Abstract
Background
Among adolescents uric acid is associated with insulin resistance, hypertension and the metabolic syndrome (MetS) and in adults high uric acid levels are an independent risk factor for cardiovascular disease and diabetes.
Objective
Determine whether the relationship of uric acid with MetS varies in adolescents by race/ethnicity and gender.
Methods
We used linear regression to evaluate associations between uric acid and other MetS-associated clinical and laboratory measures among 3,296 non-Hispanic-white, non-Hispanic-black and Hispanic adolescents age 12–19y participating in the National Health and Nutrition Evaluation Survey (1999–2006).
Results
Overall, non-Hispanic-white males and females had the highest uric acid levels among the three racial/ethnic groups. In each racial/ethnic group there were higher uric acid levels for those adolescents with vs. without MetS. However, the extent of the MetS-related increase in uric acid level varied by race and gender. Among males, MetS was associated with the greatest increases in uric acid among non-Hispanic whites. However, among females, the MetS-related increase in uric acid was greatest among non-whites. Non-Hispanic-white females exhibited the lowest degrees of correlation between levels of uric acid and MetS-associated variables. Uric acid levels did not correlate with insulin levels in non-Hispanic-white females.
Conclusions
These data suggest the relationship between uric acid and MetS varies by race/ethnicity and gender. In particular, non-Hispanic-white males exhibit a strong relationship and non-Hispanic-white females exhibit a relatively poor correlation between uric acid and MetS-related factors. These data may have implications for the use of uric acid as a marker of future risk among adolescents.
Keywords: obesity, insulin resistance, hypertension
Introduction
Uric acid is a by-product of purine metabolism that is implicated in worsening insulin resistance [1–3] and appears to contribute to the development of hypertension [3–7]. Given these relationships it is not surprising that uric acid is tightly linked to the metabolic syndrome (MetS), a constellation of cardiovascular risk factors also associated with insulin resistance [8–11]. In addition, large prospective trials have demonstrated that elevations in uric acid are independently associated with future MetS [12], renal disease [13], cardiovascular disease (CVD) [14–16] and type 2 diabetes (T2DM) [17]. Even among adolescents, elevated levels of uric acid are independently associated with long-term risk for hypertension [6] and carotid artery intima media thickness [18]. This has raised the potential to use elevated levels of uric acid as marker of increased risk.
Among adolescents, uric acid levels are influenced by central obesity [8] and by the intake of fructose and sucrose [19,20]. Additionally, gender differences in uric acid are well known, with males having higher levels of uric acid than females, at least in part because estrogen increases excretion of uric acid [21,22].
However, data on racial/ethnic differences in uric acid levels among adolescents are scarce. Many features of MetS itself display racial/ethnic differences [23–25]. Non-Hispanic-black adolescents have a greater degree of hypertension and insulin resistance than non-Hispanic whites but have lower rates of MetS overall [26–30]. Our goal was to evaluate the relationship between uric acid and MetS in adolescents on a race/ethnicity- and gender-related basis. We used the National Health and Nutrition Examination Survey (NHANES) ‘99-’06 to better define these relationships and to evaluate for potential explanations for any possible gender/ethnic differences.
Methods
Data were obtained from NHANES (1999–2006), a complex, multistage probability sample of the US population. These annual cross-sectional surveys are conducted by the National Center for Health Statistics (NCHS) of the Centers for Disease Control (CDC), with randomly-selected subjects undergoing anthropometric and blood pressure measurements, answering questionnaires and undergoing phlebotomy (http://www.cdc.gov/nchs/nhanes.htm). The NCHS ethics review board reviewed and approved the survey and participants gave informed consent prior to participation.
WC, blood pressure (BP), and laboratory measures of triglycerides, HDL-C, and glucose were obtained using standardized protocols and calibrated equipment [8,31]. Serum uric acid was measured by a colorimetric method in which uric acid is oxidized by uricase to form allantoin and H2O2. For NHANES ‘99-’02 this method was used by Hitachi model 704 analyzer, Roche Diagnostics and from ‘03-’06 this was measured by Beckman Synchron LX20, Beckman Coulter, Inc. All blood samples used for analyses were obtained following a fast ≥8 hours prior to the blood draw.
MetS Classification
MetS was defined by a commonly-used pediatric/adolescent adaptation of the Adult Treatment Panel III (ATP III) criteria [8,32–34]. Participants had to meet ≥3 of the following 5 criteria: concentration of triglycerides ≥110 mg/dL, HDL-C ≤40 mg/dL, WC ≥90th percentile for age/sex (or ATP III limit of 102 cm for males and 88 cm for females, whichever was lower) [35,36], glucose concentration ≥100 mg/dL, and systolic or diastolic BP ≥90th percentile (age, height, and sex-specific) [37]. Similarly, hypertension was defined as systolic or diastolic BP ≥90th percentile for age, height, and sex.
Data from non-Hispanic-white, non-Hispanic-black, or Hispanic (Mexican-American/other Hispanic) adolescents 12–19y were analyzed. Children <12y were excluded since fasting values for triglycerides and glucose were only obtained in participants ≥12y. Subjects were excluded if they self-reported diabetes, were pregnant or taking antihyperlipidemic or anti-diabetic medications, as these are all likely to alter lipid and insulin levels in a manner that may not reflect baseline MetS-uric acid correlations. Individuals taking anti-hypertensive medication were classified as having hypertension. Following these exclusions the study sample consisted of 3,296 non-Hispanic-white, non-Hispanic-black and Hispanic adolescents age 12–19y with data for all variables tested (52% male). NHANES includes an over-sample of racial/ethnic minorities, and thus the sample included 28% non-Hispanic whites, 40% Hispanics and 32% non-Hispanic blacks. This over-sampling was accounted for using SUDAAN (version 10; Research Triangle Institute, Research Triangle Park, NC), which accounts for the survey design when estimating standard errors to obtain population-based estimates.
Statistical Analysis
Statistical significance was defined as a p-value<0.05. Statistical analysis was performed using SAS (version 9.2, Cary, NC) and SUDAAN, as mentioned previously. We combined all data sets from the 3 two-year cycles (1999–2006) for statistical analyses to increase total sample size. Prevalence rates of MetS were calculated by gender, race/ethnicity, and compared via chi-square tests. Mean uric acid levels were compared among groups using either unpaired t-tests or analysis of variance (ANOVA). Linear regression was then used to assess the effect of gender, race/ethnicity, and MetS status on levels of uric acid. All interactions of the three covariates (gender, race/ethnicity, and MetS status) were initially included in the model, but removed in a stepwise fashion if the associated interaction p-value was <0.15. We also included education [38], poverty [38], and smoking [15] in the model due to known effects on levels of uric acid. While potentially important as confounders, drug and alcohol use were not included in the model because these were not available for NHANES participants <20 y.o. Education was classified as the highest level obtained for any household member and categorized as follows: less than high school, high school, and greater than high school. Income-to-need ratio was used to measure poverty. Due to the poor reliability of self-reporting of smoking among adolescents [39], serum cotinine was used to identify smokers, with a cut-off of 15 ng/mL as recommended [40]. Because high intake of fructose has been associated with elevations in uric acid levels [19,20], we also included into the model the percent of calories from added sugars, a component of the Healthy Eating Index [41], using data collected from computer-assisted 24-hour food recall questionnaires (the Automated Multiple-Pass Method) developed by NHANES [42] and USDA [43]. Mean levels of uric acid from the final model were estimated and compared among gender and race/ethnicity, as applicable. In comparing uric acid levels and the ratio of uric acid levels among individuals with vs. without MetS, Hispanic and non-Hispanic black adolescents were combined into a single “non-white” comparator when both of these groups behaved similarly in their differences with non-Hispanic-white adolescents. Pearson correlation coefficients were computed to assess the degree of linear association between uric acid and each MetS component and ln(insulin) and the homeostasis model of insulin resistance (HOMA) [44], by race/ethnicity/gender. With the exception of the correlation estimates, all analyses incorporated the sampling weights included in NHANES.
Results
Overall Uric acid and MetS values
Values for overall uric acid, individual MetS components, insulin and HOMA are shown by race/ethnicity for all male and female subjects in Table 1. Non-Hispanic whites had the highest uric acid levels overall in males and females, as compared to Hispanics and non-Hispanic blacks. Regarding MetS components commonly associated with elevated uric acid, non-Hispanic-black males and females had the highest rates of hypertension (SBP and/or DBP >90th percent), while elevations in WC were highest overall in Hispanic males and non-Hispanic black females. Levels of fasting insulin and HOMA (as an estimate of insulin resistance) were also highest in Hispanic males and non-Hispanic-black females. The percent added sugar did not differ between racial/ethnic/gender groups (data not shown).
Table 1.
Race/ethnicity comparison of MetS components and related factors for all subjects by gender
| Male
|
Female
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Non-Hispanic White | Hispanic | Non-Hispanic Black | p-value* | Non-Hispanic White | Hispanic | Black | p-value* | |
| n | 473 | 662 | 594 | -- | 434 | 664 | 469 | |
| Mean age (95% CI), years | 15.6 (15.4, 15.8) | 15.0 (14.7, 15.3) | 15.3 (15.2, 15.5) | < 0.01 | 15.4 (15.2, 15.7) | 15.6 (15.3, 15.9) | 15.4 (15.1, 15.7) | 0.57 |
| Percent with MetS | 12.7 | 13.6 | 4.8 | <0.01 | 5.8 | 8.1 | 4.6 | 0.35 |
| Mets components | ||||||||
| Waist Circumference | ||||||||
| Mean (95% CI), cm | 81.9 (80.4,83.5 ) | 83.0 (81.0,85.0 ) | 78.3 (76.9,79.7 ) | <0.01 | 79.8 (78.2,81.4 ) | 81.8 (79.7,84.0 ) | 82.6 (81.1,84.0 ) | 0.03 |
| Percent above 90% pctile | 15.8 | 20.8 | 13.0 | 0.02 | 21.0 | 28.9 | 32.0 | <0.01 |
| Triglycerides | ||||||||
| Mean (95% CI), m/.dL | 98.0 (91.5,104.5) | 92.7 (87.5,97.9 ) | 70.3 (66.9,73.8 ) | <0.01** | 91.7 (86.4,97.0 ) | 99.4 (84.8,114.0) | 68.7 (65.0,72.4 ) | <0.01 |
| Percent above 110 | 31.0 | 26.1 | 10.6 | <0.01 | 25.2 | 24.3 | 10.5 | <0.01 |
| HDL | ||||||||
| Mean (95% CI),mg/dL | 46.7 (45.6,47.7 ) | 48.5 (47.1,49.9 ) | 54.2 (52.8,55.6 ) | <0.01 | 52.6 (51.4,53.8 ) | 51.7 (50.3,53.0 ) | 55.3 (53.7,56.9 ) | 0.01 |
| Percent below 40 | 24.0 | 22.5 | 10.1 | <0.01 | 11.7 | 14.2 | 8.7 | 0.12 |
| SBP | ||||||||
| Mean (95% CI),mmHg | 112.5 (111.3,113.8) | 111.2 (109.3,113.2) | 114.4 (113.5,115.3) | <0.01 | 106.2 (105.1,107.3) | 106.7 (105.6,107.9) | 109.4 (108.3,110.6) | <0.01 |
| Percent above 90% pctile | 8.8 | 5.7 | 12.5 | <0.01 | 3.8 | 3.9 | 6.7 | 0.14 |
| DBP | ||||||||
| Mean (95% CI),mmHg | 61.5 (60.4,62.6 ) | 59.0 (57.9,60.0 ) | 60.6 (59.4,61.8 ) | <0.01 | 63.6 (62.5,64.6 ) | 62.6 (61.5,63.7 ) | 63.3 (62.4,64.2 ) | 0.47 |
| Percent above 90% pctile | 2.3 | 2.0 | 3.0 | 0.41 | 3.2 | 2.2 | 3.7 | 0.55 |
| Fasting Glucose | ||||||||
| Mean (95% CI),mg/dL | 95.1 (94.4,95.9 ) | 95.9 (95.0,96.9 ) | 92.9 (92.2,93.7 ) | <0.01 | 91.2 (90.3,92.0 ) | 91.5 (90.8,92.3 ) | 89.4 (88.6,90.2 ) | <0.01 |
| Percent above 100 | 22.1 | 28.2 | 14.4 | <0.01 | 9.5 | 7.4 | 5.2 | 0.11 |
| MetS-related measures (Mean (95% CI)) | ||||||||
| BMI, kg/m2 | 23.0 (22.5,23.5 ) | 23.5 (22.8,24.2 ) | 23.3 (22.8,23.9 ) | 0.52 | 22.9 (22.3,23.5 ) | 24.0 (23.2,24.9 ) | 25.8 (25.2,26.3 ) | <0.01 |
| Insulin, IU/mL | 10.4 (9.3,11.5 ) | 11.6 (10.4,12.9 ) | 10.1 (9.5,10.8 ) | 0.03** | 9.8 (9.1,10.6 ) | 12.6 (11.4,13.7 ) | 13.7 (12.7,14.7 ) | <0.01** |
| HOMA score | 2.5 (2.2,2.8 ) | 2.8 (2.5,3.1 ) | 2.4 (2.2,2.5 ) | 0.02** | 2.2 (2.1,2.4 ) | 2.9 (2.6,3.2 ) | 3.1 (2.8,3.4 ) | <0.01** |
| Uric acid, mg/dL | 5.8 (5.7,6.0 ) | 5.6 (5.4,5.8 ) | 5.3 (5.2,5.4 ) | <0.01 | 4.6 (4.5,4.7 ) | 4.4 (4.3,4.6 ) | 4.4 (4.3,4.5 ) | <0.01 |
Chi-square test comparing percents, ANOVA comparing means (overall difference among the groups).
Comparison of ln(Triglyceride), ln(Insulin), ln(Homa).
Uric acid linear model
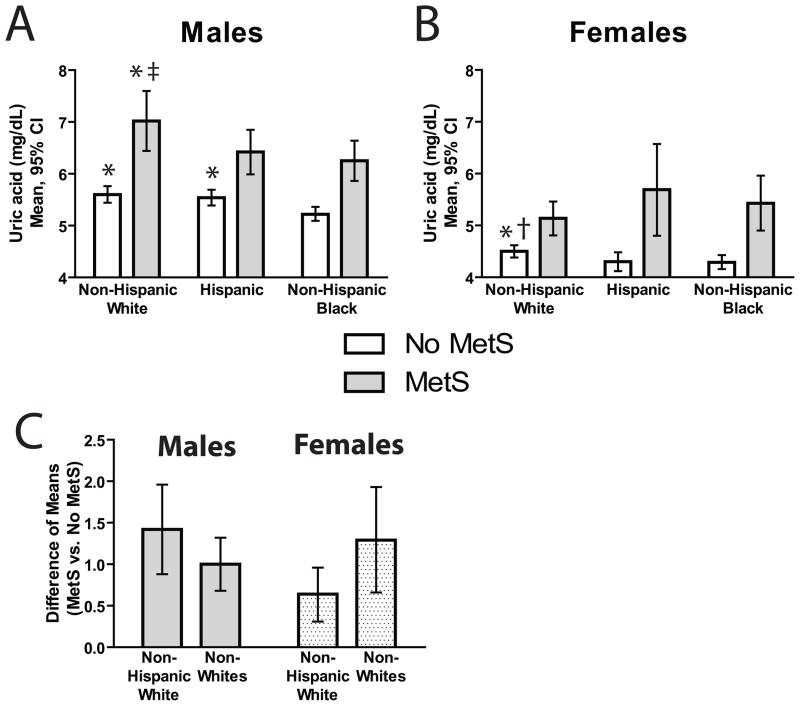
Covariates in the final model of uric acid are shown in Table 2, and mean values by race/ethnicity and gender are shown in Figure 1A–B. A three-way interaction between MetS, ethnicity, and gender was significant and thus remained in the model (p=0.0495). While the percent added sugar on its own was significantly associated with levels of uric acid, this effect was not significant after adjusting for the other covariates in the model. For each race/ethnicity/gender group, uric acid levels were higher in individuals with MetS compared to those without MetS (Figure 1A–B).
Table 2.
Linear Model Results of Uric Acid*
| Model Covariate | Estimate | 95% CI | p-value |
|---|---|---|---|
| Education** | |||
| Less than High School | 0.02 | (−0.18, 0.13) | 0.78 |
| High School | 0.02 | (−0.12, 0.16) | 0.78 |
| Added Sugar (Percent of Total Calories) | 0.005 | (−0.001, 0.011) | 0.13 |
| Income-to-Needs Ratio | 0.03 | (−0.01, 0.06) | 0.20 |
| Current Smoker | 0.13 | (−0.06, 0.32) | 0.17 |
| Females (vs. Males) | −1.10 | (−1.24, −0.96) | < 0.01 |
| Race/Ethnicity† | |||
| Hispanic | −0.06 | (−0.22, 0.11) | 0.51 |
| Non-Hispanic Black | −0.37 | (−0.52, −0.23) | < 0.01 |
| MetS vs. No MetS – Females | |||
| Non-Hispanic White | 0.64 | (0.31, 0.96) | < 0.01 |
| Hispanic | 1.38 | (0.48, 2.29) | < 0.01 |
| Non-Hispanic Black | 1.14 | (0.59, 1.69) | < 0.01 |
| MetS vs. No MetS – Males | |||
| Non-Hispanic White | 1.42 | (0.88, 1.96) | < 0.01 |
| Hispanic | 0.88 | (0.47, 1.29) | < 0.01 |
| Non-Hispanic Black | 1.03 | (0.66, 1.40) | < 0.01 |
Final model included three-way interaction among MetS, gender, and ethnicity (interaction p-value = 0.0495); model R2 = 0.321. Intercept 5.44 (95% CI 5.22–5.67).
Highest among household (person who owns/rents house or his/her spouse), values indicate differences from “more than high school” category
Values indicate difference from Non-Hispanic White
Figure 1. Comparison of Uric Acid Levels by Race/Ethnicity, Gender and MetS Status.
A and B, Adjusted means of uric acid by gender, race/ethnicity and MetS status. Estimated means (95% CI’s) for males (A) and females (B) among adolescents with and without MetS. C, Ratio of adjusted means (and 95% CI’s) of uric acid (MetS+/MetS-) for non-Hispanic whites and non-whites (non-Hispanic blacks and Hispanics combined) among males and females. The pattern of these inter-ethnic differences between whites and other ethnicities is significantly different between non-Hispanic-white males and females (p<0.05). Comparisons between ethnic groups by corresponding MetS status are: * p<0.05 vs. non-Hispanic blacks, ‡ p<0.05 vs. Hispanics and † p<0.05 vs. non-Hispanic blacks and Hispanics combined.
Levels of uric acid among adolescents by MetS status
Among males without MetS, both non-Hispanic whites and Hispanics had higher uric acid levels than non-Hispanic blacks (Figure 1A). Among males with MetS, non-Hispanic whites had higher uric acid levels than the other two race/ethnicities (both p<0.05). Among females without MetS, non-Hispanic whites had higher levels of uric acid than non-Hispanic blacks and Hispanics combined (Figure 1B; p<0.05). Among females with MetS, however, there were no significant differences in uric acid levels by race/ethnicity.
Among males, the elevation in uric acid levels between those with and without MetS was greatest with non-Hispanic whites, although the difference in these elevations was not significantly different among the racial/ethnic groups (non-Hispanic white vs. non-white p-value=0.1812; Figure 1C). Conversely, among females, non-Hispanic whites had the lowest elevation in uric acid attributable to MetS, but again the difference in these elevations were not significantly different among the racial/ethnic groups (non-Hispanic white vs. non-white p-value=0.0702; Figure 1C). However, the pattern of differences in these increases when comparing by race/ethnicity was significantly different between males and females – namely, non-Hispanic-white males had the greatest MetS-related increase while non-Hispanic-white females had the lowest MetS-related increase. This difference in the pattern of uric acid and MetS between non-Hispanic-white males and females was the cause of the significant gender-ethnicity-MetS interaction (p=0.0495) mentioned previously.
Levels of uric acid among adolescents by hypertension, obesity and insulin status
In order to investigate if individual MetS components could explain the final model and the resulting racial/ethnic/gender differences of note, we compared mean uric acid levels by gender and race/ethnicity, stratified by hypertension status, obesity status, and insulin status—as each of these indices has been particularly tightly linked to uric acid elevations. For each race/ethnicity/gender group, individuals with hypertension, elevated WC, and elevated insulin had higher uric acid levels compared to individuals with normal levels of these indices (Supplementary Table 1). Among males with and without hypertension, elevated WC and elevated insulin, non-Hispanic whites had significantly higher uric acid levels than non-Hispanic blacks (but not Hispanics). Among females without these MetS-related findings, non-Hispanic whites had the highest levels of uric acid, while among females with elevations in these MetS-related indices there were no significant race/ethnicity differences in uric acid levels. These findings were thus similar to the findings regarding uric acid levels in groups with and without MetS.
Uric acid and age
To evaluate for the possibility that differences in levels of uric acid levels were affected by inter-racial differences in the timing of puberty, we evaluated levels over the age span of adolescence (Supplementary Figure 1). For both males and females, levels of uric acid were similar between races/ethnicities at 12–13y. Non-Hispanic white males had higher uric acid levels starting at 14–15y and continuing through 18–19y, while non-Hispanic white females had higher levels starting at 16–17y and continuing to 18–19y.
Uric acid correlations with MetS components and insulin
Table 3 shows correlations of uric acid with individual components of MetS, as well as with insulin and HOMA. Among all of the components tested, uric acid correlated best with BMI and WC in all racial/ethnic/gender groups. There were higher degrees of correlation with BP indices among males compared to females. For all measures except triglycerides, non-Hispanic-white males had the highest correlation coefficients for all groups. For all measures non-Hispanic-white females had the lowest correlation coefficients of all groups. Non-Hispanic-white females were the only group for which uric acid was not correlated with levels of insulin or HOMA (Table 3).
Table 3.
Correlations between MetS components and uric acid level.*
| Gender | Ethnicity | BMI | WC | SBP | DBP | Triglycerides** | HDL | Fasting Glucose | Fasting Insulin** | HOMA** |
|---|---|---|---|---|---|---|---|---|---|---|
| Overall | Non-Hispanic White | 0.43 ( 0.37, 0.48) | 0.47 ( 0.42, 0.52) | 0.37 ( 0.31, 0.42) | 0.03 (−0.03, 0.10) | 0.22 ( 0.15, 0.28) | −035 (0.41, −0.30) | 0.21 ( 0.14, 0.27) | 0.18 ( 0.12, 0.24) | 0.20 ( 0.13, 0.26) |
| Hispanic | 0.36 ( 0.31, 0.40) | 0.41 ( 0.36, 0.45) | 0.34 ( 0.29, 0.39) | 0.00 (−0.05, 0.05) | 0.25 ( 0.20, 0.30) | −0.32 (−0.37, −0.27) | 0.20 ( 0.15, 0.25) | 0.20 ( 0.14, 0.25) | 0.21 ( 0.16, 0.26) | |
| Non-Hispanic Black | 0.35 ( 0.29, 0.40) | 0.36 ( 0.31, 0.42) | 0.33 ( 0.28, 0.38) | 0.04 (−0.02, 0.10) | 0.24 ( 0.18, 0.30) | −0.28 (−0.34, −0.23) | 0.19 ( 0.13, 0.24) | 0.16 ( 0.10, 0.22) | 0.18 ( 0.12, 0.23) | |
| Males | Non-Hispanic White | 0.54 (0.47, 0.60) | 0.55 ( 0.48, 0.61) | 0.34 ( 0.26, 0.42) | 0.13 ( 0.04, 0.22) | 0.28 ( 0.20, 0.36) | −0.37 (−0.45, −0.29) | 0.17 ( 0.08, 0.26) | 0.33 ( 0.25, 0.41) | 0.33 ( 0.25, 0.41) |
| Hispanic | 0.46 ( 0.40, 0.52) | 0.46 ( 0.40, 0.52) | 0.26 ( 0.19, 0.33) | 0.10 ( 0.02, 0.17) | 0.31 ( 0.24, 0.37) | −0.29 (−0.36, −0.22) | 0.05 (−0.03, 0.13) | 0.30 ( 0.23, 0.37) | 0.30 ( 0.23, 0.36) | |
| Non-Hispanic Black | 0.49 ( 0.43, 0.55) | 0.48 ( 0.42, 0.54) | 0.31 ( 0.23, 0.38) | 0.12 ( 0.04, 0.20) | 0.28 ( 0.20, 0.35) | −0.31 (−0.38, −0.23) | 0.12 ( 0.04, 0.20) | 0.27 ( 0.20, 0.35) | 0.27 ( 0.20, 0.35) | |
| Females | Non-Hispanic White | 0.39 ( 0.30, 0.46) | 0.39 ( 0.30, 0.46) | 0.10 ( 0.00, 0.19) | 0.01 (−0.09, 0.10) | 0.13 ( 0.04, 0.22) | −0.14 (−0.23, −0.05) | −0.02 (−0.12, 0.07) | 0.06 (−0.03, 0.16) | 0.06 (−0.04, 0.15) |
| Hispanic | 0.40 ( 0.33, 0.46) | 0.39 ( 0.32, 0.45) | 0.20 ( 0.12, 0.27) | 0.07 (−0.01, 0.14) | 0.28 ( 0.21, 0.35) | −0.27 (−0.33, −0.19) | 0.06 (−0.02, 0.13) | 0.27 ( 0.20, 0.34) | 0.26 ( 0.19, 0.33) | |
| Non-Hispanic Black | 0.45 ( 0.37, 0.52) | 0.43 ( 0.36, 0.51) | 0.18 ( 0.09, 0.27) | 0.06 (−0.03, 0.15) | 0.20 ( 0.11, 0.28) | −0.27 (−0.35, −0.18) | 0.09 ( 0.00, 0.18) | 0.30 ( 0.22, 0.38) | 0.30 ( 0.22, 0.38) |
Correlation estimates and corresponding 95% CI’s; significant correlations (p<0.05) in bold.
Natural log of variable was used to achieve normality.
Discussion
We found significant racial/ethnic- and gender differences in the relationship between uric acid and MetS. Of the racial/ethnic groups studied, non-Hispanic-white adolescents had the highest uric acid levels overall despite having both lower fasting insulin levels than Hispanics and less hypertension than non-Hispanic blacks. Interestingly, the pattern of these racial/ethnic differences in the relationship between uric acid and MetS varied between non-Hispanic-white males and females. Among males with MetS, non-Hispanic whites had the highest uric acid levels of the three ethnic groups and although not significant, a greater difference in uric acid levels between individuals with and without MetS (Figure 1C). This suggests that MetS was tightly linked to uric acid in non-Hispanic-white males, as is further supported by strong correlations between uric acid and individual MetS components among non-Hispanic-white males (Table 3). These associations are consistent with prevailing notions regarding the relationship between uric acid and insulin resistance [3].
Non-Hispanic-white females exhibited a different pattern in the relationship between uric acid and MetS. While non-Hispanic-white females had the highest uric acid levels overall among the three racial/ethnic groups, they did not exhibit an exaggerated increase in uric acid among individuals with MetS as had been seen among males. Indeed, among females with MetS, non-Hispanic whites had lower uric acid levels than the other groups. Among non-Hispanic-white females, it was the non-MetS individuals who had notably high uric acid levels. The reason for these gender differences between non-Hispanic-white males and females is unclear but may relate to differences in the relationship between uric acid and MetS between these groups.
In general, each racial/gender group exhibited strong correlations between uric acid and the individual components of MetS (Table 3), the strongest associations being with BMI and WC—as has been shown previously [8]—and the weakest with fasting glucose. It is notable, however, that with the exception of BMI and WC, the associations between uric acid and MetS components were lower among non-Hispanic-white females as compared to non-Hispanic blacks and Hispanics. Indeed, there was not a significant correlation between uric acid and fasting insulin (or HOMA) among non-Hispanic-white females. This finding is consistent with the relatively high levels of uric acid in non-Hispanic white females without MetS. Given what appear to be reciprocal relationships between uric acid and MetS [3,45] this raises the question about whether processes besides MetS itself contribute to higher uric acid levels in non-Hispanic whites.
Consequently, we investigated for several non-MetS processes that might explain higher levels of uric acid in non-Hispanic-white females, examining for potential racial/ethnic differences in added sugar intake [19,20,46–50], obesity [8] and puberty [21,22], each of which are known to affect levels of uric acid. While consumption of added sugar was associated with uric acid levels in our analysis, we found no differences in added sugar intake among female racial/ethnic groups. Regarding obesity, uric acid retained strong correlations with BMI and WC in non-Hispanic-white females (similar to the strength of correlation seen in the other ethnicities) but non-Hispanic-white females had overall less obesity compared to the other ethnicities (Table 1), which is also true of non-Hispanic white females with or without MetS [29]. Thus neither of these considerations appeared to be the cause of the higher levels of uric acid in non-Hispanic-white females without MetS.
Considerations regarding the potential effect of racial/ethnic differences in pubertal timing on uric acid levels were not as straightforward, as NHANES ‘99-’06 did not include assessment of pubertal status. This is important because estrogen is uricosuric and thus lower levels of estrogen are a potential explanation for higher levels of uric acid [21,22]. To assess for the possible effect of differences in pubertal timing, we adjusted for age in its interaction with ethnicity and gender to account for any potential impact of puberty on the ethnic difference in uric acid. Additionally, we evaluated uric acid levels across adolescence, from 12–19y (Supplementary Figure 1). Non-Hispanic-black girls frequently undergo puberty at younger ages than non-Hispanic whites and Hispanics and can have higher estradiol levels associated with these timing differences [51], though estradiol levels in adulthood have been noted to be similar between these groups [52,53] Nevertheless, most girls in each ethnic group would have completed puberty by 18–19y at which point uric acid levels remained higher in non-Hispanic whites. Thus, while we remain uncertain regarding differences in estrogen as a cause of differences in uric acid, our analysis suggests against differences in pubertal timing as the cause.
Genetics may play a role in these processes, supported by the fact that both non-Hispanic-white male and female adolescents had higher uric acid levels than other ethnicities. Surveys of uric acid levels in adults have had mixed findings with respect to racial/ethnic differences, reporting higher levels in non-Hispanic whites [54,55], no difference [56] and higher levels in non-Hispanic blacks [14], potentially owing to differences in underlying MetS-related comorbidities among these studies of adults. In many ways, adolescents represent a more logical group to test for these racial/ethnic differences, given a very low rate of these comorbidities.
Prior reports have noted racial/ethnic differences in other MetS-related factors. Non-Hispanic-black adolescents have higher levels of hsCPR and insulin than non-Hispanic whites and Hispanics and also have a greater difference in hsCRP and insulin between individuals with and without MetS [29,32]. The lower uric acid levels in non-Hispanic blacks is thus perhaps surprising, given known associations between uric acid and both hypertension [4] and insulin resistance [3]—both of which are higher in non-Hispanic blacks as compared to non-Hispanic whites [28,29]. Overall, the lower uric acid levels among non-Hispanic blacks further suggest racial/ethnic differences in the relationship between uric acid and MetS.
These data may have some bearing regarding the utility of elevated uric acid levels as a risk factor for future disease. While prospective studies have shown a strong association between uric acid levels and future hypertension [6], renal disease [13], cardiovascular disease [14–16] and diabetes [17], these relationships have not been defined on a race/ethnicity-specific basis and it is possible that the predictive nature of uric acid is more powerful among some ethnicities than others. Indeed one study revealed that the association between uric acid and CVD mortality was stronger for non-Hispanic-black men and women compared to non-Hispanic whites [14]. A clear limitation of the current study is the cross-sectional nature of NHANES ‘99-’06; future prospective studies will be necessary to further define these relationships.
In conclusion, we found higher uric acid levels in non-Hispanic-white adolescents compared to non-Hispanic blacks and Hispanics despite lower degree of insulin resistance (compared to both other ethnicities considered) and lower rates of hypertension (compared to non-Hispanic blacks). Uric acid levels were tightly linked to MetS in non-Hispanic-white males but among non-Hispanic white females uric acid exhibited lower correlations with the components of MetS, with the exception of WC. While uncertain, these data may have implications for the predictive power of uric acid by race/ethnicity, though future studies are needed.
Supplementary Material
Mean levels of uric acid by 2-year age groups for each of the three racial/ethnic groups for males (A) and females (B). * p<0.05 vs. non-Hispanic blacks.
Acknowledgments
Funding acknowledgement:
We received funding for this work from NIH grants 5K08HD060739-02 (MDD) and 1R21DK085363 (MDD and MJG)
Abbreviations
- ANOVA
analysis of variance
- ATP
Adult Treatment Panel
- BMI
body mass index
- BP
blood pressure
- CDC
Centers for Disease Control
- CVD
cardiovascular disease
- HDL-C
high density lipoprotein cholesterol
- HOMA
homeostasis model of insulin resistance
- hsCRP
high sensitivity C-reactive protein
- MetS
metabolic syndrome
- NCHS
National Center for Health Statistics
- NHANES
National Health and Nutrition Survey
- T2DM
Type 2 diabetes
- WC
waist circumference
Footnotes
Conflict of interest: The authors have no conflicts of interest to declare.
Statement of effort: Dr. DeBoer and Dr. Gurka conceived of the set of experiments. Dr. Gurka and Dr. Dong performed all of the statistical analyses. Dr. DeBoer wrote the manuscript in conjunction with Dr. Gurka.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography
- 1.Nakagawa T, Hu H, Zharikov S, et al. A causal role for uric acid in fructose-induced metabolic syndrome. Am J Physiol Renal Physiol. 2006;290:F625–31. doi: 10.1152/ajprenal.00140.2005. [DOI] [PubMed] [Google Scholar]
- 2.Baldwin W, McRae S, Marek G, et al. Hyperuricemia as a mediator of the proinflammatory endocrine imbalance in the adipose tissue in a murine model of the metabolic syndrome. Diabetes. 2011;60:1258–69. doi: 10.2337/db10-0916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johnson RJ, Perez-Pozo SE, Sautin YY, et al. Hypothesis: could excessive fructose intake and uric acid cause type 2 diabetes? Endocr Rev. 2009;30:96–116. doi: 10.1210/er.2008-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Feig DI, Kang DH, Johnson RJ. Uric acid and cardiovascular risk. N Engl J Med. 2008;359:1811–21. doi: 10.1056/NEJMra0800885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Feig DI, Soletsky B, Johnson RJ. Effect of allopurinol on blood pressure of adolescents with newly diagnosed essential hypertension: a randomized trial. JAMA. 2008;300:924–32. doi: 10.1001/jama.300.8.924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alper AB, Jr, Chen W, Yau L, et al. Childhood uric acid predicts adult blood pressure: the Bogalusa Heart Study. Hypertension. 2005;45:34–8. doi: 10.1161/01.HYP.0000150783.79172.bb. [DOI] [PubMed] [Google Scholar]
- 7.Feig DI, Johnson RJ. Hyperuricemia in childhood primary hypertension. Hypertension. 2003;42:247–52. doi: 10.1161/01.HYP.0000085858.66548.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ford ES, Li C, Cook S, Choi HK. Serum concentrations of uric acid and the metabolic syndrome among US children and adolescents. Circulation. 2007;115:2526–32. doi: 10.1161/CIRCULATIONAHA.106.657627. [DOI] [PubMed] [Google Scholar]
- 9.Liu PW, Chang TY, Chen JD. Serum uric acid and metabolic syndrome in Taiwanese adults. Metabolism. 59:802–7. doi: 10.1016/j.metabol.2009.09.027. [DOI] [PubMed] [Google Scholar]
- 10.Rho YH, Woo JH, Choi SJ, et al. Association between serum uric acid and the Adult Treatment Panel III-defined metabolic syndrome: results from a single hospital database. Metabolism. 2008;57:71–6. doi: 10.1016/j.metabol.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 11.Reaven GM. Banting lecture 1988. Role of insulin resistance in human disease. Diabetes. 1988;37:1595–607. doi: 10.2337/diab.37.12.1595. [DOI] [PubMed] [Google Scholar]
- 12.Sui X, Church TS, Meriwether RA, et al. Uric acid and the development of metabolic syndrome in women and men. Metabolism. 2008;57:845–52. doi: 10.1016/j.metabol.2008.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iseki K, Ikemiya Y, Inoue T, et al. Significance of hyperuricemia as a risk factor for developing ESRD in a screened cohort. Am J Kidney Dis. 2004;44:642–50. [PubMed] [Google Scholar]
- 14.Fang J, Alderman MH. Serum uric acid and cardiovascular mortality the NHANES I epidemiologic follow-up study, 1971–1992. National Health and Nutrition Examination Survey. JAMA. 2000;283:2404–10. doi: 10.1001/jama.283.18.2404. [DOI] [PubMed] [Google Scholar]
- 15.Hoieggen A, Alderman MH, Kjeldsen SE, et al. The impact of serum uric acid on cardiovascular outcomes in the LIFE study. Kidney Int. 2004;65:1041–9. doi: 10.1111/j.1523-1755.2004.00484.x. [DOI] [PubMed] [Google Scholar]
- 16.Tuttle KR, Short RA, Johnson RJ. Sex differences in uric acid and risk factors for coronary artery disease. Am J Cardiol. 2001;87:1411–4. doi: 10.1016/s0002-9149(01)01566-1. [DOI] [PubMed] [Google Scholar]
- 17.Kodama S, Saito K, Yachi Y, et al. Association between serum uric acid and development of type 2 diabetes. Diabetes Care. 2009;32:1737–42. doi: 10.2337/dc09-0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meyer AA, Kundt G, Steiner M, et al. Impaired flow-mediated vasodilation, carotid artery intima-media thickening, and elevated endothelial plasma markers in obese children: the impact of cardiovascular risk factors. Pediatrics. 2006;117:1560–7. doi: 10.1542/peds.2005-2140. [DOI] [PubMed] [Google Scholar]
- 19.Nguyen S, Choi HK, Lustig RH, et al. Sugar-sweetened beverages, serum uric acid, and blood pressure in adolescents. J Pediatr. 2009;154:807–13. doi: 10.1016/j.jpeds.2009.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gao X, Qi L, Qiao N, et al. Intake of added sugar and sugar-sweetened drink and serum uric acid concentration in US men and women. Hypertension. 2007;50:306–12. doi: 10.1161/HYPERTENSIONAHA.107.091041. [DOI] [PubMed] [Google Scholar]
- 21.Nicholls A, Snaith ML, Scott JT. Effect of oestrogen therapy on plasma and urinary levels of uric acid. Br Med J. 1973;1:449–51. doi: 10.1136/bmj.1.5851.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yahyaoui R, Esteva I, Haro-Mora JJ, et al. Effect of long-term administration of cross-sex hormone therapy on serum and urinary uric acid in transsexual persons. J Clin Endocrinol Metab. 2008;93:2230–3. doi: 10.1210/jc.2007-2467. [DOI] [PubMed] [Google Scholar]
- 23.DeBoer MD. Ethnicity, obesity and the metabolic syndrome: implications on assessing risk and targeting intervention. Expert Rev Endocrinol Metab. 2011;6:279–289. doi: 10.1586/eem.11.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sumner AE, Cowie CC. Ethnic differences in the ability of triglyceride levels to identify insulin resistance. Atherosclerosis. 2008;196:696–703. doi: 10.1016/j.atherosclerosis.2006.12.018. [DOI] [PubMed] [Google Scholar]
- 25.Sumner AE, Vega GL, Genovese DJ, et al. Normal triglyceride levels despite insulin resistance in African Americans: role of lipoprotein lipase. Metabolism. 2005;54:902–9. doi: 10.1016/j.metabol.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 26.Arslanian S, Suprasongsin C. Differences in the in vivo insulin secretion and sensitivity of healthy black versus white adolescents. J Pediatr. 1996;129:440–3. doi: 10.1016/s0022-3476(96)70078-1. [DOI] [PubMed] [Google Scholar]
- 27.Lieb DC, Snow RE, DeBoer MD. Socioeconomic factors in the development of childhood obesity and diabetes. Clin Sports Med. 2009;28:349–78. doi: 10.1016/j.csm.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Walker SE, Gurka MJ, Oliver MN, et al. Racial/ethnic discrepancies in the metabolic syndrome begin in childhood and persist after adjustment for environmental factors. Nutr Metab Cardiovasc Dis. 2010 doi: 10.1016/j.numecd.2010.05.006. e-pub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.DeBoer MD, Dong L, Gurka MJ. Racial/ethnic and gender differences in the ability of metabolic syndrome criteria to predict elevations in fasting insulin in adolescents: An Analyses of NHANES 1999–2008. Journal of Pediatrics. 2011 doi: 10.1016/j.jpeds.2011.05.023. e-pub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.DeBoer MD. Underdiagnosis of Metabolic Syndrome in Non-Hispanic Black Adolescents: A Call for Ethnic-Specific Criteria. Curr Cardiovasc Risk Rep. 2010;4:302–310. doi: 10.1007/s12170-010-0104-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li C, Ford ES, McGuire LC, et al. Trends in hyperinsulinemia among nondiabetic adults in the U.S. Diabetes Care. 2006;29:2396–402. doi: 10.2337/dc06-0289. [DOI] [PubMed] [Google Scholar]
- 32.DeBoer MD, Gurka MJ, Sumner AE. Diagnosis of the Metabolic Syndrome Is Associated With Disproportionately High Levels of High-Sensitivity C-Reactive Protein in Non-Hispanic Black Adolescents: An analysis of NHANES 1999–2008. Diabetes Care. 2011;34:734–40. doi: 10.2337/dc10-1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Johnson WD, Kroon JJ, Greenway FL, et al. Prevalence of risk factors for metabolic syndrome in adolescents: National Health and Nutrition Examination Survey (NHANES), 2001–2006. Arch Pediatr Adolesc Med. 2009;163:371–7. doi: 10.1001/archpediatrics.2009.3. [DOI] [PubMed] [Google Scholar]
- 34.DeBoer MD, Gurka MJ. Ability among adolescents for the metabolic syndrome to predict elevations in factors associated with type 2 diabetes and cardiovascular disease: data from the national health and nutrition examination survey 1999–2006. Metab Syndr Relat Disord. 2010;8:343–53. doi: 10.1089/met.2010.0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fernandez JR, Redden DT, Pietrobelli A, et al. Waist circumference percentiles in nationally representative samples of African-American, European-American, and Mexican-American children and adolescents. J Pediatr. 2004;145:439–44. doi: 10.1016/j.jpeds.2004.06.044. [DOI] [PubMed] [Google Scholar]
- 36.Grundy SM, Brewer HB, Jr, Cleeman JI, et al. Definition of metabolic syndrome: Report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation. 2004;109:433–8. doi: 10.1161/01.CIR.0000111245.75752.C6. [DOI] [PubMed] [Google Scholar]
- 37.The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics. 2004;114:555–76. [PubMed] [Google Scholar]
- 38.Saha N, Banerjee B. Social class and serum-uric-acid. Lancet. 1970;1:1182. doi: 10.1016/s0140-6736(70)91261-4. [DOI] [PubMed] [Google Scholar]
- 39.Malcon MC, Menezes AM, Assuncao MC, et al. Agreement between self-reported smoking and cotinine concentration in adolescents: a validation study in Brazil. J Adolesc Health. 2008;43:226–30. doi: 10.1016/j.jadohealth.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 40.SRNT Subcommittee on Biochemical Verification. Biochemical verification of tobacco use and cessation. Nicotine Tob Res. 2002;4:149–59. doi: 10.1080/14622200210123581. [DOI] [PubMed] [Google Scholar]
- 41.Guenther PM, Reedy J, Krebs-Smith SM, et al. Development and Evaluation of the Healthy Eating Index-2005: Technical Report. [accessed May 9, 2011]; http://www.cnpp.usda.gov/HealthyEatingIndex.htm.
- 42.CDC. [accessed May 9, 2011.];Dietary Interview Component, NHANES. http://www.cdc.gov/nchs/data/nhanes/nhanes_07_08/dietaryrecall_e.pdf.
- 43.USDA. Features of AMPM. http://www.ars.usda.gov/Services/docs.htm?docid=7711.
- 44.Schwartz B, Jacobs DR, Jr, Moran A, et al. Measurement of insulin sensitivity in children: comparison between the euglycemic-hyperinsulinemic clamp and surrogate measures. Diabetes Care. 2008;31:783–8. doi: 10.2337/dc07-1376. [DOI] [PubMed] [Google Scholar]
- 45.Borges RL, Ribeiro AB, Zanella MT, et al. Uric acid as a factor in the metabolic syndrome. Curr Hypertens Rep. 2010;12:113–9. doi: 10.1007/s11906-010-0098-2. [DOI] [PubMed] [Google Scholar]
- 46.Perheentupa J, Raivio K. Fructose-induced hyperuricaemia. Lancet. 1967;2:528–31. doi: 10.1016/s0140-6736(67)90494-1. [DOI] [PubMed] [Google Scholar]
- 47.Raben A, Vasilaras TH, Moller AC, et al. Sucrose compared with artificial sweeteners: different effects on ad libitum food intake and body weight after 10 wk of supplementation in overweight subjects. Am J Clin Nutr. 2002;76:721–9. doi: 10.1093/ajcn/76.4.721. [DOI] [PubMed] [Google Scholar]
- 48.Sanchez-Lozada LG, Tapia E, Jimenez A, et al. Fructose-induced metabolic syndrome is associated with glomerular hypertension and renal microvascular damage in rats. Am J Physiol Renal Physiol. 2007;292:F423–9. doi: 10.1152/ajprenal.00124.2006. [DOI] [PubMed] [Google Scholar]
- 49.Stavric B, Johnson WJ, Clayman S, et al. Effect of fructose administration on serum urate levels in the uricase inhibited rat. Experientia. 1976;32:373–4. doi: 10.1007/BF01940847. [DOI] [PubMed] [Google Scholar]
- 50.Stirpe F, Della Corte E, Bonetti E, et al. Fructose-induced hyperuricaemia. Lancet. 1970;2:1310–1. doi: 10.1016/s0140-6736(70)92269-5. [DOI] [PubMed] [Google Scholar]
- 51.Casazza K, Goran MI, Gower BA. Associations among insulin, estrogen, and fat mass gain over the pubertal transition in African-American and European-American girls. J Clin Endocrinol Metab. 2008;93:2610–5. doi: 10.1210/jc.2007-2776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Randolph JF, Jr, Sowers M, Bondarenko IV, Harlow SD, et al. Change in estradiol and follicle-stimulating hormone across the early menopausal transition: effects of ethnicity and age. J Clin Endocrinol Metab. 2004;89:1555–61. doi: 10.1210/jc.2003-031183. [DOI] [PubMed] [Google Scholar]
- 53.Richards RJ, Svec F, Bao W, et al. Steroid hormones during puberty: racial (black-white) differences in androstenedione and estradiol--the Bogalusa Heart Study. J Clin Endocrinol Metab. 1992;75:624–31. doi: 10.1210/jcem.75.2.1639961. [DOI] [PubMed] [Google Scholar]
- 54.Palmer IM, Schutte AE, Huisman HW. Uric acid and the cardiovascular profile of African and Caucasian men. J Hum Hypertens. 2010;24:639–45. doi: 10.1038/jhh.2010.1. [DOI] [PubMed] [Google Scholar]
- 55.Palmer IM, Schutte AE, Huisman HW, et al. A comparison of uric acid levels in Black African vs Caucasian women from South Africa: the POWIRS study. Ethn Dis. 2007;17:676–81. [PubMed] [Google Scholar]
- 56.Rathmann W, Funkhouser E, Dyer AR, et al. Relations of hyperuricemia with the various components of the insulin resistance syndrome in young black and white adults: the CARDIA study. Coronary Artery Risk Development in Young Adults. Ann Epidemiol. 1998;8:250–61. doi: 10.1016/s1047-2797(97)00204-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Mean levels of uric acid by 2-year age groups for each of the three racial/ethnic groups for males (A) and females (B). * p<0.05 vs. non-Hispanic blacks.