To the Editors
Programmed death-1 (PD-1/CD279) cell-surface protein, an inhibitory member of the CD28 costimulatory receptor superfamily, is expressed mainly on a subset of activated T lymphocytes including helper T cells located in the follicle germinal centers.1,2 PD-1 inhibits T-cell activity3 by providing a second signal to T cells in conjunction with signaling through the T-cell receptor for antigen. Two cell-membrane proteins designated PD-L1 (B7-H1CD274) and PD-L2 (B7-DC/CD273) have been identified as ligands for PD-14,5. Interactions between PD-1 and the ligands control the induction and maintenance of peripheral T-cell tolerance during normal immune responses. These interactions may play a role also in immune evasion in malignancy,6–8 since PD-1 expression tends to be upregulated on tumor infiltrating lymphocytes9 and cancer cells of various types aberrantly express PD-L16.
In this report, we present results of the immunohistochemical examination of the PD-1 and PD-L1 expression at the patch/plaque, tumor, and large cell transformation stages of CTCL. CTCL cases for the study were retrieved from the Departments of Pathology and Laboratory Medicine and Dermatopathology at the University of Pennsylvania. The diagnosis of different stages of CTCL was established based on the clinical presentation and histologic and immunohistochemical evaluation. To perform the PD-1 and PD-L1 stains, the formalin-fixed paraffin-embedded CTCL tissue specimen slides were heat-treated for antigen retrieval by boiling in 10 mM citrate buffer pH 6 for 20 min. The sections were blocked with the peroxidase blocking system (Dako, Carpinteria, CA) for 10 min and 2% normal horse serum for 20 min at room temperature (RT). To detect PD1 expression, the slides were incubated with the primary PD-1 mouse monoclonal antibody (Abcam, Cambridge, MA) at 1:200 dilution (overnight at 4°C); secondary biotinylated anti-mouse antibody at 1:250 dilution (30 min at RT); the streptavidin-biotinylated horseradish peroxidase complex reagent (Dako) (30 min at RT); and three 5 min washes in buffer after each incubations. To detect the PD-L1 expression, the slides were incubated at RT with the rabbit PD-L1 antibody (Lifespan Biosciences, Seattle, WA) at 1:200 dilution for 30 min and an anti-rabbit-HRP polymer for 30 min followed by washes. The PD-1- or PD-L1-antibody stained slides were exposed to the DAB plus chromagen (Dako) for 5 min at RT and counterstained with hematoxylin. The results of this evaluation are summarized in Table 1 and the representative images are presented in Fig. 1. The retrieved 26 specimens were classified as 9 patch/plaque, 6 tumor, and 9 large cell transformation stage lesions.
Table 1.
Expression of immunosuppressive proteins PD1 and PDL-1 at the depicted clinical-histological stages of CTCL/MF
| PD1 | PDL-1 | |||
|---|---|---|---|---|
| Patch/plaque stage | ||||
| 0–2 (% of atypical lymphocytes) | 0 (number of cases) | 0 | ||
| 3–25% | 2 | 1 | ||
| 26–50% | 4 | 1 | ||
| >50% | 3 | 7 | ||
| Tumor stage | ||||
| 0–2% | 1 | 0 | ||
| 3–25% | 2 | 0 | ||
| 26–50% | 2 | 0 | ||
| >50% | 1 | 6 | ||
| Large cell transformation | Small atypical lymphocytes | Large transformed lymphocytes | Small atypical lymphocytes | Large transformed lymphocytes |
| 0–2% | 1 | 9 | 0 | 0 |
| 3–25% | 4 | 2 | 0 | 0 |
| 26–50% | 3 | 0 | 0 | 0 |
| >50% | 3 | 0 | 11 | 11 |
FIGURE 1.
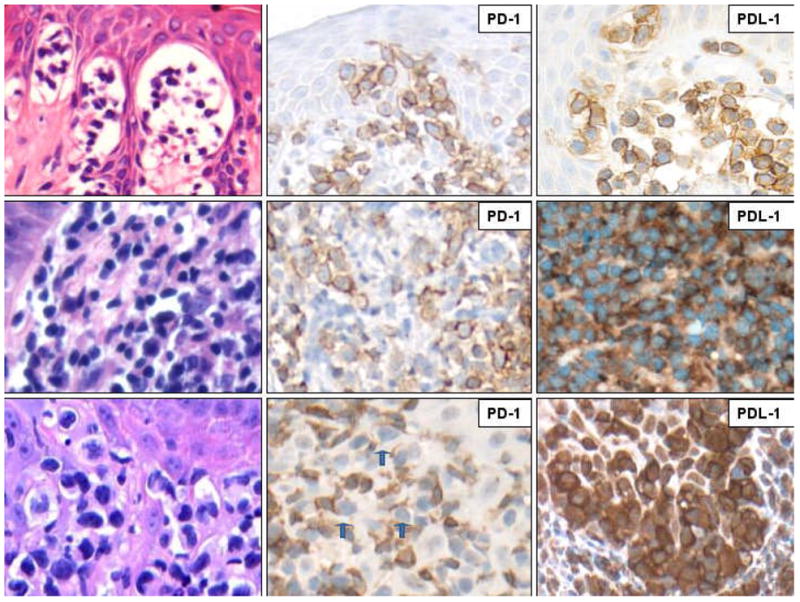
PD-1 and PD-L1 expression at various stages of CTCL/MF: patch/plague (upper row), tumor (middle row), and large cell transformation (lower row). The formalin-fixed, paraffin-embedded skin biopsy tissue sections were stained with H&E (left column) or antibodies against PD-1 (middle column) or PD-L1 (right column). The depicted images are representative of the 26 CTCL cases examined and were captured at the 400X magnification. The blue arrows in the PD-1 stain of the large cell transformation case highlight the negative large lymphocytes.
PD-1 was frequently expressed at the early, patch and plaque stages of CTCL (Fig. 1, upper panel in the middle column). PD-1 was detected in all nine patch and plaque cases, in seven of the nine cases more than 25% of the atypical lymphocytes expressed PD-1 including three with staining was present in at least 50% of the lymphocytes (Table 1, middle column, rows 3–6). By comparison, PD-1 was expressed less frequently at the tumor stage of the lymphoma (Fig. 1, middle panel/middle column and Table 1, middle column, rows 8–11). Accordingly, PD-1-positive cells exceeded 25% in three of the six cases examined and in one they comprised more than 50%. Of note, the PD-1 expression loss preferentially affected lymphocytes that have undergone large cell transformation. (Fig. 1, lower panel/middle column and Table 1, middle columns, rows 13–16); while the small atypical cells were frequently PD-1 positive, in 9 of the 11 cases examined PD-1-expressing large cells were extremely rare in none of the cases they comprised more than 25% of the large cell population. In contrast to PD-1, all stages of CTCL cases showed staining for PD-L1 in the majority of the atypical lymphocytes. If anything, PD-L1 expression actually seemed to increase with the lymphoma progression with essentially all large transformed lymphocytes strongly expressing the protein on their surface (Fig. 1, right panel column and Table 1, right column).
While the progressive nature of immunosuppression in CTCL is well recognized, the mechanisms that underlie the immune impairment remain essentially unknown. Our recent study demonstrated expression by the atypical lymphocytes in CTCL of FoxP3, the transcription factor defining the key type of regulatory T cells11. However, similar to PD-1, the FOXP3-expressing tumor cells were common at the patch and plaque stages but their frequency was profoundly diminished at the tumor stage and after the large cell transformation. These combined findings strongly suggest that the PD-1 and FoxP3 dependent immunosuppression is important at the early but not later stages of CTCL. As mentioned, PD-1 downregulation seems to result mainly from the large cell transformation of the CTCL cells; the FoxP3 loss appears to occur primarily at transition to the tumor stage11. Of note, the studies on the angioimmunoblastic T-cell lymphoma and other types of the nodal based peripheral T-cell lymphomas suggested that PD-1 expression is highly characteristic for, and most likely related to the follicle germinal center T helper cell derivation of the lymphoma cells.1,12 Although CTCL cells share also another marker with follicular T helper cells: CXCL13,13 they clearly are distinct from these cells given the lack of CD10 and BCL-6 expression as well as lack of follicle formation contrasted by the presence of epidermotropism in the typical CTCL lesions. Therefore, our observation that CTCL cells express PD-1 as well as the one made by others1 regarding the primary cutaneous CD4+ pleomorphic T-cell lymphoma indicate that non-follicular T-cell lymphoma cells are also capable of expressing the receptor. The mechanisms leading to PD-1 expression by the various types of malignant T cells remain to be explored. Studies in animal model showed that PD-L1 expressed by tumors inhibits T cell activation, protects tumor cells from lysis, and in some cases induces death of the tumor-specific T cells.14,15 Wilcox et al documented the importance of PD-L1 in the suppression of host immunity in the subset human T-cell lymphoproliferative disorders including CTCL and peripheral T cell lymphoma PTCL by showing that PD-L1 inhibits immune T-cells and promotes the induction of FoxP3+ regulatory T cells.16 Our current findings also point to PD-L1 as the likely key immunosuppressive protein in the advanced CTCL. Of note, the large T-cell lymphoma expressing anaplastic lymphoma kinase (ALK) is also PD-L1 positive,10,17 suggesting some common pathogenic aspects for these two types of lymphoma. It may be related to the persistent activation of the transcription factor STAT3 since PD-L1 gene is induced by the factor in the ALK-positive T-cell lymphomas10 and the activated, tyrosine-phosphorylated form of STAT3 is expressed at all stages of CTCL18 with some evidence suggesting particularly strong expression in advanced stage of the disease like tumor stage than MF19. Finally, our findings may have potential therapeutic implications for CTCL. Thus, antibody-induced blockade of PD-16 may improve the anti-tumor immunity and, in principle, could be used as an adjuvant to standard therapy, in particular at the early stages of CTCL when expression of this immunosuppressive receptor is the most pronounced. Similarly antibody-mediated inhibition of PD-L16,20 may increase the number and effectiveness of the tumor-specific immune T cells. This may be particularly important at the more advanced stages of CTCL, when the PD-L1 expression seems the strongest, the immunosuppression the most pronounced, and the current therapies are the least effective in controlling the lymphoma.
References
- 1.Rodriguez Pinilla SM, Roncador G, et al. Primary cutaneous CD4+ small/medium-sized pleomorphic T-cell lymphoma expresses follicular T-cell markers. Am J Surg Pathol. 2009;33:81–90. doi: 10.1097/PAS.0b013e31818e52fe. [DOI] [PubMed] [Google Scholar]
- 2.Nishimura H, Agata Y, Kawasaki A, et al. Developmentally regulated expression of the PD-1 protein on the surface of double-negative (CD4−CD8−) thymocytes. Int Immunol. 1996;8:773–780. doi: 10.1093/intimm/8.5.773. [DOI] [PubMed] [Google Scholar]
- 3.Riley JL, June CH. The CD28 family: a T-cell rheostat for therapeutic control of T-cell activation. Blood. 2005;105:13–21. doi: 10.1182/blood-2004-04-1596. [DOI] [PubMed] [Google Scholar]
- 4.Chen L. Co-inhibitory molecules of the B7-CD28 family in the control of T-cell immunity. Nat Rev Immunol. 2004;4:336–7. doi: 10.1038/nri1349. [DOI] [PubMed] [Google Scholar]
- 5.Yamamoto R, Nishikori M, Kitawaki T, et al. PD-1–PD-1 ligand interaction contributes to immunosuppressive microenvironment of Hodgkin lymphoma. Blood. 2008;111:3220–3224. doi: 10.1182/blood-2007-05-085159. [DOI] [PubMed] [Google Scholar]
- 6.Keir ME, Butte MJ, Freeman GJ, et al. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dong H, Chen L. B7–H1 pathway and its role in the evasion of tumor immunity. J Mol Med. 2003;81:281–287. doi: 10.1007/s00109-003-0430-2. [DOI] [PubMed] [Google Scholar]
- 8.Okazaki T, Honjo T. PD-1 and PD-1 ligands: from discovery to clinical application. Intern Immunol. 2007;19:813–824. doi: 10.1093/intimm/dxm057. [DOI] [PubMed] [Google Scholar]
- 9.Blank C, Brown I, Marks R, et al. Absence of programmed death receptor 1 alters thymic development and enhances generation of CD4/CD8 double-negative TCR-transgenic T cells. J Immunol. 2003;171:4574–4581. doi: 10.4049/jimmunol.171.9.4574. [DOI] [PubMed] [Google Scholar]
- 10.Marzec M, Zhang Q, Goradia A, et al. Oncogenic kinase NPM/ALK induces through STAT3 expression of immunosuppressive protein CD274 (PD-L1, B7-H1) Proc Natl Acad Sci USA. 2008;105:20852–20857. doi: 10.1073/pnas.0810958105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kasprzycka M, Zhang Q, Witkiewicz A, et al. Gamma c-Signaling Cytokines Induce a Regulatory T cell Phenotype in Malignant CD4+ T Lymphocytes. J Immunol. 2008;181:2506–2512. doi: 10.4049/jimmunol.181.4.2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dorfman DM, Brown JA, Shahsafaei A, et al. Programmed Death (PD-1) is a marker of germinal center associated T cells and angioimmunoblastic T cell lymphoma. Am J Surg Pathol. 2006;30:802–810. doi: 10.1097/01.pas.0000209855.28282.ce. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Picchio MC, Scala E, Pomponi D, et al. CXCL13 is highly produced by Sézary cells and enhances their migratory ability via a synergistic mechanism involving CCL19 and CCL21 chemokines. Cancer Res. 2008;68:7137–7146. doi: 10.1158/0008-5472.CAN-08-0602. [DOI] [PubMed] [Google Scholar]
- 14.Dong H, Strome SE, Salomao DR, et al. Tumour associated B7-H1 promotes T cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8:793–800. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- 15.Hirano F, Kaneko K, Tamura H, et al. Blockade of B7-H1 and PD-1 by monoclonal antibodies potentiates cancer therapeutic immunity. Cancer Res. 2005;65:1089–1096. [PubMed] [Google Scholar]
- 16.Wilcox RA, Feldman AL, Wada DA, et al. B7-H1 (PD-L1, CD274) suppresses host immunity in T-cell lymphoproliferative disorders. Blood. 2009;114:2149–2158. doi: 10.1182/blood-2009-04-216671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brown JA, Dorfman DM, Ma FR, et al. Blockade of programmed death-1 ligands on dendritic cells enhances T cell activation and cytokine production. J Immunol. 2003;170:1257–1266. doi: 10.4049/jimmunol.170.3.1257. [DOI] [PubMed] [Google Scholar]
- 18.Witkiewicz A, Raghunath P, Wasik A, et al. Loss of SHP-1 tyrosine phosphatase expression correlates with the advanced stages of cutaneous T-cell lymphoma. Human Pathology. 2007;38:462–467. doi: 10.1016/j.humpath.2006.09.012. [DOI] [PubMed] [Google Scholar]
- 19.Sommer VH, Clemmensen OJ, Nielsen O, et al. In vivo activation of STAT3 in cutaneous T-cell lymphoma. Evidence for an antiapoptotic function of STAT3. Leukemia. 2004;18:1288–1295. doi: 10.1038/sj.leu.2403385. [DOI] [PubMed] [Google Scholar]
- 20.Iwai Y, Ishida M, Tanaka Y, et al. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc Natl Acad Sci USA. 2002;99:12293–12297. doi: 10.1073/pnas.192461099. [DOI] [PMC free article] [PubMed] [Google Scholar]


