Abstract
Objective
Metabolic disorders increase MCP-1-induced monocyte chemotaxis in mice. The goal of this study was to determine the molecular mechanisms responsible for the enhanced responsiveness of monocytes to chemoattractants induced by metabolic stress.
Methods and Results
Chronic exposure of monocytes to diabetic conditions induced by human low-density lipoproteins plus high D-glucose concentrations (LDL+HG) promoted Nox4 expression, increased intracellular H2O2 formation, stimulated protein S-glutathionylation, and increased chemotaxis in response to MCP-1, PDGF-B and RANTES. Both, H2O2 added exogenously and overexpression of Nox4 mimicked LDL+HG-induced monocyte priming, whereas Nox4 knockdown protected monocytes against metabolic stress-induced priming and accelerated chemotaxis. Exposure of monocytes to LDL+HG promoted the S-glutathionylation of actin, decreased the F-actin/G-actin ratio and increased actin remodeling in response to MCP-1. Preventing LDL+HG-induced protein S-glutathionylation by overexpressing glutaredoxin 1 (Grx1) prevented monocyte priming and normalized monocyte chemotaxis in response to MCP-1. Induction of hypercholesterolemia and hyperglycemia in C57BL/6 mice promoted Nox4 expression and protein-S-glutathionylation in macrophages, and increased macrophage recruitment into MCP-1-loaded Matrigel plugs implanted subcutaneous in these mice.
Conclusions
By increasing actin-S-glutathionylation and remodeling, metabolic stress primes monocytes for chemoattractant-induced transmigration and recruitment to sites of vascular injury. This Nox4-dependent process provides a novel mechanism through which metabolic disorders promote atherogenesis.
Keywords: Chemotaxis, Macrophages, Metabolic disorders, Nox4, Glutaredoxin
INTRODUCTION
Atherosclerosis is a chronic inflammatory disease induced by metabolic disorders and associated with the recruitment of mononuclear cells into the arterial wall. Monocytes are intimately involved in the initiation and progression of atherosclerotic lesions and both blood monocyte counts and the relative distribution of subsets within the monocyte population appear to be critical determinants of disease progression. Blood monocyte counts are a well-established independent risk factor in human vascular disease 1–3 and a number of animal studies have clearly demonstrated that lowering blood monocyte counts reduces the severity of atherosclerosis 1, 4. More recent evidence suggests that monocyte subsets, which are primarily distinguished by their expression of cell surface antigens, including chemokine receptors, may also be functionally distinct. In addition, different monocyte subsets may be recruited at different stages of plaque development and possibly contribute to atherogenesis through distinct mechanisms 5, 6.
The recruitment of monocytes into the “injured” vessel wall is regulated by cell adhesion molecules and chemoattractants, and their receptors. Adhesion molecules are upregulated in both animal models of atherosclerosis and in humans 7–9, and deficiency or pharmacological targeting of adhesion molecules including integrins, selectins and P-selectin glycoprotein ligand reduces disease severity 10–14. Numerous chemokines and chemokine receptors contribute to the recruitment of monocytes in atherosclerosis, including MCP-1/CCL2 and its receptor CCR2, RANTES/CCL5 and its receptor CCR5, fraktalkine/CX3CL1 and its receptor CX3CR1, and PDGF-B and its receptor PDGFR-β15–21. The fact that the combined genetic targeting of three chemokine/chemokine receptor pairs practically abolishes atherosclerosis in dyslipidemic mice, underscores the critical importance and rate-limiting nature of monocyte recruitment to the development of atherosclerotic lesions 22.
Dyslipidemia stimulates monocytosis and promotes a shift in the monocyte subset distribution 23, 24. While increased monocyte counts and changes in subset distribution are likely to affect monocyte recruitment – the two main monocyte subsets in mice differ in their expression pattern of chemokine receptors 6 – this does not appear to be the sole mechanism underlying increased monocyte recruitment associated with metabolic disorders. Studies by Quehenberger and colleagues demonstrated that monocytes from hypercholesterolemic patients show increased expression of CCR2 and that exposure of cultured THP-1 monocytes to human LDL induces CCR2 expression and increases their chemotactic responsiveness to MCP-1 25, 26. We recently reported that exposing LDL-R−/− mice to moderate metabolic stress increases 2.6-fold macrophage chemotactic activity in vivo 27. Macrophage recruitment increased 9.8–fold in severely metabolically stressed diabetic LDL-R−/− mice, yet blood monocyte counts increased by less than 20%. We went on to show that the glutathione reduction potential of peritoneal macrophages isolated from these mice not only was a strong predictor of atherosclerotic lesion size and macrophage content in these lesions, the macrophage thiol redox state also strongly correlated with the rate of macrophage chemotaxis in these mice. Taken together, these findings suggest that not only hypercholesterolemia, but metabolic stress in general may accelerate macrophage recruitment and atherogenesis by increasing the responsiveness of monocytes to chemoattractants. This process appears to be sensitive to thiol redox regulation, but the molecular details of the underlying mechanisms were not known. In the current study, we demonstrate for the first time that metabolic stress primes monocytes for activation by chemotactic stimuli. The transformation of monocytes into this hyper-responsive phenotype requires the induction of Nox4 and increased H2O2 production. Furthermore, we provide evidence that a major target of Nox4-derived H2O2 in monocytes is actin, and that S-glutathionylation of actin appears to be responsible for the enhanced actin remodeling and increased chemotactic activity we observed in monocyte primed by metabolic stress.
METHODS
A detailed description of all methods is available in the Supplemental Materials section.
LDL was freshly isolated by ultracentrifugation from pooled plasma from healthy blood donors and purified by gel-filtration chromatography, filter-sterilized and characterized as described previously 28, 29. To mimic metabolic disorders in vitro, THP-1 monocytes were cultured at 37°C for 20 h in RPMI 1640 medium containing 10% FBS, 5 mM D-glucose and supplemented with either vehicle, freshly isolated native human LDL (100 μg/ml), D-glucose (HG, 20 mM), or LDL plus HG. Intracellular oxidative stress and thiol oxidation in the absence of LDL or HG was induced by incubating THP-1 monocytes for 2 – 5 h with H2O2 (0.1 – 1 mM) in RPMI 1640 medium with 2% FBS. Monocyte chemotaxis was measured in 48-well modified Boyden chambers (NeuroProbe). C57BL/6J mice were obtained from The Jackson Laboratories (Bar Harbor, ME). After one week on a maintenance diet (MD, AIN-93G, F3156, BioServ), mice were randomized into two groups and fed either a MD or a high-fat diet (HFD; 60 kcal% saturated fat; F3282, BioServ) for 10 weeks. Body weights and fasted blood glucose levels were monitored every other week and at the end of the study. In vivo macrophage chemotaxis was measured using the Matrigel Plug assay described previously 27. Resident peritoneal cells were harvested by lavage and plated 30. Protein-bound glutathione was quantified by HPLC as described elsewhere 31.
RESULTS
Enhanced monocyte chemotaxis induced by metabolic stress correlates with increased cellular H2O2 formation but not with CCR2 surface-expression
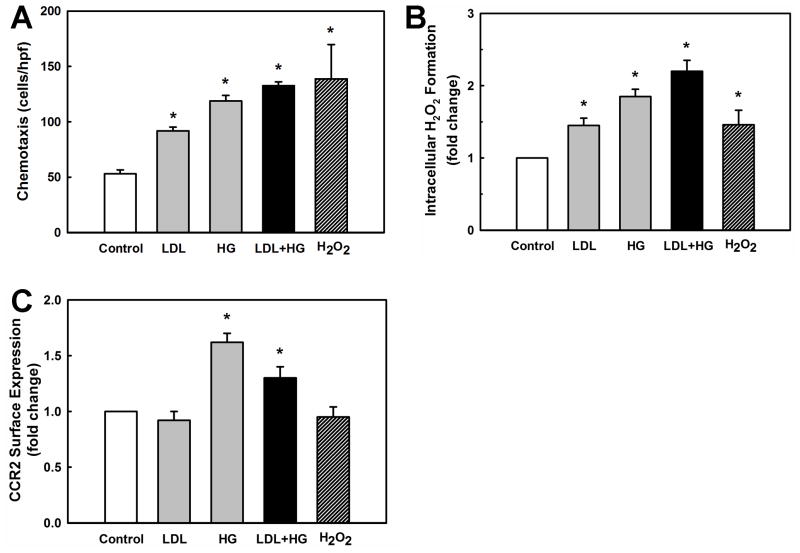
Metabolic stress in atherosclerosis-prone LDL-R−/− mice amplifies monocyte chemotactic responses and increases macrophage recruitment to sites of vascular injury 27. To examine whether we could recapitulate the effects of metabolic stress on monocytes in vitro, we exposed THP-1 monocytes for 20 h with freshly isolated human low-density lipoprotein (LDL; 100μg/ml), high D-glucose (HG) concentrations (25 mM, final concentration) or both (LDL+HG). Chronic exposure to a hypercholesterolemic (LDL) or hyperglycemic (HG) environment sensitized THP-1 monocytes to the chemoattractant MCP-1, resulting in a 1.7-fold and 2.2-fold respective increase in monocyte migration (Fig. 1A). Combining LDL and HG further sensitized monocytes to MCP-1, increasing monocyte migration 2.6-fold. Exposure to LDL and/or HG did not increases monocyte migration in the absence of MCP-1 stimulation, indicating that metabolic stress “primes” monocytes and increases their response to subsequent activation by chemoattractants. We did not observed monocyte priming to MCP-1-induced chemotaxis in cells treated with L-glucose instead of D-glucose (Supplemental Figure I), demonstrating that the priming effect is not caused by changes in osmotic pressure.
Figure 1. Effects of metabolic stress on chemotaxis, intracellular H2O2 production and CCR2 surface expression in THP-1 monocytes.
THP-1 monocytes in RPMI 1640 medium with 10% FBS were stimulated for 20 h with 100 μg/ml native LDL, high D-glucose (HG) concentrations (25 mM), LDL+HG, or vehicle (Control; 5 mM D-glucose, no LDL) or for 5 h with H2O2 (1 mM). Cells were washed and (A) monocyte chemotaxis in response to MCP-1 (2 nM), (B) intracellular H2O2 production, and (C) CCR2 surface expression were determined as described under Methods. Results shown are means ± SD of three independent experiments. *:P < 0.05 versus Control; (open bar, n=3).
Increased chemotaxis induced by chronic metabolic stress was paralleled by and correlated with an increase in intracellular H2O2 formation (Fig. 1B). Importantly, short-term exposure (5 h) of THP-1 monocytes to exogenous H2O2 at concentrations (1 mM) that generated similar levels of intracellular H2O2 as LDL or HG (Fig. 1B), also resulted in enhanced chemotaxis in response to MCP-1 (Fig. 1A). This result suggests that the metabolic stress-induced sensitization of THP-1 monocytes to MCP-1 might be mediated by an increase in intracellular H2O2 formation.
One possible mechanism that could account for the enhanced chemotactic response of metabolically stressed THP-1 monocytes is an increase in cell-surface expression of the receptor for MCP-1, CCR2. Increased CCR2 mRNA expression and MCP-1 binding was reported in both monocytes treated with human LDL ex vivo and in monocytes isolated from hypercholesterolemic patients 25, 26. CCR2 surface expression was not directly analyzed in these studies. FACS analysis of metabolically-stressed THP-1 monocytes revealed that while HG induced a 1.6-fold increase in CCR2 expression, neither LDL nor exogenously added H2O2 affected CCR2 surface expression (Fig. 1C). This finding suggests that CCR2 upregulation is not the likely common mechanism underlying the enhanced chemotactic response of metabolically stressed THP-1 monocytes.
Metabolic stress enhances monocyte chemotaxis in response to MCP-1, PDGF-B and RANTES
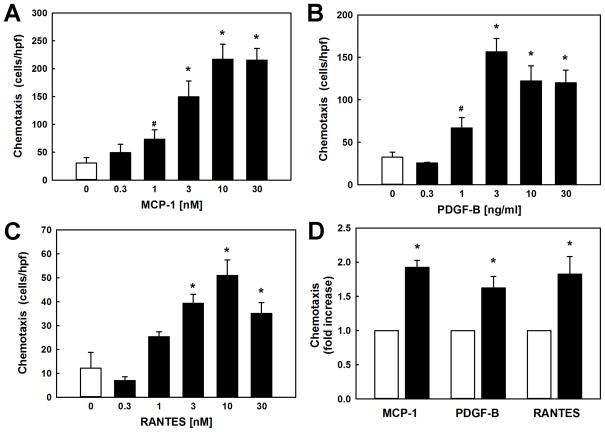
To determine if enhanced chemotaxis induced by metabolic stress was limited to MCP-1, we also measured monocyte chemotaxis in response to PDGF-B and RANTES. PDGF-B, a chemokine involved in wound healing, plays a critical role in atherosclerosis and kidney injury and may also be responsible for recruiting macrophages into sites of vascular and renal injury 21, 32, 33. RANTES (CCL5), a potent macrophage chemoattractant like MCP-1, also plays an important role in the recruitment of macrophages to sites of vascular injury and the development of atherosclerosis 34. Like MCP-1 (Fig. 2A), both PDGF-B and RANTES stimulate THP-1 monocyte chemotaxis (Fig. 2B and 2C). We found that metabolic stress (LDL+HG, 24 h) sensitized monocytes to all three chemoattractants, increasing chemotactic responses to PDGF-B 1.6-fold and to RANTES 1.8-fold. These results confirm that the priming effect of metabolic stress on monocyte chemotaxis is not limited to MCP-1 and appears to represent a more general phenomenon affecting other chemoattractants. Our data also suggest that metabolic stress-induced priming appears to target processes downstream of each of these three distinct signaling pathways, specifically processes that control cytoskeleton turnover and cell motility.
Figure 2. Metabolic stress increases chemotaxis by THP-1 monocytes in response to monocyte chemoattractants MCP-1, PDGF-B and RANTES.
THP-1 monocytes (2 × 106/ml) were loaded into multi-well chemotaxis chambers and allowed to migrate for 3 h against gradients of increasing concentrations of (A) rMCP-1, (B) rPDGF-B or (C) RANTES. (D) Metabolic stress and hyper-reactivity of THP-1 monocytes was induced by pretreating monocytes for 20 h with RPMI 1640 medium with 10% FBS supplemented with 100 μg/ml native LDL plus 20 mM D-glucose (LDL+HG) prior to inducing chemotaxis with either rMCP-1 (2 nM), rPDGF-B (2 ng/ml; 0.08 nM) or RANTES (5 nM). Results shown are mean ± SE of 4–6 independent experiments. *: P < 0.05 versus vehicle (“0”; open bars).
Priming of monocytes by metabolic stress to MCP-1-induced chemotaxis is mediated by Nox4
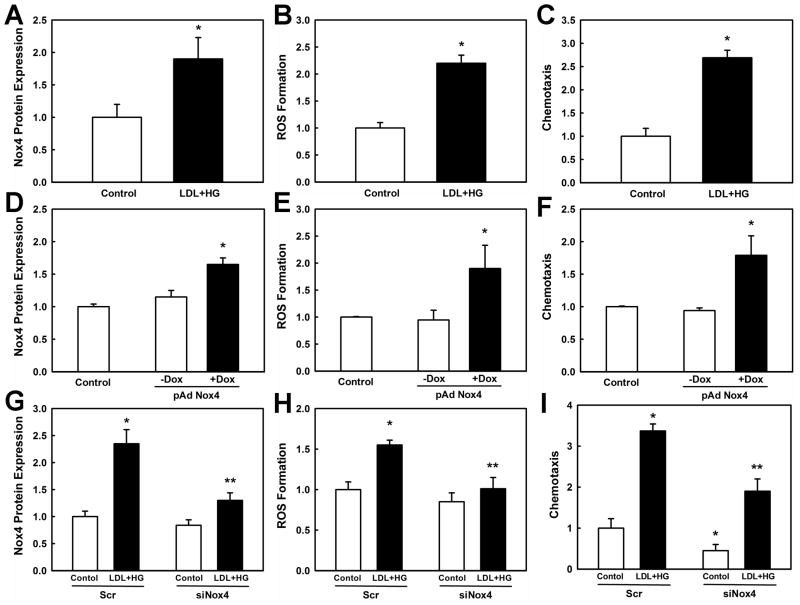
Next, we examined whether the increase in H2O2 formation induced by LDL+HG treatment was causally related to increased chemotactic activity and responsible for mediating the sensitizing effects of metabolic stress. We recently identified a novel inducible NADPH oxidase, Nox4, in human monocytes and macrophages 35. Because Nox4 is both rapidly inducible by oxidatively modified LDL 35 and generates primarily H2O2 36, 37, we explored whether Nox4 is also induced by metabolic stress and thus might be the source of the intracellular H2O2 we detected in metabolically stressed monocytes. THP-1 monocytes exposed for 20 h to LDL+HG showed a 1.9-fold increase in Nox4 expression (Fig. 3A and Supplemental Fig. IIA), which coincided with a 2.2-fold increase in H2O2 production (Fig. 3B) and a 2.5–fold increase in monocyte chemotaxis (Fig. 3C). To examine whether increased expression of Nox4 alone could account for monocyte priming and the increased chemotactic activity of metabolically-stressed monocytes, we overexpressed human Nox4 in THP-1 monocytes using a doxycyline (Dox)-inducible adenoviral vector. Compared to uninduced virus-infected monocytes (−Dox), cells treated with Dox (1 μg/ml, +Dox) showed a 1.6-fold increase in Nox4 levels (Fig. 3D and Supplemental Fig. IIB), a 2-fold increase in H2O2 production (Fig. 3E), and a 1.8-fold increase in chemotactic activity in response to MCP-1 (Fig. 3F). Thus, overexpression of Nox4 recapitulated the priming effects of metabolic stress in monocytes. Viral infection alone did not significantly alter monocyte Nox4 levels and H2O2 production, and did not affect MCP-1-induced chemotaxis.
Figure 3. Nox4 mediates increased H2O2 formation and hyper-responsiveness of THP-1 monocytes to MCP-1-stimulated chemotaxis induced by metabolic stress.
(A – C) THP-1 monocytes were pretreated for 20 h with either medium alone or medium supplemented with 100 μg/ml native LDL plus 20 mM D-glucose (LDL+HG) to induce metabolic stress. (D – F) THP-1 monocytes were infected with an inducible adenoviral vector carrying human Nox4 and Nox4 expression was induced by adding doxycycline (DOX, 1 μg/ml). (G – I) THP-1 monocytes were transfected with either scrambled siRNA (Scr) or siRNA directed against Nox4 (siNox4) and subsequently pretreated for 20 h with medium (Control) or medium supplemented with 100 μg/ml native LDL plus 20 mM D-glucose (LDL+HG). Nox4 protein expression, DCF-sensitive ROS formation and chemotaxis were determined as described under Methods. Results shown are mean ± SE of at least 3 independent experiments. *: P < 0.05 versus Control (open bars); **: P < 0.05 versus Scr/LDL+HG.
To further establish a causal link between Nox4-derived H2O2 formation and enhanced chemotactic activity in metabolically stress monocytes, we targeted endogenous Nox4 with siRNA specific for Nox4. This particular siRNA did not affect expression levels of Nox2, the superoxide-generating subunit of the phagocytic NADPH oxidase complex (not shown). Nox4 induction in response to metabolic stress was inhibited by 64% in THP-1 cells that received Nox4-targeting siRNA (Fig. 3G and Supplemental Fig. IIC). The Nox4-targeting siRNA reduced Nox4 mRNA levels in THP-1 monocytes by 72%, without affecting Nox2 levels (Supplemental Fig. IID). However, analogous to our previous findings in human monocyte-derived macrophages, endogenous Nox4 protein levels in THP-1 monocytes were relatively resistant to siRNA knockdown (−20%, P=0.09), suggesting that monocytic Nox4 has a long half-live and may be resistant to proteolytic degradation 35. These findings also imply that the siRNA-mediated reduction in Nox4 protein levels we observed in metabolically stressed THP-1 monocytes was primarily due to the inhibition of de novo synthesized Nox4. Blunting Nox4 induction by LDL+HG also inhibited metabolic stress-induced H2O2 formation by 71% (Fig. 3H) and blocked the exaggerated chemotactic response of metabolically stressed monocytes to MCP-1 by 60% (Fig. 3I). Of note, the 20% reduction in Nox4 protein also reduced MCP-1-induced chemotaxis in healthy monocytes, supporting a physiological role for Nox4 in the regulation of monocyte migration. Together these results strongly suggest that monocyte priming by metabolic stress for increased chemotactic responses is mediated by Nox4-derived H2O2.
H2O2 mimics the priming effects of metabolic stress on monocyte chemotaxis
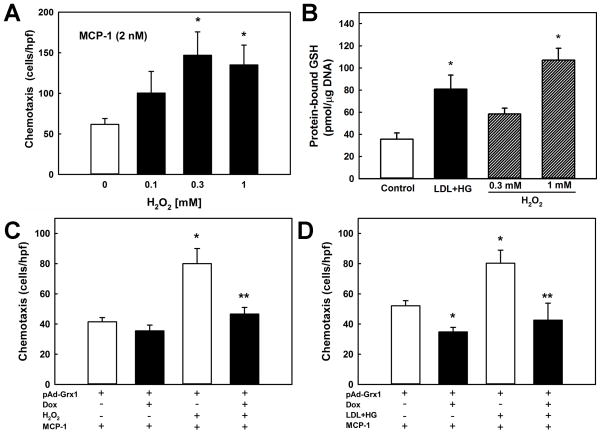
To further examine whether H2O2 mediates metabolic stress-induced priming of monocytes to chemoattractants, we exposed THP-1 cells for short periods of time (5 h) to increasing doses of H2O2. This brief treatment allows lipophilic H2O2 to diffuse into the cells and increase intracellular H2O2 levels to those measured in cells exposed to “chronic” metabolic stress, i.e. LDL+HG for 20 h (Fig. 1B). Pretreatment of THP-1 cells with increasing concentrations of H2O2 accelerated monocyte chemotaxis induced by either MCP-1 (2 nM, Fig. 4A) or PDGF-B (2 ng/ml or 0.08 nM, not shown) in a dose-dependent manner, maximal chemotaxis being observed at 0.3 mM H2O2. These findings provide further support for H2O2 as the likely second messenger responsible for mediating the priming effects of metabolic stress on monocyte chemotaxis.
Figure 4. Protein-S-glutathionylation mediates the metabolic stress-induced hyper-responsiveness of monocytes towards MCP-1.
(A): THP-1 monocytes (2 × 106/ml) were pretreated for 3 h with H2O2 at the indicated concentration before being loaded into multi-well chemotaxis chambers. Chemotaxis was induced for 3 h with rMCP-1 (2 nM). (B) THP-1 monocytes (2 × 106/ml) were either cultured for 20 h in culture medium (RPMI 1640 medium with 10% FBS; Control) or culture medium supplemented with 100 μg/ml native LDL plus 20 mM D-glucose concentrations (LDL+HG), or pretreated for 3 h with H2O2 (0.3 or 1 mM). Cellular levels of protein-S-glutathionylation were determined as described under Methods. (C and D) Overexpression of Grx1 in THP-1 monocytes was achieved using a doxycycline (Dox)-inducible adenoviral vector carrying the sequence for a Grx1-EGFP fusion protein (pAd; see Methods). Grx1 expression was induced with 1 μg/ml Dox (24 h; see Supplemental Fig. II). THP-1 monocytes (2 × 106/ml) were either pretreated for 3 h with H2O2 (0.3 mM). (C) or metabolically stressed for 24 h in culture medium supplemented with 100 μg/ml native LDL plus 20 mM D-glucose concentrations (LDL+HG, D). Monocyte chemotaxis in response to MCP-1 (2 nM) was measured in uninduced (open bars) and Dox-induced (solid bars) monocytes. Results shown are mean ± SE of 3–6 independent experiments. *: P < 0.05 versus control (unstressed, uninduced); **: P < 0.05 versus uninduced, H2O2 or LDL+HG-treated monocytes.
Overexpression of glutaredoxin 1 protects monocytes against protein-S-glutathionylation and the sensitization to chemoattractants induced by H2O2 and metabolic stress
Within cells, primary targets of H2O2 are reactive thiols 38, 39. Increased intracellular H2O2 formation is known to promote the formation of protein-glutathione mixed disulfides (PSSG) 40, 41, an indicator of intracellular thiol oxidative stress and a posttranslational modification involved in redox signaling 41, 42. THP-1 monocytes exposed to exogenously added H2O2 (1 mM) showed a 3.1-fold increase in PSSG formation (Fig 4B). A 1.6-fold increase in PSSG levels was induced by 0.3 mM H2O2, but this increase did not quite reach statistical significance (P=0.11). Importantly, metabolically stressed THP-1 monocytes (LDL+HG; 20 h) showed a 2.3-fold increase in PSSG levels (Fig 4B), suggesting that monocyte priming induced by metabolic stress may involve S-glutathionylation of proteins that control and regulate monocyte as priming with LDL+HG migration. In further support of our hypothesis that Nox4 induction is sufficient to promote monocyte priming, we found that the controlled, 1.5-fold to 2-fold overexpression of Nox4 in THP-1 monocytes (see Fig. 3D) also increased total cellular PSSG levels by 1.4-fold over infected but uninduced cells (Supplemental Fig. IIIA).
Under physiological conditions, deglutathionylation and restoration of the free protein thiols within cells is catalyzed by glutaredoxins 43. To determine whether protein-S-glutathionylation mediates the priming effects of H2O2 and metabolic stress on monocyte chemotaxis, THP-1 monocytes were infected with inducible adenoviruses carrying a human cytosolic glutaredoxin 1 (Grx1)-EGFP fusion construct. No EGFP expression was observed in adenovirus-infected THP-1 cells in the absence of Dox. However, EGFP fluorescence increased in the cytosol of all cells with increasing doses of Dox (0.1 – 1 μg/ml) added to the cell supernatant, indicating that the Grx1-EGFP transgene was expressed. Induction of Grx1 transgene expression with 1 μg/ml Dox increased transgenic Grx1 expression (Supplemental Fig. IIIB) and completely blocked H2O2-induced protein-S-glutathionylation (Supplemental Fig. IIIC). Interestingly, Grx1 overexpression reduced basal PSSG levels, but by only 10%, suggesting that the majority of these S-glutathionylated proteins may not be accessible to the cytosolic Grx1-EGFP fusion protein. Nevertheless, Grx1 overexpression also reduced MCP-1-induced chemotaxis in unstressed THP-1 monocytes by 15% – 35% (Fig. 4C and 4D, 1st solid bar), providing further evidence that MCP-1 signaling pathways are redox-sensitive. Importantly, overexpression of Grx1 completely blocked monocyte priming and accelerated chemotaxis induced by either short-term H2O2-treatment (Fig. 4C, 2nd solid bar) or 20 h of metabolic stress, i.e. LDL+HG (Fig. 4D, 2nd solid bar). Similar results were obtained for PDGF-B-stimulated chemotaxis (not shown). Taken together, these results show that the priming effects of metabolic stress (and H2O2) on monocyte chemotaxis are mediated by protein-S-glutathionylation.
Metabolic stress promotes actin-S-glutathionylation and enhances MCP-1-induced F-actin disassembly in monocytes
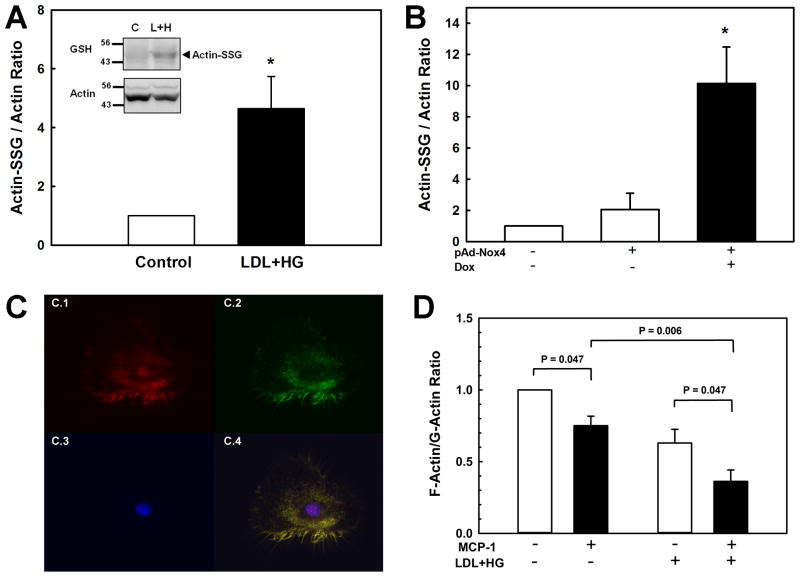
Monocyte migration requires increased turnover, i.e. the continuous assembly and disassembly of the actin cytoskeleton, a process regulated by reversible protein S-glutathionylation 44,45. Under resting conditions, a fraction of actin is S-glutathionylated, dramatically reducing the ability of G-actin to polymerize into F-actin 44. In response to physiological stimuli such as EGF, actin is de-glutathionylated, resulting in an increased rate of polymerization and F-actin formation. De-glutathionylation of actin is catalyzed by Grx1, but the mechanism involved in the formation of actin-glutathione mixed disulfides is not known 43. We hypothesized that the increased production of H2O2 we observed in monocytes primed by metabolic stress might increase actin-S-glutathionylation, thereby increasing actin turnover and decreasing the F-actin/G-actin ratio. Increased actin turnover would allow monocytes to respond more effectively to chemoattractant signals. We therefore investigated whether monocyte priming by metabolic stress promotes actin-S-glutathionylation. Indeed, pretreatment of THP-1 monocytes with LDL+HG increased the ratio of S-glutathionylated actin to actin 4.6-fold (Fig. 5A). Actin S-glutathionylation induced by LDL+HG was blocked in monocytes that overexpress Grx1 (Supplemental Fig. IVA). Because monocyte priming appears to require induction of Nox4, we also examined whether overexpression of Nox4 is sufficient to promote actin-S-glutathionylation. As shown in figure 5B, THP-1 monocytes that overexpress Nox4 showed a 10-fold increase in actin-S-glutathionylation (Fig. 5B), suggesting that Nox4 may be required for the S-glutathionylation of actin. Indeed, Nox4 knockdown blocked LDL+HG-induced actin-S-glutathionylation (Supplemental Fig. IVB), confirming the essential role of Nox4 in the S-glutathionylation of actin induced by metabolic stress. As indicated above, for a H2O2-based mechanism for S-glutathionylation to be both protein-specific and minimize non-specific thiol oxidation, we would predict that Nox4 would have to associate with or at least be in close proximity to actin. To test this hypothesis, we stained human monocyte-derived macrophages with the actin marker phalloidin and a highly specific monoclonal antibody directed against Nox4 35. Analysis of confocal images taken of these cells revealed a high degree of colocalization between actin and Nox4 (Fig. 5C, Pearson coefficient > 0.84), suggesting that Nox4 may associates with actin.
Figure 5. Metabolic stress promotes actin-S-glutathionylation and increases actin remodeling and MCP-1-induced actin turnover.
(A) THP-1 monocytes (2 × 106/ml) were cultured for 20 h in culture medium (RPMI 1640 medium with 10% FBS; (Control) or culture medium supplemented with 100 μg/ml native LDL plus 20 mM D-glucose concentrations (LDL+HG), and stimulated with rMCP-1 (2 nM) for the times indicated. Actin levels and acti-S-glutathionylation were assessed by Western blot analysis using an anti-actin and anti glutathione antibodies (see insert). Results are shown as the ratio of S-glutathionylated actin/total actin and are means ± SE of four independent experiments. *: P < 0.05 versus control. (B): THP-1 monocytes were infected with an inducible adenoviral vector carrying human Nox4 and Nox4 expression was induced by adding doxycycline (DOX, 1 μg/ml) as described in figure 3. Levels of actin and S-glutathionylated actin were determined as described in (A). Results shown are mean ± SE of four independent experiments.. *: P < 0.05 versus infected but uninduced control (grey bar). (C) Confocal micrographs were taken of human monocyte-derived macrophages stained with anti-Nox4 antibodies (red, C.1); the F-actin stain phalloidin (green, C.2) and the nuclear stain DAPI (blues, C.3). Colocalization of Nox4 with actin is shown in the overlay in yellow (C.4). (D): THP-1 monocytes were cultured for 20 h in culture medium or culture medium supplemented with LDL+HG, and stimulated with rMCP-1 (2 nM, solid bars) for 30 min. F- and G-actin levels were measured as described under Methods. Results are shown as F-actin/G-actin ratios and are means ± SE of 5 independent experiments.
To examine whether metabolic stress-induced actin-S-glutathionylation promotes the dissolution of actin filaments in monocytes, we measured the ratio of filamentous (F) to monomeric (G) actin in healthy and metabolically primed monocytes. As expected, stimulating monocyte chemotaxis with MCP-1 resulted in a 25% decrease in the F-actin/G-actin ratio (Fig. 5C), indicating increased actin turnover associated with cell migration. Even prior to MCP-1 stimulation, the F-actin/G-actin ratio of LDL+HG-treated monocytes was already 37% lower than in healthy cells, yet these primed cells showed an even more pronounced decrease (-43%) in response to MCP-1 activation. Our data therefore suggest that metabolic stress enhances both basal and MCP-1-stimulated actin turnover in THP-1 monocytes. These findings are in good agreement with the concept that increased actin-S-glutathionylation induced by metabolic stress facilitates the dissolution of actin fibers, increasing the pools of monomeric actin required as substrate for rapid reassembly at the leading edge and focal adhesions of primed monocytes to support their accelerated migratory response.
Metabolic syndrome in mice induces Nox4 expression and protein-S-glutathionylation in macrophages and primes monocytes to MCP-1-induced chemotaxis
Previously we reported that monocytes in dyslipidemic or diabetic atherosclerosis-prone LDL-R−/− mice convert into a hyper-chemotactic phenotype 27. We also demonstrated that this hyper-responsiveness to MCP-1-induced chemotaxis tightly correlated with the macrophage thiol redox state in these mice. To examine if this novel, potentially proatherogenic effect of metabolic stress on monocytes was limited to atherosclerosis-prone mice or a more general phenomenon associated with metabolic disorders, we measured monocyte chemotaxis in a mouse model of diet-induced obesity and metabolic syndrome 46. After 10 weeks on HFD (60 kcal% fat), these mice were obese and had developed hyperlipidemia and hyperglycemia (Table 1). Three days prior to sacrifice, all mice received Matrigel plugs loaded with either vehicle or MCP-1 (300 ng/ml) in their right and left flank, respectively. In pilot studies we had determined that after 3 days, more than 93% of cells recruited into the MCP-1-loaded plugs were macrophages. To quantify the number of macrophages recruited into the Matrigel plugs, the plugs were surgically removed after 3 days, dissolved in dispase, and cells were counted. Mice fed a HFD recruited 2.5-fold more macrophages into MCP-1-loaded Matrigel plugs than healthy control mice fed a MD (Fig. 6A, solid bars), confirming that metabolic stress is sufficient to sensitize blood monocytes to MCP-1-induced chemotaxis, even in the absence of established atherosclerosis. Macrophage recruitment into vehicle-loaded Matrigel plugs was low in both groups, but interestingly, even in control plugs we observed a 3.9–fold higher macrophage count in HFD-fed mice than control mice (Fig. 6A, open bars). These data confirm that monocytes from metabolically stressed mice are primed and hyper-responsive to MCP-1 and show increased chemotactic responses.
Table 1. Blood and Plasma Parameters for Mildly (MD) and Modestly (HFD) Metabolically-Stressed C57BL/J6 Mice.
Results are expressed as mean ± SE for 10 mice.
| Parameter | MD (n=10) | HFD (n=10) |
|---|---|---|
| Weight (g) | 26.9 ± 2.4 | 42.8 ± 3.7* |
| Plasma total cholesterol (mg/dl) | 148.5 ± 8.5 | 186.1 ± 11.9* |
| Plasma triglycerides (mg/dl) | 53.3 ± 7.8 | 65.4 ± 10.0 |
| Blood Glucose (mg/dl) | 105.3 ± 48.6 | 209.0 ± 82.7** |
: P < 0.01 versus MD;
: P < 0.05 versus MD. Body weights were determined after overnight fasting. Lipid measurements were performed in plasma samples from overnight-fasted mice. Glucose was measured in whole blood with a glucometer after overnight fasting.
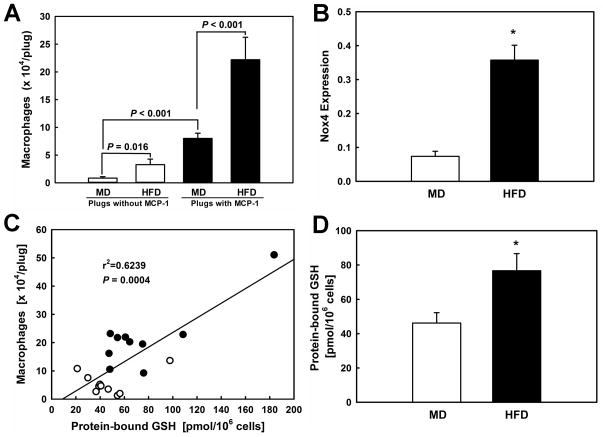
Figure 6. Metabolic stress induced by HFD-feeding promotes macrophage protein-S-glutathionylation and Nox4 expression and enhances macrophage recruitment into MCP-1-loaded Matrigel plugs.
(A) Matrigel supplemented with either vehicle (open bars) or MCP-1 (300 ng/ml; closed bars) was injected into the left and right flank, respectively, of mice fed either a maintenance diet (MD; n=10) or a high-fat diet (HFD; n=10) for 10 weeks. Matrigel plugs were surgically removed and dissolved and macrophage content was determined in a fluorescent-based cell counter. (B): Nox4 expression in macrophages isolated from Matrigel plugs from normolipidemic (MD) and metabolically-stressed mice (HFD) was determined by real-time RT-PCR. Nox4 expression was normalized to GAPDH mRNA levels. (C): Protein-S-glutathionylation was determined in peritoneal macrophages isolated from these control (MD) and metabolically stressed mice (HFD), as described under Methods. Results shown are means ± SE. * P < 0.05. (D): Correlation between protein-S-glutathionylation in peritoneal macrophages and macrophage counts in Matrigel plugs.
We showed previously that Nox4 is expressed in monocyte-derived macrophages with atherosclerotic lesions in mice 35. To examine if metabolic stress upregulates Nox4 expression in monocytes in vivo, we isolated monocyte-derived macrophages from the MCP-1-loaded plugs and determined Nox4 expression levels by real-time PCR. Compared to Matrigel plug-derived macrophages from healthy control mice, macrophages from HFD-fed mice showed a 4.9-fold increase in Nox4 expression (Fig. 6B). These results confirm our in vitro findings and suggest that priming of monocytes by metabolic stress in vivo also appears to be mediated by Nox4-derived H2O2. We therefore predicted that macrophages isolated from these metabolically stressed mice should also show increased levels of protein-S-glutathionylation. The numbers of macrophages we isolated from the Matrigel plugs were too low for an accurate assessment of their PSSG levels. However, in our previous studies in dyslipidemic and diabetic LDL-R−/− mice, we found that metabolic stress shifts the thiol redox state of peritoneal macrophages toward a more oxidized state and that changes in the thiol redox state of peritoneal macrophages correlate with the extent of monocyte dysfunction, i.e. enhanced chemotactic activity in vivo 27. We therefore isolated peritoneal macrophages from the same MD and HFD-fed C57BL/6 mice from which we had removed the Matrigel plugs, and measured macrophage PSSG level as a surrogate marker for protein-S-glutathionylation in blood monocytes. Peritoneal macrophages isolated from metabolically stressed mice showed PSSG levels that were 1.7-fold higher than those found in macrophages isolated from healthy control mice (Fig. 6D). More importantly, we observed a highly significant correlation (r2 = 0.624, P = 0.0004) between the levels of PSSG in peritoneal macrophages and the number of macrophages recruited into the Matrigel plugs (Fig. 6C) from healthy and metabolically stressed mice. It should be noted that the metabolic stress in this mouse model is milder with regard to changes in blood glucose, cholesterol and triglycerides than the changes induced by LDL+HG in vitro. Nevertheless, we were able to recapitulate all key findings obtained with our in vitro model (Nox4 induction, increase protein-S-glutathionylation and accelerated chemotaxis) in this mouse model.
Collectively, these data support our hypothesis that the priming and hyper-responsiveness of blood monocytes to MCP-1-induced transmigration observed in metabolically-stressed mice requires the induction of monocytic Nox4 and is mediated by protein S-glutathionylation.
DISCUSSION
The aim of this study was to examine the mechanisms underlying the hyper-responsiveness of monocytes in vivo to the chemoattractant MCP-1 we recently reported in dyslipidemic and diabetic mice 27. Here we show that metabolic stress primes monocytes and induces a gain-of-function phenotype that is characterized by enhanced chemotactic activity. The hyper-responsiveness of monocytes was not limited to MCP-1, but was also observed in response to PDGF-B and RANTES, suggesting a more fundamental change in the intracellular signaling that controls monocyte migration. We show that the transformation of monocytes by metabolic stress into this pro-inflammatory and pro-atherogenic phenotype requires the induction of Nox4, an NADPH oxidase we recently discovered in monocytes and macrophages 35. Induction of Nox4, which generates primarily H2O2 37, was associated with increased formation of intracellular H2O2, implicating H2O2 as a critical second messenger of metabolic stress-induced monocyte priming. The requirement for increased intracellular H2O2 production is supported by the fact that both overexpression of Nox4 and exposure of monocytes to extracellular, membrane-permeable H2O2 mimicked the priming effects of metabolic stress on the monocytes’ responsiveness to chemoattractants. Furthermore, blocking the induction of Nox4 with siRNA normalized intracellular H2O2 levels and completely prevented monocyte dysfunction induced by metabolic stress. Metabolic stress also promoted the formation of mixed disulfides between protein thiols and GSH, the main low molecular weight thiol antioxidant present in cells at millimolar concentrations 47. In cells, the reduction of these mixed disulfides, i.e. the deglutathionylation of protein thiols to the corresponding free thiols, is catalyzed by glutaredoxins 43. Overexpression of cytosolic Grx1 not only prevented the increase in protein-S-glutathionylation induced by metabolic stress, it also protected monocytes from converting into the hyper-chemotactic phenotype. Collectively, these data support the concept that chronic oxidative modifications of reactive protein thiols by Nox4-derived H2O2 are responsible for the phenotypic transformation observed in monocytes exposed to metabolic stress.
Nox4 is expressed in a number of cell types, including endothelial cells 48, 49, fibroblasts, vascular smooth muscle cells 50, and monocytes and macrophages 35. The role of Nox4 appears to be specific to the cell type. For example, in smooth muscle cells, Nox4 is required for the maintenance of the differentiated cell phenotype 51, whereas in preadipocytes the enzyme promotes the switch from insulin-induced proliferation to differentiation 52. We showed that in human macrophages, Nox4 mediates OxLDL-induced oxidative stress and cell death 35. As part of our current studies, we have now uncovered a completely new role for Nox4. Here we provide evidence that in human monocytes, Nox4 plays a role in the regulation of cell migration and appears to mediate the signaling events that in response to metabolic stress, promote monocyte dysfunction and transform monocytes into a hyper-chemotactic, pro-atherogenic phenotype.
A major difference between Nox4 and other Nox family members such as Nox1 and Nox2 is that the major ROS generated by Nox4 is H2O2, not superoxide 37. The primary targets of H2O2 in biological systems are thiols, although there is considerable debate whether H2O2–mediated thiol oxidation in cells can occur spontaneously or requires enzyme-mediated catalysis 39, 53. The uncatalyzed oxidation of a thiolate anion with H2O2 occurs with a rate constant of 18–26 M−1s−1 54, but the activation energy for this reaction would be too high to be physiologically relevant. However, if a basic amino acid residue is present in close proximity of the targeted thiol, the thiol becomes acidic, which is the case for protein thiols known to be S-glutathionylated, and the oxidation of the thiolate anion to the corresponding sulfenic acid (S-OH) occurs much faster 53. Sulfenic acids are highly reactive and rapidly form disulfides. Because GSH is the most abundant thiol in cells, this reaction leads to the formation of mixed disulfides between protein thiols and GSH.
Protein S-glutathionylation has been proposed as a mechanism involved in redox signal transduction 43, 55. However, for H2O2-mediated S-glutathionylation to function as a signaling mechanism would require the generation of micromolar H2O2 concentrations in close proximity to the redox-sensitive target in order to overcome the slow reaction rate and to ensure signal specificity 39. This would only seem possible if the source of H2O2 can be recruited to and localized at the redox-regulated protein. The importance of Nox4 localization for the specificity of ROS-mediated signal transduction was illustrated by Chen and co-workers in human aortic endothelial cells, where localization of Nox4 in the endoplasmic reticulum was found to be critical for the redox-mediated regulation of the cysteine-based protein tyrosine phosphatase 1B 56. We now provide evidence that Nox4 not only localizes to actin fibers but that increased Nox4 expression, either induced by metabolic stress or via adenovirus-mediated overexpression of transgenic Nox4, increases actin-S-glutathionylation and promotes actin turnover. These findings suggest that Nox4 may indeed be recruited to specific sites of redox-regulation. Nox4 is therefore a strong candidate for the elusive enzyme responsible for the S-glutathionylation of actin and possibly other redox-regulated proteins and signaling complexes.
Monocyte priming by metabolic stress could occur at the level of chemokine receptor activation and internalization. For example, PDGF-B-induced activation of PDGFRβ is counter-regulated by the cysteine-based low molecular weight protein tyrosine phosphatase (LMW-PTP), which dephosphorylates and inactivates PDGFRβ 57. Protein tyrosine phosphatases are well-known targets of thiol oxidative stress and protein S-glutathionylation 43, and LMW-PTP itself is under the regulation of Grx1 58. However, in the case of CCR2, the receptor for MCP-1, we observed no correlation between CCR2 surface expression and chemotactic activity. Furthermore, the fact that we observed approximately the same extent of monocyte priming in response to three different chemoattractants, makes it more likely that in metabolically stressed monocytes, Nox4-derived H2O2 targets molecules in signaling pathways common to all three chemoattractants. Redox-sensitive proteins involved in actin remodeling turnover are logical candidates, and our data strongly implicate actin itself as a major target. Cell motility requires the well-coordinated spatial and temporal reorganization of the actin cytoskeleton, i.e. the tight regulation of actin polymerization and depolymerization. Work by several groups demonstrated that these processes are redox sensitive and regulated by S-glutathionylation of actin on Cys374 44,45. S-glutathionylation decreases the stability of actin filaments and reduces the ability of actin to polymerize, whereas Grx1-mediated deglutathionylation of actin monomers accelerates actin polymerization and fiber formation. RNAi-mediated knockdown of Grx1 in NIH-3T3 cells inhibits actin deglutathionylation and blocked growth factor induced actin polymerization 59. Conversely, blocking actin S-glutathionylation in these cells, either by depleting GSH or expression of a redox-insensitive C374A mutant, also prevented cell spreading and the formation of stress fibers 60, demonstrating the dynamic nature of both actin remodeling and its redox regulation.
In summary, we identified a novel, redox-sensitive mechanism by which metabolic stress primes monocytes for chemokine activation and enhances monocyte chemotaxis and transmigration. To our knowledge, this is the first report to 1) identify a gain-of-function phenotype for monocytes associated with metabolic disorders, 2) provide a NADPH oxidase-dependent mechanism for the formation of actin-glutathione mixed disulfides in cells, and to 3) demonstrate a role for Nox4 in monocyte migration and macrophage recruitment - both key processes involved in the onset and progression of atherosclerosis.
Supplementary Material
Acknowledgments
ACKNOWLEDGEMENTS
None
FUNDING SOURCES
This work was supported by grants to R.A. from the NIH (HL-70963) and the AHA (0855011F). C.F.L was supported by a Predoctoral Fellowship (10PRE3460002) from the AHA. S.U. was supported by a fellowship from the Translational Science Training (TST) Across Disciplines program at the University of Texas Health Science Center at San Antonio, with funding provided by the University of Texas System’s Graduate Programs Initiative. Confocal images were generated in the Core Optical Imaging Facility which is supported by UTHSCSA, NIH-NCI P30 CA54174 (San Antonio Cancer Institute), NIH-NIA P30 AG013319 (Nathan Shock Center) and (NIH-NIA P01AG19316).
ABBREVIATIONS
- DCFH-DA
2′,7′-dichlorodihydrofluorescin diacetate
- Dox
doxycycline
- Grx
glutaredoxin
- GSH
reduced glutathione
- HFD
high-fat diet
- HG
high D-glucose
- HPLC
high-performance liquid chromatography
- LDL
low-density lipoprotein
- MCP-1
monocyte chemoattractant protein-1
- MD
maintenance diet
- NG
normal glucose
- Nox4
NADPH Oxidase 4
- PDGF-B
platelet-derived growth factor B
- PSSG
protein-glutathione mixed disulfide
- ROS
reactive oxygen species
Footnotes
DISCLOSURES
There are no conflicts to disclose.
References
- 1.Stoneman V, Braganza D, Figg N, Mercer J, Lang R, Goddard M, Bennett M. Monocyte/macrophage suppression in CD11b diphtheria toxin receptor transgenic mice differentially affects atherogenesis and established plaques. Circ Res. 2007;100(6):884–93. doi: 10.1161/01.RES.0000260802.75766.00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nasir K, Guallar E, Navas-Acien A, Criqui MH, Lima JA. Relationship of monocyte count and peripheral arterial disease: results from the National Health and Nutrition Examination Survey 1999–2002. Arterioscler Thromb Vasc Biol. 2005;25(9):1966–71. doi: 10.1161/01.ATV.0000175296.02550.e4. [DOI] [PubMed] [Google Scholar]
- 3.Johnsen SH, Fosse E, Joakimsen O, Mathiesen EB, Stensland-Bugge E, Njolstad I, Arnesen E. Monocyte count is a predictor of novel plaque formation: a 7-year follow-up study of 2610 persons without carotid plaque at baseline the Tromso Study. Stroke. 2005;36(4):715–9. doi: 10.1161/01.STR.0000158909.07634.83. [DOI] [PubMed] [Google Scholar]
- 4.Smith JD, Trogan E, Ginsberg M, Grigaux C, Tian J, Miyata M. Decreased atherosclerosis in mice deficient in both macrophage colony-stimulating factor (op) and apolipoprotein E. Proc Natl Acad Sci U S A. 1995;92(18):8264–8. doi: 10.1073/pnas.92.18.8264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Swirski FK, Weissleder R, Pittet MJ. Heterogeneous in vivo behavior of monocyte subsets in atherosclerosis. Arterioscler Thromb Vasc Biol. 2009;29(10):1424–32. doi: 10.1161/ATVBAHA.108.180521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gautier EL, Jakubzick C, Randolph GJ. Regulation of the migration and survival of monocyte subsets by chemokine receptors and its relevance to atherosclerosis. Arterioscler Thromb Vasc Biol. 2009;29(10):1412–8. doi: 10.1161/ATVBAHA.108.180505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johnson-Tidey RR, McGregor JL, Taylor PR, Poston RN. Increase in the adhesion molecule P-selectin in endothelium overlying atherosclerotic plaques. Coexpression with intercellular adhesion molecule-1. Am J Pathol. 1994;144(5):952–61. [PMC free article] [PubMed] [Google Scholar]
- 8.Wood KM, Cadogan MD, Ramshaw AL, Parums DV. The distribution of adhesion molecules in human atherosclerosis. Histopathology. 1993;22(5):437–44. doi: 10.1111/j.1365-2559.1993.tb00157.x. [DOI] [PubMed] [Google Scholar]
- 9.Zibara K, Chignier E, Covacho C, Poston R, Canard G, Hardy P, McGregor J. Modulation of expression of endothelial intercellular adhesion molecule-1, platelet-endothelial cell adhesion molecule-1, and vascular cell adhesion molecule-1 in aortic arch lesions of apolipoprotein E-deficient compared with wild-type mice. Arterioscler Thromb Vasc Biol. 2000;20(10):2288–96. doi: 10.1161/01.atv.20.10.2288. [DOI] [PubMed] [Google Scholar]
- 10.Dong ZM, Chapman SM, Brown AA, Frenette PS, Hynes RO, Wagner DD. The combined role of P- and E-selectins in atherosclerosis. J Clin Invest. 1998;102(1):145–52. doi: 10.1172/JCI3001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nageh MF, Sandberg ET, Marotti KR, Lin AH, Melchior EP, Bullard DC, Beaudet AL. Deficiency of inflammatory cell adhesion molecules protects against atherosclerosis in mice. Arterioscler Thromb Vasc Biol. 1997;17(8):1517–20. doi: 10.1161/01.atv.17.8.1517. [DOI] [PubMed] [Google Scholar]
- 12.Cybulsky MI, Iiyama K, Li H, Zhu S, Chen M, Iiyama M, Davis V, Gutierrez-Ramos JC, Connelly PW, Milstone DS. A major role for VCAM-1, but not ICAM-1, in early atherosclerosis. J Clin Invest. 2001;107(10):1255–62. doi: 10.1172/JCI11871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Collins RG, Velji R, Guevara NV, Hicks MJ, Chan L, Beaudet AL. P-Selectin or intercellular adhesion molecule (ICAM)-1 deficiency substantially protects against atherosclerosis in apolipoprotein E-deficient mice. J Exp Med. 2000;191(1):189–94. doi: 10.1084/jem.191.1.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.An G, Wang H, Tang R, Yago T, McDaniel JM, McGee S, Huo Y, Xia L. P-selectin glycoprotein ligand-1 is highly expressed on Ly-6Chi monocytes and a major determinant for Ly-6Chi monocyte recruitment to sites of atherosclerosis in mice. Circulation. 2008;117(25):3227–37. doi: 10.1161/CIRCULATIONAHA.108.771048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boring L, Gosling J, Cleary M, Charo IF. Decreased lesion formation in CCR2−/− mice reveals a role for chemokines in the initiation of atherosclerosis. Nature. 1998;394(6696):894–7. doi: 10.1038/29788. [DOI] [PubMed] [Google Scholar]
- 16.Gu L, Okada Y, Clinton SK, Gerard C, Sukhova GK, Libby P, Rollins BJ. Absence of monocyte chemoattractant protein-1 reduces atherosclerosis in low density lipoprotein receptor-deficient mice. Mol Cell. 1998;2(2):275–81. doi: 10.1016/s1097-2765(00)80139-2. [DOI] [PubMed] [Google Scholar]
- 17.Koenen RR, von Hundelshausen P, Nesmelova IV, Zernecke A, Liehn EA, Sarabi A, Kramp BK, Piccinini AM, Paludan SR, Kowalska MA, Kungl AJ, Hackeng TM, Mayo KH, Weber C. Disrupting functional interactions between platelet chemokines inhibits atherosclerosis in hyperlipidemic mice. Nat Med. 2009;15(1):97–103. doi: 10.1038/nm.1898. [DOI] [PubMed] [Google Scholar]
- 18.Zernecke A, Liehn EA, Gao JL, Kuziel WA, Murphy PM, Weber C. Deficiency in CCR5 but not CCR1 protects against neointima formation in atherosclerosis-prone mice: involvement of IL-10. Blood. 2006;107(11):4240–3. doi: 10.1182/blood-2005-09-3922. [DOI] [PubMed] [Google Scholar]
- 19.Teupser D, Pavlides S, Tan M, Gutierrez-Ramos JC, Kolbeck R, Breslow JL. Major reduction of atherosclerosis in fractalkine (CX3CL1)-deficient mice is at the brachiocephalic artery, not the aortic root. Proc Natl Acad Sci U S A. 2004;101(51):17795–800. doi: 10.1073/pnas.0408096101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Combadiere C, Potteaux S, Gao JL, Esposito B, Casanova S, Lee EJ, Debre P, Tedgui A, Murphy PM, Mallat Z. Decreased atherosclerotic lesion formation in CX3CR1/apolipoprotein E double knockout mice. Circulation. 2003;107(7):1009–16. doi: 10.1161/01.cir.0000057548.68243.42. [DOI] [PubMed] [Google Scholar]
- 21.Raines EW. PDGF and cardiovascular disease. Cytokine Growth Factor Rev. 2004;15(4):237–54. doi: 10.1016/j.cytogfr.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 22.Combadiere C, Potteaux S, Rodero M, Simon T, Pezard A, Esposito B, Merval R, Proudfoot A, Tedgui A, Mallat Z. Combined inhibition of CCL2, CX3CR1, and CCR5 abrogates Ly6C(hi) and Ly6C(lo) monocytosis and almost abolishes atherosclerosis in hypercholesterolemic mice. Circulation. 2008;117(13):1649–57. doi: 10.1161/CIRCULATIONAHA.107.745091. [DOI] [PubMed] [Google Scholar]
- 23.Swirski FK, Libby P, Aikawa E, Alcaide P, Luscinskas FW, Weissleder R, Pittet MJ. Ly-6Chi monocytes dominate hypercholesterolemia-associated monocytosis and give rise to macrophages in atheromata. J Clin Invest. 2007;117(1):195–205. doi: 10.1172/JCI29950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu H, Gower RM, Wang H, Perrard XY, Ma R, Bullard DC, Burns AR, Paul A, Smith CW, Simon SI, Ballantyne CM. Functional role of CD11c+ monocytes in atherogenesis associated with hypercholesterolemia. Circulation. 2009;119(20):2708–17. doi: 10.1161/CIRCULATIONAHA.108.823740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Han KH, Han KO, Green SR, Quehenberger O. Expression of the monocyte chemoattractant protein-1 receptor CCR2 is increased in hypercholesterolemia. Differential effects of plasma lipoproteins on monocyte function. J Lipid Res. 1999;40(6):1053–63. [PubMed] [Google Scholar]
- 26.Han KH, Tangirala RK, Green SR, Quehenberger O. Chemokine receptor CCR2 expression and monocyte chemoattractant protein-1-mediated chemotaxis in human monocytes. A regulatory role for plasma LDL. Arterioscler Thromb Vasc Biol. 1998;18(12):1983–91. doi: 10.1161/01.atv.18.12.1983. [DOI] [PubMed] [Google Scholar]
- 27.Qiao M, Zhao Q, Lee CF, Tannock L, Smart EJ, LeBaron RG, Phelix CF, Rangel Y, Asmis R. Thiol Oxidative Stress Induced by Metabolic Disorders Amplifies Macrophage Chemotactic Responses and Accelerates Atherogenesis and Kidney Injury in LDL Receptor-Deficient Mice. Arterioscler Thromb Vasc Biol. 2009;29:1779–86. doi: 10.1161/ATVBAHA.109.191759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Asmis R, Jelk J. Large variations in human foam cell formation in individuals. A fully autologous in vitro assay based on the quantitative analysis of cellular neutral lipids. Atherosclerosis. 2000;148(2):243–53. doi: 10.1016/s0021-9150(99)00268-3. [DOI] [PubMed] [Google Scholar]
- 29.Wintergerst ES, Jelk J, Rahner C, Asmis R. Apoptosis induced by oxidized low density lipoprotein in human monocyte-derived macrophages involves CD36 and activation of caspase-3. Eur J Biochem. 2000;267(19):6050–8. doi: 10.1046/j.1432-1327.2000.01682.x. [DOI] [PubMed] [Google Scholar]
- 30.Qiao M, Kisgati M, Cholewa J, Zhu W, Smart EJ, Sulistio MS, Asmis R. Increased expression of cytosolic and mitochondrial glutathione reductase in macrophages inhibits atherosclerotic lesion development in LDL receptor-deficient mice. Arterioscler Thromb Vasc Biol. 2007;27:1375–82. doi: 10.1161/ATVBAHA.107.142109. [DOI] [PubMed] [Google Scholar]
- 31.Asmis R, Wang Y, Xu l, Kisgati M, Begley JG, Mieyal JJ. A novel thiol oxidation-based mechanism for adriamycin-induced cell injury in human macrophages. FASEB J. 2005;19:1866–8. doi: 10.1096/fj.04-2991fje. [DOI] [PubMed] [Google Scholar]
- 32.DiPietro LA. Wound healing: the role of the macrophage and other immune cells. Shock. 1995;4(4):233–40. [PubMed] [Google Scholar]
- 33.Hirschberg R, Wang S. Proteinuria and growth factors in the development of tubulointerstitial injury and scarring in kidney disease. Curr Opin Nephrol Hypertens. 2005;14(1):43–52. doi: 10.1097/00041552-200501000-00008. [DOI] [PubMed] [Google Scholar]
- 34.Grone HJ, Weber C, Weber KS, Grone EF, Rabelink T, Klier CM, Wells TN, Proudfood AE, Schlondorff D, Nelson PJ. Met-RANTES reduces vascular and tubular damage during acute renal transplant rejection: blocking monocyte arrest and recruitment. FASEB J. 1999;13(11):1371–83. [PubMed] [Google Scholar]
- 35.Lee CF, Qiao M, Schroder K, Zhao Q, Asmis R. Nox4 is a Novel Inducible Source of Reactive Oxygen Species in Monocytes and Macrophages and Mediates Oxidized Low Density Lipoprotein-Induced Macrophage Death. Circ Res. 2010;106(9):1489–97. doi: 10.1161/CIRCRESAHA.109.215392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dikalov SI, Dikalova AE, Bikineyeva AT, Schmidt HH, Harrison DG, Griendling KK. Distinct roles of Nox1 and Nox4 in basal and angiotensin II-stimulated superoxide and hydrogen peroxide production. Free Radic Biol Med. 2008;45(9):1340–51. doi: 10.1016/j.freeradbiomed.2008.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Helmcke I, Heumuller S, Tikkanen R, Schroder K, Brandes RP. Identification of structural elements in Nox1 and Nox4 controlling localization and activity. Antioxid Redox Signal. 2009;11(6):1279–87. doi: 10.1089/ars.2008.2383. [DOI] [PubMed] [Google Scholar]
- 38.Adimora NJ, Jones DP, Kemp ML. A model of redox kinetics implicates the thiol proteome in cellular hydrogen peroxide responses. Antioxid Redox Signal. 2010;13(6):731–43. doi: 10.1089/ars.2009.2968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Winterbourn CC, Hampton MB. Thiol chemistry and specificity in redox signaling. Free Radic Biol Med. 2008;45(5):549–61. doi: 10.1016/j.freeradbiomed.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 40.Klatt P, Lamas S. Regulation of protein function by S-glutathiolation in response to oxidative and nitrosative stress. Eur J Biochem. 2000;267(16):4928–44. doi: 10.1046/j.1432-1327.2000.01601.x. [DOI] [PubMed] [Google Scholar]
- 41.Shelton MD, Mieyal JJ. Regulation by reversible S-glutathionylation: molecular targets implicated in inflammatory diseases. Mol Cells. 2008;25(3):332–46. [PMC free article] [PubMed] [Google Scholar]
- 42.Dalle-Donne I, Rossi R, Giustarini D, Colombo R, Milzani A. S-glutathionylation in protein redox regulation. Free Radic Biol Med. 2007;43(6):883–98. doi: 10.1016/j.freeradbiomed.2007.06.014. [DOI] [PubMed] [Google Scholar]
- 43.Shelton MD, Chock PB, Mieyal JJ. Glutaredoxin: Role of reversible protein-S-glutathionylation and regulation of redox signal transduction and protein translocation. Antioxid Redox Signal. 2005;7(3–4):348–66. doi: 10.1089/ars.2005.7.348. [DOI] [PubMed] [Google Scholar]
- 44.Wang J, Boja ES, Tan W, Tekle E, Fales HM, English S, Mieyal JJ, Chock PB. Reversible glutathionylation regulates actin polymerization in A431 cells. J Biol Chem. 2001;276(51):47763–6. doi: 10.1074/jbc.C100415200. [DOI] [PubMed] [Google Scholar]
- 45.Dalle-Donne I, Giustarini D, Rossi R, Colombo R, Milzani A. Reversible S-glutathionylation of Cys374 regulates actin filament formation by inducing structural changes in the actin molecule. Free Radic Biol Med. 2003;34(1):23–32. doi: 10.1016/s0891-5849(02)01182-6. [DOI] [PubMed] [Google Scholar]
- 46.Jiang T, Wang Z, Proctor G, Moskowitz S, Liebman SE, Rogers T, Lucia MS, Li J, Levi M. Diet-induced obesity in C57BL/6J mice causes increased renal lipid accumulation and glomerulosclerosis via a sterol regulatory element-binding protein-1c-dependent pathway. J Biol Chem. 2005;280(37):32317–25. doi: 10.1074/jbc.M500801200. [DOI] [PubMed] [Google Scholar]
- 47.Meister A, Griffith OW, Novogrodsky A, Tate SS. New aspects of glutathione metabolism and translocation in mammals. Ciba Found Symp. 1979;(72):135–61. doi: 10.1002/9780470720554.ch9. [DOI] [PubMed] [Google Scholar]
- 48.Hwang J, Ing MH, Salazar A, Lassegue B, Griendling K, Navab M, Sevanian A, Hsiai TK. Pulsatile versus oscillatory shear stress regulates NADPH oxidase subunit expression: implication for native LDL oxidation. Circ Res. 2003;93(12):1225–32. doi: 10.1161/01.RES.0000104087.29395.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ago T, Kitazono T, Ooboshi H, Iyama T, Han YH, Takada J, Wakisaka M, Ibayashi S, Utsumi H, Iida M. Nox4 as the major catalytic component of an endothelial NAD(P)H oxidase. Circulation. 2004;109(2):227–33. doi: 10.1161/01.CIR.0000105680.92873.70. [DOI] [PubMed] [Google Scholar]
- 50.Wingler K, Wunsch S, Kreutz R, Rothermund L, Paul M, Schmidt HH. Upregulation of the vascular NAD(P)H-oxidase isoforms Nox1 and Nox4 by the renin-angiotensin system in vitro and in vivo. Free Radic Biol Med. 2001;31(11):1456–64. doi: 10.1016/s0891-5849(01)00727-4. [DOI] [PubMed] [Google Scholar]
- 51.Clempus RE, Sorescu D, Dikalova AE, Pounkova L, Jo P, Sorescu GP, Schmidt HH, Lassegue B, Griendling KK. Nox4 is required for maintenance of the differentiated vascular smooth muscle cell phenotype. Arterioscler Thromb Vasc Biol. 2007;27(1):42–8. doi: 10.1161/01.ATV.0000251500.94478.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schroder K, Wandzioch K, Helmcke I, Brandes RP. Nox4 acts as a switch between differentiation and proliferation in preadipocytes. Arterioscler Thromb Vasc Biol. 2009;29(2):239–45. doi: 10.1161/ATVBAHA.108.174219. [DOI] [PubMed] [Google Scholar]
- 53.Forman HJ, Maiorino M, Ursini F. Signaling functions of reactive oxygen species. Biochemistry. 2010;49(5):835–42. doi: 10.1021/bi9020378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Winterbourn CC, Metodiewa D. Reactivity of biologically important thiol compounds with superoxide and hydrogen peroxide. Free Radic Biol Med. 1999;27(3–4):322–8. doi: 10.1016/s0891-5849(99)00051-9. [DOI] [PubMed] [Google Scholar]
- 55.Janssen-Heininger YM, Mossman BT, Heintz NH, Forman HJ, Kalyanaraman B, Finkel T, Stamler JS, Rhee SG, Van DV. Redox-based regulation of signal transduction: principles, pitfalls, and promises. Free Radic Biol Med. 2008;45(1):1–17. doi: 10.1016/j.freeradbiomed.2008.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chen K, Kirber MT, Xiao H, Yang Y, Keaney JF., Jr Regulation of ROS signal transduction by NADPH oxidase 4 localization. J Cell Biol. 2008;181(7):1129–39. doi: 10.1083/jcb.200709049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ronnstrand L, Heldin CH. Mechanisms of platelet-derived growth factor-induced chemotaxis. Int J Cancer. 2001;91(6):757–62. doi: 10.1002/1097-0215(200002)9999:9999<::aid-ijc1136>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 58.Kanda M, Ihara Y, Murata H, Urata Y, Kono T, Yodoi J, Seto S, Yano K, Kondo T. Glutaredoxin modulates platelet-derived growth factor-dependent cell signaling by regulating the redox status of low molecular weight protein-tyrosine phosphatase. J Biol Chem. 2006;281(39):28518–28. doi: 10.1074/jbc.M604359200. [DOI] [PubMed] [Google Scholar]
- 59.Wang J, Tekle E, Oubrahim H, Mieyal JJ, Stadtman ER, Chock PB. Stable and controllable RNA interference: Investigating the physiological function of glutathionylated actin. Proc Natl Acad Sci U S A. 2003;100(9):5103–6. doi: 10.1073/pnas.0931345100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fiaschi T, Cozzi G, Raugei G, Formigli L, Ramponi G, Chiarugi P. Redox regulation of beta-actin during integrin-mediated cell adhesion. J Biol Chem. 2006;281(32):22983–91. doi: 10.1074/jbc.M603040200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.