Abstract
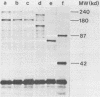
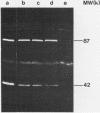
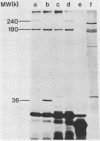
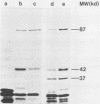
The sog gene of the large plasmid ColIdrd-1 has previously been shown to encode a DNA primase and a smaller antigenically related polypeptide. Genesis of these two products has been examined using Sog+ recombinant plasmids. Effects of amber mutations, isolated after in vitro mutagenesis, and deletions into or within sog suggest that the smaller polypeptide is a separate translation product which is encoded by DNA specifying the C-terminal region of the larger protein. Under control of the lac promotor, synthesis of both polypeptides is reduced when transcription is repressed. These findings imply that transcription of sog yields a single transcript which is translated from two initiation sites.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Backman K., Ptashne M. Maximizing gene expression on a plasmid using recombination in vitro. Cell. 1978 Jan;13(1):65–71. doi: 10.1016/0092-8674(78)90138-1. [DOI] [PubMed] [Google Scholar]
- Barrell B. G., Air G. M., Hutchison C. A., 3rd Overlapping genes in bacteriophage phiX174. Nature. 1976 Nov 4;264(5581):34–41. doi: 10.1038/264034a0. [DOI] [PubMed] [Google Scholar]
- Borck K., Beggs J. D., Brammar W. J., Hopkins A. S., Murray N. E. The construction in vitro of transducing derivatives of phage lambda. Mol Gen Genet. 1976 Jul 23;146(2):199–207. doi: 10.1007/BF00268089. [DOI] [PubMed] [Google Scholar]
- Boseley P. G., Moss T., Birnstiel M. L. 5'-Labeling and poly(dA) tailing. Methods Enzymol. 1980;65(1):478–494. [PubMed] [Google Scholar]
- Boulnois G. J., Wilkins B. M. A novel priming system for conjugal synthesis of an IncI alpha plasmid in recipients. Mol Gen Genet. 1979 Oct 1;175(3):275–279. doi: 10.1007/BF00397227. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Deininger P., Esty A., LaPorte P., Friedmann T. Nucleotide sequence and genetic organization of the polyoma late region: features common to the polyoma early region and SV40. Cell. 1979 Nov;18(3):771–779. doi: 10.1016/0092-8674(79)90130-2. [DOI] [PubMed] [Google Scholar]
- Dougan G., Sherratt D. The transposon Tn1 as a probe for studying ColE1 structure and function. Mol Gen Genet. 1977 Mar 7;151(2):151–160. doi: 10.1007/BF00338689. [DOI] [PubMed] [Google Scholar]
- Edge M. D., Green A. R., Heathcliffe G. R., Meacock P. A., Schuch W., Scanlon D. B., Atkinson T. C., Newton C. R., Markham A. F. Total synthesis of a human leukocyte interferon gene. Nature. 1981 Aug 20;292(5825):756–762. doi: 10.1038/292756a0. [DOI] [PubMed] [Google Scholar]
- Eichenlaub R. Mutants of the mini-F plasmid pML31 thermosensitive in replication. J Bacteriol. 1979 May;138(2):559–566. doi: 10.1128/jb.138.2.559-566.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg A. L., St John A. C. Intracellular protein degradation in mammalian and bacterial cells: Part 2. Annu Rev Biochem. 1976;45:747–803. doi: 10.1146/annurev.bi.45.070176.003531. [DOI] [PubMed] [Google Scholar]
- Heyneker H. L., Shine J., Goodman H. M., Boyer H. W., Rosenberg J., Dickerson R. E., Narang S. A., Itakura K., Lin S., Riggs A. D. Synthetic lac operator DNA is functional in vivo. Nature. 1976 Oct 28;263(5580):748–752. doi: 10.1038/263748a0. [DOI] [PubMed] [Google Scholar]
- Holmes D. S., Quigley M. A rapid boiling method for the preparation of bacterial plasmids. Anal Biochem. 1981 Jun;114(1):193–197. doi: 10.1016/0003-2697(81)90473-5. [DOI] [PubMed] [Google Scholar]
- Lanka E., Scherzinger E., Günther E., Schuster H. A DNA primase specified by I-like plasmids. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3632–3636. doi: 10.1073/pnas.76.8.3632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linney E., Hayashi M. Two proteins of gene A of psiX174. Nat New Biol. 1973 Sep 5;245(140):6–8. doi: 10.1038/newbio245006a0. [DOI] [PubMed] [Google Scholar]
- Miller J. H., Calos M. P., Galas D., Hofer M., Büchel D. E., Müller-Hill B. Genetic analysis of transpositions in the lac region of Escherichia coli. J Mol Biol. 1980 Nov 25;144(1):1–18. doi: 10.1016/0022-2836(80)90212-0. [DOI] [PubMed] [Google Scholar]
- Pratt J. M., Boulnois G. J., Darby V., Orr E., Wahle E., Holland I. B. Identification of gene products programmed by restriction endonuclease DNA fragments using an E. coli in vitro system. Nucleic Acids Res. 1981 Sep 25;9(18):4459–4474. doi: 10.1093/nar/9.18.4459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prentki P., Karch F., Iida S., Meyer J. The plasmid cloning vector pBR325 contains a 482 base-pair-long inverted duplication. Gene. 1981 Sep;14(4):289–299. doi: 10.1016/0378-1119(81)90161-x. [DOI] [PubMed] [Google Scholar]
- Reddy V. B., Dhar R., Weissman S. M. Nucleotides sequence of the genes for the simian virus 40 proteins VP2 and VP3. J Biol Chem. 1978 Jan 25;253(2):621–630. [PubMed] [Google Scholar]
- Shaw J. E., Murialdo H. Morphogenetic genes C and Nu3 overlap in bacteriophage lambda. Nature. 1980 Jan 3;283(5742):30–35. doi: 10.1038/283030a0. [DOI] [PubMed] [Google Scholar]
- Smith H. O., Birnstiel M. L. A simple method for DNA restriction site mapping. Nucleic Acids Res. 1976 Sep;3(9):2387–2398. doi: 10.1093/nar/3.9.2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M., Brown N. L., Air G. M., Barrell B. G., Coulson A. R., Hutchison C. A., 3rd, Sanger F. DNA sequence at the C termini of the overlapping genes A and B in bacteriophage phi X174. Nature. 1977 Feb 24;265(5596):702–705. doi: 10.1038/265702a0. [DOI] [PubMed] [Google Scholar]
- Smith R. A., Parkinson J. S. Overlapping genes at the cheA locus of Escherichia coli. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5370–5374. doi: 10.1073/pnas.77.9.5370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson R., Achtman M. The control region of the F sex factor DNA transfer cistrons: physical mapping by deletion analysis. Mol Gen Genet. 1979 Jan 16;169(1):49–57. doi: 10.1007/BF00267544. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twigg A. J., Sherratt D. Trans-complementable copy-number mutants of plasmid ColE1. Nature. 1980 Jan 10;283(5743):216–218. doi: 10.1038/283216a0. [DOI] [PubMed] [Google Scholar]
- Wilkins B. M., Boulnois G. J., Lanka E. A plasmid DNA primase active in discontinuous bacterial DNA replication. Nature. 1981 Mar 19;290(5803):217–221. doi: 10.1038/290217a0. [DOI] [PubMed] [Google Scholar]