Abstract
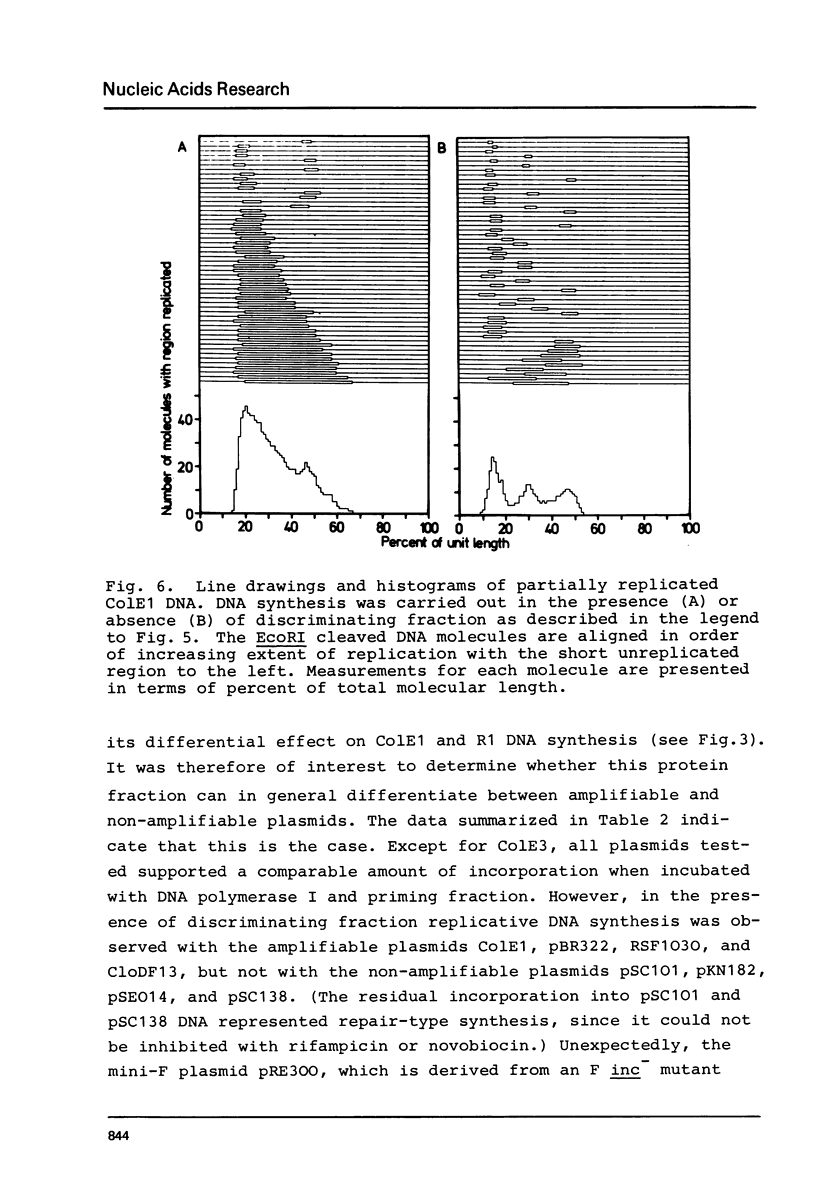
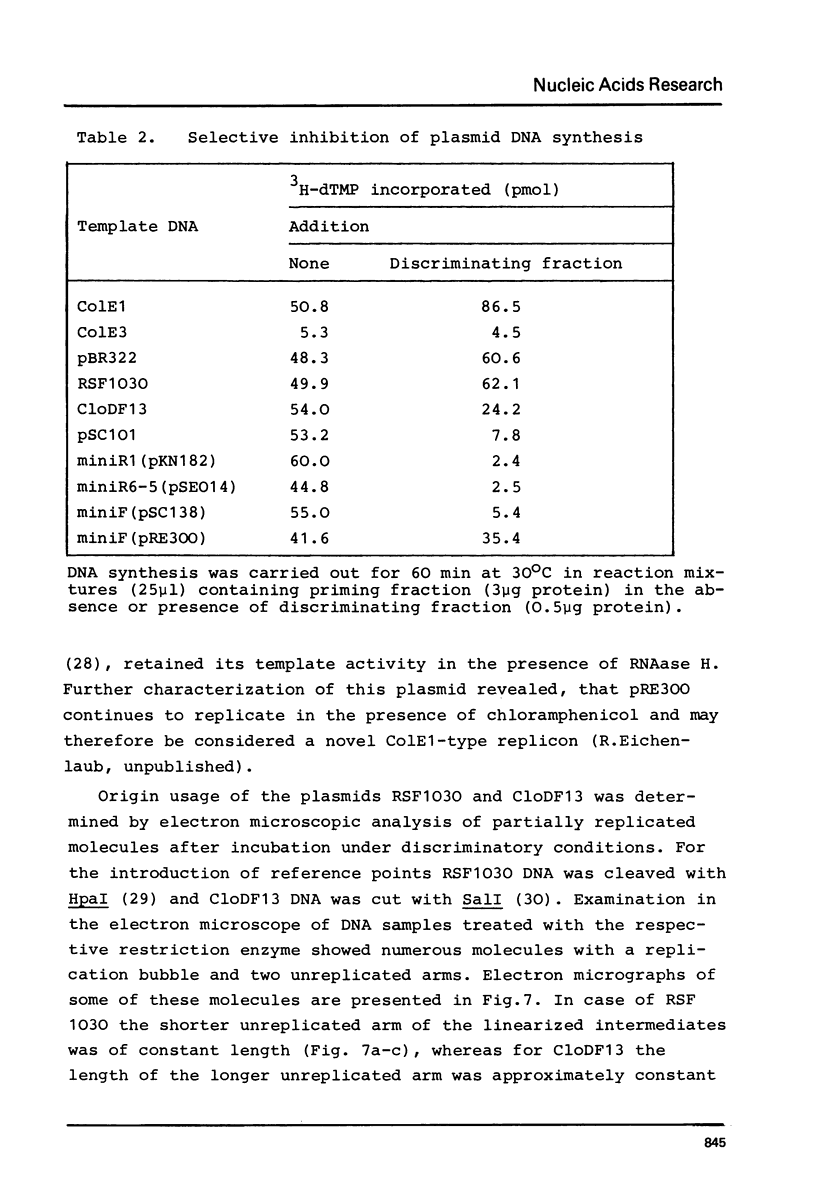
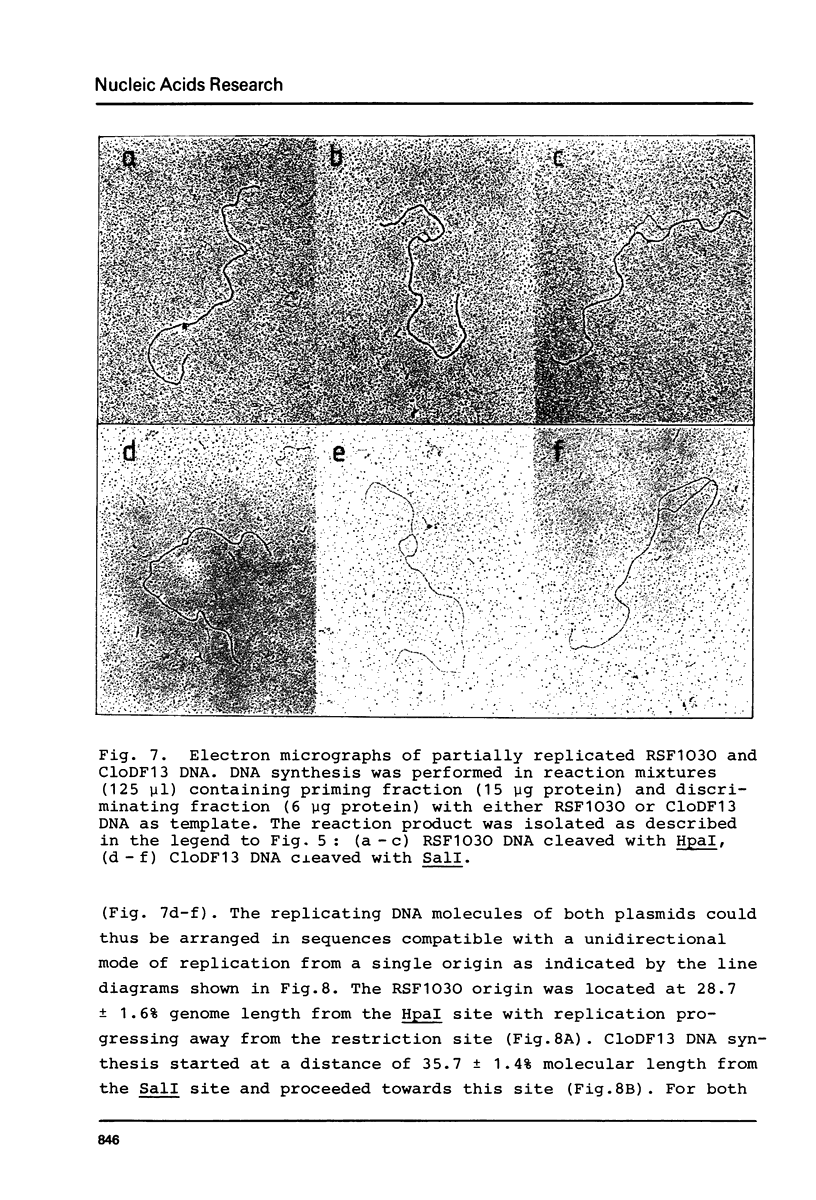
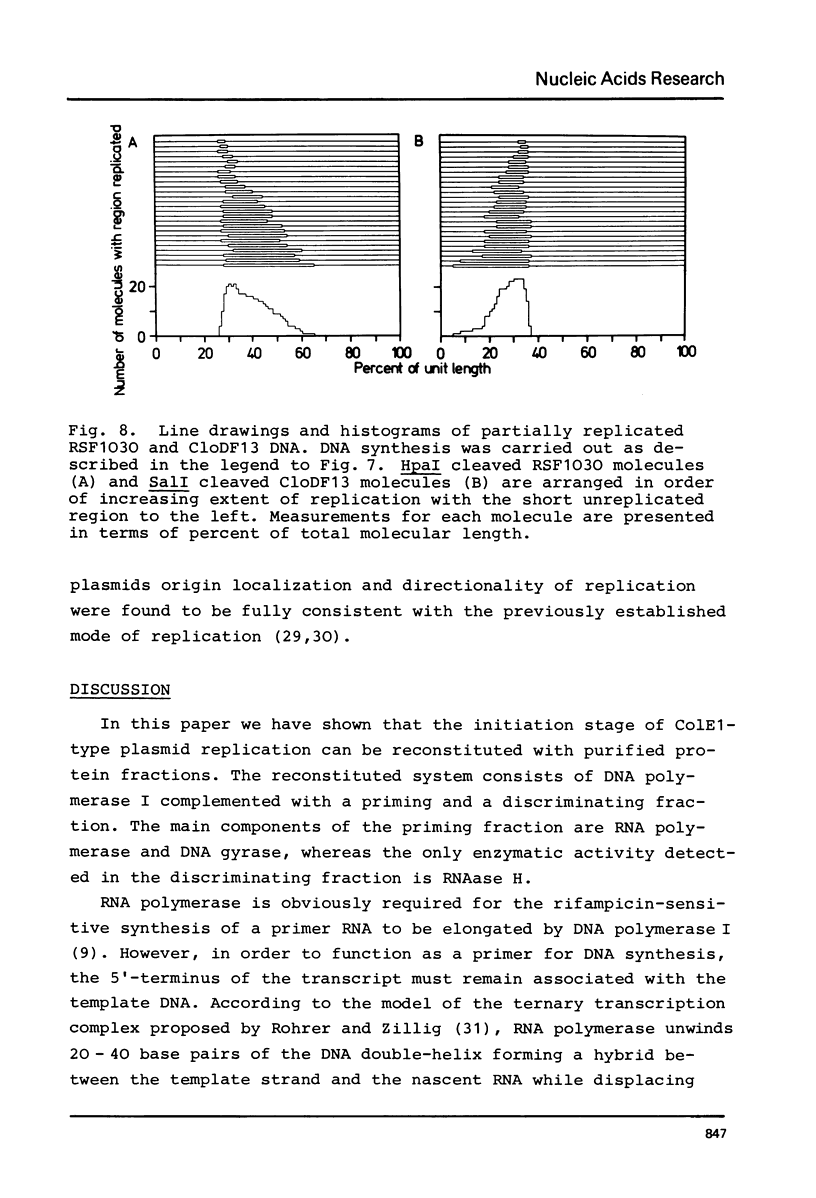
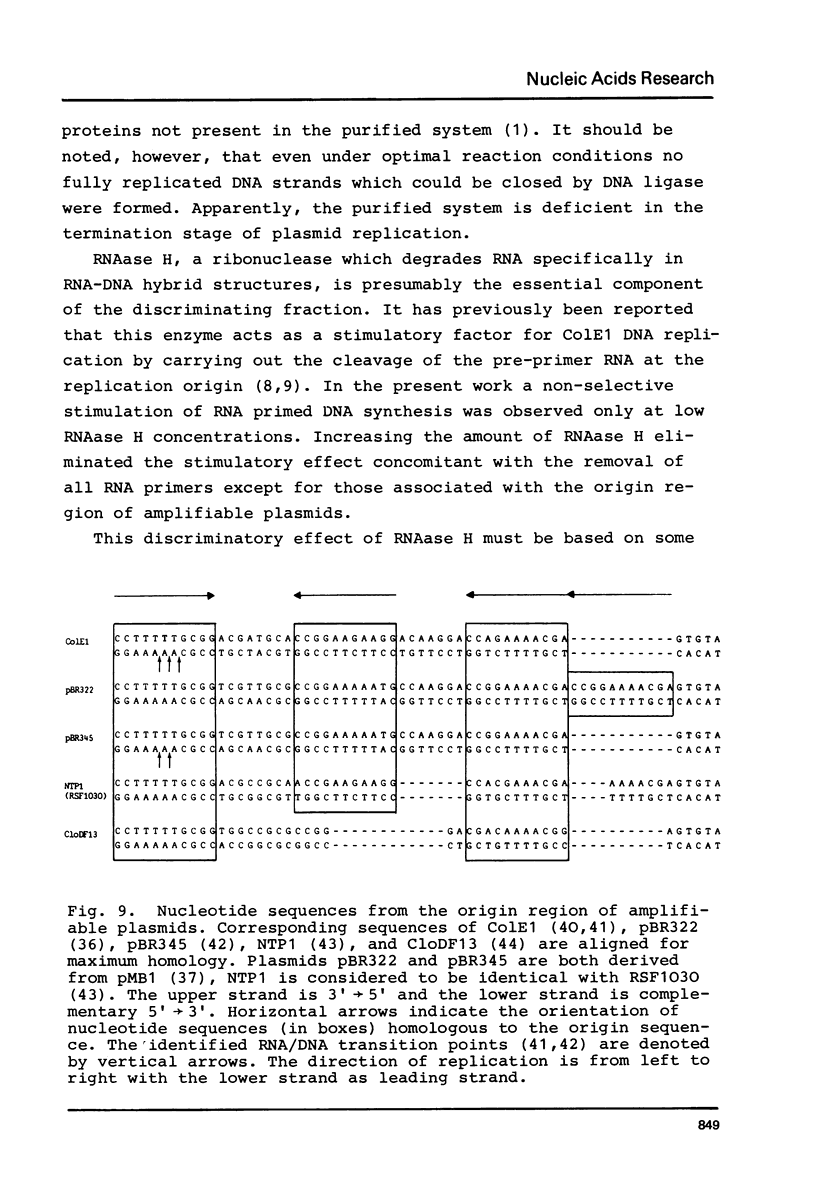
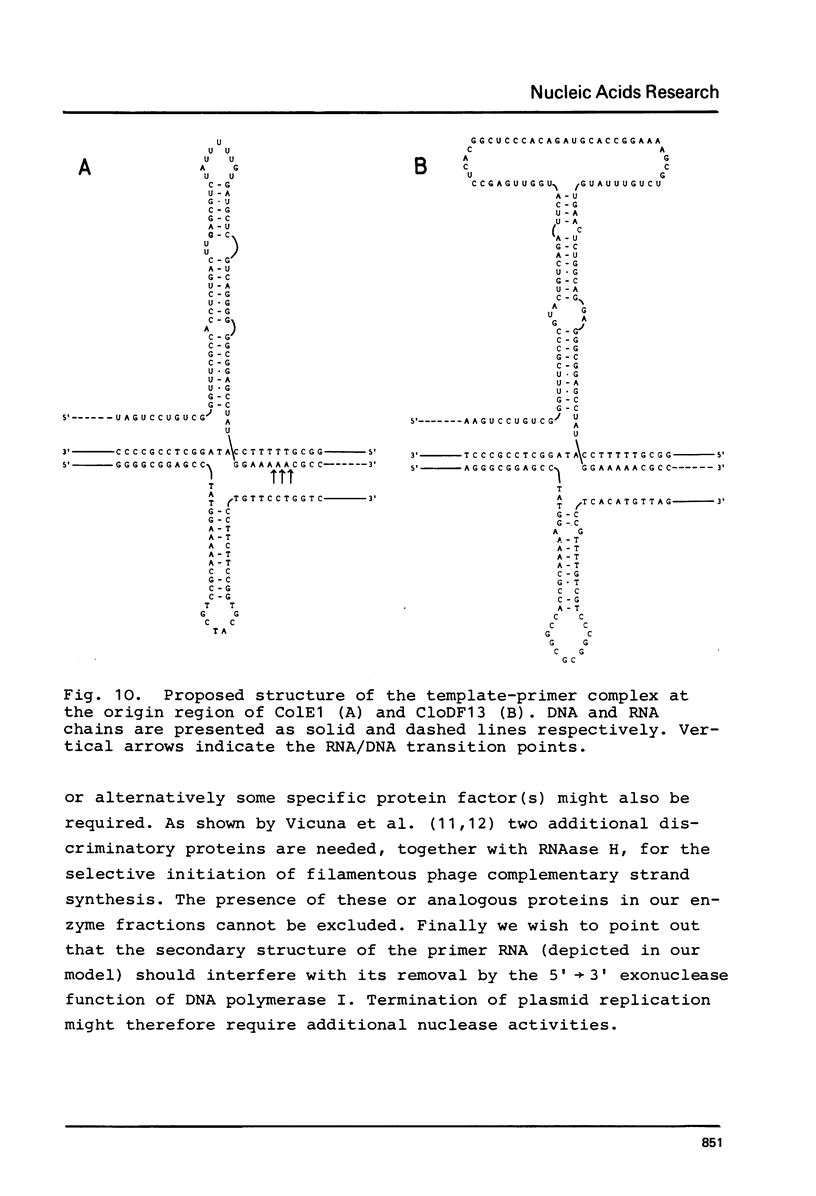
The initiation stage of ColE1-type plasmid replication was reconstituted with purified protein fractions from Escherichia coli. The reconstituted system included DNA polymerase I, DNA ligase, RNA polymerase, DNA gyrase, and a discriminating activity copurifying with RNAase H (but free of RNAase III). Initiation of DNA synthesis in the absence of RNAase H did not occur at the normal replication origin and was non-selective with respect to the plasmid template. In the presence of RNAase H the system was selective for ColE1-type plasmids and could not accept the DNA of non-amplifiable plasmids. Electron microscopic analysis of the reaction product formed under discriminatory conditions indicated that origin usage and directionally of ColE1, RSF1030, and CloDF13 replication were consistent with the normal replication pattern of these plasmids. It is proposed that the initiation of ColE1-type replication depends on the formation of an extensive secondary structure in the origin primer RNA that prevents its degradation by RNAase H.
Full text
PDF





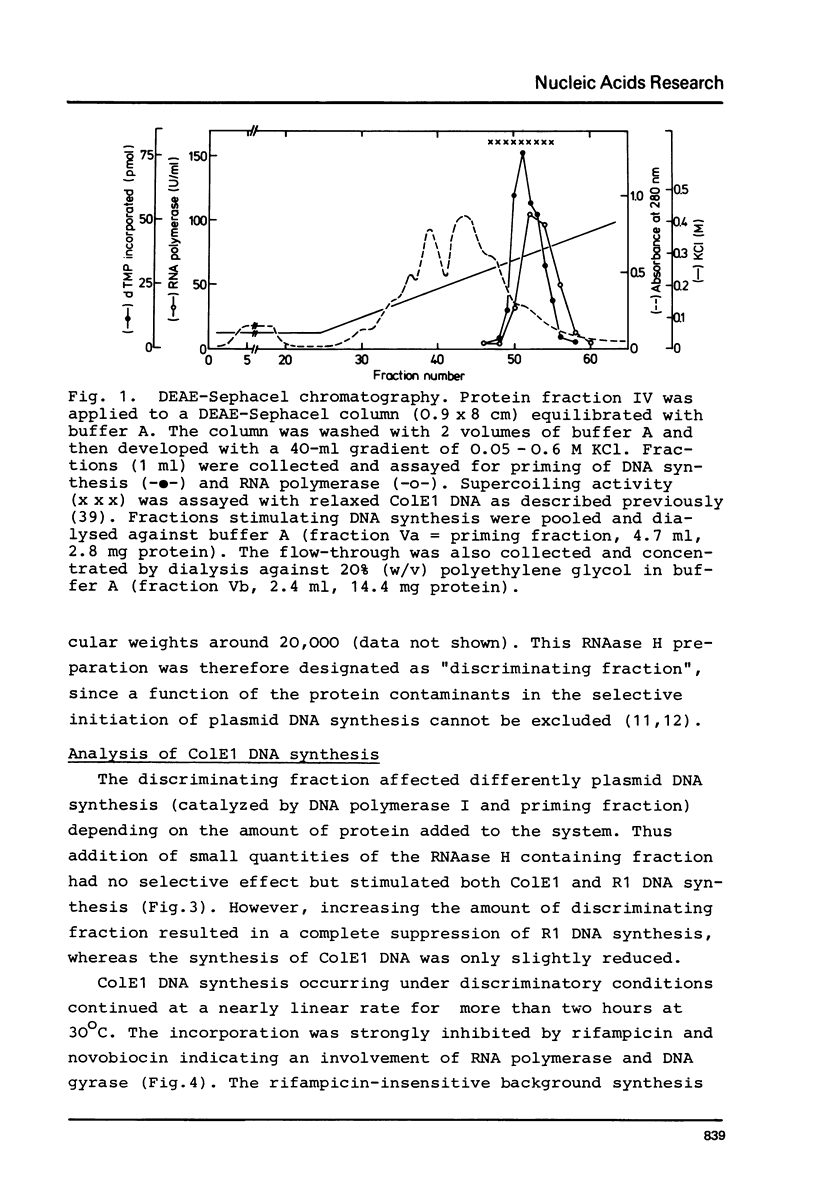
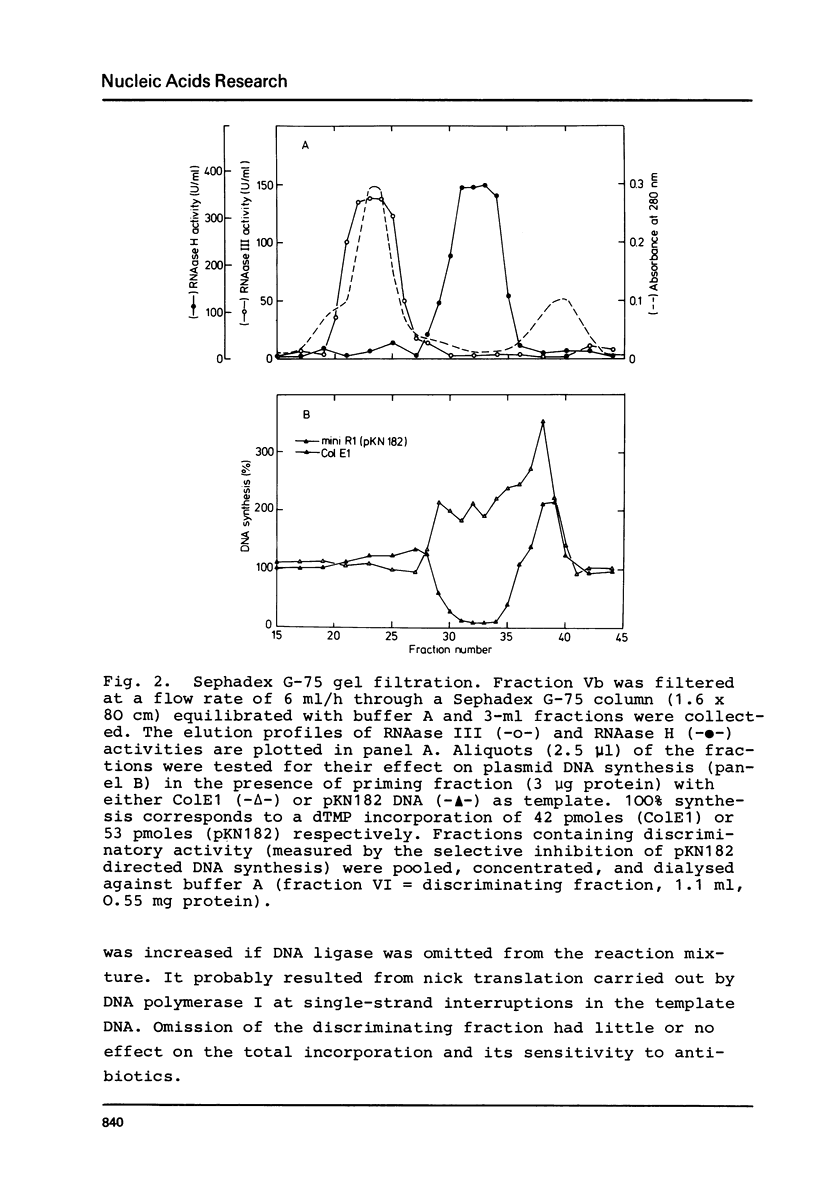
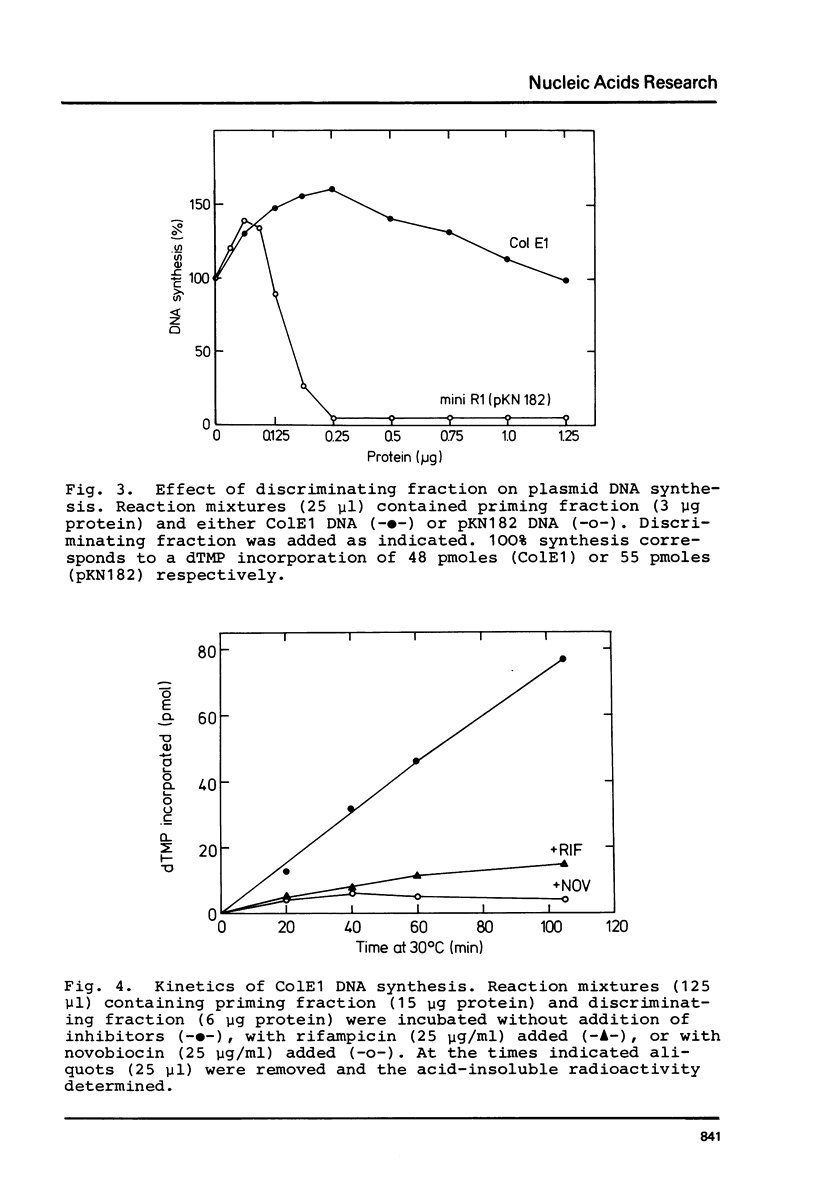

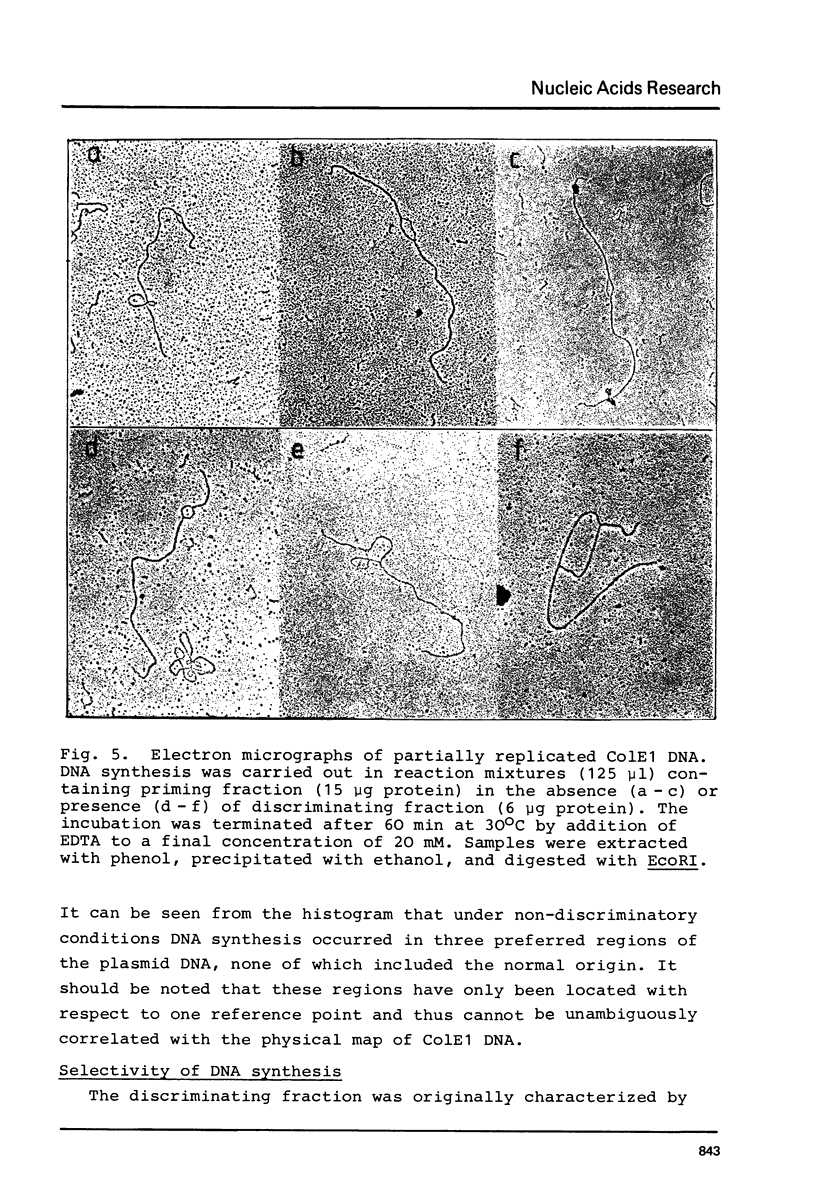




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Backman K., Betlach M., Boyer H. W., Yanofsky S. Genetic and physical studies on the replication of ColE1-type plasmids. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 1):69–76. doi: 10.1101/sqb.1979.043.01.012. [DOI] [PubMed] [Google Scholar]
- Bastia D. The nucleotide sequence surrounding the origin of DNA replication of Col E1. Nucleic Acids Res. 1977 Sep;4(9):3123–3142. doi: 10.1093/nar/4.9.3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolivar F., Betlach M. C., Heyneker H. L., Shine J., Rodriguez R. L., Boyer H. W. Origin of replication of pBR345 plasmid DNA. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5265–5269. doi: 10.1073/pnas.74.12.5265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouché J. P., Zechel K., Kornberg A. dnaG gene product, a rifampicin-resistant RNA polymerase, initiates the conversion of a single-stranded coliphage DNA to its duplex replicative form. J Biol Chem. 1975 Aug 10;250(15):5995–6001. [PubMed] [Google Scholar]
- Champoux J. J., McConaughy B. L. Priming of superhelical SV40 DNA by Escherichia coli RNA polymerase for in vitro DNA synthesis. Biochemistry. 1975 Jan 28;14(2):307–316. doi: 10.1021/bi00673a017. [DOI] [PubMed] [Google Scholar]
- Conrad S. E., Campbell J. L. Characterization of an improved in vitro DNA replication system for Escherichia coli plasmids. Nucleic Acids Res. 1979 Jul 25;6(10):3289–3304. doi: 10.1093/nar/6.10.3289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad S. E., Campbell J. L. Role of plasmid-coded RNA and ribonuclease III in plasmid DNA replication. Cell. 1979 Sep;18(1):61–71. doi: 10.1016/0092-8674(79)90354-4. [DOI] [PubMed] [Google Scholar]
- Conrad S. E., Wold M., Campbell J. L. Origin and direction of DNA replication of plasmid RSF1030. Proc Natl Acad Sci U S A. 1979 Feb;76(2):736–740. doi: 10.1073/pnas.76.2.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVries J. K., Maas W. K. Description of an incompatibility mutant of Escherichia coli. J Bacteriol. 1973 Jul;115(1):213–220. doi: 10.1128/jb.115.1.213-220.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz R., Nordström K., Staudenbauer W. L. Plasmid R1 DNA replication dependent on protein synthesis in cell-free extracts of E. coli. Nature. 1981 Jan 22;289(5795):326–328. doi: 10.1038/289326a0. [DOI] [PubMed] [Google Scholar]
- Ely S., Staudenbauer W. L. Regulation of plasmid DNA synthesis: isolation and characterization of copy number mutants of miniR6-5 and miniF plasmids. Mol Gen Genet. 1981;181(1):29–35. doi: 10.1007/BF00339001. [DOI] [PubMed] [Google Scholar]
- Fisher L. M., Mizuuchi K., O'Dea M. H., Ohmori H., Gellert M. Site-specific interaction of DNA gyrase with DNA. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4165–4169. doi: 10.1073/pnas.78.7.4165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gellert M., Mizuuchi K., O'Dea M. H., Nash H. A. DNA gyrase: an enzyme that introduces superhelical turns into DNA. Proc Natl Acad Sci U S A. 1976 Nov;73(11):3872–3876. doi: 10.1073/pnas.73.11.3872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grindley J. N., Nakada D. The nucleotide sequence of the replication origin of plasmid NTP1. Nucleic Acids Res. 1981 Sep 11;9(17):4355–4366. doi: 10.1093/nar/9.17.4355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerry P., LeBlanc D. J., Falkow S. General method for the isolation of plasmid deoxyribonucleic acid. J Bacteriol. 1973 Nov;116(2):1064–1066. doi: 10.1128/jb.116.2.1064-1066.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphreys G. O., Willshaw G. A., Anderson E. S. A simple method for the preparation of large quantities of pure plasmid DNA. Biochim Biophys Acta. 1975 Apr 2;383(4):457–463. doi: 10.1016/0005-2787(75)90318-4. [DOI] [PubMed] [Google Scholar]
- Inselburg J. Replication of colicin E1 plasmid DNA in minicells from a unique replication initiation site. Proc Natl Acad Sci U S A. 1974 Jun;71(6):2256–2259. doi: 10.1073/pnas.71.6.2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh T., Tomizawa J. Formation of an RNA primer for initiation of replication of ColE1 DNA by ribonuclease H. Proc Natl Acad Sci U S A. 1980 May;77(5):2450–2454. doi: 10.1073/pnas.77.5.2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh T., Tomizawa J. Initiation of replication of plasmid ColE1 DNA by RNA polymerase, ribonuclease H, and DNA polymerase I. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 1):409–417. doi: 10.1101/sqb.1979.043.01.047. [DOI] [PubMed] [Google Scholar]
- Kahn M. L., Figurski D., Ito L., Helinski D. R. Essential regions for replication of a stringent and a relaxed plasmid in Escherichia coli. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 1):99–103. doi: 10.1101/sqb.1979.043.01.015. [DOI] [PubMed] [Google Scholar]
- Kolter R., Helinski D. R. Regulation of initiation of DNA replication. Annu Rev Genet. 1979;13:355–391. doi: 10.1146/annurev.ge.13.120179.002035. [DOI] [PubMed] [Google Scholar]
- Lovett M. A., Katz L., Helinski D. R. Unidirectional replication of plasmid ColE1 DNA. Nature. 1974 Sep 27;251(5473):337–340. doi: 10.1038/251337a0. [DOI] [PubMed] [Google Scholar]
- Morris C. F., Sinha N. K., Alberts B. M. Reconstruction of bacteriophage T4 DNA replication apparatus from purified components: rolling circle replication following de novo chain initiation on a single-stranded circular DNA template. Proc Natl Acad Sci U S A. 1975 Dec;72(12):4800–4804. doi: 10.1073/pnas.72.12.4800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr E., Staudenbauer W. L. An Escherichia coli mutant thermosensitive in the B subunit of DNA gyrase: effect on the structure and replication of the colicin E1 plasmid in vitro. Mol Gen Genet. 1981;181(1):52–56. doi: 10.1007/BF00339004. [DOI] [PubMed] [Google Scholar]
- Rohrer H., Zillig W. Studies on the transcription complex of Escherichia coli RNA polymerase. Eur J Biochem. 1977 Oct 3;79(2):401–409. doi: 10.1111/j.1432-1033.1977.tb11822.x. [DOI] [PubMed] [Google Scholar]
- Schekman R., Weiner J. H., Weiner A., Kornberg A. Ten proteins required for conversion of phiX174 single-stranded DNA to duplex form in vitro. Resolution and reconstitution. J Biol Chem. 1975 Aug 10;250(15):5859–5865. [PubMed] [Google Scholar]
- Schuster H., Mikolajczyk M., Rohrschneider J., Geschke B. phiX174 DNA-dependent DNA synthesis in vitro: requirement for P1 ban protein in dnaB mutant extracts of Escherichia coli. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3907–3911. doi: 10.1073/pnas.72.10.3907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector T. Refinement of the coomassie blue method of protein quantitation. A simple and linear spectrophotometric assay for less than or equal to 0.5 to 50 microgram of protein. Anal Biochem. 1978 May;86(1):142–146. doi: 10.1016/0003-2697(78)90327-5. [DOI] [PubMed] [Google Scholar]
- Staudenbauer W. L., Orr E. DNA gyrase: affinity chromatography on novobiocin-Sepharose and catalytic properties. Nucleic Acids Res. 1981 Aug 11;9(15):3589–3603. doi: 10.1093/nar/9.15.3589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staudenbauer W. L. Replication of the ampicillin resistance plasmid RSF1030 in extracts of Escherichia coli: separation of the replication cycle into early and late stages. Mol Gen Genet. 1977 Nov 4;156(1):27–34. doi: 10.1007/BF00272248. [DOI] [PubMed] [Google Scholar]
- Staudenbauer W. L. Structure and replication of the colicin E1 plasmid. Curr Top Microbiol Immunol. 1978;83:93–156. doi: 10.1007/978-3-642-67087-9_3. [DOI] [PubMed] [Google Scholar]
- Stuitje A. R., Veltkamp E., Maat J., Heyneker H. L. The nucleotide sequence surrounding the replication origin of the cop3 mutant of the bacteriocinogenic plasmid Clo DF13. Nucleic Acids Res. 1980 Apr 11;8(7):1459–1473. doi: 10.1093/nar/8.7.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuitje A. R., Veltkamp E., Weijers P. J., Nijkamp H. J. Origin and direction of replication of the bacteriocinogenic plasmid Clo DF13. Nucleic Acids Res. 1979 Jan;6(1):71–80. doi: 10.1093/nar/6.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutcliffe J. G. Complete nucleotide sequence of the Escherichia coli plasmid pBR322. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 1):77–90. doi: 10.1101/sqb.1979.043.01.013. [DOI] [PubMed] [Google Scholar]
- Timmis K., Cabello F., Cohen S. N. Cloning, isolation, and characterization of replication regions of complex plasmid genomes. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2242–2246. doi: 10.1073/pnas.72.6.2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomizawa J. I., Ohmori H., Bird R. E. Origin of replication of colicin E1 plasmid DNA. Proc Natl Acad Sci U S A. 1977 May;74(5):1865–1869. doi: 10.1073/pnas.74.5.1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomizawa J., Selzer G. Initiation of DNA synthesis in Escherichia coli. Annu Rev Biochem. 1979;48:999–1034. doi: 10.1146/annurev.bi.48.070179.005031. [DOI] [PubMed] [Google Scholar]
- Veltkamp E., Stuitje A. R. Replication and structure of the bacteriocinogenic plasmids Clo DF13 and CoI E1. Plasmid. 1981 Jan;5(1):76–99. doi: 10.1016/0147-619x(81)90078-0. [DOI] [PubMed] [Google Scholar]
- Vicuna R., Hurwitz J., Wallace S., Girard M. Selective inhibition of in vitro DNA synthesis dependent on phiX174 compared with fd DNA. I. Protein requirements for selective inhibition. J Biol Chem. 1977 Apr 25;252(8):2524–2533. [PubMed] [Google Scholar]
- Vicuna R., Ikeda J. E., Hurwitz J. Selective inhibition of phiX RFII compared with fd RFII DNA synthesis in vitro. II. Resolution of discrimination reaction into multiple steps. J Biol Chem. 1977 Apr 25;252(8):2534–2544. [PubMed] [Google Scholar]
- Wang J. C. The degree of unwinding of the DNA helix by ethidium. I. Titration of twisted PM2 DNA molecules in alkaline cesium chloride density gradients. J Mol Biol. 1974 Nov 15;89(4):783–801. doi: 10.1016/0022-2836(74)90053-9. [DOI] [PubMed] [Google Scholar]





