Abstract
Background
Preterm infants are at high risk of encountering oral feeding difficulties. Early sensorimotor interventions may improve oral feeding skills in preterm infants.
Aim
To further explore the effects of an oral (O), tactile/kinesthetic (T/K), and combined (O+T/K) sensorimotor intervention on preterm infants’ nutritive sucking, swallowing and their coordination with respiration.
Study design
Seventy-five infants (29 [0.3, standard error of mean, SEM] weeks gestation, 49 males/26 females) were randomly assigned to an O group involving sensorimotor input to the oral structures; a T/K group involving sensorimotor input to the trunk and limbs; a combined (O+T/K) group; and a control group.
Outcome measures
Stage of sucking, suction and expression amplitudes (mmHg), suck-swallow ratio, stability of suck-swallow interval, and swallow-respiration patterns.
Results
The O group had significantly more advanced sucking stages, and greater suction and expression amplitudes than controls [p≤0.035, effect size (ES) >0.6]. The suck-swallow ratio and stability of suck-swallow intervals did not significantly differ among groups (p≥0.181, ES≤0.3). The three interventions led to fewer swallows bracketed by prolonged respiratory pauses compared to controls (pause-swallow-pause, p≤0.044, ES≥0.7). The T/K and combined (O+T/K) groups had greater occurrence of swallows bracketed by expiration than the control and O groups (expiration-swallow-expiration, p≤0.039, ES ≥0.3)
Conclusion
The O intervention enhanced specific components of nutritive sucking. All three interventions resulted in improved swallow-respiration coordination. Sensorimotor interventions have distributed beneficial effects that go beyond the specific target of input.
Keywords: Oral stimulation, tactile/kinesthetic stimulation, bottle feeding, feeding difficulty, respiration-swallow coordination
1. Introduction
Oral feeding in the neonatal period requires precise coordination between sucking, swallowing and breathing1. Preterm infants often have difficulty in establishing this key coordinative relationship1. Hence, they are tube fed and are kept in hospital until they are able to safely meet their nutritional requirements orally, while maintaining adequate daily weight gain without cardiorespiratory compromise2. Our studies suggest that early sensorimotor interventions may improve oral feeding skills and facilitate suck-swallow-respiration in preterm infants3, 4.
Many current interventions to improve sucking, swallowing, and their coordination with respiration focus on promoting the neural maturation of these processes3, 4, 5. Our earlier work has shown that an oral (O), tactile/kinesthetic (T/K), and combined oral and tactile/kinesthetic (O+T/K) sensorimotor intervention, administered before the start of oral feeding, accelerated the transition from introduction to independent oral feeding4. Specifically, all three interventions improved proficiency (percent volume taken in first 5- minutes), volume transfer (percent total volume taken) and rate of transfer (ml/min), compared to controls4. Nutritive suck, suck-swallow and swallow-respiration coordination appear to be key components underlying these improved oral feeding outcomes4. Knowledge on how these underlying mechanisms mediate these vital coordinative functions is very limited. In our previous work, we have found that oral sensorimotor input improves nutritive sucking3, however the impact of oral and particularly non-oral sensorimotor input (tactile/kinesthetic sensorimotor input to the trunk and limbs) on sucking, swallowing, and respiration and their coordination has not been investigated. Furthermore, given multiple points of interaction in the nervous system6, we hypothesize that multiple stimulation sites may potentially impact common underlying systems or may in fact provide multiplicative effects on these coordinative functions.
Therefore, the purpose of this current study was to further explore whether: 1) preterm infants who receive an O, T/K, or combined (O+T/K) intervention, before the introduction of oral feeding, will demonstrate more advanced nutritive sucking, suck-swallow and swallow-respiration coordination than controls. Specifically, 1a) they will exhibit more mature sucking stages, and greater suction and expression amplitudes, 1b) they will demonstrate an equal suck to swallow ratio and a stable suck-swallow interval, and 1c) they will have a more mature swallow-respiration pattern than controls. 2) Preterm infants who receive a combined (O+T/K) intervention will demonstrate more advanced nutritive sucking, suck-swallow and swallow-respiration coordination than those who receive an O or T/K intervention singly.
2. Methods
2.1 Participants
Participants were recruited from the nursery at Texas Children’s Hospital, Houston, TX. Eligibility included infants: 1) born between 26–32 weeks gestational age (GA); 2) of appropriate size for GA; 3) receiving only tube feedings; 4) with no congenital anomalies; and 5) with no chronic medical complications including severe bronchopulmonary dysplasia, intraventricular hemorrhages III or IV, periventricular leukomalacia, or necrotizing enterocolitis. The study was approved by the Institutional Review Board for Human Subjects at Baylor College of Medicine and Affiliated Hospitals.
2.2 Procedures
After parental consent was obtained, infants were randomized into an O, T/K, combined (O+T/K), or control group using a stratified blocked randomization. A block stratification by GA (26–29 and 30–32 weeks GA) was used to ensure that all four groups had equal GA distribution, and stratification by time (three month intervals) was done to make certain each group had balanced distribution of attending neonatologists. All participants were followed from the start of the study until hospital discharge.
Similar to our previous investigations3, 4 the O intervention consisted of sensorimotor input to the oral structures: specifically, perioral stimulation to the cheeks, lips, and jaw for 7 minutes, intraoral stimulation to the gums and tongue for 5 minutes, and nonnutritive sucking on a pacifier for 3 minutes3, 4. The 15-minute O intervention was administered twice a day (total: 30 minutes per day). The infants were in supine position in the incubator throughout the O intervention. The T/K intervention consisted of stroking the body starting from the head, followed by the neck, shoulders, back, legs, and arms for 10 minutes and passive range of motion of the arms and legs for 5 minutes8. The 15-minute T/K intervention was administered twice a day (total: 30 minutes per day). The infants were in the incubator, in prone and supine position, respectively, during the T/K intervention. This regimen was selected because at least 15 minutes/day of O or T/K intervention had beneficial effects on oral feeding performance and motor activity, respectively3, 4, 8. The combined (O+T/K) intervention consisted of the same 15 minutes of O and 15 minutes of T/K intervention, described above. Each type of intervention was administered once a day (total: 30 minutes per day), in random order. For the control intervention the researcher (SF) placed her hands in the incubator but did not touch the infant for 15 minutes, twice a day (total: 30 minutes per day). The control intervention was designed to eliminate possible effects of the daily presence of the researcher at the bedside.
All interventions were commenced 48 hours following discontinuation of nasal continuous positive airway pressure, and administered for 10 days, within a 14-day period. Interventions were provided 30 minutes prior to a tube feeding, with a minimum 3-hour interval between each daily session, and when infants were clinically stable determined by nurses’ recommendation. Interventions were stopped if infants had an episode of apnea, bradycardia, oxygen desaturation, fussing, crying, or emesis. All interventions were administered in the incubator by the same researcher. A screen was placed around the bedside in order to ‘blind’ family members and caregivers to group assignment.
Sucking, swallowing and respiration were monitored once during three oral feeding sessions, when infants were taking 1–2, 3–5, and 6–8 oral feedings per day. The management of oral feeding was left to the attending neonatologists’ discretion. Nurses were responsible for feeding the infants in their customary manner. Oral feeding sessions were no longer than 20 minutes, per nursery protocol.
2.3 Outcomes
Nutritive sucking skills were assessed using a 5-point stage of sucking scale defined by Lau et al9. Stage 1, represents an immature/disorganized sucking pattern with no suction, arrhythmic expression, and/or arrhythmic alternation of suction/expression. Stages 2–4, represent more mature sucking patterns, with suction emerging, more rhythmic expression, and/or more rhythmic alternation of suction/expression. Stage 5, represents a mature sucking pattern with rhythmic alternation of suction/expression, similar to that of full term infants. Suction and expression amplitudes (mmHg) were also monitored.
Suck-swallow coordination was assessed using suck to swallow ratio defined as number of expressions over number of swallows, and stability of the suck-swallow interval defined as the time (seconds) from peak expression to swallow, using the coefficient of variation (standard deviation of the mean interval divided by the mean interval). These outcomes were selected because a 1:1 suck-swallow ratio and a stable suck-swallow interval (i.e. smaller coefficient of variation) reflect more mature suck-swallow coordination10–12.
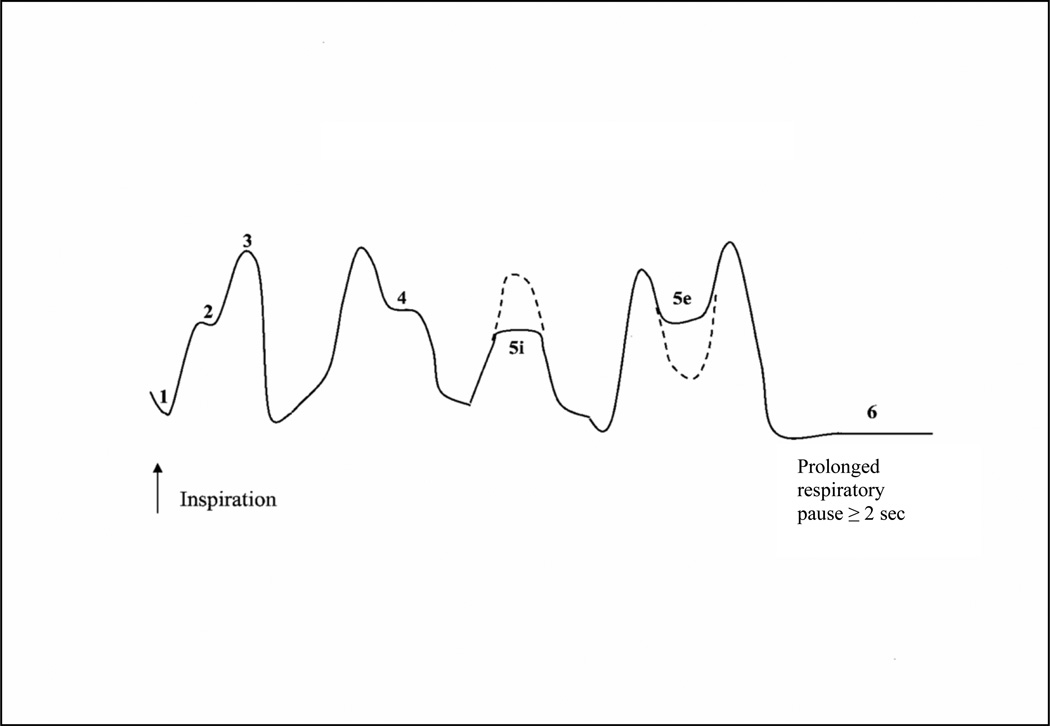
Respiration is always inhibited to accommodate the swallow in all species including infant and adult humans13. Swallow-respiration coordination was assessed by identifying the respiratory phase that immediately preceded and followed the swallow. The mean percent occurrence of particular swallow-respiration patterns was monitored using a 6-pattern classification developed by Lau et al.,14 which included: 1) end expiration-swallow-start inspiration (end E-Sw- start I); 2) inspiration-swallow-inspiration (I-Sw-I); 3) end inspiration-swallow-start expiration (end I-Sw-start E); 4) expiration-swallow-expiration (E-Sw-E); 5i) swallow interrupt inspiration (Sw-interrupt I); 5e) swallow interrupt expiration (Sw-interrupt E); and 6) swallow occurring during a prolonged respiratory pause of ≥ 2.0 seconds (P-Sw-P, Figure 1). We operationally defined a prolonged respiratory pause as ≥ 2.0 seconds, based on work by Bamford and colleagues who found normal breath-to-breath intervals ranged between 1.2–2.0 seconds during feeding epochs in term infants10.
Figure 1.
Swallow-respiration patterns. Pattern 1 - start inspiration-swallow-end expiration (start I-Sw-end E); pattern 2 - inspiration-swallow-inspiration (I-Sw-I); pattern 3 - end inspiration-swallow-start expiration (end I-Sw-start E); pattern 4 - expiration-swallow-expiration (E-Sw-E); pattern 5i - swallow interrupts inspiration (Sw-interrupt I); pattern 5e - swallow interrupts expiration (Sw-interrupt E); and pattern 6 - pause-swallow-pause (P-Sw-P, swallows occurring at cessation of respiration ≥ 2 seconds).
The following potential covariates were considered: severity of illness using the Nursery Neurobiologic Risk Score15, number of infants receiving all or partial breast feeding, number of co-interventions (occupational, physical and/or speech therapy), and number of parental visits. Postmenstrual age (PMA), days of life, weight, behavioral state during feeding using a 3-point scale (1=asleep, 2= drowsy/awake, 3=fussy/crying), and episodes of apnea, bradycardia and/or oxygen desaturation at the three monitored oral feeding sessions, were also recorded.
2.4 Instrumentation
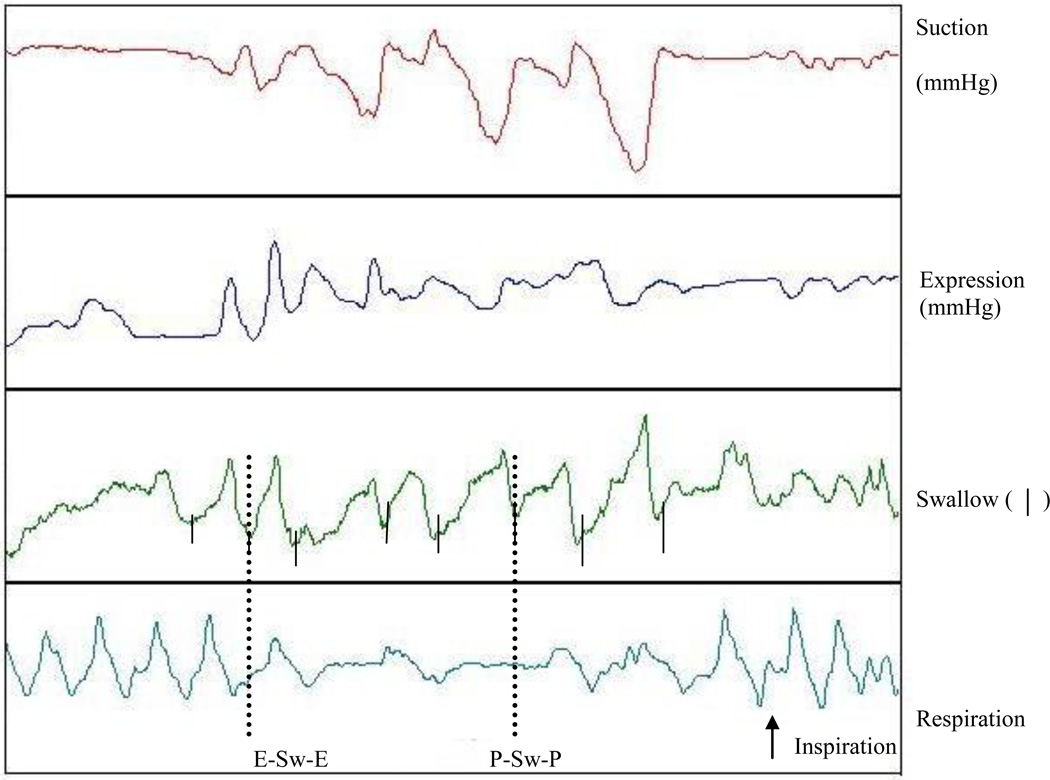
A nipple-bottle apparatus that simultaneously recorded suck (suction and expression components), swallow, and respiration was used16. A Mikro-tip sensor transducer (model SPR-524, Miller Instruments, Houston, TX) inserted through a catheter flush with the tip of the nipple was used to measure the suction component. The expression component was measured via an identical sensor inserted through a silastic tube 0.5 cm from nipple tip and positioned upward against the hard palate. Similac Infant Nipples (Ross Laboratories, Columbus, OH) were used for all participants. Swallowing was monitored via an air pressure drum held snugly onto the hyoid/hyolaryngeal region16. There is a consistent upward and anterior movement of the larynx during swallowing resulting in a pressure change inside the drum. We used the initial peak pressure deflection as a marker of swallowing in our pattern analysis of swallow-respiration coordination16,17. Respiratory movements were monitored using an air pressure drum taped to the midline of the thoraco-abdominal junction16,17. Inspiratory and expiratory movements result in positive and negative changes in the drum, respectively (Figure 2). All signals were recorded using Biopac MP 100 WSP software (Biopac Systems, Inc., Santa Barbara, CA). Data were stored for analyses using the Acknowledge software program version 3.9.1.
Figure 2.
Sample tracing of swallow-respiratory movements (pressure changes during respiration) recorded from one infant during oral feeding, demonstrating, the pause-swallow-pause pattern (P-Sw-P), and expiration-swallow-expiration pattern (E-Sw-E).
2.5 Data reduction and statistical analyses
A weighted average was used for the analyses of nutritive suck, suck-swallow and swallow-respiration outcomes14. This weighted average was calculated from three sucking bursts occurring at the first, second and final third of the feeding session, and was computed as follows: (T1*B1 + T2*B2 + T3*B3) / (T1 + T2 +T3), whereby T1, T2, T3 correspond to the duration (sec) of the respective sucking bursts, and B1, B2, B3 relate to the average value of a particular measure. Sucking bursts were delineated by pauses ≥1.5 seconds. The three sucking bursts analyzed were selected so that they were representative of those sucking stages and sucking burst durations within each respective feeding period.
Figure 2, illustrates a sample tracing of sucking, swallowing and respiratory movements. Suction and expression amplitudes were identified with a change in pressure greater than 1 mmHg. Suck-swallow ratio was calculated by taking the total number of expression components over the number of swallows. Suck-swallow intervals were measured as the time interval (sec) from peak expression to swallow. The respiratory phase (inspiratory or expiratory) that preceded and followed each swallow were used to determine swallow-respiration pattern.
To assess the effect of O, T/K, and combined (O+T/K) interventions on all outcomes 2-way repeated measures ANOVA was used to compare between and within group differences over time. In case of statistical significance post-hoc Tukey tests were used. Effect sizes (ES) were calculated using Cohen’s definition18. ES of 0.2, 0.5, and 0.8 were considered small, moderate and large effects, respectively18. Significance was set at 0.05. Sample size estimation was adequate to detect differences at the p=0.05 level, with a power of 0.804. Analyses were performed using the Statistical Program for Social Sciences software version 18.0 (SPSS Inc, Chicago, IL).
3. Results
3.1 Study population
Eighty-four participants were enrolled. After enrollment, 4 infants were transferred to another hospital, 4 developed NEC and 1 had a congenital heart defect. These conditions were diagnosed prior to the onset of the study. Therefore, a total of 75 infants (29 [0.3] weeks GA) completed the study. Baseline characteristics were comparable for infants in all four groups (Table 1). The ethnic distribution is reflective of Harris County, Houston, TX, where the study was conducted. The covariates were equally distributed among all groups.
Table 1.
Baseline characteristics of preterm infants in the four groups.
| O n=19 |
T/K n=18 |
O+T/K n=18 |
Control n=20 |
Pa | |
|---|---|---|---|---|---|
| Gestational age (wks) | 29.6 (0.4) | 29.1 (0.3) | 29.0 (0.3) | 29.4 (0.1) | 0.689 |
| Infant distribution | |||||
| 26–29 weeks | 10 | 8 | 11 | 9 | 0.266b |
| 30–32 weeks | 9 | 10 | 7 | 11 | |
| Birth weight (g) | 1359.7 (78.2) | 1325.4 (53.3) | 1329.6 (39.1) | 1346.6 (39.3) | 0.988 |
| Gender distribution | |||||
| Male | 12 | 11 | 10 | 16 | 0.057b |
| Female | 7 | 7 | 8 | 4 | |
| Apgar score (5 min) | 8.1 (0.1) | 8.5 (0.1) | 8.3 (0.1) | 8.3 (0.1) | 0.089 |
| Ethnic Distribution | |||||
| African American | 8 | 3 | 4 | 5 | 0.010b |
| Cucasian | 6 | 4 | 9 | 3 | |
| Hispanic | 5 | 11 | 5 | 12 |
Data presented as means (SEM, standard error of mean) or number of participants,
One-way ANOVA unless otherwise indicated,
Fisher’s exact test
3.2 Nutritive sucking skills
There was a significant group (F3,69=5.222, p=0.007), time (F2,138=12.059, p<0.001), and group by time interaction effect (F6,141=2.930, p=0.022) in stage of sucking (Table 2). Post-hoc group tests indicated that only the O group achieved a significantly more advanced stage of sucking compared to controls (p=0.003, ES: O=1.2). All other group comparisons were not significant (p≥0.350, ES≤0.4). Post-hoc time tests indicated that stage of sucking improved significantly over time from 1–2 to 3–5 and 6–8 oral feedings/day (p<0.001, ES≥0.2). Post-hoc group by time tests indicated that the O group had significantly greater stages of sucking than controls at 1–2 oral feedings/day (p<0.001 ES: O=1.3); there was no significant difference between groups at 3–5 oral feedings/day, but at 6–8 oral feedings/day the O group had greater stages of sucking than the control, T/K and combined (O+T/K) groups (p<0.039, ES≥0.3).
Table 2.
Stages of sucking, suction amplitude, and expression amplitude at the three monitored oral feeding sessions
| O n=19 |
T/K n=18 |
O+T/K n=18 |
Control n=20 |
|
|---|---|---|---|---|
| Stage of sucking | ||||
| 1–2 PO/day | 3.3 (0.1)a | 3.1 (0.1) | 3.2 (0.1) | 2.8 (0.1) |
| 3–5 PO/day | 3.3 (0.1) | 3.2 (0.1) | 3.1 (0.1) | 3.0 (0.1) |
| 6–8 PO/day | 3.7 (0.1)a,b, c | 3.3 (0.1) | 3.2 (0.1) | 3.1 (0.1) |
| Group mean | 3.4 (0.1)a | 3.2 (0.1) | 3.2 (0.1) | 3.0 (0.1) |
| Suction amplitude | ||||
| 1–2 PO/day | −35.3 (5.0) | −34.8 (6.3) | −28.8 (4.5) | −24.0 (3.4) |
| 3–5 PO/day | −53.4 (5.5) | −42.4 (8.7) | −41.8 (8.1) | −35.9 (5.0) |
| 6–8 PO/day | −46.4 (5.5) | −46.4 (6.7) | −46.7 (8.8) | −38.3 (5.8) |
| Group mean | −45.0 (5.3)a,c | −41.2 (7.2) | −39.1 (7.1) | −32.7 (4.7) |
| Expression amplitude | ||||
| 1–2 PO/day | 6.5 (1.9)a, b | 1.9 (0.6) | 6.7 (1.5) | 1.5 (0.3) |
| 3–5 PO/day | 3.4 (1.2) | 1.9 (0.4)a | 3.4 (1.0) | 3.7 (0.6) |
| 6–8 PO/day | 15.7 (2.6)a,b,c | 3.2 (0.7) | 2.9 (0.9) | 2.5 (0.5) |
| Group mean | 8.5 (1.9)a,b | 2.3 (0.6) | 4.3 (1.1) | 2.6 (0.5) |
PO-oral feedings/day, Data presented as means (SEM) or number of infants, Repeated measures ANOVA followed by post-hoc Tukey tests
p < 0.05 vs. control,
p < 0.05 vs. T/K,
p < 0.05 vs. O+T/K
In suction amplitude (Table 2) there was a significant group effect (F3,69=4.804, p=0.011), but no time (F2,138=3.272, p=0.076) nor group by time effect (F6,141=0.633, p=0.674). Post-hoc tests indicated that suction amplitude was significantly greater in the O compared to the control and combined (O+T/K) groups (p≤0.035, ES O≥0.2). No other group comparisons were significant (p≥0.540, ES≤0.2).
In expression amplitude (Table 2), there was a significant group (F3,69=8.347, p=0.006), time (F2,138=8.434, p=0.007), and group by time interaction effect (F6,141=11.360, p=0.001). Post-hoc group tests indicated that the O group had significantly greater expression amplitudes than control and T/K groups (p≤0.026, ES≥0.82). All other group comparisons were not significant (p≥0.370, ES≤0.4). Post-hoc time tests indicated that expression amplitude increased significantly over time from 1–2 and 3–5 to 6–8 oral feedings/day (p≤0.040, ES≥0.5). Post-hoc group by time tests indicated that the T/K group had significantly smaller expression amplitudes than controls at 3–5 oral feedings/day (p=0.034, ES=0.2), and the O group had significantly greater expression amplitudes than control, T/K, and combined (O+T/K) groups at 6–8 oral feedings/day (p<0.003, ES≥0.6).
3.2 Suck-swallow coordination
There were no significant group, time, nor group by time interaction effects for ratio of sucks/swallows (mean 1.1 [0.04]) and stability of suck-swallow interval (mean 0.3 [0.2], p≥0.181, ES≤0.3).
3.3 Swallow-respiration coordination
There was only a significant group effect in percent occurrence of the P-Sw-P pattern (Table 3, F3,69=4.615, p=0.009) and E-Sw-E pattern (F3,69=8.938, p=0.001). Post-hoc tests indicated that all three interventions groups had significantly fewer P-Sw-P patterns than the control group (p≤0.044, ES≥0.7), and the T/K and combined (O+T/K) groups had a significantly greater occurrence of the E-Sw-E pattern than either the control or O groups (p≤0.03, ES≥0.3).
Table 3.
Mean percent occurrence of swallow-respiration patterns in the study groups at the three monitored oral feeding sessions
| O n=19 |
T/K n=18 |
O+T/K n=18 |
Control n=20 |
|
|---|---|---|---|---|
| Start I-Sw-End E | ||||
| 1–2 PO/day | 18.4 (2.1) | 11.0 (2.4) | 16.1 (1.9) | 12.1 (3.4) |
| 3–5 PO/day | 19.2 (2.5) | 12.5 (1.9) | 18.2 (2.5) | 14.4 (2.1) |
| 6–8 PO/day | 17.1 (1.5) | 13.7 (1.7) | 15.7 (3.2) | 16.2 (2.6) |
| Group mean | 18.2 (2.0) | 12.4 (2.0) | 16.7 (2.5) | 14.2 (2.7) |
| I-Sw-I | ||||
| 1–2 PO/day | 21.7 (1.9) | 25.9 (5.8) | 23.2 (3.3) | 19.8 (2.1) |
| 3–5 PO/day | 21.9 (2.7) | 26.8 (2.8) | 16.9 (2.2) | 20.8 (3.3) |
| 6–8 PO/day | 19.1 (2.5) | 20.4 (2.5) | 22.2 (3.0) | 14.2 (2.7) |
| Group mean | 20.9 (2.4) | 24.4 (3.7) | 20.8 (2.8) | 18.3 (2.7) |
| End I-Sw-Start E | ||||
| 1–2 PO/day | 16.0 (2.0) | 14.2 (4.9) | 13.5 (2.8) | 12.8 (1.3) |
| 3–5 PO/day | 17.2 (2.3) | 14.0 (1.9) | 14.1 (2.7) | 11.1 (1.9) |
| 6–8 PO/day | 15.2 (1.8) | 17.3 (1.8) | 13.9 (1.9) | 14.2 (3.0) |
| Group mean | 16.1 (2.0) | 15.2 (2.9) | 13.8 (2.5) | 12.7 (2.1) |
| E-Sw-E | ||||
| 1–2 PO/day | 13.0 (2.0) | 20.7 (8.3) | 17.2 (2.1) | 9.1 (2.3) |
| 3–5 PO/day | 11.1 (3.0) | 20.7 (2.6) | 19.8 (2.9) | 10.2 (3.1) |
| 6–8 PO/day | 15.1 (1.6) | 18.0 (2.5) | 21.1 (3.0) | 9.0 (4.9) |
| Group mean | 13 (2.2) | 19.8(4.5)a, b | 19.4(2.7)a, b | 9.4 (3.4) |
| Sw interrupt I | ||||
| 1–2 PO/day | 6.2 (1.4) | 6.1 (3.1) | 6.4 (2.1) | 5.9 (1.2) |
| 3–5 PO/day | 7.0 (1.6) | 6.3 (1.1) | 2.7 (0.7) | 2.0 (0.5) |
| 6–8 PO/day | 8.3 (1.6) | 4.3 (0.9) | 3.1 (0.8) | 6.2 (1.3) |
| Group mean | 7.2 (1.5) | 5.6 (1.7) | 4.1 (1.2) | 4.7 (1.0) |
| Sw interrupt E | ||||
| 1–2 PO/day | 6.0 (1.1) | 5.6 (2.6) | 3.8 (0.8) | 3.3 (2.2) |
| 3–5 PO/day | 6.5 (1.6) | 5.6 (0.9) | 5.1 (1.1) | 9.1 (2.1) |
| 6–8 PO/day | 4.1 (0.9) | 6.1 (0.9) | 3.0 (1.4) | 6.2 (1.2) |
| Group mean | 5.5 (1.2) | 5.8 (1.5) | 4.0(1.1) | 6.2 (1.8) |
| P-Sw-P | ||||
| 1–2 PO/day | 18.7 (3.9) | 16.5 (2.6) | 17.8 (5.4) | 38.8 (6.0) |
| 3–5 PO/day | 17.2 (4.0) | 14.2 (4.0) | 23.2 (4.1) | 32.5 (6.0) |
| 6–8 PO/day | 21.1 (4.3) | 20.3 (4.7) | 21.0 (5.0) | 34.0 (5.3) |
| Group mean | 19.0 (4.1)a | 17.0 (3.8)a | 20.7 (6.5)a | 35.1 (5.8) |
I-inspiration, Sw-swallow, E-expiration, PO-oral feedings/day, P-pause, Data presented as means (SEM) Repeated measures ANOVA followed by post-hoc Tukey tests
p < 0.05 vs. control,
p < 0.05 vs. O
4. Discussion
This study investigated the effect of oral and non-oral sensorimotor interventions on sucking, swallowing, and respiration coordination in preterm infants. Results demonstrated that all three interventions impacted these coordinative functions, to some degree.
The O intervention was the only one that resulted in more advanced nutritive sucking skills compared to controls. Specifically, it led to more mature nutritive sucking pattern and improved expression amplitude at 1–2 and 6–8 oral feedings/day, and overall greater suction amplitude compared to controls. These improvements may be due to the direct sensorimotor input to the oral musculoskeletal system involved in sucking3. Interestingly, the O group had significantly better nutritive sucking skills at 1–2 and 6–8 oral feedings/day only, yet they were able to achieve better proficiency at 1–2 and 3–5 oral feedings/day, volume transfer at 1–2, 3–5 and 6–8 oral feedings/day compared to controls in our earlier study4. Moreover, the T/K and combined (O+T/K) did not lead to better nutritive sucking skills than controls. However, they improved proficiency, volume intake and rate of transfer compared to the controls in our earlier work4. These findings demonstrate that sucking is one of many factors involved in oral feeding19, and that suck- swallow-respiration coordination is a critical factor in achieving safe and successful oral feeding in preterm infants.
We also observed that 30 minutes of O intervention, in this study improved stages of sucking as well as suction and expression amplitudes. In our earlier work3, 15 minutes of the same O intervention increased sucking stages and the expression component only. It appears that the suction component requires longer input than the expression component, most likely due to the higher complexity of muscle activity involved in its generation. These data suggest that duration of the sensorimotor intervention is an important determinant for the achievement of specific nutritive sucking skills.
Suck-swallow coordination outcomes, including suck to swallow ratio and stability of the suck-swallow interval did not significantly differ among the groups. These results are likely due to infants having already established a coordinated suck-swallow rhythm by the time of their first testing (mean age 34 weeks PMA). A stable 1:1 suck-swallow ratio and interval is attained by 31 and 33 weeks PMA, respectively12, 20.
For swallow-respiration coordination, all three interventions reduced the occurrence of P-Sw-P pattern, and the T/K and combined (O+T/K) groups had greater occurrence of the E-Sw-E pattern compared to controls. These findings are indicative of more advanced swallow-respiration coordination11, 14, 21. The swallow-respiration coordinative pattern is influenced by degree of prematurity11, 14, 21. The predominant swallow-respiration pattern in preterm infants 34–35 weeks PMA is P-Sw-P11, 14. As preterm infants mature P-Sw-P decreases and E-Sw-E increases11, 14, 22. The E-Sw-E pattern is potentially “the safest” for airway protection and may facilitate laryngeal elevation and cricopharygeal sphincter opening10, 11, 13, 23. Our finding that O, T/K, and combined (O+T/K) interventions improved swallow-respiration coordination is novel and intriguing. At this moment, we can only speculate as to why both oral and non-oral interventions had a positive impact on swallow-respiration coordination. Perhaps these interventions have distributed effects that go beyond their site of intervention and/or they impact underlying neuronal and musculoskeletal structures that in turn improve swallow-respiration coordination. Looking specifically at their direct effects, the O which only targeted the oral structures, enhanced nutritive sucking, which may have led to increased bolus control and decreased transit times, reducing the requirements for prolonged respiratory pauses to accommodate the swallow. The T/K, which targeted the whole body likely enhanced postural control of head, neck, and trunk, allowing for a more stable base for swallow-respiration24, leading not only to a reduction in the pause-swallow-pause phase, but also increased the expiration-swallow-expiration phase. These data support the growing body of experimental and clinical literature that there are multiple points of interaction in developing sensorimotor systems6.
The combined (O+T/K) intervention when compared to the O or T/K intervention did not lead to additive/synergistic benefits in sucking, swallowing and respiration. This may have been due to the shorter duration of each intervention. Investigating dosage and cross-system interventions would be an interesting next step in this research.
Contrary to other studies, we did not find a consistent increase in nutritive sucking, suck-swallow and swallow-respiration outcomes over time (i.e. oral feeding progression)11, 22. Results may be due to the differences in methodologies where the other studies monitored suck-swallow-respiration coordination at specific PMAs’22, while we monitored these outcomes at three oral feeding sessions.
5. Conclusion
The O intervention improved specific components of nutritive sucking skills. All three interventions reduced the occurrence of P-Sw-P, but only the T/K and combined (O+T/K) interventions led to greater occurrence of E-Sw-E, “safer swallows.” Sensorimotor input appears to have distributed effects that go beyond their specific system target, thereby enhancing sucking, swallowing, and respiration coordination.
Acknowledgements
The authors would like to thank all the nurses at Texas Children’s Hospital for their collaboration in the data collection. This study was supported by the Fonds de la Recherche en Santé du Québec, and a National Institute of Child Health and Human Development grant R01-HD 044469. The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of the National Institute of Child Health and Human Development or the National Institutes of Health.
LIST OF ABBREVIATIONS
- ES
Effect size
- E
Expiration
- GA
Gestational age
- I
Inspiration
- O
Oral
- P
Pause
- PMA
Postmenstrual age
- SEM
Standard error of mean
- Sw
Swallow
- T/K
Tactile/kinesthetic
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
The authors do not have any conflict of interest to disclose.
References
- 1.McGrath JM, Braescu AV. State of the science: feeding readiness in the preterm infant. J Perinat Neonatal Nurs. 2004;18:353–368. doi: 10.1097/00005237-200410000-00006. [DOI] [PubMed] [Google Scholar]
- 2.Collins CT, Makrides M, McPhee AJ. Early discharge with home support of gavage feeding for stable preterm infants who have not established full oral feeds. Cochrane Database Syst Rev. 2003;4 doi: 10.1002/14651858.CD003743. CD003743. [DOI] [PubMed] [Google Scholar]
- 3.Fucile S, Gisel EG, Lau C. Effect of an oral stimulation program on sucking skill maturation of preterm infants. Dev Med Child Neurol. 2005;47:158–162. doi: 10.1017/s0012162205000290. [DOI] [PubMed] [Google Scholar]
- 4.Fucile S, Gisel EG, McFarland DH, Lau C. Oral and non-oral sensorimotor interventions enhance feeding performance in preterm infants. Dev Med Child Neurol. 2011;53:829–835. doi: 10.1111/j.1469-8749.2011.04023.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barlow SM, Dusick A, Finan DS, Coltart S, Biswas A. Mechanically evoked perioral reflexes in premature and term human infants. Brain Res. 2001;899:251–254. doi: 10.1016/s0006-8993(01)02239-9. [DOI] [PubMed] [Google Scholar]
- 6.McFarland DH, Tremblay P. Clinical implications of cross-systsem interactions. Semin Speech Lang. 2006;27:300–309. doi: 10.1055/s-2006-955119. [DOI] [PubMed] [Google Scholar]
- 7.Fucile S, Gisel E, Lau C. Oral stimulation accelerates the transition from tube to oral feeding in preterm infants. J Pediatr. 2002;141:230–236. doi: 10.1067/mpd.2002.125731. [DOI] [PubMed] [Google Scholar]
- 8.Field TM, Schanberg SM, Scafidi F, Bauer CR, Vega-Lahr N, Garcia R, et al. Tactile/kinesthetic stimulation effects on preterm neonates. Pediatrics. 1986;77:654–658. [PubMed] [Google Scholar]
- 9.Lau C, Alagugurusamy R, Schanler RJ, Smith EO, Shulman RJ. Characterization of the developmental stages of sucking in preterm infants during bottle feeding. Acta Paediatr. 2000;89:846–852. [PubMed] [Google Scholar]
- 10.Bamford O, Taciak V, Gewolb IH. The relationship between rhythmic swallowing and breathing during suckle feeding in term neonates. Pediatr Re. 1992;31:619–624. doi: 10.1203/00006450-199206000-00016. [DOI] [PubMed] [Google Scholar]
- 11.Gewolb IH, Vice FL. Maturational changes in the rhythms, patterning, and coordination of respiration and swallow during feeding in preterm and term infants. Dev Med Child Neuro. 2006;48:589–594. doi: 10.1017/S001216220600123X. [DOI] [PubMed] [Google Scholar]
- 12.Gewolb IH, Vice FL, Schwietzer-Kenney EL, Taciak VL, Bosma JF. Developmental patterns of rhythmic suck and swallow in preterm infants. Dev Med Child Neurol. 2001;43:22–27. doi: 10.1017/s0012162201000044. [DOI] [PubMed] [Google Scholar]
- 13.McFarland DH, Lund JP, Gagner M. Effects of posture on the coordination of respiration and swallowing. J Neurophysiol. 1994;72:2431–2437. doi: 10.1152/jn.1994.72.5.2431. [DOI] [PubMed] [Google Scholar]
- 14.Lau C, Smith EO, Schanler RJ. Coordination of suck-swallow and swallow- respiration in preterm infants. Acta Paediatr. 2003;92:721–727. [PubMed] [Google Scholar]
- 15.Brazy JE, Eckerman CO, Oehler JM, Goldstein RF, O'Rand AM. Nursery Neurobiologic Risk Score: important factor in predicting outcome in very low birth weight infants. J Pediatr. 1991;118:783–792. doi: 10.1016/s0022-3476(05)80047-2. [DOI] [PubMed] [Google Scholar]
- 16.Lau C, Schanler RJ. Oral motor function in the neonate. Clin Perinatol. 1996;23:161–178. [PubMed] [Google Scholar]
- 17.Tarrant SC, Ellis RE, Flack FC, Selley WG. Comparative review of techniques for recording respiratory events at rest and during deglutition. Dysphagia. 1997;12:24–38. doi: 10.1007/pl00009515. [DOI] [PubMed] [Google Scholar]
- 18.Portney LG, Watkins MP. Foundations of clinical research: applications to practice. 2nd edn. Upper Saddle River, NJ: Prentice Hall; 2003. [Google Scholar]
- 19.Scheel CE, Schanler RJ, Lau C. Does the choice of bottle nipple affect the oral feeding performance of very-low-birthweight (VLBW) infants? Acta Paediatr. 2005;94:1266–1272. doi: 10.1080/08035250510027255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Amaizu N, Shulman R, Schanler R, Lau C. Maturation of oral feeding skills in preterm infants. Acta Paediatr. 2008;97:61–67. doi: 10.1111/j.1651-2227.2007.00548.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mizuno K, Ueda A. The maturation and coordination of sucking, swallowing, and respiration in preterm infants. J Pediatr. 2003;142:36–40. doi: 10.1067/mpd.2003.mpd0312. [DOI] [PubMed] [Google Scholar]
- 22.Nixon GM, Charbonneau I, Kermack AS, Brouillette RT, McFarland DH. Respiratory-swallowing interactions during sleep in premature infants at term. Respir Physiol Neurobiol. 2007;160:76–82. doi: 10.1016/j.resp.2007.08.010. [DOI] [PubMed] [Google Scholar]
- 23.Kawasaki M, Ogura JH. Interdependence of deglutition with respiration. Ann Otol Rhinol Laryngol. 1968;77:906–913. doi: 10.1177/000348946807700509. [DOI] [PubMed] [Google Scholar]
- 24.Redstone F, West JF. The importance of postural control for feeding. Pediatr Nurs. 2004;30:97–100. [PubMed] [Google Scholar]