADPH oxidase in cultured cortical neurons and astrocytes. J Neurosci 2000;20:RC111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jang HJ, Hwang S, Cho KY. Kim DK, Chay KO, Kim JK. Taxol induces oxidative neuronal cell death by enhancing the activity of NADPH oxidase in mouse cortical cultures. Neurosci Lett 2008;443:17–22 [DOI] [PubMed] [Google Scholar]
- 68.Dennis KE, Aschner JL, Milatovic D, Schmidt JW, Aschner M, Kaplowitz MR, Zhang Y, Fike CD. NADPH oxidases and reactive oxygen species at different stages of chronic hypoxia-induced pulmonary hypertension in newborn piglets. Am J Physiol Lung Cell Mol Physiol 2009;297:L596–L607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rathore R, Zheng YM, Niu CF, Liu QH, Korde A, Ho YS, Wang YX. Hypoxia activates NADPH oxidase to increase [ROS]i and [Ca2+]i through the mitochondrial ROS-pkcepsilon signaling axis in pulmonary artery smooth muscle cells. Free Radic Biol Med 2008;45:1223–1231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.He L, Liu X, Chen J, Dinger B, Stensaas L, Fidone S. Modulation of chronic hypoxia-induced chemoreceptor hypersensitivity by NADPH oxidase subunits in rat carotid body. J Appl Physiol 2010;108:1304–1310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gharib S, Dayyat E, Khalyfa A, Kim JK, Clair H, Kucia M, Gozal D. Intermittent hypoxia mobilizes bone marrow-derived very small embryonic-like stem cells and activates developmental transcriptional programs in mice. Sleep 2010;11:1439–1446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ohayon MM. The effects of breathing-related sleep disorders on mood disturbances in the general population. J Clin Psychiatry 2003;64:1195–1200; quiz, 1274–1196 [DOI] [PubMed] [Google Scholar]
- 73.File SE. Behavioural detection of anxiolytic action. : Elliott JM, Heal DJ, Marsden CA, Experimental approaches to anxiety and depression. Chichester, UK: Wiley; 1992. pp. 25–44 [Google Scholar]
- 74.D'Almeida V, Lobo LL, Hipolide DC, de Oliveira AC, Nobrega JN, Tufik S. Sleep deprivation induces brain region-specific decreases in glutathione levels. Neuroreport 1998;9:2853–2856 [DOI] [PubMed] [Google Scholar]
- 75.Singh R, Kiloung J, Singh S, Sharma D. Effect of paradoxical sleep deprivation on oxidative stress parameters in brain regions of adult and old rats. Biogerontology 2008;9:153–162 [DOI] [PubMed] [Google Scholar]
- 76.Khadrawy YA, Nour NA, Aboul Ezz HS. Effect of oxidative stress induced by paradoxical sleep deprivation on the activities of Na+, K+-ATPase and acetylcholinesterase in the cortex and hippocampus of rat. Transl Res 2011;157:100–107 [DOI] [PubMed] [Google Scholar]
- 77.Hovatta I, Juhila J, Donner J. Oxidative stress in anxiety and comorbid disorders. Neurosci Res 2010;68:261–275 [DOI] [PubMed] [Google Scholar]
- 78.Pellow S, Chopin P, File SE, Briley M. Validation of open: closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J Neurosci Methods 1985;14:149–167 [DOI] [PubMed] [Google Scholar]
- 79.Hui-guo L, Kui L, Yan-ning Z, Yong-jian X. Apocynin attenuate spatial learning deficits and oxidative responses to intermittent hypoxia. Sleep Med 2010;11:205–212 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.