Abstract
Breast cancer metastasis is a major clinical problem. The molecular basis of breast cancer progression to metastasis remains poorly understood. PELP1 is an estrogen receptor (ER) coregulator that has been implicated as a proto-oncogene whose expression is deregulated in metastatic breast tumors and whose expression is retained in ER-negative tumors. We examined the mechanism and significance of PELP1-mediated signaling in ER-negative breast cancer progression using two ER-negative model cells (MDA-MB231 and 4T1 cells) that stably express PELP1-shRNA. These model cells had reduced PELP1 expression (75% of endogenous levels) and exhibited less propensity to proliferate in growth assays in vitro. PELP1 down regulation substantially affected migration of ER-negative cells in Boyden chamber and invasion assays. Using mechanistic studies, we found that PELP1 modulated expression of several genes involved in the epithelial mesenchymal transition EMT including MMPs, Snail1, Twist, and ZEB. In addition, PELP1 knockdown reduced the in vivo metastatic potential of ER-negative breast cancer cells and significantly reduced lung metastatic nodules in a xenograft assay. These results implicate PELP1 as having a role in ER-negative breast cancer metastasis, reveal novel mechanism of coregulator regulation of metastasis via promoting cell motility / EMT by modulating expression of genes, and suggest PELP1 may be a potential therapeutic target for metastatic ER-negative breast cancer.
Keywords: ER-coactivators, Proto-oncogene, Breast cancer, Metastasis, PELP1
Introduction
Endocrine therapy has been shown to have a positive effect on the treatment of advanced metastatic disease (1, 2). Despite these positive effects, initial or acquired resistance to endocrine therapies frequently occurs with tumors recurring as metastasis, which is the leading cause of death from breast cancer. Tumor metastasis comprises a series of discrete biological processes that move tumor cells from the primary neoplasm to a distant location (3) and involves a multi-step cascade of coordinated cell adhesion and contractility as well as proteolytic remodeling of the extra-cellular matrix (4, 5). The process of migration is orchestrated through the activation of biochemical pathways that involve multiple cytoskeleton proteins (6). Even though substantial information is available on the process of metastasis, a critical need to identify novel targets that can be used to curb the progression of breast cancer metastasis still exists.
During the past 20 years, studies have extensively focused on the role of two nuclear receptors, the estrogen receptor (ER) and the progesterone receptor (PR). The presence of ER and PR in ER-positive tumors can explain the tumor’s biology; but, what drives ER-negative metastatic tumors is not known (7). With the recent advances in detection technologies, the potential importance of several additional nuclear receptor (NRs) including estrogen-related receptor alpha (ERRα), Glucocorticoid receptor (GR), Androgen receptor (AR) are being appreciated in breast cancer (8–10). Emerging evidence also suggests that NR action is complex and requires functional interactions with coregulators (11, 12). As a modulator of NR functions, coregulators are likely to play a role in breast cancer progression. Coregulators function as “master genes” sensing the physiological signals and activating the appropriate set of genes and thus have the potential to control the expression of subsets of genes to produce a desired function such as cell growth (13). With the enormous potential of coregulators as master regulators, their deregulation is likely to provide cancer cells an advantage in growth and metastasis (14). Understanding how NR coregulators play a role in metastasis will be useful in maximizing treatment opportunities for metastatic breast cancer.
Proline glutamic acid-rich protein (PELP1) was initially identified as an ER coregulator (15). Recent studies showed that PELP1 functions as a general coregulator for a number nuclear receptors including ERβ, ERR, GR, and AR (16). In the nuclear compartment PELP1 interacts with histones and histone-modifying enzymes, and thus plays a role in chromatin remodeling (17). PELP1 also couples NRs to several cytosolic signaling axes, such as Src-MAPK, PI3K-Akt, and EGFR/Her2 (16), thus functioning as a mediator of NR-extranuclear actions. PELP1 expression is deregulated in breast cancer, and exhibits oncogenic potential(18). Its expression is maintained in ER-negative breast tumors (18, 19). Although these studies suggested that PELP1 has potential to participate in hormonal-driven pathologies, whether PELP1 plays a role in initiation and progression of ER-negative breast cancer remains unknown.
In this study, we examined whether the proto-oncogene PELP1 contributes to the metastatic potential of ER-negative breast cancer cells. Using in vitro and in vivo xenograft models, we provide evidence that demonstrates PELP1 plays a role in ER-negative breast cancer invasion and metastasis by modulating expression of the genes involved in EMT. Our results suggest that PELP1 plays a critical role in promoting cell motility / EMT and suggest PELP1 may be a potential therapeutic target for metastatic ER-negative breast cancer.
Materials and Methods
Cell cultures and reagents
MDA-MB-231 and 4T1 cells were purchased from the American-Type Culture Collection (ATCC) and maintained in RPMI 1640 supplemented with 10% fetal bovine serum (FBS; Hyclone Laboratories Ltd, Logan, UT). The PELP1 antibody was purchased from Bethyl laboratories (Montgomery, TX).
Generation of PELP1-shRNA model cells
Breast cancer cells stably expressing PELP1-shRNA were generated using validated human and mouse specific Lentiviral PELP1-shRNA particles (Sigma, Indianapolis, IN) for use on MDAMB231 and 4T1 cells respectively. Stable clones were selected with puromycin selection (1 μg/mL) and pooled clones were used for all the studies. Lentiviral particles expressing non-targeted shRNA were used to generate control cells. Transient knockdown of PELP1 was achieved using On-TargetPlusSMARTpool siRNA (L-004463-00-0050) purchased from Thermoscientific (Waltham, MA) and by using oligofectamine transfection (Invitrogen, Carlsbad, CA).
Microarray studies
The Human Epithelial to Mesenchymal Transition (EMT) RT2 Profiler PCR Array that profiles the expression of 84 key genes was purchased from SABiosciences (Frederick, MD). Total RNA isolated from the MDA-MB-231 cells was used for screening by real-time PCR as per the manufacturer’s instructions. Target genes whose expression was differentially regulated (at least 2 fold difference) by PELP1 under expression were selected and validated using real-time PCR with MDA-MB-231 and 4T1 cells. All real-time PCR primers used for validation of PELP1-regulated genes were purchased from RealTimePrimers (Elkins Park, PA).
Cell migration, invasion and MMP reporter gene assays
The cell migration and invasion assays were performed by using the calorimetric cell migration assay kit (Chemicon, Billerica, MA) and the BD Biocat growth factor reduced Matrigel invasion chamber kit (BD Biosciences, San Diego, CA), respectively, as described (20). The PGL3-MMP9-Luc and MMP2-Luc plasmids were described earlier (20). Reporter gene assays were performed by transient transfection using the FuGENE6 method (Roche Indianapolis, IN) as described (21). Each transfection was carried out in 6-well plates in triplicate and normalized with the β-gal activity and total protein concentration.
Gelatin Zymography
The model cells expressing control or PELP1-shRNA were cultured in a low percentage of serum containing RPMI medium and then the culture supernatant was used to determine the enzymatic activity of MMP2 and MMP9 by using SDS-PAGE gelatin zymography as described (22) using Novex Zymogram Gels (Invitrogen, Carlsbad, CA). Recombinant MMP2 and MMP9 were purchased from R&D systems (Minneapolis, MN) and used as positive controls.
Nude mice studies
Model cells (1 × 105 ) in serum-free medium were injected into left cardiac ventricle of 6-week-old female athymic nude mice (n = 10 per group) as described (22). MDA-MB-231 cells and MDA-MB231-PELP1-shRNA cells were transfected with GFP-Luc plasmid to monitor metastasis with whole animal imaging (23). The mice were monitored daily for adverse affects and the body weight was recorded every three days. The Xenogen Small-Animal Imaging System was used for subcellular imaging in live mice once a week. On day 21, mice were euthanized, and the total weight and the number of micro-metastastic tumor nodules in the lungs were counted with an inverted microscope. Bone tissues (tibia and femora) were fixed in 10% neutral buffered formalin (Fisher Scientific) for 48 hours at room temperature, decalcified in 10% EDTA and embedded in paraffin. Sections were stained with hematoxylin, eosin, orange G and phloxine. Histomorphometrical analysis was carried out to obtain the trabecular bone volume and tumor burden in the tibia using BioQuant Osteo, 2010, version V10.3.6 software.
Statistical analysis
Statistical differences among groups were analyzed with either t-test or ANOVA as appropriate using SPSS software. P values <0.05 were considered significant.
Results
PELP1 knockdown reduces cell proliferation of ER-negative breast cancer cells
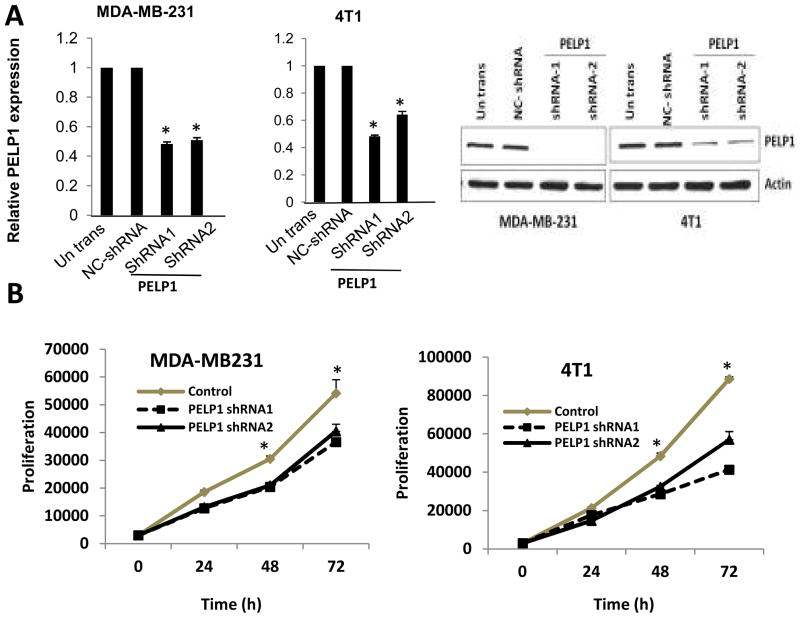
We used two ER-negative model cells: MDA-MB231 (human) and 4T1 (mouse). Earlier studies showed that these models cells metastasize efficiently to sites affected in human breast cancer (24, 25) and both cells express high levels of PELP1. To establish the significance of the PELP1 axis, we knocked down PELP1 expression using lentiviral-mediated transduction of PELP1-shRNA. Pooled clones stably expressing PELP1-shRNA were selected by puromycin. qRTPCR and Western analysis showed that PELP1 expression in MDA-MB231-PELP1shRNA and 4T1-PELP1shRNA model cells was reduced by 70–80% (Fig. 1A). We next examined whether PELP1 down regulation affects proliferation of breast cancer cells in vitro using the Cell Titer-Glo assay. Both PELP1shRNA model cells showed substantially less cellular proliferation than the control shRNA-transfected cells (Fig.1B). We also validated these results using another PELP1 siRNA that targeted a different site on PELP1 than the PELP1-shRNA targeted site. Transient knockdown using PELP1 siRNA resulted in a 80% reduction of endogenous PELP1 (Fig. S1). In proliferation assays, transient knockdown of PELP1 expression also substantially decreased the proliferation of MDA-MB231 cells (Fig. S1). Collectively, these results indicate that the proto-oncogene PELP1 has potential to regulate the cell proliferation of ER-negative breast epithelial cells.
Figure 1.
Effect of PELP1 knockdown on cell proliferation of ER-negative breast cancer cells. A, MDA-MB231 and 4T1 cells untransfected, and or stably expressing negative control (NC) shRNA vector or PELP1-shRNA were lysed and the expression of PELP1 was analyzed by real-time PCR analysis and Western blotting . B, Cell proliferation potential of the MDA-MB231 and 4T1 cells -PELP1-shRNA and control shRNA clones were measured in vitro by using a Cell Titer-Glo assay. Mean and SDs are from 3 independent experiments. *, p ≤ 0.05, t test.
PELP1 signaling axis is need for optimal cell migration and invasion of ER-negative breast cancer cells
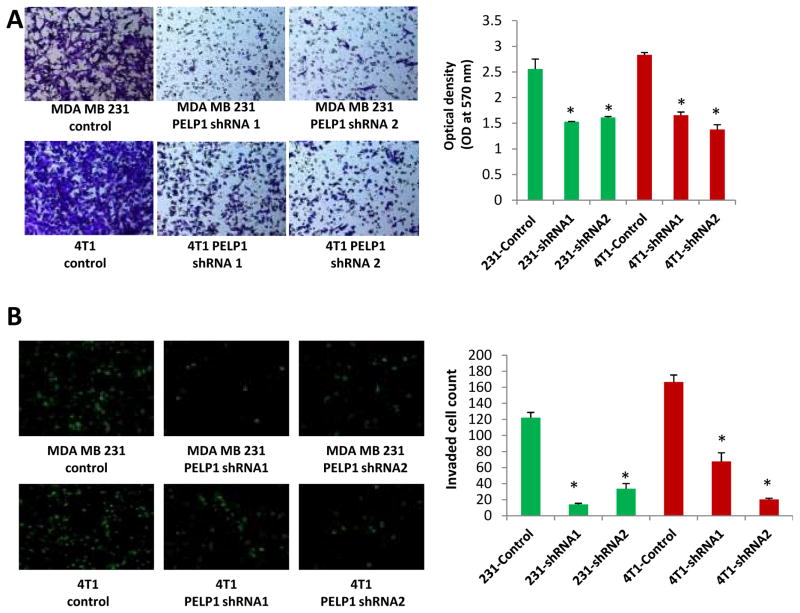
PELP1 expression is deregulated in metastatic tumors (18). However, whether PELP1 plays a role in the metastatsis of ER-negative cells remains unknown. To examine the significance of PELP1 in ER-negative cell metastasis, we performed in vitro migration assays and an invasion assays using Boyden chamber assay. In the migration assays, PELP1 knock down resulted in significantly less migration in both the MDA-MB231 and 4T1 cells than in the control vector-transfected cells (Fig. 2A). PELP1 knock down also significantly reduced the invasion potential of both the MDA-MB231 and 4T1 cells (Fig. 2B). The results observed in PELP1-shRNA stable clones were also validated by using transient knock down of PELP1 by siRNA that targeted a different site on PELP1 than the PELP1-shRNA target site. PELP1 siRNA but not control siRNA treatment significantly reduced the ability of MDA-MD231 cells to migrate in the Boyden chamber assays (Fig. S2). Collectively these results suggest that PELP1 has the potential to modulate migration and invasion of ER-negative breast cancer cells.
Figure 2.
PELP1 knockdown affects the migration and invasion of ER-negative breast cancer cells. A, Cell migration potential of the MDA-MB231 and 4T1 cells transfected with control shRNA or PELP1-shRNA was analyzed by using a Boyden chamber assay. Photomicrographs of migrated cells in various treatments are shown. B, Cell invasion potential of the MDA-MB231 and 4T1 cells transfected with control shRNA or PELP1-shRNA was analyzed by using Matrigel invasion chamber assays. Photomicrographs of invaded cells in various treatments are shown. Mean and SDs are from 3 independent experiments. *,p ≤ 0.05 t test.
PELP1 knock down promotes alterations in the expression of the EMT markers
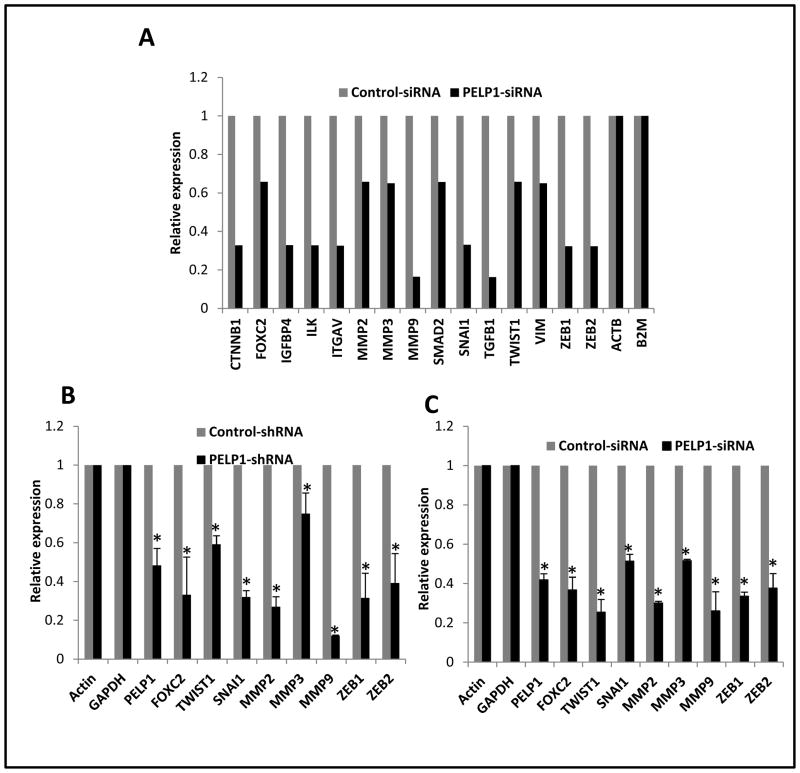
EMT is a key process that is implicated in tumor metastases. PELP1 is a coregulator that plays a critical role in NR genomic functions via chromatin remodeling and by modulating the histone code at the NR target promoters (17). Since PELP1 knockdown ER-negative breast cancer cells exhibited less migration and invasion, we examined whether the lack of expression of PELP1 affects the expression of genes involved in EMT by using a focused microarray approach. The EMT microarray contains 84 genes including cell surface receptors; cytoskeletal genes mediating cell adhesion, migration, motility, and morphogenesis; genes controlling cell differentiation, development, growth, and proliferation; and signal transduction and transcription factor genes that cause EMT and all of its associated processes. Total RNA isolated from the MDA-MB231 and MDA-MB231-PELP1siRNA cells were used for the array analysis. The results from the screen suggested that PELP1 down regulation substantially reduced the expression of a number of genes, including MMP2, MMP3, MMP9, SMAD2, SNAIL, TWIST1, VIM, ZEB1 and ZEB2 compared to their expression in control shRNA-transfected cells (Fig. 3A). We validated the changes seen in the array study by measuring gene expression of the top eight genes that had significant reductions by performing real-time qPCR in the MDA-MB231-PELP1-shRNA stable clones (Fig. 3B). PELP1 regulation of EMT-related genes was also independently confirmed in the MDA-MB231 cells by the transient expression of PELP1 siRNA (Fig. 3C). Further, qRTPCR analysis using the 4T1 cells expressing PELP1siRNA cells also showed that PELP1 down regulation significantly affected the expression of EMT genes compared to the genes in the control siRNA cells (Fig. S3A). Further, RTqPCR and Western analysis of 4T1 model cells showed upregulation of Ecadherin (CDH1) under conditions of PELP1 knockdown (Fig. S3B). These results suggest that PELP1 has potential to modulate expression of genes involved in the EMT/metastasis.
Figure 3.
PELP1 knockdown affects expression of selective genes involved in EMT. A, RNA isolated from MDA-MB231-siRNA negative control and MDA-MB231-PELP1siRNA-expressing cells was hybridized to the human EMT Array. Changes in the gene expression were analyzed by using SABioscience software with actin as a control for normalization. Representative genes downregulated upon PELP1 depletion are shown. B, Real-time qPCR validation of the changes in gene expression in MDA-MB231 cells stably expressing negative control shRNA or PELP1-shRNA. C, MDA-MB231 cells were transiently transfected with control or PELP1-specific siRNA and the expression of indicated genes was analyzed by real-time qPCR. *, p ≤ 0.05, t test.
PELP1 knock down modulates expression and activities of MMPs
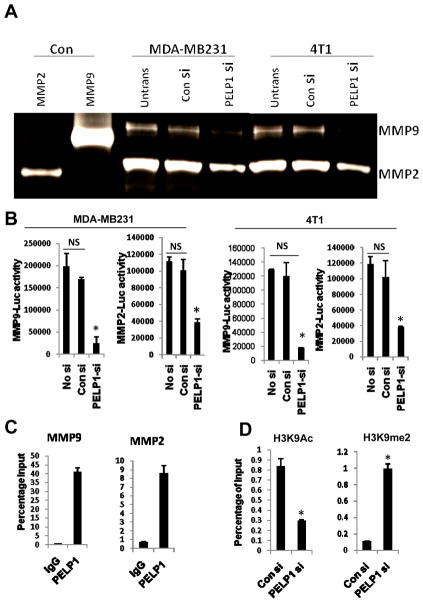
Since PELP1 down regulation altered expression of matrix metalloproteinase (MMPs) in ER-negative breast cancer and because MMPs are involved in EMT and all of its associated processes (18), we examined whether the reduced expression of MMPs correlates with functional activity. We determined MMP activity in both the MDA-MB231 and 4T1 cells expressing control or PELP1-siRNA by using gelatin zymography. MMP2 and MMP9 activities were lower in PELP1 knockdown cells than in control cells (Fig. 4A). To elucidate the mechanism by which PELP1 regulates MMP2 and MMP9 expression, we performed promoter-Luc assays using the previously published MMP2 and MMP9 reporter genes (26, 27). In both the MDA-MB231 and 4T1 model cells, knock down of PELP1 significantly reduced the MMP2 and MMP9 reporter gene activation (Fig. 4B). We examined further whether the PELP1-mediated regulation of MMPs is due to PELP1 recruitment to the promoter regions of MMPs by using a ChIP-based assay. Our ChIP results showed that PELP1 is uniquely recruited to the proximal promoter region of MMP9 and MMP2 (Fig. 4C) and no recruitment in the distal regions was observed (data not shown). PELP1 associates with acetylases (p300, CBP) and demethylases (KDM1) and participates in epigenetic modifications that are required for optimal transcriptional activation (16). To examine whether lack of PELP1 promotes inhibitory marks, we examined the status of epigenetic modifications at the MMP9 proximal promoter. ChIP analysis revealed that PELP1 knock down enhances the repressive mark H3K9me2 and decreases the activation mark H3K9ac (Fig. 4D). These results suggest that PELP1-mediated genomic actions may play a role in PELP1 modulation of the expression and function of MMPs.
Figure 4.
PELP1 regulates the expression and activities of MMPs in ER-negative cells A, Gelatin zymography analysis of activity of MMP2 and MMP9 in the MDA-MB231 and 4T1 cells that were transfected with control siRNA or PELP1-siRNA. B, cells transfected with MMP9-Luc and MMP2-Luc vectors, treated with control siRNA or PELP1-siRNA and the reporter gene activity was measured after 72 hours. C, ChIP assay was done using the DNA isolated from the MDA MB-231 cells and by using antibodies specific for PELP1 or isotype rabbit IgG control. DNA recovered from ChIP or input controls was subjected to real-time quantitative PCR that spanned the MMP9 and MMP2 promoter region. D, MDA-MB-231 cells were transfected with control or PELP1-siRNA and the ChIP assay was done using antibodies specific for H3K9Ac or H3K9me2. DNA recovered from ChIP or input controls was subjected to real-time quantitative PCR using primers that detect proximal MMP9 promoter region. The promoter occupancy was calculated based on the ratio of ChIP/input control. *,p ≤ 0.05**,p ≤ 0.001, t test.
Significance of the PELP1 axis in the metastatic potential of ER-negative cells in vivo
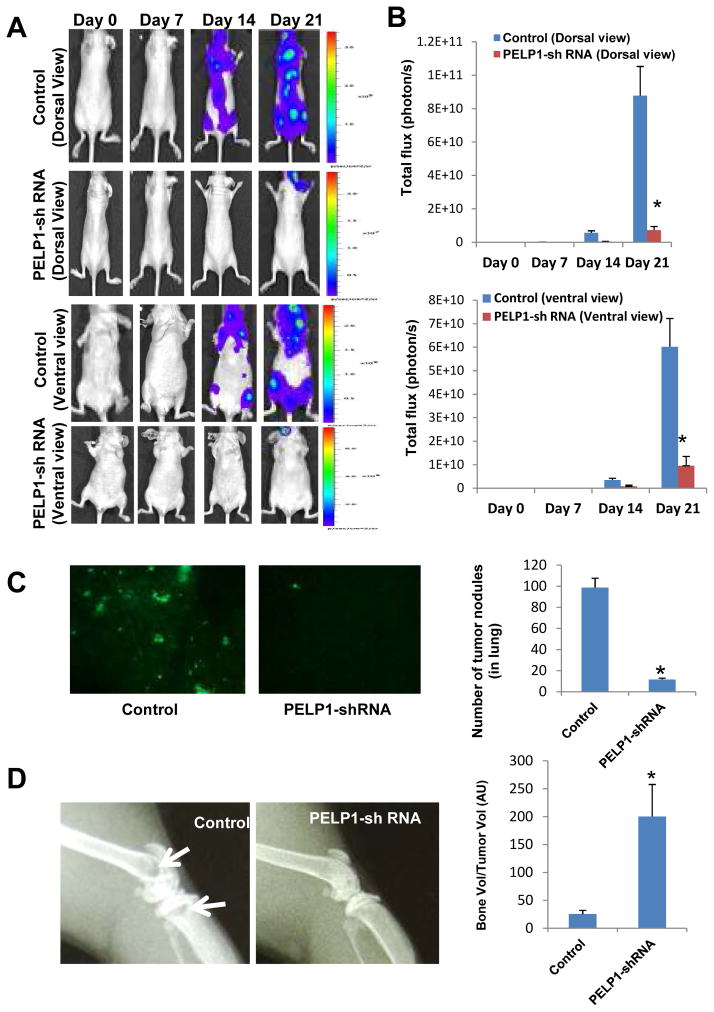
To examine whether blockage of PELP1 axis in vivo reduces metastasis potential of ER-negative breast cancer cells, we performed studies in nude mice using the MDA-MB231 and MDA-MB231-PELP1shRNA cells that were transfected with GFP-Luciferase vectors (Fig. S4). For this assay, model cells (1 × 105 in 0.1 ml PBS/mouse) were injected into the left cardiac ventricle of nude mice (10 animals each group, total 20 mice) to monitor metastasis with whole animal imaging. The Xenogen non-invasive optical imaging system was used for whole animal imaging on days 0, 7, 14 and 21. The presence of the luciferase signal within the whole animal indicated that there was a greater propensity of metastasis signal both in the dorsal and ventral views (Fig. 5A) of mice injected with control MDA-MB231 cells than with the corresponding MDA-MB231-PELP1shRNA 2. Compared to control MDA-MB231shRNA cells injected mice, nude mice injected with PELP1-shRNA cells had a significant reduction in tumor metastatic signal (91%, p < 0.0001) in the dorsal view and a reduction in the ventral view (84%, p < 0.0001) (Fig. 5B). The body weights were not significantly different between control shRNA and PELP1shRNA treatment groups (data not shown), however, mice injected with the control MDA-MB231shRNA cells had slower mobility (probably due to metastasis) than the mice injected with PELP1shRNA cells on day 21. To further examine the role of PELP1 in dissemination of tumor cells in vivo, the lungs and bones were collected after day 21 and histologically examined for metastasis. Subcellular imaging of whole lung GFP fluorescence revealed that a significant reduction (88%, p < 0.0001) in tumor nodule formation in the PELP1shRNA treatment groups compared to the control group (Fig. 5C). X-ray imaging and corresponding quantitation of tibia and femur bones analysis elucidated that a portion of bone loss was observed in the MDA-MB231 control mice and not in the PELP1shRNA groups (Fig. 5D). Collectively, these results suggest that PELP1 knockdown cells exhibited less propensity to metastasize to distant organs.
Figure 5.
PELP1downregulation reduces the metastatic potential of ER-negative cells in vivo. A, whole animal imaging analysis of mice (n=10/group) treated with MDA-MB231 and MDA-MB231-PELP1shRNA. Both dorsal and ventral views are shown. B, Luciferase signals were quantitated by using the software Living Image 3.2. C, Number of tumor nodules in the lung was measured in the control and PELP1-shRNA treated mice groups (n=10/group). D, Bone loss was detected by X-ray imaging analysis and bone and tumor volumes in sections of tibiae were quantified with histomorphometry analysis as described in Materials and Methods.
Discussion
Breast cancer is the most frequently diagnosed cancer in women. Tumor metastasis remains a significant problem and is the main cause of patient fatality. In this study, we examined whether the proto-oncogene PELP1 contributes to metastatic potential of ER-negative breast cancer cells and determined whether blocking the PELP1 signaling axis has a therapeutic effect. We found that (a) PELP1 knockdown affected the cell proliferation of ER-negative breast cancer cells; (b) PELP1 knockdown substantially affected the ability of both MDA-MB231 and 4T1 cells to migrate in Boyden chamber and invasion assays; (c) down regulation of PELP1 reduced the activity of MMPs in ER-negative metastatic cells; (d) PELP1 modulated the expression of genes involved in EMT; and (e) down regulation of PELP1 in vivo by PELP1-shRNA significantly reduced ER-negative breast metastasis in a nude mice model. Collectively, these results suggest that PELP1 signaling confers growth and metastatic advantages to ER-negative breast epithelial cells.
To appreciate the mechanisms by which breast cancers develop into metastasis, it is necessary to understand the molecular mechanism(s) involved in metastasis. Recent advances suggest that nuclear receptor interacting coregulators may play roles in growth and metastasis of ER-negative tumors by modulating transcription of target genes (28, 29). For example the ER coregulator AIB1 can promote breast cancer metastasis by the activation of PEA3-mediated MMP2 and MMP9 expression (30). PELP1 was initially identified as a novel ER coregulator (15). Recent studies showed that PELP1 functions as a general coregulator for a number of nuclear receptors including ER, ERR, PR, GR, AR and transcription factors such as E2F, FHL2, and STAT3 (16). The ability of PELP1 to interact with a wide variety of nuclear receptors and transcription factors suggest that PELP1 deregulation in metastatic cells may provide advantage by modulating a set of genes required for metastasis. Accordingly, the results from our gene array experiment indicated that PELP1 plays a critical role in the expression of a number of genes involved in EMT and metastasis.
PELP1 expression is deregulated in breast cancer. Our earlier studies using breast tumor prognostic arrays demonstrated that node-positive and metastatic tumors have greater PELP1 expression than in node-negative specimens (18). Curiously, in this study, PELP1overexpression is equally observed in both in ER -positive and negative Grade2/3, node-positive and metastatic tumors, suggesting that PELP1 may have functions independent of the ER in metastatic cells (18). A recent study of a larger number of patients (n=1,162) with invasive breast cancers found that high PELP1 expression is associated with tumor clinical parameters, and implicated PELP1 protein expression as an independent prognostic predictor of shorter breast cancer-specific survival (19). In this study, using in vitro and in vivo models, we have provided the first evidence that PELP1 signaling plays a critical role in ER-negative breast cancer proliferation and metastasis. Earlier studies using ovarian cancer xenograft model indicated that PELP1 knockdown reduces primary tumorigenesis (20). Our findings that PELP1 reduces proliferation and metastasis of ER-ve model cells suggests that PELP1 signaling can also be potentially used to therapeutically target ER-negative breast cancers.
In the nuclear compartment PELP1 interacts with histones and histone-modifying enzymes, and thus plays a role in chromatin remodeling activities at target genes (31). Previous studies from our lab showed that PELP1 facilitates target gene activation by histone modification via lysine demethylase 1 (LSD1/KDM1) (17). Our results using the MMP9 and MMP2 promoter reporter gene assays suggest that PELP1 has potential to modulate transcription of these genes. ChIP analysis showed that PELP1 is recruited to the MMP9 and MMP2 promoters and that PELP1 status has potential to influence the inhibitory histone marks at MMP promoter. Further, EMT gene array analysis indicated that PELP1 has potential to modulate several regulatory genes involved in the EMT process including TWIST, Snail and Zeb. These findings suggest that PELP1 modulation of genes involved in EMT and invasion as one possible mechanism by which it promotes tumor progression in ER-negative breast metastasis.
Earlier studies showed that PELP1 interacts with several proteins involved in cytoskeleton remodeling, including Src kinase (32), PI3K (33), four-and-a-half LIM protein 2 (34), and ILK1 (35). PELP1 modulates functions of metastasis associated antigen 1 (MTA1), a protein implicated in metastasis. PELP1 also interacts with the MTA1-associated co-activator and promotes ER-transactivation functions in a synergistic manner (36). PELP1 is shown to modulate expression of MTA3, a gene implicated in the invasive growth of human breast cancers. These earlier studies suggest that PELP1 interactions with the ER, cytoskeletal kinases and MTA family members contribute to PELP1-driven ER-positive metastasis. Our current results add new information that PELP1 also has potential to modulate migratory properties of ER-negative breast cancer cells. In vitro migration and invasion assays using two different model cells showed that PELP1 signaling is essential for optimal cell migration. Further, in vivo xenograft-based assays demonstrated that PELP1 plays a critical role in in vivo metastasis of ER-negative breast cancer cells.
In summary, our results demonstrate that the NR coregulator PELP1 plays a critical role in ER-negative breast cancer cell migration and modulates expression of several genes involved in EMT/metastasis. Even though earlier studies found that PELP1 plays a role in the proliferation of hormone-driven tumors, this study demonstrates that it also has the potential to promote metastasis of ER-negative breast epithelial cells. Further, our study provides the first in vivo evidence that the PELP1 axis is a potential therapeutic for blocking ER-negative breast cancer metastasis. Exploring the role of the PELP1 axis in metastasis is a novel area and understanding how PELP1 plays a role in metastasis will be useful in maximizing treatment opportunities for metastatic breast cancer.
Supplementary Material
Acknowledgments
This study was supported by the NIH/NCI grant CA095681 (R.K.V.), NIH T32CA148724 (SSR) and CA075253 (L-Z. S.) and Cancer Center Support Grant P30CA054174.
Reference List
- 1.Lang K, Drell TL, Zaenker KS, Entschladen F. Inhibitors for metastasis development. Recent Pat Anticancer Drug Discov. 2006;1:69–80. doi: 10.2174/157489206775246511. [DOI] [PubMed] [Google Scholar]
- 2.Utsumi T, Kobayashi N, Hanada H. Recent perspectives of endocrine therapy for breast cancer. Breast Cancer. 2007;14:194–9. doi: 10.2325/jbcs.959. [DOI] [PubMed] [Google Scholar]
- 3.Steeg PS. Tumor metastasis: mechanistic insights and clinical challenges. Nat Med. 2006;12:895–904. doi: 10.1038/nm1469. [DOI] [PubMed] [Google Scholar]
- 4.Stetler-Stevenson WG, Liotta LA, Kleiner DE., Jr Extracellular matrix 6: role of matrix metalloproteinases in tumor invasion and metastasis. FASEB J. 1993;7:1434–41. doi: 10.1096/fasebj.7.15.8262328. [DOI] [PubMed] [Google Scholar]
- 5.Friedl P, Wolf K. Tumour-cell invasion and migration: diversity and escape mechanisms. Nat Rev Cancer. 2003;3:362–74. doi: 10.1038/nrc1075. [DOI] [PubMed] [Google Scholar]
- 6.Friedl P, Wolf K. Tumour-cell invasion and migration: diversity and escape mechanisms. Nat Rev Cancer. 2003;3:362–74. doi: 10.1038/nrc1075. [DOI] [PubMed] [Google Scholar]
- 7.Hudis CA, Gianni L. Triple-negative breast cancer: an unmet medical need. Oncologist. 2011;16(Suppl 1):1–11. doi: 10.1634/theoncologist.2011-S1-01. [DOI] [PubMed] [Google Scholar]
- 8.Conzen SD. Minireview: nuclear receptors and breast cancer. Mol Endocrinol. 2008;22:2215–28. doi: 10.1210/me.2007-0421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gucalp A, Traina TA. Triple-negative breast cancer: role of the androgen receptor. Cancer J. 2010;16:62–5. doi: 10.1097/PPO.0b013e3181ce4ae1. [DOI] [PubMed] [Google Scholar]
- 10.Ni M, Chen Y, Lim E, Wimberly H, Bailey ST, Imai Y, et al. Targeting androgen receptor in estrogen receptor-negative breast cancer. Cancer Cell. 2011;20:119–31. doi: 10.1016/j.ccr.2011.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Green KA, Carroll JS. Oestrogen-receptor-mediated transcription and the influence of co-factors and chromatin state. Nat Rev Cancer. 2007;7:713–22. doi: 10.1038/nrc2211. [DOI] [PubMed] [Google Scholar]
- 12.McKenna NJ, Lanz RB, O'Malley BW. Nuclear receptor coregulators: cellular and molecular biology. Endocr Rev. 1999;20:321–44. doi: 10.1210/edrv.20.3.0366. [DOI] [PubMed] [Google Scholar]
- 13.Rosenfeld MG, Lunyak VV, Glass CK. Sensors and signals: a coactivator/corepressor/epigenetic code for integrating signal-dependent programs of transcriptional response. Genes Dev. 2006;20:1405–28. doi: 10.1101/gad.1424806. [DOI] [PubMed] [Google Scholar]
- 14.O'Malley BW. Coregulators: from whence came these “master genes”. Mol Endocrinol. 2007;21:1009–13. doi: 10.1210/me.2007-0012. [DOI] [PubMed] [Google Scholar]
- 15.Vadlamudi RK, Wang RA, Mazumdar A, Kim Y, Shin J, Sahin A, et al. Molecular cloning and characterization of PELP1, a novel human coregulator of estrogen receptor alpha. J Biol Chem. 2001;276:38272–9. doi: 10.1074/jbc.M103783200. [DOI] [PubMed] [Google Scholar]
- 16.Vadlamudi RK, Kumar R. Functional and biological properties of the nuclear receptor coregulator PELP1/MNAR. Nucl Recept Signal. 2007;5:e004. doi: 10.1621/nrs.05004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nair SS, Nair BC, Cortez V, Chakravarty D, Metzger E, Schule R, et al. PELP1 is areader of histone H3 methylation that facilitates oestrogen receptor-alpha target gene activation by regulating lysine demethylase 1 specificity. EMBO Rep. 2010;11:438–44. doi: 10.1038/embor.2010.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rajhans R, Nair S, Holden AH, Kumar R, Tekmal RR, Vadlamudi RK. Oncogenic Potential of the Nuclear Receptor Coregulator Proline-, Glutamic Acid-, Leucine-Rich Protein 1/Modulator of the Nongenomic Actions of the Estrogen Receptor. Cancer Res. 2007;67:5505–12. doi: 10.1158/0008-5472.CAN-06-3647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Habashy HO, Powe DG, Rakha EA, Ball G, Macmillan RD, Green AR, et al. The prognostic significance of PELP1 expression in invasive breast cancer with emphasis on the ER-positive luminal-like subtype. Breast Cancer Res Treat. 2009 doi: 10.1007/s10549-009-0419-9. [DOI] [PubMed] [Google Scholar]
- 20.Chakravarty D, Roy SS, Babu CR, Dandamudi R, Curiel TJ, Vivas-Mejia P, et al. Therapeutic Targeting of PELP1 Prevents Ovarian Cancer Growth and Metastasis. Clin Cancer Res. 2011;17:2250–9. doi: 10.1158/1078-0432.CCR-10-2718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nair BC, Nair SS, Chakravarty D, Challa R, Manavathi B, Yew PR, et al. Cyclin-dependent kinase-mediated phosphorylation plays a critical role in the oncogenic functions of PELP1. Cancer Res. 2010;70:7166–75. doi: 10.1158/0008-5472.CAN-10-0628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lengyel E, Gum R, Juarez J, Clayman G, Seiki M, Sato H, et al. Induction of M(r) 92,000 type IV collagenase expression in a squamous cell carcinoma cell line by fibroblasts. Cancer Res. 1995;55:963–7. [PubMed] [Google Scholar]
- 23.Bandyopadhyay A, Wang L, Agyin J, Tang Y, Lin S, Yeh IT, et al. Doxorubicin in combination with a small TGFbeta inhibitor: a potential novel therapy for metastatic breast cancer in mouse models. PLoS One. 2010;5:e10365. doi: 10.1371/journal.pone.0010365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aslakson CJ, Miller FR. Selective events in the metastatic process defined by analysis of the sequential dissemination of subpopulations of a mouse mammary tumor. Cancer Res. 1992;52:1399–405. [PubMed] [Google Scholar]
- 25.Tavazoie SF, Alarcon C, Oskarsson T, Padua D, Wang Q, Bos PD, et al. Endogenous human microRNAs that suppress breast cancer metastasis. Nature. 2008;451:147–52. doi: 10.1038/nature06487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Qin L, Liao L, Redmond A, Young L, Yuan Y, Chen H, et al. The AIB1 oncogene promotes breast cancer metastasis by activation of PEA3-mediated matrix metalloproteinase 2 (MMP2) and MMP9 expression. Mol Cell Biol. 2008;28:5937–50. doi: 10.1128/MCB.00579-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Qin H, Sun Y, Benveniste EN. The transcription factors Sp1, Sp3, and AP-2 are required for constitutive matrix metalloproteinase-2 gene expression in astroglioma cells. J Biol Chem. 1999;274:29130–7. doi: 10.1074/jbc.274.41.29130. [DOI] [PubMed] [Google Scholar]
- 28.O'Malley BW, Kumar R. Nuclear receptor coregulators in cancer biology. Cancer Res. 2009;69:8217–22. doi: 10.1158/0008-5472.CAN-09-2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lydon JP, O'Malley BW. Minireview: steroid receptor coactivator-3: a multifarious coregulator in mammary gland metastasis. Endocrinology. 2011;152:19–25. doi: 10.1210/en.2010-1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yan J, Erdem H, Li R, Cai Y, Ayala G, Ittmann M, et al. Steroid receptor coactivator-3/AIB1 promotes cell migration and invasiveness through focal adhesion turnover and matrix metalloproteinase expression. Cancer Res. 2008;68:5460–8. doi: 10.1158/0008-5472.CAN-08-0955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nair SS, Mishra SK, Yang Z, Balasenthil S, Kumar R, Vadlamudi RK. Potential role of a novel transcriptional coactivator PELP1 in histone H1 displacement in cancer cells. Cancer Res. 2004;64:6416–23. doi: 10.1158/0008-5472.CAN-04-1786. [DOI] [PubMed] [Google Scholar]
- 32.Vadlamudi RK, Manavathi B, Balasenthil S, Nair SS, Yang Z, Sahin AA, et al. Functional implications of altered subcellular localization of PELP1 in breast cancer cells. Cancer Res. 2005;65:7724–32. doi: 10.1158/0008-5472.CAN-05-0614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dimple C, Nair SS, Rajhans R, Pitcheswara PR, Liu J, Balasenthil S, et al. Role of PELP1/MNAR signaling in ovarian tumorigenesis. Cancer Res. 2008;68:4902–9. doi: 10.1158/0008-5472.CAN-07-5698. [DOI] [PubMed] [Google Scholar]
- 34.Nair SS, Guo Z, Mueller JM, Koochekpour S, Qiu Y, Tekmal RR, et al. Proline-, glutamic acid-, and leucine-rich protein-1/modulator of nongenomic activity of estrogen receptor enhances androgen receptor functions through LIM-only coactivator, four-and-a-half LIM-only protein 2. Mol Endocrinol. 2007;21:613–24. doi: 10.1210/me.2006-0269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chakravarty D, Nair SS, Santhamma B, Nair BC, Wang L, Bandyopadhyay A, et al. Extranuclear Functions of ER Impact Invasive Migration and Metastasis by Breast Cancer Cells. Cancer Res. 2010;70:4092–101. doi: 10.1158/0008-5472.CAN-09-3834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mishra SK, Mazumdar A, Vadlamudi RK, Li F, Wang RA, Yu W, et al. MICoA, a novel metastasis-associated protein 1 (MTA1) interacting protein coactivator, regulates estrogen receptor-alpha transactivation functions. J Biol Chem. 2003;278:19209–19. doi: 10.1074/jbc.M301968200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.