Abstract
Objective
Peroxisome proliferator activated receptor γ (PPARγ) ligands attenuate angiotensin II (AngII)-induced atherosclerosis through interactions with vascular smooth muscle cells (VSMC)-specific PPARγ in hypercholesterolemic mice. Therefore, the purpose of this study was to determine the mechanism of AngII-mediated intracellular regulation of PPARγ in VSMCs.
Methods and Results
Incubation of cultured mouse aortic VSMCs with AngII for 24 hours reduced abundance of PPARγ protein, mRNA, and transcriptional activity (p<0.001). This effect was attenuated by a angiotensin type 1 (AT1) receptor antagonist, losartan. AngII-induced PPARγ reduction was dependent on stimulation of TGF-β1 as demonstrated using either a neutralizing antibody or siRNA. AngII-induced TGF-β1 secretion was dependent on EGFR kinase activation through reactive oxygen species production. Inhibition of p38 MAPK by SB 203580 or siRNA inhibited both AngII and TGF-β1- induced PPARγ reduction. Blockade of TGF-β1 decreased p38 phosphorylation induced by AngII. SiRNA mediated inhibition of HDAC3 attenuated p38-mediated reductions in PPARγ abundance.
Conclusion
These findings suggest that AngII decreases PPARγ abundance in cultured VSMCs via an AT1 receptor dependent manner secretion of TGF-β1 via phosphorylation of p38 MAPK and HDAC3.
Keywords: angiotensin II, nuclear receptors, signaling
INTRODUCTION
Peroxisome proliferator activated receptor γ (PPARγ) is a transcription factor belonging to the nuclear hormone receptor superfamily.1 PPARγ is predominantly expressed in adipose tissue and has been characterized as an important regulator of adipocyte differentiation and glucose homeostasis.2 PPARγ is also expressed in macrophages, endothelial cells, and vascular smooth muscle cells (VSMCs) where it regulates gene expression of key proteins involved in vascular inflammation and proliferation.3
PPARγ ligands, namely the thiazolidinedione (TZD) compounds reduce hypercholesterolemia-induced and angiotensin II (AngII)enhanced atherosclerosis in apolipoprotein E4 and LDL receptor deficient mice.5,6 We showed recently that a TZD compound, pioglitazone, attenuated AngII-induced atherosclerosis through interactions with PPARγ expressed in VSMC.6 Conversely, PPARγ ligands also modulate AngII signaling in VSMCs both at the receptor level and downstream of AT1 receptors.7,8 AngII has been demonstrated to reduce PPARγ transcriptional activity through the activation of BCR kinase in VSMCs.9 However, the effect of AngII on PPARγ gene expression, and the mechanisms by which AngII interacts with PPARγ in VSMCs remain poorly understood.
Transforming growth factor β (TGF-β) molecules are a family of cytokines with multiple effects on growth and development.10 AngII induces autocrine production of TGF-β1 in VSMCs that mediates its mitogenic effects.11 The primary downstream effectors of TGF-β signal transduction are Smad signaling proteins. In addition to Smad proteins, mitogen-activated protein kinases (MAPKs) have also been invoked as downstream effectors of TGF-β signaling.12,13
In VSMCs, PPARγ and TGF-β1 signal transduction pathways have mutual regulatory mechanisms. PPARγ activation was initially shown to inhibit TGF-β1-induced connective tissue growth factor expression in human aortic VSMCs.14 Subsequently, it was demonstrated that TGF-β1 had a biphasic effect by exerting a rapid induction followed by a strong repression on PPARγ gene transcription in aortic VSMCs.15
Collectively, in the literature, it has been known that (i) AngII reduces PPARγ transcriptional activity in VSMCs through BCR kinase activation, (ii) TGF-β1 represses PPARγ transcription in VSMCs, and (iii) AngII activates p38 MAPK in VSMCs via the activation of EGFR kinase and ROS production. In this study, we provide novel evidence that (i) AngII reduces PPARγ protein and mRNA in aortic VSMCs associated with reduced transcriptional activity via AT1 receptors. In addition, the present study showed new evidence that (ii) AngII activates p38 MAPK via EGFR kinase mediated induction of TGF-β1 and causes a reduction in expression of PPARγ protein. (iii) Furthermore, AngII-activated p38 MAPK decreases PPARγ protein in a HDAC3 dependent manner. (iv) AngII-TGF-β1-p38 MAPK mediated reduction of PPARγ in VSMCs is independent of AngII-induced Bcr kinase activation. (v) Furthermore, we show that TGF-β1 mediates its effect on PPARγ independent of the Smad-2 pathway.
MATERIALS AND METHODS
A detailed description of all methods is presented in the Supplemental Materials.
Isolation and culture of VSMCs
Mouse aortas were sectioned into thoracic, suprarenal, and infrarenal regions, and VSMCs were isolated as described previously.16 All procedures were approved by the University of Kentucky Institutional Animal Care and Use Committee.
Cell culture
VSMCs were incubated with either saline or AngII (1 μM) for 24 hours to measure PPARγ expression and transcriptional activity, or 0 - 60 minutes to measure MAPK activity and TGF-β1 expression. Pharmacological inhibitors were added 30 minutes prior to addition of AngII. Incubation with recombinant TGF-β1 (10 ng/ml) or active p38 (100 nM) for 30 minutes or 24 hours was used to measure MAPK activity or PPARγ mRNA abundance.
Western blot analyses
Total cell lysates were used for Western blot analyses.
Real time PCR
Real time PCR were performed using an iCycler (Bio-Rad) as described previously.17
Transient Transfection Assays
PPARγ1 promoter luciferase activities were analyzed using transient transfection assays as described previously.18
RNA Silencing Experiments
SiRNA experiments were performed using the On-target plus SMARTpool technology.
Statistical Analyses
All data are reported as means ± SEM. Statistic analyses were perform as appropriate for number of group and the parametric or non parametric nature of the data.
RESULTS
AngII decreased expression of PPARγ in aortic VSMCs via interaction with AT1 receptors
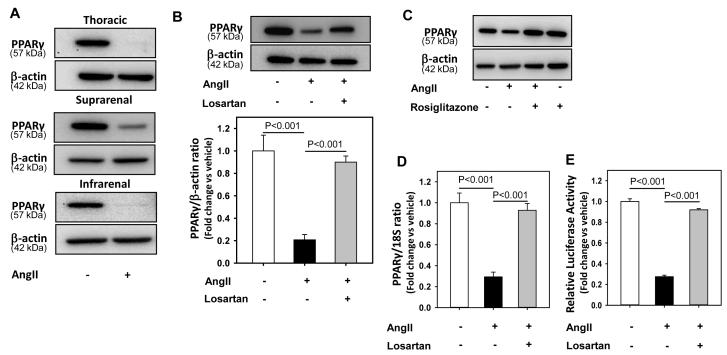
To investigate if AngII regulates expression of PPARγ, VSMCs isolated from thoracic, suprarenal, and infrarenal aortic regions were incubated with AngII (1 μM) for 24 hours. AngII decreased PPARγ protein abundance in VSMCs from all regions (Figure 1A). All subsequent experiments were conducted with cells obtained from the suprarenal region. To examine the role of AT1 receptors in AngII-induced PPARγ regulation, VSMCs were pre-incubated with losartan for 30 minutes followed by AngII incubation for 24 hours. Blockade of AT1 receptors with losartan prevented AngII-induced decreases in PPARγ protein abundance (P < 0.001, Figure 1B). In addition, activation of PPARγ by ligand, rosiglitazone (10 μM), completely inhibited AngII-induced reduction in PPARγ implicating that AngII mediated its effect on PPARγ via inhibition of PPARγ transcriptional activity (Figure 1C).
Figure 1. AngII decreased PPARγ protein, mRNA abundance and transcriptional activity.
A: Thoracic, suprarenal, and infrarenal aorta-derived VSMCs were incubated with either vehicle (saline) or AngII (1 μM) for 24 hours, lysed, and proteins were resolved by SDS-PAGE. Western analyses were performed to detect PPARγ protein (n = 3). B: VSMCs were incubated with either vehicle (saline), AngII (1 μM) or AngII + losartan (10 μM) for 24 hours. Total cell lysates were analyzed by Western blotting using antibodies to PPARγ (n = 5). C: VSMCs were pre-incubated with either vehicle (DMSO) or rosiglitazone (10 μM) for 24 hours followed by incubation with either saline or AngII (1 μM) for 24 hours. Total cell lysates were analyzed by Western blotting using antibodies to PPARγ (n = 3). Abundance of β-actin was used as an internal control. D: VSMCs were incubated with either vehicle (saline), AngII (1 μM) or AngII + losartan (10 μM) for 24 hours. Total RNA was extracted from these cells and then subjected to qRT-PCR analyses. 18S was used as an internal control (n = 4). E: VSMCs were transiently transfected with PPARγ promoter luciferase reporter construct along with plasmid encoding renilla luciferase. After transfection, cells were serum-deprived and incubated with either vehicle (saline) or AngII for 24 hours. Transfection efficiency was adjusted by normalizing firefly luciferase activities to renilla luciferase activities (n = 4). Results are represented as means ± SEMs; Data were analyzed by one-way ANOVA with a Holm-Sidak multiple comparison post-hoc test.
AngII decreased PPARγ by a transcriptional mechanism via AT1 receptors
To assess if AngII-induced reduction in PPARγ abundance occurred by a transcriptional mechanism, VSMCs were incubated with either vehicle or AngII for 24 hours. AngII reduced PPARγ mRNA abundance in aortic VSMCs via AT1 receptors (P < 0.001, Figure 1D). To assess whether AngII-induced reduction in PPARγ expression was associated with changes in transcriptional activity, VSMCs were transiently transfected with a PPARγ promoter construct and then incubated with AngII for 24 hours. As shown in Figure 1E, AngII inhibited PPARγ transcriptional activity via AT1 receptors.
Involvement of TGF-β1 signaling in PPARγ regulation
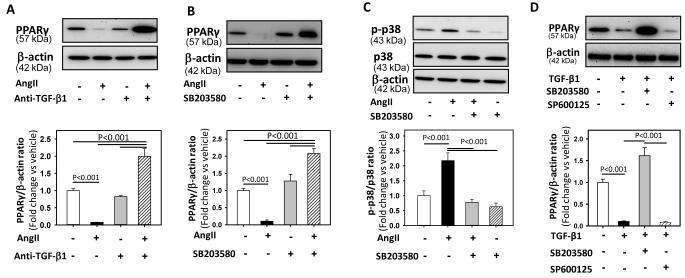
TGF-β1 regulates PPARγ expression in several cell types, including VSMCs.15 AngII also activates TGF-β1 in VSMCs.19 To study if activation of TGF-β1 regulates PPARγ, VSMCs were pre-incubated with a TGF-β1 neutralizing antibody for 30 minutes, followed by incubation with AngII for 24 hours. Neutralization of TGF-β1 activity completely reversed AngII-induced decreases in PPARγ protein abundance (Figure 2A). In addition, AngII stimulation, after blocking TGF-β1 activity, increased abundance of PPARγ protein (P < 0.001, Figure 2A).
Figure 2. AngII decreased PPARγ via the activation of TGF-β1 and p38 MAPK.
A: VSMCs were incubated with either vehicle (saline), AngII (1 μM) or AngII + anti-TGF-β1 antibody (2 μg/ml) for 24 hours. Total cell lysates were analyzed by Western blotting using antibodies to PPARγ (n = 4). B: VSMCs were incubated with vehicle (DMSO), AngII (1 μM) or AngII + SB-203580 (10 μM) for 24 hours. Total cell lysates were analyzed by Western blotting using antibodies to PPARγ (n = 4). C: VSMCs were pre-incubated with SB-203580 (10 μM for 30 minutes) followed by AngII incubation for 30 minutes. Total cell lysates were analyzed by Western blot using antibodies to phospho p38. D: VSMCs were incubated with vehicle (DMSO), TGF-β1 (10 ng/ml) or TGF-β1 + SB-203580 (10 μM) / SP600125 (10 μM) for 24 hours. Total cell lysates were analyzed by Western blotting using antibodies to PPARγ. β-actin was used as an internal control (n = 4). Results are represented as means ± SEMs; Data were analyzed by Student’s t test or one-way ANOVA with a Holm-Sidak multiple comparison post-hoc test.
Involvement of p38 MAPK in regulation of PPARγ expression
Members of the MAPK superfamily are known to regulate PPARγ expression in VSMCs.20,21 To study if MAPKs activation (p38, ERK and JNK) plays a role in AngII-induced PPARγ regulation, VSMCs were pre-incubated with SP600125 (JNK inhibitor), SB203580 (p38 inhibitor), or PD98059 (ERK inhibitor) for 30 minutes, followed by incubation with AngII for 24 hours. Pre-treatment with SB203580, a p38 inhibitor, completely prevented the reduction of PPARγ protein (P < 0.001, Figure 2B), whereas incubation with either SP600125 or PD98059 had no effect on AngII-induced decreases of PPARγ protein (Supplementary Figure I). In addition, AngII incubation after p38 MAPK inhibition significantly increased PPARγ protein abundance (P < 0.001, Figure 2B) similar to that of neutralization of TGF-β1 activity (Figure 2A). To examine the specificity of SB203580 compound on its target p38 MAPK, VSMCs were pre-incubated with SB203580 for 30 minutes followed by incubation with AngII for 30 minutes. SB203580 inhibited p38 phosphorylation induced by AngII (P < 0.001, Figure 2C).
The role of TGF-β1 and p38 on AngII-induced PPARγ reductions was further confirmed by using siRNA-mediated reductions of either TGF-β1 or p38 in VSMCs. Silencing of both TGF-β1 (Supplemental Figure IIA) or p38 (Supplementary Figure IIB) completely prevented AngII-induced PPARγ protein reduction (P < 0.001; Supplemental Figure IIIA), suggesting that AngII-induced effects were mediated by TGF-β1 and p38. In addition, AngII incubation after siRNA-induced reductions of either TGF-β1 or p38 significantly increased PPARγ protein abundance (P <0.001; Supplemental Figure IIIA) similar to neutralization of TGF-β1 activity (Figure 2A). To further understand whether AngII mediates super-induction of PPARγ through ERK or JNK MAPK, VSMCs were pre-incubated with either PD98059 or SP600125 in combination with a TGF-β1 neutralizing antibody for 30 minutes, followed by incubation with AngII. PD98059 or SP600125 did not influence PPARγ super-induction (Supplemental Figure IIIB), suggesting that neither ERK nor JNK are involved in AngII-induced super-induction of PPARγ after TGF-β1 neutralization.
TGF-β1 downregulated PPARγ via activation of p38 MAPK
Inhibition of either TGF-β1 or p38 MAPK prevented the AngII-induced reduction in PPARγ protein abundance in VSMCs. To examine whether TGF-β1 activation mediated its effect via p38 MAPK, VSMCs were pre-incubated with SB203580 for 30 minutes and then incubated with recombinant TGF-β1 for 24 hours. TGF-β1 incubation significantly decreased PPARγ protein via p38 MAPK (P < 0.001; Figure 2D). The involvement of p38 was further confirmed with siRNA-mediated knockdown of p38 in VSMCs. Silencing of p38 completely prevented the reduction of PPARγ protein (P < 0.001; Supplemental Figure IV), which further confirmed that the effects of TGF-β1 are mediated by p38.
Kinetics of AngII activation of p38 MAPK via TGF-β1
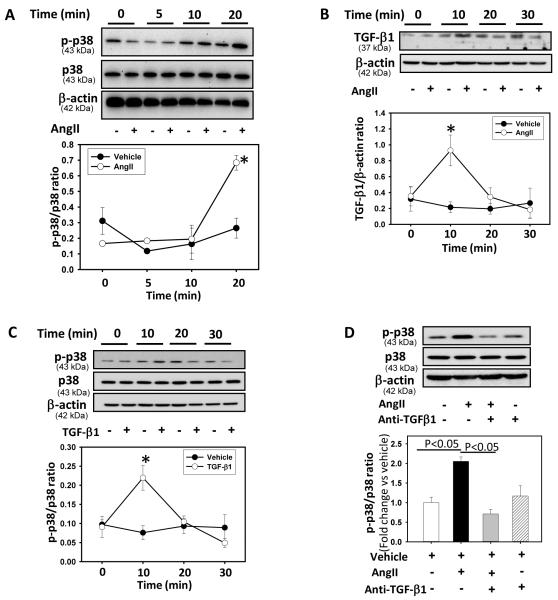
We determined the kinetics of AngII activation of p38 and TGF-β1 in VSMCs by incubating cells with AngII for 0 to 60 minutes. Western blot analyses demonstrated that AngII incubation led to p38 phosphorylation that was maximal after 20 minutes (P < 0.05; Figure 3A) and returned to basal level after 45 minutes (data not shown). In addition, AngII incubation also increased TGF-β1 protein expression significantly within 10 minutes of incubation (P < 0.05; Figure 3B).
Figure 3. AngII increased p38 phosphorylation and TGF-β1 expression.
A: VSMCs were incubated with AngII (1 μM) for selected time intervals (0 - 20 minutes). Total cell lysates were analyzed by Western blot using antibodies to phospho-p38 or p38. * Denotes P < 0.05 comparing AngII versus vehicle (n = 3). B: VSMCs were incubated with AngII (1 μM) for selected time intervals (0 - 30 minutes). Total cell lysates were analyzed by Western blotting using a TGF-β1 antibody. β-actin was used as an internal control. * Denotes P < 0.05 comparing AngII versus vehicle and TGF-β1 antibody + AngII (n = 3). Results are represented as means ± SEMs. C: VSMCs were incubated with vehicle or TGF-β1 (10 ng/ml) for selected intervals (0 - 30 minutes). Total cell lysates were analyzed by Western blotting using antibodies to phospho-p38 or p38. * Denotes P < 0.05 for TGF-β1 vs vehicle (n = 3). Results are represented as means ± SEMs; Statistical significances between vehicle and AngII / TGF-β1 at different intervals were analyzed by Student’s t-test. D: VSMCs were incubated with either vehicle (saline), AngII (1 μM) or AngII + anti-TGF-β1 antibody (2 μg/ml) for 30 minutes. Total cell lysates were analyzed by Western blot using antibodies to phospho-p38 or p38 (n = 3). Results are represented as means ± SEMs; Data were analyzed by one-way ANOVA with a Holm-Sidak multiple comparison post-hoc test.
TGF-β1 has also been shown to mediate its downstream effect via MAPK activation.13 To examine if TGF-β1 activated p38 MAPK in VSMCs, cells were incubated with recombinant TGF-β1 for selected time intervals (0 - 60 minutes). Western blot analyses demonstrated increased p38 phosphorylation after 10 minutes incubation with TGF-β1 (P < 0.05; Figure 3C). VSMCs were pre-incubated with a TGF-β1 neutralizing antibody for 30 minutes, followed by incubation with AngII for 30 minutes, to determine whether AngII induced MAPKs activation through TGF-β1 stimulation. Inhibition of TGF-β1 decreased the p38 phosphorylation induced by AngII, (P < 0.05, Figure 3D) but had no effect on phosphorylation of JNK and ERK (Supplemental Figure V).
EGF receptor and reactive oxygen species inhibition suppressed AngII not TGF-β1-induced p38 MAPK activation
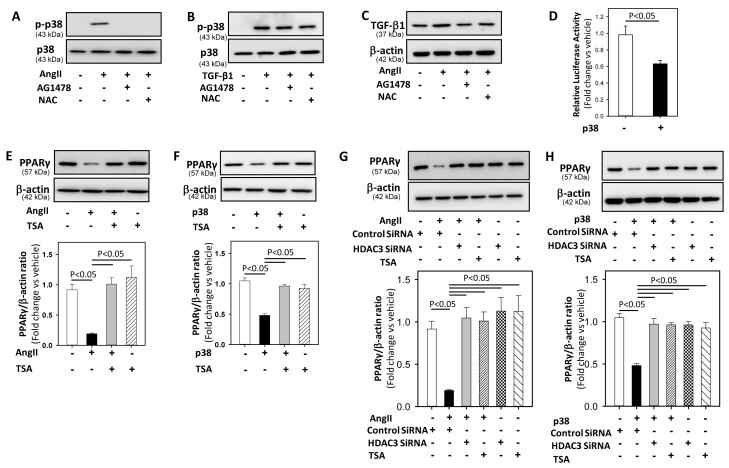
AngII induced trans-activation of EGF receptors (EGFR) with reactive oxygen species (ROS) production has been shown to mediate p38 activation in VSMCs.22 In order to understand whether EGFR and ROS production are involved in AngII-induced p38 MAPKs activation (either upstream or downstream of TGF-β1), VSMCs were pre-incubated with EGFR kinase inhibitor, AG1478, or an antioxidant, N-acetyl L-cysteine (NAC), for 30 minutes followed by incubation with either AngII or recombinant TGF-β1 for 20 and 10 minutes, respectively. Inhibition of EGFR and ROS significantly blunted AngII-induced p38 activation (vehicle: 1.4 ± 0.2; AngII: 173.7 ± 45.0; AngII+AG1478: 0.4 ± 0.003; AngII+NAC:1.6 ± 0.4; P <0.05 versus AngII; Figure 4A) but had no effect on TGF-β1-induced p38 activation (vehicle: 1.0 ± 0.7; TGF-β1: 133.0 ± 32.8; TGF-β1+AG1478: 122.0 ± 31.1; TGF-β1+ NAC:125.9 ± 9.9; P <0.05 versus vehicle; Figure 4B). In addition, EGFR and ROS inhibition also significantly decreased AngII-induced TGF-β1 protein in VSMCs (vehicle: 1.0 ± 0.05; AngII: 1.8 ± 0.21; AngII+AG1478: 1.08 ± 0.11; AngII+ NAC:1.02 ± 0.06; P <0.05 versus AngII; Figure 4C), indicating that EGFR and ROS are acting upstream of TGF-β1 and involved in AngII-induced TGF-β1 production.
Figure 4. P38 MAPK mediated AngII-induced reduction in PPARγ via HDAC-3.
A, B: VSMCs were pre-incubated with AG1478 (100 nM), or NAC (10 mM) for 30 minutes followed by incubation with either vehicle (saline) or AngII (A) / recombinant TGF-β1 (B) for 20 and 10 minutes, respectively. Total cell lysates were analyzed by Western blot using antibodies to phospho-p38 or p38 (n = 3). C: VSMCs were pre-incubated with AG1478 (100 nM), or NAC (10 mM) for 30 minutes followed by incubation with either vehicle (saline) or AngII for 10 minutes. Total cell lysates were analyzed by Western blot using antibodies to TGF-β1 (n = 3). D: VSMCs were transiently transfected with a PPARγ promoter luciferase reporter construct along with plasmid encoding renilla luciferase. After transfection, cells were serum deprived and stimulated with vehicle (saline) or recombinant active p38 (100 nM) for 24 hours. Transfection efficiency was adjusted by normalizing firefly luciferase activities to renilla luciferase activities (n = 4). E, F: VSMCs were pre-incubated with TSA (1 μM) for 30 minutes followed by incubation with either vehicle (saline) or AngII (E) / recombinant active p38 (F) for 24 hours. Total cell lysates were analyzed by Western blot using antibodies to PPARγ (n = 4). G, H: VSMCs were transfected with either HDAC3 SiRNA for 48 hours or pre-incubated with TSA for 30 minutes, followed by incubation with either vehicle (saline) or AngII (G) / recombinant active p38 (H) for 24 hours. Total cell lysates were analyzed by Western blot using antibodies to PPARγ (n = 4). β-actin was used as an internal control. Results are represented as means ± SEMs; Data were analyzed by one-way ANOVA with a Holm-Sidak multiple comparison post-hoc test.
P38 downregulated PPARγ via HDAC3
To further understand the downstream signaling mechanism of p38-mediated reduction in PPARγ, first we examined the effects of recombinant active p38 protein on PPARγ protein abundance and transcriptional activity in VSMCs. VSMCs transiently transfected with a PPARγ promoter construct were incubated with either vehicle or recombinant active p38 for 24 hours. For protein expression studies, VSMCs were incubated with either vehicle or recombinant active p38 for 24 hours. Active p38 significantly inhibited PPARγ transcriptional activity and protein expression in VSMCs (P <0.05; Figures 4D and F).
Members of the histone deacetylases (HDACs) family, especially HDAC3, have been shown to repress PPARγ in complex with retinoblastoma protein.23 Since HDACs have also been shown to mediate AngII-induced VSMC hypertrophy,24 we sought to determine the involvement of HDAC in AngII-p38 mediated reduction in PPARγ in VSMCs. VSMCs were pre-incubated with a HDAC inhibitor, trichostatin A (TSA), for 30 minutes followed by incubation with either AngII or recombinant active p38 protein for 24 hours. TSA-induced HDAC inhibition prevented both AngII and p38-induced decreases in PPARγ protein abundance (P <0.05; Figure 4E & 4F). This is consistent with an involvement of HDACs downstream of p38 in AngII-mediated PPARγ reduction. In addition, Western analyses showed that incubation with either AngII or active p38 led to increased HDAC-3 protein in VSMCs (vehicle: 0.89 ± 0.06; AngII: 1.86 ± 0.2; active p38: 1.77 ± 0.02; P < 0.05 versus vehicle; Supplemental Figure VI). Next, we examined whether siRNA induced reduction of HDAC3 would mimic the effects of HDAC inhibition on AngII-p38 -induced reductions in PPARγ. VSMCs were transfected with HDAC3 siRNA for 48 hours followed by incubation with either AngII or active p38 for 24 hours. HDAC3 silencing (Supplemental Figure VII) completely prevented AngII-p38-induced reduction of PPARγ protein (P <0.05; Figure 4G & 4H), suggesting the involvement of HDAC3 (downstream of p38) in AngII-mediated PPARγ reduction in VSMCs.
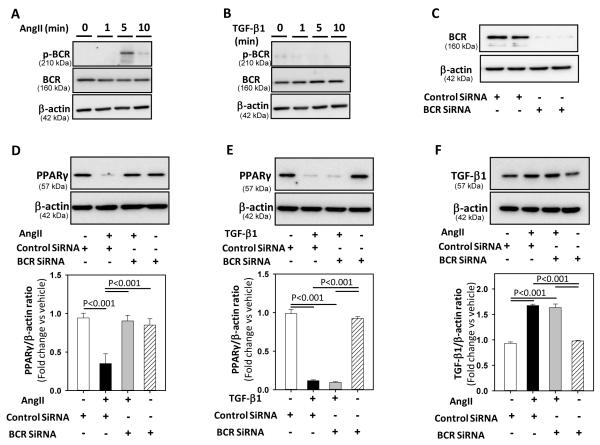
BCR kinase differentially regulated AngII- and TGF-β1-induced PPARγ reduction
Activation of BCR kinase by AngII has been shown to inhibit PPARγ transcriptional activity and induces NF-kB activation in VSMCs.9 To determine whether BCR kinase and activated NF-kB were involved in the AngII-TGF-β1-p38 MAPKs pathway to reduce PPARγ in VSMCs, first we examined BCR kinase activation by either AngII or TGF-β1. VSMCs were incubated with either AngII or TGF-β1 for 0 -10 minutes. Western blot analyses of cellular extracts using phospho-BCR kinase antibodies demonstrated that AngII incubation led to BCR kinase phosphorylation that was maximal at 5 minutes (Figure 5A) and returned to basal levels after 10 minutes. In contrast, TGF-β1 incubation has no effect on BCR kinase activation (Figure 5B). Next, we examined whether BCR kinase silencing in VSMCs would inhibit AngII-TGF-β1 - induced PPARγ reduction. VSMCs were transfected with BCR kinase siRNA for 48 hours followed by incubation with either AngII or TGF-β1 for 24 hours. BCR kinase silencing (Figure 5C) prevented AngII-induced reduction of PPARγ protein (P <0.001; Figure 5D), but did not influence PPARγ protein reduction induced by TGF-β1 (Figure 5E). In addition, BCR kinase silencing had no effect on AngII-induced TGF-β1 protein expression (Figure 5F). Neutralization of TGF-β1 did not influence AngII-induced NF-kB activation in VSMCs (Supplemental Figure VIII A). Furthermore, inhibition of NF-kB activation using two different inhibitors (SN50-cell permeable inhibitor peptide, and NF-kB activation inhibitor) had no effect on either AngII or TGF-β1 induced reduction in PPARγ protein (Supplemental Figure VIII B). These results suggest that AngII-induced BCR kinase and NF-kB activation are not involved in TGF-β1-p38 MAPK mediated down regulation of PPARγ.
Figure 5. BCR-kinase silencing prevented AngII, but not TGF-β1-induced downregulation of PPARγ.
A, B: VSMCs were incubated with vehicle or AngII (A) / TGF-β1 (B) for selected intervals (0 - 10 minutes). Total cell lysates were analyzed by Western blotting using antibodies to phospho-BCR kinase or BCR kinase (n=3). C: VSMCs were transfected with either control, or BCR kinase siRNA for 48 hours and total cell lysates were analyzed by Western blot using antibodies to BCR kinase (n = 3). D, E: VSMCs were transfected with either control or BCR kinase SiRNA for 48 hours, followed by incubation with either vehicle (DMSO) or AngII (D) / recombinant TGF-β1 (E) for 24 hours. Total cell lysates were analyzed by Western blot using antibodies to PPARγ (n = 3-4). F: VSMCs were transfected with either control or BCR kinase siRNA for 48 hours, followed by incubation with either vehicle (saline) or AngII for 10 minutes. Total cell lysates were analyzed by Western blot using antibodies to TGF-β1 (n = 3). β-actin was used as an internal control. Results are represented as means ± SEMs; Data were analyzed by one-way ANOVA with a Holm-Sidak multiple comparison post-hoc test.
Blockade of p38 MAPK did not influence TGF-β1-induced Smad phosphorylation
Smad signaling proteins are the primary downstream effectors of TGF-β signal transduction. To determine if TGF-β1-induced Smad activation is affected by p38 MAPK inhibition, VSMCs were pre-incubated with SB203580 for 30 minutes followed by incubation with recombinant TGF-β1 for 24 hours. TGF-β1 promoted phosphorylation of Smad-2 in the presence of a p38 MAPK inhibitor (Supplemental Figure IX), indicative that this pathway was not involved in AngII-induced reduction of PPARγ.
DISCUSSION
In the present study, we examined PPARγ regulation by AngII in mouse aortic VSMCs. The findings of this study demonstrate that AngII decreases expression of PPARγ via AT1 receptors in VSMCs. Furthermore, this AngII-induced decrease in PPARγ occurs via TGF-β1 and the p38 MAPK pathway. AngII activated p38 via TGF-β1 and decreased PPARγ through HDAC3 without involving Smad signaling.
In this study, blockade of AT1 receptors with losartan inhibited AngII induced PPARγ reduction. AT1 receptors mediate most responses to AngII, and this receptor subtype is predominantly expressed in VSMCs.25,26 AT1 receptor antagonists have been shown to activate PPARγ in various cell types including adipocytes and VSMCs.27,28 Among the various AT1 receptor antagonists, telmisartan has been shown to activate PPARγ within a 10 μM concentration,28 whereas the losartan activates PPARγ only at a high concentration (100 μM).29 In our study, losartan at 10 μM concentration inhibited AngII-induced PPARγ reduction, but it did not increase the abundance of PPARγ over the basal level, consistent with a PPARγ-independent effect of the drug.
Antibody-induced neutralization of TGF-β1 activity completely ablated AngII-induced reductions in PPARγ abundance. Conversely, addition of exogenous recombinant TGF-β1 to aortic VSMCs downregulated PPARγ protein expression. Previous studies have highlighted an inter-relationship between AngII and TGF-β1 in selected cell types, including VSMCs.19,30 TGF-β1 has also been shown to directly regulate PPARγ gene expression in VSMCs.15 Furthermore, in our study, AngII stimulation after neutralization of TGF-β1 activity significantly increased abundance of PPARγ protein which indicates that TGF-β1 activity blockade synergizes with AngII and upregulates PPARγ.
AngII is a powerful activator of the MAPK cascade system in both cultured VSMCs and in intact arteries.31,32 Activation of p38 MAPK contributes to AngII induced DNA synthesis33 and migration in VSMCs.34 Our results demonstrate that both AngII and TGF-β1 activate p38 MAPK to reduce PPARγ abundance in aortic VSMCs. Our data clearly indicate that AngII activates p38 MAPK through TGF-β1 signaling and thereby downregulates PPARγ in VSMCs. Consistent with these findings, a recent study showed that TGF-β1-induced p38 MAPK mediated induction of miR 143/145 in human coronary artery VSMCs.35 Thus, while AngII activates other MAPKs (e.g., JNK and ERK1/2)22 in VSMCs, p38 MAPK is the primary mediator of AngII-induced PPARγ downregulation.
AngII-induced EGFR kinase trans-activation and ROS production has been shown to mediate p38 MAPK activation in VSMCs.22 Consistent with this report, inhibition of EGFR kinase by AG1478 and scavenging of ROS using NAC abolished AngII-induced p38 activation with no effect on TGF-β1-induced p38 activation. These data suggest that EGFR kinase and ROS are act as upstream signals of TGF-β1 in AngII-induced p38 activation. Our data further showed that EGFR kinase and ROS are involved in AngII-induced TGF-β1 production confirming the involvement of TGF-β1 in AngII mediated p38 activation in VSMCs.
HDAC3, a member of class I HDAC family, is well known to be a repressor of PPARγ. In adipocytes, activation of HDAC3 represses PPARγ in complex with retinoblastoma protein and inhibits adipocyte differentiation.23 In mice, liver-specific deletion of HDAC3 resulted in a significant upregulation of PPARγ, mainly through nuclear hormone receptor mediated regulation.36 Our current study demonstrated that HDAC activation is involved in both AngII and p38- mediated reduction in PPARγ. In support of this finding, recent studies have reported increased HDAC activity in mice infused with AngII,37 and pharmacological inhibition of HDACs prevented AngII-induced AAAs,38 and cardiac hypertrophy in mice.39 The observed reduction in PPARγ may be due to increased repression by HDAC3, induced by AngII via the activation of TGF-β1 and p38 MAPKs.
In a recent study, AngII (200 nM) inhibited PPARγ transcriptional activity, as defined by transcriptional activity using luciferase reporter constructs, via the activation of BCR kinase in rat and mouse VSMCs.9 Our current study confirmed this report and also determined the effects of AngII on PPARγ mRNA and protein abundance in addition to its effect on transcriptional activity. Activation of BCR kinase was shown to inhibit PPARγ transcriptional activity mainly by PPARγ phosphorylation without influencing the ERK1/2 and JNK activity.9 Our data demonstrates that AngII-induced TGF-β1-P38 MAPKs activation is independent of BCR kinase activation, and further demonstrates that AngII-p38 MAPKs mediated reduction in PPARγ abundance is HDAC3 dependent. These results suggest that AngII-induced reduction in PPARγ via the activation of TGF-β1-p38 MAPKs is produced by potentiating HDAC3 mediated repression of PPARγ in SMCs.
Smad proteins are the primary TGF-β1 receptor substrates capable of signal transduction.40 Although Smad-dependent responses represent one of the main signaling systems utilized by TGF-β1 receptors, alternative signaling components such as MAPKs are able to mediate TGF-β1-induced biological effects. An earlier report also suggest that in mouse VSMCs, p38 MAPK plays an essential and non-redundant role in TGF-β1 dependent growth inhibition.41 In addition, another recent study showed that p38 MAPK mediates TGF-β1-induced induction of miR 143/145 in human coronary artery smooth muscle cells.35 Consistent with these reports, our data also demonstrate that TGF-β1 mediated its effect on PPARγ via p38 MAPK. In addition, our data also confirmed that TGF-β1 induced Smad-2 phosphorylation was not influenced by p38 MAPK inhibition, which confirms that TGF-β1-induced Smad-2 activation was not involved in AngII-induced reduction of PPARγ.
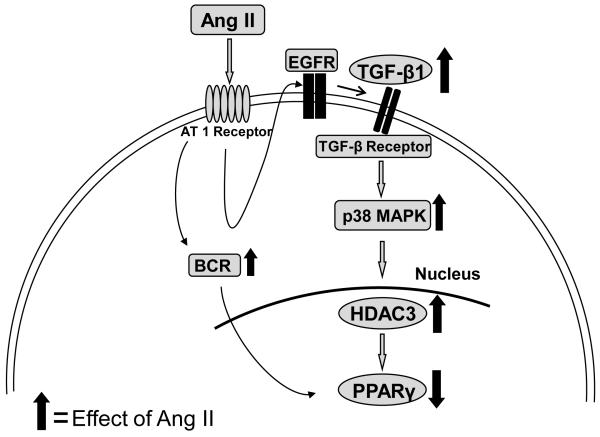
In summary, these data provide evidence that AngII inhibits expression of PPARγ in VSMCs. This supports a novel link between AngII and TGF-β1, in the regulation of PPARγ induced by AngII (Figure 6). In addition, the data also suggests that PPARγ protein may be involved in AngII-induced structural changes of the vascular wall.
Figure 6. Proposed schematic representation of signaling pathways involved in the regulation of AngII induced decreases in PPARγ in aortic VSMCs.
AngII-induced decrease in PPARγ protein was regulated via AT1 receptor activation of TGF-β1 leading to subsequent induction of p38 MAPK mediated HDAC3.
Supplementary Material
Figure I. Blockade of JNK, and ERK MAPK did not affect AngII-induced PPARγ downregulation. VSMCs were incubated with vehicle (DMSO) or AngII (1 μM) and inhibitors of JNK (SP-600125; 25 μM), ERK (PD-98059; 20 μM) and p38 (SB-203580; 10 μM) for 24 hours. Total cell lysates were analyzed by Western blotting using an anti-PPARγ antibody. β-actin was used as an internal control (n = 3). Representative blots show blockade of p38 MAPK rescued PPARγ in VSMCs incubated with AngII.
Figure II. SiRNA mediated silencing of TGF-β1or p38 in VSMCs. VSMCs were transfected with either control, TGF-β1, or p38 SiRNA for 48 hours and total cell lysates were analyzed by Western blot using antibodies to TGF-β1(A) or p38 (B).
Figure III. A: SiRNA mediated silencing of TGF-β1 or p38 attenuated AngII-induced PPARγ reduction in VSMCs. VSMCs were transfected with either control, TGF-β1, or p38 SiRNA for 48 hours, followed by incubation with either vehicle (saline) or AngII (1 μM) for 24 hours. Total cell lysates were analyzed by Western blot using antibodies to PPARγ (n = 4). B: Super-induction of PPARγ post TGF-β1 blockade is not mediated by ERK or JNK MAPKs. VSMCs were pre-incubated with anti-TGF-β1 antibody (2 μg/ml) + PD98059 (10 μM) / SP600125 (20 μM) for 30 minutes followed by AngII incubation for 24 hours. Total cell lysates were analyzed by Western blot using antibodies to PPARγ (n = 4). Results are represented as means ± SEMs; Data were analyzed by one-way ANOVA with a Holm-Sidak multiple comparison post-hoc test.
Figure IV. SiRNA mediated silencing of p38 attenuated TGF-β1-induced PPARγ reduction in VSMCs. VSMCs were transfected with either control or p38 SiRNA for 48 hours, followed by incubation with either vehicle (DMSO) or TGF-β1 (10 ng/ml) for 24 hours. Total cell lysates were analyzed by Western blot using antibodies to PPARγ (n = 3). Results are represented as means ± SEMs; Data were analyzed by one-way ANOVA with a Holm-Sidak multiple comparison post-hoc test.
Figure V. Neutralization of TGF-β1 did not effect AngII-induced JNK, or ERK phosphorylation. VSMCs were incubated with either vehicle (saline), AngII (1 μM) or AngII + anti-TGF-β1 antibody (2 μg/ml) for 30 minutes. Total cell lysates were analyzed by Western blotting using antibodies to p-JNK, JNK, p-ERK and ERK (n = 3).
Figure VI. AngII / active p38 increased HDAC3 protein in VSMCs. VSMCs were incubated with either vehicle (DMSO), AngII (1 μM) or recombinant active p38 (100 nM) for 24 hours. Total cell lysates were analyzed by Western blotting using antibodies to HDAC3 (n = 3).
Figure VII. SiRNA mediated silencing of HDAC3 in VSMCs. VSMCs were transfected with either control, or HDAC3 siRNA for 48 hours and total cell lysates were analyzed by Western blot using antibodies to HDAC3.
Figure VIII. NF-kB inhibition had no effect on either AngII- or TGF-β1- induced reduction in PPARγ protein. A: VSMCs were pre-incubated with anti-TGF-β1 (2 mg/ml) / SN50 (10 μM) / NF-kB activation inhibitor (NF-kBi 10 μM) for 30 minutes followed by incubation with either vehicle (saline) or AngII for 24 hours. Total cell lysates were analyzed by Western blot using antibodies to phospho-NF-kB p65 / NF-kB p65 (n=3). B: VSMCs were pre-incubated with SN50 (10 μM) / NF-kB activation inhibitor (NFkBi 10 μM) for 30 minutes followed by incubation with either vehicle (DMSO) or AngII / recombinant TGF-β1 for 24 hours. Total cell lysates were analyzed by Western blot using antibodies to PPARγ (n = 4). β-actin was used as an internal control.
Figure IX. Inhibition of p38 did not effect TGF-β1-induced Smad-2 activation. VSMCs were incubated with vehicle, TGF-β1 (10 ng/ml) or TGF-β1 + SB-203580 (10 μM) for 24 hours. Total cell lysates were analyzed by Western blot using anti-pSmad-2 antibodies (n=3-5). Results are represented as means ± SEMs. Data were analyzed by one-way ANOVA with a Holm-Sidak multiple comparison post-hoc test.
ACKNOWLEDGMENTS
We acknowledge the skilled technical assistance of Talha Ijaz.
SOURCES OF FUNDING The studies were supported by grants from the National Heart Lung and Blood Institute (HL80010 to JG and HL80100 to AD) and an American Heart Association Great Rivers Affiliate Postdoctoral Fellowship (0825592D) to VS.
Footnotes
DISCLOSURES None
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Schoonjans K, Martin G, Staels B, Auwerx J. Peroxisome proliferator-activated receptors, orphans with ligands and functions. Current Opinion in Lipidol. 1997;8:159–166. doi: 10.1097/00041433-199706000-00006. [DOI] [PubMed] [Google Scholar]
- 2.Olefsky JM. Treatment of insulin resistance with peroxisome proliferator-activated receptor gamma agonists. J Clin Invest. 2000;106:467–472. doi: 10.1172/JCI10843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hsueh WA, Bruemmer D. Peroxisome proliferator-activated receptor gamma: implications for cardiovascular disease. Hypertension. 2004;43:297–305. doi: 10.1161/01.HYP.0000113626.76571.5b. [DOI] [PubMed] [Google Scholar]
- 4.Levi Z, Shaish A, Yacov N, Levkovitz H, Trestman S, Gerber Y, Cohen H, Dvir A, Rhachmani R, Ravid M, Harats D. Rosiglitazone (PPARgamma-agonist) attenuates atherogenesis with no effect on hyperglycaemia in a combined diabetes-atherosclerosis mouse model. Diabetes Obes Metab. 2003;5:45–50. doi: 10.1046/j.1463-1326.2003.00240.x. [DOI] [PubMed] [Google Scholar]
- 5.Li AC, Brown KK, Silvestre MJ, Willson TM, Palinski W, Glass CK. Peroxisome proliferator-activated receptor gamma ligands inhibit development of atherosclerosis in LDL receptor-deficient mice. J Clin Invest. 2000;106:523–531. doi: 10.1172/JCI10370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Subramanian V, Golledge J, Ijaz T, Bruemmer D, Daugherty A. Pioglitazone-induced reductions in atherosclerosis occur via smooth muscle cell-specific interaction with PPAR{gamma} Circ Res. 2010;107:953–958. doi: 10.1161/CIRCRESAHA.110.219089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Takeda K, Ichiki T, Tokunou T, Funakoshi Y, Iino N, Hirano K, Kanaide H, Takeshita A. Peroxisome proliferator-activated receptor gamma activators downregulate angiotensin II type 1 receptor in vascular smooth muscle cells. Circulation. 2000;102:1834–1839. doi: 10.1161/01.cir.102.15.1834. [DOI] [PubMed] [Google Scholar]
- 8.Xi XP, Graf K, Goetze S, Fleck E, Hsueh WA, Law RE. Central role of the MAPK pathway in ang II-mediated DNA synthesis and migration in rat vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 1999;19:73–82. doi: 10.1161/01.atv.19.1.73. [DOI] [PubMed] [Google Scholar]
- 9.Alexis JD, Wang N, Che W, Lerner-Marmarosh N, Sahni A, Korshunov VA, Zou Y, Ding B, Yan C, Berk BC, Abe J. Bcr kinase activation by angiotensin II inhibits peroxisome-proliferator-activated receptor gamma transcriptional activity in vascular smooth muscle cells. Circ Res. 2009;104:69–78. doi: 10.1161/CIRCRESAHA.108.188409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Du BH, Fu CZ, Kent KC, Bush H, Schulick AH, Kreiger K, Collins T, McCaffrey TA. Elevated Egr-1 in human atherosclerotic cells transcriptionally represses the transforming growth factor-beta type II receptor. J Biol Chem. 2000;275:39039–39047. doi: 10.1074/jbc.M005159200. [DOI] [PubMed] [Google Scholar]
- 11.Gibbons GH, Pratt RE, Dzau VJ. Vascular smooth muscle cell hypertrophy vs. hyperplasia. Autocrine transforming growth factor-beta 1 expression determines growth response to angiotensin II. J Clin Invest. 1992;90:456–461. doi: 10.1172/JCI115881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Massague J, Wotton D. Transcriptional control by the TGF-beta/Smad signaling system. EMBO J. 2000;19:1745–1754. doi: 10.1093/emboj/19.8.1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yu L, Hebert MC, Zhang YE. TGF-beta receptor-activated p38 MAP kinase mediates Smad-independent TGF-beta responses. EMBO J. 2002;21:3749–3759. doi: 10.1093/emboj/cdf366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fu M, Zhang J, Zhu X, Myles DE, Willson TM, Liu X, Chen YE. Peroxisome proliferator-activated receptor gamma inhibits transforming growth factor beta-induced connective tissue growth factor expression in human aortic smooth muscle cells by interfering with Smad3. J Biol Chem. 2001;276:45888–45894. doi: 10.1074/jbc.M105490200. [DOI] [PubMed] [Google Scholar]
- 15.Fu M, Zhang J, Lin Y, Zhu X, Zhao L, Ahmad M, Ehrengruber MU, Chen YE. Earlystimulation and late inhibition of peroxisome proliferator-activated receptor gamma (PPAR gamma) gene expression by transforming growth factor beta in human aortic smooth muscle cells: role of early growth-response factor-1 (Egr-1), activator protein 1 (AP1) and Smads. Biochem J. 2003;370:1019–1025. doi: 10.1042/BJ20021503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ray JL, Leach R, Herbert JM, Benson M. Isolation of vascular smooth muscle cells from a single murine aorta. Methods Cell Sci. 2001;23:185–188. doi: 10.1023/a:1016357510143. [DOI] [PubMed] [Google Scholar]
- 17.Daugherty A, Rateri DL, Lu H, Inagami T, Cassis LA. Hypercholesterolemia stimulates angiotensin peptide synthesis and contributes to atherosclerosis through the AT1A receptor. Circulation. 2004;110:3849–3857. doi: 10.1161/01.CIR.0000150540.54220.C4. [DOI] [PubMed] [Google Scholar]
- 18.Ogawa D, Nomiyama T, Nakamachi T, Heywood EB, Stone JF, Berger JP, Law RE, Bruemmer D. Activation of peroxisome proliferator-activated receptor gamma suppresses telomerase activity in vascular smooth muscle cells. Circ Res. 2006;98:e50–59. doi: 10.1161/01.RES.0000218271.93076.c3. [DOI] [PubMed] [Google Scholar]
- 19.Siegert A, Ritz E, Orth S, Wagner J. Differential regulation of transforming growth factor receptors by angiotensin II and transforming growth factor-beta1 in vascular smooth muscle. J Mol Med. 1999;77:437–445. doi: 10.1007/s001090050374. [DOI] [PubMed] [Google Scholar]
- 20.Syrovets T, Schule A, Jendrach M, Buchele B, Simmet T. Ciglitazone inhibits plasmin-induced proinflammatory monocyte activation via modulation of p38 MAP kinase activity. Thromb Haemost. 2002;88:274–281. [PubMed] [Google Scholar]
- 21.Benkirane K, Amiri F, Diep QN, El Mabrouk M, Schiffrin EL. PPAR-gamma inhibits ANG II-induced cell growth via SHIP2 and 4E-BP1. Am J Physiol Heart Circ Physiol. 2006;290:H390–H397. doi: 10.1152/ajpheart.00662.2005. [DOI] [PubMed] [Google Scholar]
- 22.Eguchi S, Dempsey PJ, Frank GD, Motley ED, Inagami T. Activation of MAPKs byangiotensin II in vascular smooth muscle cells. Metalloprotease-dependent EGF receptor activation is required for activation of ERK and p38 MAPK but not for JNK. J Biol Chem. 2001;276:7957–7962. doi: 10.1074/jbc.M008570200. [DOI] [PubMed] [Google Scholar]
- 23.Fajas L, Egler V, Reiter R, Hansen J, Kristiansen K, Debril MB, Miard S, Auwerx J. The retinoblastoma-histone deacetylase 3 complex inhibits PPARgamma and adipocyte differentiation. Dev Cell. 2002;3:903–910. doi: 10.1016/s1534-5807(02)00360-x. [DOI] [PubMed] [Google Scholar]
- 24.Xu X, Ha CH, Wong C, Wang W, Hausser A, Pfizenmaier K, Olson EN, McKinsey TA, Jin ZG. Angiotensin II stimulates protein kinase D-dependent histone deacetylase 5 phosphorylation and nuclear export leading to vascular smooth muscle cell hypertrophy. Arterioscler Thromb Vasc Biol. 2007;27:2355–2362. doi: 10.1161/ATVBAHA.107.151704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhuo J, Moeller I, Jenkins T, Chai SY, Allen AM, Ohishi M, Mendelsohn FA. Mapping tissue angiotensin-converting enzyme and angiotensin AT1, AT2 and AT4 receptors. J Hypertens. 1998;16:2027–2037. doi: 10.1097/00004872-199816121-00026. [DOI] [PubMed] [Google Scholar]
- 26.Allen AM, Zhuo J, Mendelsohn FA. Localization and function of angiotensin AT1 receptors. Am J Hypertens. 2000;13:31S–38S. doi: 10.1016/s0895-7061(99)00249-6. [DOI] [PubMed] [Google Scholar]
- 27.Schupp M, Janke J, Clasen R, Unger T, Kintscher U. Angiotensin type 1 receptor blockers induce peroxisome proliferator-activated receptor-gamma activity. Circulation. 2004;109:2054–2057. doi: 10.1161/01.CIR.0000127955.36250.65. [DOI] [PubMed] [Google Scholar]
- 28.Imayama I, Ichiki T, Inanaga K, Ohtsubo H, Fukuyama K, Ono H, Hashiguchi Y, Sunagawa K. Telmisartan downregulates angiotensin II type 1 receptor through activation of peroxisome proliferator-activated receptor gamma. Cardiovasc Res. 2006;72:184–190. doi: 10.1016/j.cardiores.2006.07.014. [DOI] [PubMed] [Google Scholar]
- 29.Schupp M, Lee LD, Frost N, Umbreen S, Schmidt B, Unger T, Kintscher U. Regulation of peroxisome proliferator-activated receptor gamma activity by losartan metabolites. Hypertension. 2006;47:586–589. doi: 10.1161/01.HYP.0000196946.79674.8b. [DOI] [PubMed] [Google Scholar]
- 30.Rosenkranz S. TGF-beta1 and angiotensin networking in cardiac remodeling. Cardiovasc Res. 2004;63:423–432. doi: 10.1016/j.cardiores.2004.04.030. [DOI] [PubMed] [Google Scholar]
- 31.Epstein AM, Throckmorton D, Brophy CM. Mitogen-activated protein kinase activation: an alternate signaling pathway for sustained vascular smooth muscle contraction. J Vasc Surg. 1997;26:327–332. doi: 10.1016/s0741-5214(97)70196-4. [DOI] [PubMed] [Google Scholar]
- 32.Schiffrin EL, Park JB, Intengan HD, Touyz RM. Correction of arterial structure and endothelial dysfunction in human essential hypertension by the angiotensin receptor antagonist losartan. Circulation. 2000;101:1653–1659. doi: 10.1161/01.cir.101.14.1653. [DOI] [PubMed] [Google Scholar]
- 33.Won SM, Park YH, Kim HJ, Park KM, Lee WJ. Catechins inhibit angiotensin II-induced vascular smooth muscle cell proliferation via mitogen-activated protein kinase pathway. Exp Mol Med. 2006;38:525–534. doi: 10.1038/emm.2006.62. [DOI] [PubMed] [Google Scholar]
- 34.Lee HM, Lee CK, Lee SH, Roh HY, Bae YM, Lee KY, Lim J, Park PJ, Park TK, Lee YL, Won KJ, Kim B. p38 mitogen-activated protein kinase contributes to angiotensin II-stimulated migration of rat aortic smooth muscle cells. J Pharmacol Sci. 2007;105:74–81. doi: 10.1254/jphs.fp0070770. [DOI] [PubMed] [Google Scholar]
- 35.Long X, Miano JM. TGF{beta}1 utilizes distinct pathways for the transcriptional activation of microRNA 143/145 in human coronary artery smooth muscle cells. J Biol Chem. 2011;286:30119–30129. doi: 10.1074/jbc.M111.258814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Knutson SK, Chyla BJ, Amann JM, Bhaskara S, Huppert SS, Hiebert SW. Liver-specific deletion of histone deacetylase 3 disrupts metabolic transcriptional networks. EMBO J. 2008;27:1017–1028. doi: 10.1038/emboj.2008.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Song R, Van Buren T, Yosypiv IV. Histone deacetylases are critical regulators of the renin-angiotensin system during ureteric bud branching morphogenesis. Pediatr Res. 2010;67:573–578. doi: 10.1203/PDR.0b013e3181da477c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vinh A, Gaspari TA, Liu HB, Dousha LF, Widdop RE, Dear AE. A novel histone deacetylase inhibitor reduces abdominal aortic aneurysm formation in angiotensin II-infused apolipoprotein E-deficient mice. J Vasc Res. 2008;45:143–152. doi: 10.1159/000110041. [DOI] [PubMed] [Google Scholar]
- 39.Kee HJ, Sohn IS, Nam KI, Park JE, Qian YR, Yin Z, Ahn Y, Jeong MH, Bang YJ, Kim N, Kim JK, Kim KK, Epstein JA, Kook H. Inhibition of histone deacetylation blocks cardiac hypertrophy induced by angiotensin II infusion and aortic banding. Circulation. 2006;113:51–59. doi: 10.1161/CIRCULATIONAHA.105.559724. [DOI] [PubMed] [Google Scholar]
- 40.Javelaud D, Mauviel A. Mammalian transforming growth factor-betas: Smad signaling and physio-pathological roles. Int J Biochem Cell Biol. 2004;36:1161–1165. doi: 10.1016/S1357-2725(03)00255-3. [DOI] [PubMed] [Google Scholar]
- 41.Seay U, Sedding D, Krick S, Hecker M, Seeger W, Eickelberg O. Transforming growth factor-beta-dependent growth inhibition in primary vascular smooth muscle cells is p38-dependent. J Pharmacol Exp Ther. 2005;315:1005–1012. doi: 10.1124/jpet.105.091249. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure I. Blockade of JNK, and ERK MAPK did not affect AngII-induced PPARγ downregulation. VSMCs were incubated with vehicle (DMSO) or AngII (1 μM) and inhibitors of JNK (SP-600125; 25 μM), ERK (PD-98059; 20 μM) and p38 (SB-203580; 10 μM) for 24 hours. Total cell lysates were analyzed by Western blotting using an anti-PPARγ antibody. β-actin was used as an internal control (n = 3). Representative blots show blockade of p38 MAPK rescued PPARγ in VSMCs incubated with AngII.
Figure II. SiRNA mediated silencing of TGF-β1or p38 in VSMCs. VSMCs were transfected with either control, TGF-β1, or p38 SiRNA for 48 hours and total cell lysates were analyzed by Western blot using antibodies to TGF-β1(A) or p38 (B).
Figure III. A: SiRNA mediated silencing of TGF-β1 or p38 attenuated AngII-induced PPARγ reduction in VSMCs. VSMCs were transfected with either control, TGF-β1, or p38 SiRNA for 48 hours, followed by incubation with either vehicle (saline) or AngII (1 μM) for 24 hours. Total cell lysates were analyzed by Western blot using antibodies to PPARγ (n = 4). B: Super-induction of PPARγ post TGF-β1 blockade is not mediated by ERK or JNK MAPKs. VSMCs were pre-incubated with anti-TGF-β1 antibody (2 μg/ml) + PD98059 (10 μM) / SP600125 (20 μM) for 30 minutes followed by AngII incubation for 24 hours. Total cell lysates were analyzed by Western blot using antibodies to PPARγ (n = 4). Results are represented as means ± SEMs; Data were analyzed by one-way ANOVA with a Holm-Sidak multiple comparison post-hoc test.
Figure IV. SiRNA mediated silencing of p38 attenuated TGF-β1-induced PPARγ reduction in VSMCs. VSMCs were transfected with either control or p38 SiRNA for 48 hours, followed by incubation with either vehicle (DMSO) or TGF-β1 (10 ng/ml) for 24 hours. Total cell lysates were analyzed by Western blot using antibodies to PPARγ (n = 3). Results are represented as means ± SEMs; Data were analyzed by one-way ANOVA with a Holm-Sidak multiple comparison post-hoc test.
Figure V. Neutralization of TGF-β1 did not effect AngII-induced JNK, or ERK phosphorylation. VSMCs were incubated with either vehicle (saline), AngII (1 μM) or AngII + anti-TGF-β1 antibody (2 μg/ml) for 30 minutes. Total cell lysates were analyzed by Western blotting using antibodies to p-JNK, JNK, p-ERK and ERK (n = 3).
Figure VI. AngII / active p38 increased HDAC3 protein in VSMCs. VSMCs were incubated with either vehicle (DMSO), AngII (1 μM) or recombinant active p38 (100 nM) for 24 hours. Total cell lysates were analyzed by Western blotting using antibodies to HDAC3 (n = 3).
Figure VII. SiRNA mediated silencing of HDAC3 in VSMCs. VSMCs were transfected with either control, or HDAC3 siRNA for 48 hours and total cell lysates were analyzed by Western blot using antibodies to HDAC3.
Figure VIII. NF-kB inhibition had no effect on either AngII- or TGF-β1- induced reduction in PPARγ protein. A: VSMCs were pre-incubated with anti-TGF-β1 (2 mg/ml) / SN50 (10 μM) / NF-kB activation inhibitor (NF-kBi 10 μM) for 30 minutes followed by incubation with either vehicle (saline) or AngII for 24 hours. Total cell lysates were analyzed by Western blot using antibodies to phospho-NF-kB p65 / NF-kB p65 (n=3). B: VSMCs were pre-incubated with SN50 (10 μM) / NF-kB activation inhibitor (NFkBi 10 μM) for 30 minutes followed by incubation with either vehicle (DMSO) or AngII / recombinant TGF-β1 for 24 hours. Total cell lysates were analyzed by Western blot using antibodies to PPARγ (n = 4). β-actin was used as an internal control.
Figure IX. Inhibition of p38 did not effect TGF-β1-induced Smad-2 activation. VSMCs were incubated with vehicle, TGF-β1 (10 ng/ml) or TGF-β1 + SB-203580 (10 μM) for 24 hours. Total cell lysates were analyzed by Western blot using anti-pSmad-2 antibodies (n=3-5). Results are represented as means ± SEMs. Data were analyzed by one-way ANOVA with a Holm-Sidak multiple comparison post-hoc test.