Abstract
Deleted in liver cancer 1 (DLC1) is a GTPase activating protein (GAP) domain containing tumor suppressor that localizes to focal adhesions. In cancer cells, loss of DLC1 is known to enhance cancer cell migration. However, the role of DLC1 in normal cell migration has not been well studied. Here, we show that silencing of DLC1 (shDLC1) in normal prostate epithelial cells reduces cell migration in both transwell and wound healing assays. This migration defect is mainly due to upregulation of plasminogen activator inhibitor 1 (PAI-1). Silencing of PAI-1 rescues the shDLC1 reduced migration phenotype. Re-expression of DLC1 suppresses PAI-1 and restores the migration defect as well. In contrast, DLC1-K714E (GAP inactive) mutant neither decreases the PAI-1 level nor rescues the shDLC1 migration defect. Interestingly, DLC1-Y442F (tensin-binding and focal adhesion-localizing defective) mutant is able to suppress PAI-1 expression but does not restore the migration defect. Furthermore, PAI-1 upregulation in shDLC1 cells is EGFR-MEK pathway dependent and is able to promote in vitro angiogenesis. Together, our results demonstrate that at least two new mechanisms are involved in DLC1-mediated normal cell migration. (I) DLC1 modulates the expression of PAI-1, which is a negative regulator for cell migration, in a GAP domain and EGFR-MEK dependent manner. (II) Independent of PAI-1, the interaction of DLC1 with tensin members positively regulates cell migration.
Keywords: DLC, PAI-1, cell migration, focal adhesion, prostate
Introduction
Deleted in liver cancer 1 (DLC1) is a focal adhesion protein that contains multiple domains including the SAM (sterile alpha motif), GAP (GTPase activating protein), and START (steroidogenic acute regulatory (StAR)-related lipid transfer) domains. It was initially isolated as a potential tumor suppressor gene often deleted in hepatocellular carcinoma (1). Further studies have indicated that reduced expression of DLC1, either by genomic deletion or DNA methylation, is associated with a variety of cancers, including that of the prostate, lung, breast, kidney, colon, uterus, ovary, and stomach (2-3). Mutations in DLC1 that attenuate its expression and function have been identified in prostate and colon cancer (4). The function of DLC1 has been linked to the regulation of cytoskeleton organization, cell migration, proliferation, and apoptosis (2-3). Recently, a new role of DLC1 in modulating angiogenesis has been discovered (5), extending the understanding of its functions.
The role of DLC1 in cancer cell migration has been reported in numerous studies. Overall, ectopic expression of DLC1 suppresses breast, liver, and kidney cancer cell migration (6-8). Meanwhile, silencing of endogenous DLC1 in breast and colon cancer cells promotes migration (9-10). The regulatory mechanism of DLC1 in cell migration as well as other cellular events has been linked to its negative regulation of the RhoA pathway through the GAP domain. RhoA is known to promote cell migration through the activation of ROCK (Rho associated coiled-coil kinase), and mDia1 (mammalian homolog of Drosophila diaphanous) pathways (11). On the other hand, it has been shown that focal adhesion localization is also essential for DLC1's function in suppression of tumor cell growth (12-13), although how this focal adhesion localization relates to migration is not known.
However, the role of DLC1 in cell migration in non-cancer cells is relatively unexplored. Therefore, the purpose of this study was to investigate the role of DLC1 in cell migration in normal prostate epithelial cells. Here we demonstrate that silencing of DLC1 in normal cells reduces cell migration in a non-RhoA or VEGF dependent manner. Instead, migration is mediated through up-regulation of PAI-1 (plasminogen activator inhibitor 1), and is dependent on DLC1's GAP activity, as well as the EGFR-MEK pathway. Interestingly, re-expression of a DLC1 mutant that lacks tensin-binding activity also suppresses PAI-1 expression, but does not rescue the migration defect. This indicates that tensin-binding is also essential for DLC1-mediated cell migration.
Materials and Methods
Cell culture and reagents
Stable shGFP or shDLC1 cells were generated by shRNA lentivirus infection and maintained in keratinocyte serum free medium containing puromycin (2.5ug/ml) (Invitrogen) as described (5). Human vascular endothelium cells (HUVEC) from American Type Culture Collection (CRL-1730) were cultured in endothelial cell growth medium (Genlantis). Human PrEC cells from Lonza were cultured in PrEGM™ prostate epithelial cell growth medium (Lonza). Cells were used within 3 months after receipt or reconstituted from frozen aliquots. No additional test was done specifically for this study. PAI-1 ELISA kit (R&D Systems) was used to determine PAI-1 levels in conditioned media. The siPAI-1 5′AAGCAGCUAUGGGAUUCAAtt-3′ was obtained from Ambion. The control siRNA (sc-37007), RhoA siRNA (sc-29471), VEGF siRNA (sc-29520), DLC1 siRNA (sc-43725) and anti-PAI-1 antibody (sc-5297) were purchased from Santa Cruz Biotechnology. Anti-VEGF antibody was from R&D Systems (clone 26503).
Cell migration
For the transwell migration assay, shGFP and shDLC1 cells were trypsinized, resuspended in keratinocyte serum-free medium, and transferred to the upper chamber (1×105 cells in 200ul); 400ul of keratinocyte completed medium was added to the lower chamber. After incubation for 16h, cells on the upper surface of the filter were removed with a cotton swab and cells on the low surface were fixed, stained, and photographed.
For the wound healing assay, cells were seeded on six-well plates and cultured to confluence. The confluent monolayer was then wounded with a pipette tip and cells were allowed to migrate for 24h. Photographs were taken and migration distances were measured by ImageJ software (NIH).
In vitro angiogenesis tube formation assay
Growth factor–reduced Matrigel was used to coat 96 wells plate (50ul/well) and HUVECs (20,000 cells/well) were seeded with conditioned medium (200ul). After 4h of incubation, capillary-like structures were scored by measuring lengths of tubules per field in each well at 100× magnification with Image J software (NIH).
Adenoviruses
A silent mutation (856cysteine: TGT to TGC) was introduced to human DLC1 cDNA in a pENTR1A vector to generate the shRNA resistant expression construct. This DLC1/pENTR1A construct was used to generate Y442F and K714E mutants. These DLC1 inserts were moved into a pAD/CMV/V5-DEST vector by site-directed recombination reactions (Invitrogen). The adenoviral expression vectors were transfected into 293A cells. After 10-12 days, the crude viral lysates were harvested and used for infection.
Results
Down-regulation of DLC1 in normal epithelial cells reduces cell migration
Previous studies from other groups have shown that overexpression of DLC1 in various cancer cell lines reduces cell migration and invasion (6-8), suggesting a negative regulation of DLC1 on cancer cell migration. Recently, we established DLC1 knockdown (shDLC1) cells using two non-malignant prostate epithelial cell lines, RWPE1 and MLC-SV40 and demonstrated a role for DLC1 in regulating angiogenesis through VEGF expression (5). With these cell systems, we further examined the effect of loss of DLC1 on cell migration. As analyzed by in vitro wound healing and transwell migration assays (Figure 1A&B), silencing of DLC1 in both cell lines significantly reduces cell migration. Reduction of cell migration resulting from DLC1 down-regulation by either siRNA transfection or shRNA lentivirus infection was also observed in normal primary prostate epithelial cells (PrEC) (Figure 1C). Since the DLC1 siRNA and shRNA targeted different regions of DLC1, the experiment also ruled out potential off-target effects.
Figure 1. Silencing of DLC1 suppresses cell migration.
(A) GFP (shGFP) or DLC1 (shDLC1) knockdown cells were grown to confluence for 24 h wound healing assays. (B) shGFP and shDLC1 cells were used for 16h transwell migration assay. (C) PrEC cells silenced with either DLC1 siRNA transfection or shRNA lentivirus infection were subjected to transwell migration assays. Data are mean SD from triplicate experiments (*, P < 0.05).
DLC1 negatively regulates plasminogen activator inhibitor 1 expression
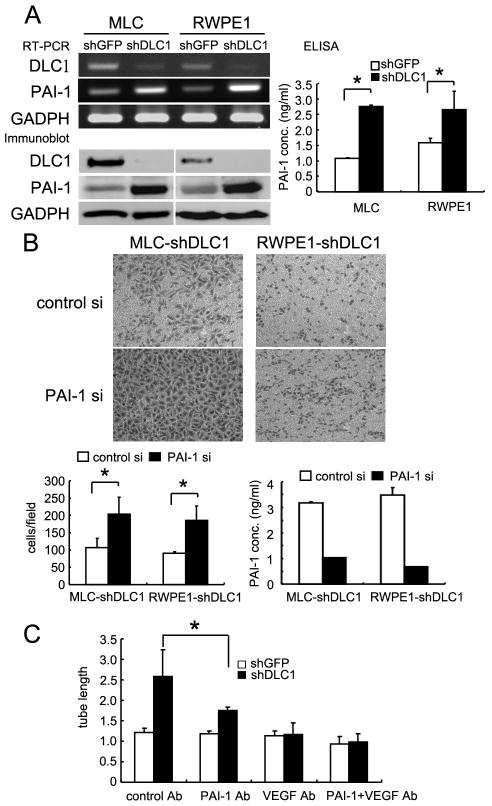
Since DLC1 suppresses RhoA GTPase activity through its GAP domain and RhoA regulates cell migration, we examined the role of RhoA in shDLC1 cell migration. Interestingly, instead of rescuing cell migration, silencing of RhoA further reduced cell migration (Figure 2). Because VEGF was up-regulated in shDLC1 cells (5), we tested whether VEGF was responsible for the migration defect in shDLC1 cells. Again, down-regulation of VEGF by siRNA did not restore, but further impaired migration (Figure 2). To understand the potential mechanism involving DLC1 mediated cell migration, gene expression profiles in these cells were studied. In addition to the previously identified VEGF, the mRNA and protein levels of PAI-1 were significantly up-regulated in shDLC1 cells (Figure 3A). PAI-1 levels in the conditioned media were also increased (Figure 3A). Since PAI-1 is known to regulate cell migration (14) and is upregulated in shDLC1 cells, we examined whether PAI-1 was the key factor in suppressing cell migration in shDLC1 cells. By silencing PAI-1 in MLC-SV40 and RWPE1 shDLC1 cells, the migration defects were rescued in both cell systems (Figure 3B). In addition, because PAI-1 is a known pro-angiogenic factor, we tested the effects of shDLC1 conditioned media on endothelial tube formation in the presence of PAI-1 and VEGF neutralizing antibodies (Figure 3C). VEGF in the conditioned media appeared to be the key angiogenic factor but PAI-1 also contributed to the tube formation. When both neutralizing antibodies were applied, the tube formation activity was reduced to baseline level.
Figure 2. Silencing of RhoA or VEGF further reduces shDLC1 cell migration.
(A) MLC shGFP transfected with scramble control siRNA (a) or shDLC1 treated with siRNAs for control (b), RhoA (c), or VEGF (d) for 48h, were used for 16h transwell migration assays. (B) The same experiments were performed in RWPE1 cell system. Data are from triplicate experiments.
Figure 3. Up-regulation of PAI-1 in shDLC1 cells suppresses cell migration and promotes tube formation.
(A) PAI-1 and DLC1 mRNA, protein, and secreted protein levels in shDLC1 cells were determined by RT-PCR, immunoblot, and ELISA assays, respectively. Note that DLC1 was first immunoprecipitated from 300 ug of protein lystate and immunoblotted against DLC1 antibodies. PAI-1 and GADPH were detected from 20 ug of protein lysate with anti-PAI-1 and GADPH antibodies. (B) shDLC1 cells transfected with scramble control siRNA or PAI-1 siRNA for 48h were used for 16h transwell migration assays. The expression levels of PAI-1 were confirmed by ELISA. (C) Conditioned media from shGFP, shDLC1, or shDLC1 cells incubated with VEGF or PAI-1 blocking antibody were cultured with HUVEC cells for 4h. The capillary-like structures were scored by measuring lengths of tubules per field in each well at 100× magnification with Image J software (NIH). (*, P < 0.05).
Rescue experiments reveal PAI-1 dependent and independent migration in shDLC1 cells
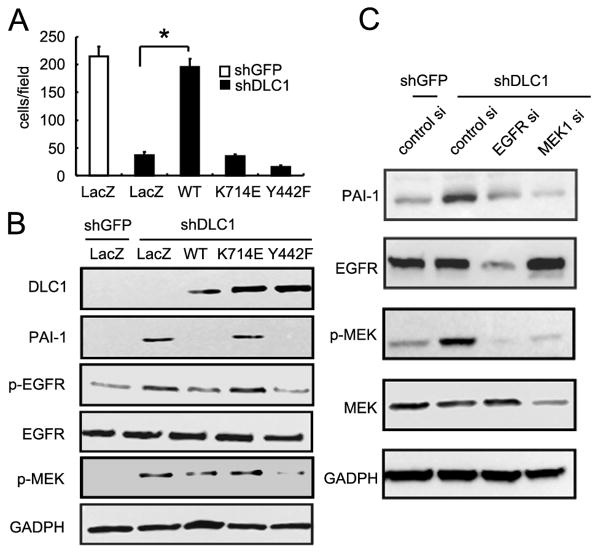
To further investigate the functional interaction among DLC1, PAI-1 and cell migration, we re-expressed various shRNA resistant DLC1 constructs in shDLC1 cells and monitored PAI-1 expression as well as cell migration. As shown in Figure 4, re-expression of wild-type or Y442F (tensin-binding defective) DLC1 constructs in shDLC1 cells suppressed PAI-1 levels. However, expression of K714E (GAP inactive) DLC1 mutant had no effect on reducing PAI-1 expression. Nonetheless, only wild-type DLC1 was able to restore cell migration in these cell lines, suggesting that DLC1 regulates cell migration through GAP domain/activity mediated PAI-1 expression, and tensin-binding mediated pathway that is independent of PAI-1 expression. Both components are critical to DLC1's function in regulating cell migration.
Figure 4. The GAP and tensin-binding activities of DLC1 are required for rescuing shDLC1 cell migration defect.
(A) shDLC1 cells infected with adenoviruses carrying lacZ, DLC1-WT, DLC1-K714E, or DLC1-Y442F for 24h were then seeded (105 cells) for 16h transwell migration assays. (B) Protein lysates (20 ug) from infected shDLC1 cells were analyzed by immunoblotting with indicated antibodies. (C) Protein lysates (20 ug) from shDLC1 transfected with EGFR or MEK1 siRNAs were analyzed by immunoblotting with indicated antibodies.
Up-regulation of PAI-1 in shDLC1 cells is EGFR-MEK pathway dependent
Since we previously found VEGF up-regulation in shDLC1 cells was mediated through the EGFR-MEK pathway (5) and this pathway is also known to promote PAI-1 expression in other cell systems (15), we tested whether EGFR and MEK activities were required for PAI-1 expression in our cell systems. Silencing of either EGFR or MEK led to down-expression of PAI-1 protein (Figure 4C), indicating that up-regulation of PAI-1 in shDLC1 cells is EGFR-MEK pathway dependent. The rescue experiments also demonstrate that the GAP domain, but not the tensin-binding domain, of DLC1 is required for suppressing EGFR and MEK activities (Figure 4B).
Discussion
In this report, we have demonstrated that DLC1 acts as a positive regulator on normal prostate epithelial cell migration. This positive regulation effect seemingly contradicts previous reports that have shown a negative role on cell migration. Those studies were often based on the observation that ectopic expression of DLC1 suppressed cancer cell migration (6-8) or silencing of DLC1 enhanced cancer cell migration (9-10). The observed difference may result from the use of cancer cells vs. normal cells. Cancer cell lines are known to contain various mutations and dysregulated signaling pathways, which may alter the effect of DLC1 on cancer cell migration.
Our current studies demonstrate that both GAP domain and tensin-binding activities contribute to DLC1-mediated cell migration under different mechanisms. The GAP domain is required to inactivate EGFR. Without DLC1's GAP activity, EGFR activates its downstream signaling and enhances PAI-1 expression, which reduces normal cell migration. On the other hand, interaction with tensins and/or localization to focal adhesions is not essential for regulating EGFR activity but is required for DLC1 mediated migration. In addition to our findings, it has been reported that binding of eukaryotic elongation factor 1A1 to the SAM domain of DLC1 suppresses cell migration (16). The SAM domain also interacts with PTEN, which reduces cell migration by suppressing focal adhesion kinase activity (17).
As mentioned above, DLC1's GAP domain regulates PAI-1 expression through the EGFR-MEK pathway. This is in agreement with other findings showing that TGFβ1-induced PAI-1 expression requires EGFR signaling (15). However, how DLC1 suppresses EGFR activity is currently not clear. It is known that deformation of cytoskeleton activates receptor tyrosine kinases, such as EGFR, leading to PAI-1 expression (18-19). It is possible that DLC1 represses a yet-to-be identified small GTPase that regulates cytoskeleton networks. Lack of DLC1 activates this GTPase, which reorganizes cytoskeleton leading to EGFR activation. We are currently testing this possibility.
The finding of PAI-1 regulated by DLC1 suggests that DLC1 may participate in fibrinolysis, which plays a critical role in dissolution of blood fibrin clots (20). Plasminogen is cleaved into the active form plasmin by plasminogen activators (either tissue-type or urokinase-type), which are negatively regulated by PAI-1. Plasmin then degrades fibrin into soluble fibrin degradation products that are removed by other proteases or by the kidney and liver. Interestingly, a recent report showed a direct interaction between DLC1 and p11 (aka S100A10 or annexin 2 light chain) and this binding promoted p11 protein degradation, which attenuated plasminogen activation (21). Therefore, DLC1 negatively regulates both PAI-1 and p11 protein levels, leading to opposite effects on plasminogen activation. How these two mechanisms coordinate within the process remains to be investigated. Since PAI-1 is also involved in obesity, diabetes, inflammation, and renal injury (20), the role of DLC1 in these conditions warrants more attention.
Recently, we have demonstrated that DLC1 negatively regulates angiogenesis by suppressing VEGF expression in epithelial cells. Our current finding has added the regulation of PAI-1, another pro-angiogenic factor, to the equation and further strengthens DLC1's function in regulating angiogenesis. Furthermore, we have identified a novel role of DLC2, another family member, by showing enhanced angiogenic response in DLC2 null endothelial cells and null mice (22). Altogether, these findings indicate DLC1 and DLC2 regulate angiogenesis through distinct mechanisms in various cell types.
Acknowledgments
Grant Support: National Institutes of Health (CA102537, CA151366) to SHL. YPS is a T32 fellowship recipient (T32CA108459).
Footnotes
No potential conflicts of interest
References
- 1.Yuan BZ, Miller MJ, Keck CL, Zimonjic DB, Thorgeirsson SS, Popescu NC. Cloning, characterization, and chromosomal localization of a gene frequently deleted in human liver cancer (DLC-1) homologous to rat RhoGAP. Cancer Res. 1998;58:2196–9. [PubMed] [Google Scholar]
- 2.Liao YC, Lo SH. Deleted in liver cancer-1 (DLC-1): a tumor suppressor not just for liver. Int J Biochem Cell Biol. 2008;40:843–7. doi: 10.1016/j.biocel.2007.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Durkin ME, Yuan BZ, Zhou X, et al. DLC-1:a Rho GTPase-activating protein and tumour suppressor. J Cell Mol Med. 2007;11:1185–207. doi: 10.1111/j.1582-4934.2007.00098.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liao YC, Shih YP, Lo SH. Mutations in the focal adhesion targeting region of deleted in liver cancer-1 attenuate their expression and function. Cancer Res. 2008;68:7718–22. doi: 10.1158/0008-5472.CAN-08-2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shih YP, Liao YC, Lin Y, Lo SH. DLC1 negatively regulates angiogenesis in a paracrine fashion. Cancer Res. 2010;70:8270–5. doi: 10.1158/0008-5472.CAN-10-1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goodison S, Yuan J, Sloan D, et al. The RhoGAP protein DLC-1 functions as a metastasis suppressor in breast cancer cells. Cancer Res. 2005;65:6042–53. doi: 10.1158/0008-5472.CAN-04-3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wong CM, Yam JW, Ching YP, et al. Rho GTPase-activating protein deleted in liver cancer suppresses cell proliferation and invasion in hepatocellular carcinoma. Cancer Res. 2005;65:8861–8. doi: 10.1158/0008-5472.CAN-05-1318. [DOI] [PubMed] [Google Scholar]
- 8.Zhang T, Zheng J, Jiang N, et al. Overexpression of DLC-1 induces cell apoptosis and proliferation inhibition in the renal cell carcinoma. Cancer Lett. 2009;283:59–67. doi: 10.1016/j.canlet.2009.03.025. [DOI] [PubMed] [Google Scholar]
- 9.Holeiter G, Heering J, Erlmann P, Schmid S, Jahne R, Olayioye MA. Deleted in liver cancer 1 controls cell migration through a Dia1-dependent signaling pathway. Cancer Res. 2008;68:8743–51. doi: 10.1158/0008-5472.CAN-08-0984. [DOI] [PubMed] [Google Scholar]
- 10.Jin Y, Tian X, Shang Y, Huang P. Inhibition of DLC-1 gene expression by RNA interference in the colon cancer LoVo cell line. Oncol Rep. 2008;19:669–74. [PubMed] [Google Scholar]
- 11.Narumiya S, Tanji M, Ishizaki T. Rho signaling, ROCK and mDia1, in transformation, metastasis and invasion. Cancer Metastasis Rev. 2009;28:65–76. doi: 10.1007/s10555-008-9170-7. [DOI] [PubMed] [Google Scholar]
- 12.Liao YC, Si L, Devere White RW, Lo SH. The phosphotyrosine-independent interaction of DLC-1 and the SH2 domain of cten regulates focal adhesion localization and growth suppression activity of DLC-1. J Cell Biol. 2007;176:43–9. doi: 10.1083/jcb.200608015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Qian X, Li G, Asmussen HK, et al. Oncogenic inhibition by a deleted in liver cancer gene requires cooperation between tensin binding and Rho-specific GTPase-activating protein activities. Proc Natl Acad Sci U S A. 2007;104:9012–7. doi: 10.1073/pnas.0703033104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Czekay RP, Loskutoff DJ. Unexpected role of plasminogen activator inhibitor 1 in cell adhesion and detachment. Exp Biol Med (Maywood) 2004;229:1090–6. doi: 10.1177/153537020422901102. [DOI] [PubMed] [Google Scholar]
- 15.Kutz SM, Higgins CE, Samarakoon R, et al. TGF-beta 1-induced PAI-1 expression is E box/USF-dependent and requires EGFR signaling. Exp Cell Res. 2006;312:1093–105. doi: 10.1016/j.yexcr.2005.12.027. [DOI] [PubMed] [Google Scholar]
- 16.Zhong D, Zhang J, Yang S, et al. The SAM domain of the RhoGAP DLC1 binds EF1A1 to regulate cell migration. J Cell Sci. 2009;122:414–24. doi: 10.1242/jcs.027482. [DOI] [PubMed] [Google Scholar]
- 17.Heering J, Erlmann P, Olayioye MA. Simultaneous loss of the DLC1 and PTEN tumor suppressors enhances breast cancer cell migration. Exp Cell Res. 2009;315:2505–14. doi: 10.1016/j.yexcr.2009.05.022. [DOI] [PubMed] [Google Scholar]
- 18.Samarakoon R, Higgins CE, Higgins SP, Higgins PJ. TGF-beta1-Induced Expression of the Poor Prognosis SERPINE1/PAI-1 Gene Requires EGFR Signaling: A New Target for Anti-EGFR Therapy. J Oncol. 2009;2009:342391. doi: 10.1155/2009/342391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Samarakoon R, Higgins PJ. Pp60c-src mediates ERK activation/nuclear localization and PAI-1 gene expression in response to cellular deformation. J Cell Physiol. 2003;195:411–20. doi: 10.1002/jcp.10247. [DOI] [PubMed] [Google Scholar]
- 20.Ghosh AK, Vaughan DE. PAI-1 in Tissue Fibrosis. J Cell Physiol. 2011 doi: 10.1002/jcp.22783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang X, Popescu NC, Zimonjic DB. DLC1 Interaction with S100A10 Mediates Inhibition of In Vitro Cell Invasion and Tumorigenicity of Lung Cancer Cells through a RhoGAP-Independent Mechanism. Cancer Res. 2011;71:2916–25. doi: 10.1158/0008-5472.CAN-10-2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin Y, Chen NT, Shih YP, Liao YC, Xue L, Lo SH. DLC2 modulates angiogenic responses in vascular endothelial cells by regulating cell attachment and migration. Oncogene. 2010;29:3010–6. doi: 10.1038/onc.2010.54. [DOI] [PMC free article] [PubMed] [Google Scholar]