Summary
Background
Striking parallels are observed when comparing the literature on the 5-HTTLPR of the serotonin transporter gene (SLC6A4) to the testosterone (T) literature on measures of stress reactivity and neural activity. Short (S) allele carriers and individuals higher in testosterone levels show exaggerated stress responses, amygdala hyperactivity, and reduction of amygdala-prefrontal cortex coupling when exposed to threat.
Methods
Three studies tested the hypothesis that higher T, S carriers would show increased cortisol responses to threat.
Results
Supporting the hypothesis, a T × 5-HTTLPR interaction was obtained across all studies. Threats to status via social exclusion (Study 1), cognitive/perceptual failure (Study 2), and physical competence (Study 3) all produced elevated cortisol levels in S carriers with higher T levels. An unexpected result was that 5-HTTLPR long (L) allele homozygotes with higher T showed lower cortisol levels in response to threat--a pattern of response that closely parallels that reported for psychopathic individuals. Finally, combining effect sizes across studies showed that the likelihood that these effects were due to Type 1 errors was quite low.
Conclusions
What emerges from these studies is a novel yet reliable, and synergistic relationship between 5-HTTLPR genotype and testosterone on stress reactivity, possibly conferring vulnerability for multiple neuropsychiatric disorders.
Keywords: 5-HTTLPR, testosterone, cortisol, stress, depression, psychopathy
One of the most intensively studied individual characteristics predicting stress-reactivity is a common functional polymorphism in the promoter region of the serotonin transporter (5-HTT) gene (SLC6A4). The so-called 5-HTT linked polymorphic region (5-HTTLPR) has been shown to moderate the influence of stress on depression (Caspi et al., 2010) but see also (Belsky et al., 2009, Chiao & Blizinsky, 2010). 5-HTTLPR short (S) allele carriers with a history of stress (e.g., recent life events, childhood maltreatment) are at increased risk to be diagnosed with major depressive disorder (MDD). A dysregulated stress response in S carriers is assumed to underlie the 5-HTTLPR-depression relationship, but only recently has the S allele been linked to acute stress reactivity in humans (Gotlib et al., 2008, Way & Taylor, 2010).
Recent findings have identified a possible neuroanatomical locus for S carrier-linked stress reactivity. A meta-analysis of 17 studies (14 published) (Munafò, Brown, & Hariri, 2008) concluded that “heightened amygdala activation to environmental threat [operationalized in a majority of studies as angry and/or fearful faces] may mediate the association between the 5-HTTLPR S allele and … risk for mood and anxiety disorders.” Further, Pezawas et al., 2005 found that S carriers showed a decrease in functional connectivity between the amygdala and the anterior cingulate cortex (ACC), which may lead to amygdala dysregulation in response to threatening stimuli.
The literature describing testosterone's connection to amygdala activity and stress sensitivity in response to threat shares a striking similarity to the 5-HTTLPR literature. Males with high endogenous levels of testosterone, and middle-aged women administered testosterone showed increased amygdala activation to threat stimuli similar to those used in many of the 5-HTTLPR studies (e.g., fearful and angry faces) (van Wingen et al., 2008; Derntl et al., 2009). Responses in the orbitofrontal cortex (OFC) were also negatively correlated with testosterone levels (van Wingen et al., 2010). In addition to endophenotypic evidence, the relationship between testosterone and OFC activity has also been observed in a measure of economic decision behavior (Mehta & Beer, 2011), implicating the OFC in mediating the relationship between testosterone and interpersonal behavior.
Other studies have documented that exaggerated stress responses (Mehta, Jones & Josephs, 2008), negative affect (Josephs et al., 2006), and changes in blood pressure and heart rate (Newman, Sellers, & Josephs, 2005; Josephs et al., 2006) only manifest when high testosterone subjects are threatened by a potential loss of status, dominance, or power. Indeed, marital and employment status, arguably two critical sources of social status, protected high testosterone men from developing depression (Booth, Johnson, & Granger, 1999). Loss of these sources of status predicted depression onset, but only in high testosterone men.
To date, the possible combined effect of testosterone and the 5-HTTLPR on sensitivity to threat has not been examined. However, there is a biologically plausible reason to believe that an interaction may exist between testosterone and the 5-HTTLPR genotype. In addition to the findings presented, there is evidence that testosterone acts as a 5-HT agonist. Testosterone increases dopaminergic and serotonergic activity in the nucleus accumbens shell (de Souza Silva et al., 2009), which has extensive connections to prefrontal cortex and limbic structures, including the amygdala. Testosterone can also excite amygdala response directly, or indirectly through upregulation of the androgen receptor in limbic structures including the amygdala (Menard & Harlan, 1993). A recent human imaging study found that concentrations of salivary testosterone in healthy men was positively correlated with threat-related amygdala activity (Manuck et al., 2010). Finally, linking status threat to S carrier status, an interesting study of rhesus monkeys showed that relocation stress (conceptually similar to the stress induced by the loss of status caused by social eviction or exclusion) produced increased amygdala reactivity in S (SLC6A4) carriers (Kalin et al., 2008).
In the current set of three studies, participants were exposed to a status threat, and stress-reactivity (measured by change in cortisol) was assessed. In the 5-HTTLPR literature, amygdala responses are more extreme among S carriers upon viewing angry and fearful faces, and during public speaking tasks (Hariri & Holmes, 2006). Interestingly, among behavioral endocrinologists, responses to angry and fearful faces have long been thought to reflect observed dominance-submissive behavior (van Honk et al., 1999), based on the link between testosterone levels (both endogenous and administered) and the selective attention to angry faces observed as a function of testosterone concentration (van Honk et al., 1999; 2001, Wirth & Schultheiss, 2007). Other studies have found that status-threatening cues do not have to be exclusively social in nature to evoke a negative reaction. Thus, individuals higher in testosterone have been shown to react negatively to a variety of status-threatening cues, including poor medical diagnoses (Liening, Josephs, & Ristvedt, 2011) and poor academic performance (Josephs et al., 2003; Newman, Sellers, & Josephs, 2005; Josephs et al., 2006; Mehta, Jones, & Josephs, 2008) because these cues block one's ability to achieve high status.
Based on the connections between these two literatures, we chose to threaten status using three techniques that differ methodologically, but are conceptually equivalent. Thus, if similar patterns are obtained across studies, this inter-study method variance will bolster the argument for a robust effect and help to rule out an artifactual alternative explanation.
In the first study, status was threatened using a social exclusion task (Twenge, et al., 2001). Exclusion or eviction from a group is one of the most effective ways an individual can be denied access to valuable resources (Williams, 2007). Once ostracized, the individual lacks support, protection, resource exchange, and mating possibilities. Consequently, social exclusion, with the likely loss of social status, is highly distressing to many people and activates a physiologic response involving the sympathetic nervous system and the HPA axis, resulting in catecholamine and cortisol release.
In the second study, status was threatened by preventing subjects' from completing a cognitive/perceptual task. In most social species, status and rank are determined primarily through dominance battles and dominance displays. Unique to humans, however, is the opportunity to be granted high status through recognition of an individual's eminence in a particular domain (Kemper, 1990). For example, as a result of outstanding scholarship (in the case of a student or professional academic) or birthright (in the case of royalty), an individual may be granted high status. Thus, failure at a cognitive/perceptual task in a population in which intellectual performance is highly valued (e.g., a college population) may be threatening, insofar as it may signal that one's ability to achieve a valued position of high status is at risk (Josephs et al., 2006).
In the third study, status was threatened by compromising individuals' physical competence through an increase in CO2 intake. On its face, impairing an individual's physical state should be highly threatening to those sensitive to threat and to those concerned with status, as a deficit in physical function might impair one's ability to succeed in a competitive dominance challenge. In support of this, there is evidence that S carriers and individuals with higher testosterone levels exhibit a higher degree of defensiveness in the face of a physical health threat, compared to L homozygotes and individuals with lower testosterone levels .
Each study tested the hypothesis that S carriers with higher T would show an exaggerated stress response to these threats, as measured by cortisol levels, relative to all other groups (i.e., low T, S carriers; high T, L homozygotes; and low T, L homozygotes). The IRB at UT-Austin approved all study procedures.
Methods: Study 1
Participants were twenty-nine students (68% male) with a Beck Depression Inventory-II (BDI-II) (Beck et al., 1988) score of 12 or less, free of endocrine disorders, and not taking antidepressants, stimulants, or steroid hormones participated to fulfill a course requirement (see Table 1 for demographic information). This study took approximately 90 minutes to complete and was conducted between 1300h and 1600h to minimize hormone variability due to diurnal hormone cycles.
Table 1.
Demographic characteristics for participants in Studies 1, 2, and 3.
| Study 1: Social exclusion | LL (n = 14) | S carrier (n = 11) | Test Statistic |
|---|---|---|---|
| Age (mean years, and SD) | 19.43 (2.28) | 19.09 (1.38) | t (23) = 0.433, p = NS |
| Gender (M/F) | 21% / 79% | 45% / 55% | χ2 (1) = 1.63, p = NS |
| Ethnicity (Hispanic / Other) | 21% / 79% | 27% / 73% | χ2 (1) = 0.12, p = NS |
| Race (Caucasian / Other) | 50% / 50% | 55% / 45% | χ2 (1) = 0.05, p = NS |
| Depressive symptoms (BDI-II; mean score, and SD) | 4.79 (2.75) | 5.91 (5.39) | t (14.1) = -.629, p = NS |
| Study 2: Mirror Tracing | LL (n = 15) | S carrier (n = 42) | Test statistic |
| Age (mean years, and SD) | 18.67 (0.98) | 19.12 (1.17) | t (55) = -1.336, p = NS |
| Gender (M/F) | 27% / 73% | 38% / 62% | χ2 (1) = 0.63, p = NS |
| Ethnicity (Hispanic / Other) | 20% / 80% | 21% / 79% | χ2 (1) = 0.01, p = NS |
| Race (Caucasian / Other) | 33% / 67% | 38% / 62% | χ2 (1) = 0.11, p = NS |
| Depressive symptoms (BDI-II; mean score, and SD) | 8.80 (6.53) | 8.60 (4.98) | t (55) = .126, p = NS |
| Study 3: CO2* | LL (n = 33) | S carrier (n = 91) | Test Statistic |
| Age (mean years, and SD) | 25.51 (5.42) | 24.99 (6.09) | F(1,122) = 0.21, p = NS |
| Gender (M/F) | 85% / 15% | 85% / 15% | χ2 (1) = 0.00, p = NS |
| Ethnicity (Hispanic / Other) | 15% / 85% | 24% / 76% | χ2 (1) = 1.07, p = .30 |
| Race (Caucasian / Other) | 69% / 31% | 69% / 31% | χ2 (1) = 0.00, p = NS |
| Depressive symptoms (CES-D; mean score, and SD) | 11.15 (8.50) | 10.51 (7.65) | F(1,122) = 0.18, p = NS |
Participants provided buccal cells via a cheek swab/mouthwash procedure for genotyping, provided demographic information, and completed the BDI-II. After an initial 20-minute resting period, participants provided the first of two 3 mL saliva samples collected via passive drool (time 1).
Social exclusion
Ten minutes after the first saliva sample had been collected (time 1), social exclusion was manipulated using a get acquainted task (Twenge et al., 2001). A same-sex group of 4 or 6 participants was given name tags and asked to socialize for 10 minutes (prompts were given to inspire conversation). Then, participants were separated into individual rooms and told that the researchers were interested in forming groups based on individuals who like and respect each other. They were then asked to write down the names of the two people with whom they most want to work with. The researcher collected all nominations and then told each participant: “I hate to tell you this, but no one in the group wanted to work with you.” The second saliva sample was collected twenty minutes after this feedback (time 2), after which participants were debriefed and asked to sign a post-hoc consent form to include their data (100% of participants agreed).
Hormone assays
Salivary cortisol and testosterone (T) concentrations were analyzed in-house with commercially available Salimetrics enzyme immunoassay kits (State College, PA, USA). Saliva samples were completely thawed and centrifuged for 10 minutes at 3000 rpm immediately prior to assay. All cortisol and T samples were assayed in duplicate. The intra-assay coefficient of variance (CV) for cortisol (1.8% CV) and T (2.8% CV) and the inter-assay CV for cortisol (5.2% CV) and T (2.6% CV) were within the acceptable tolerance. T concentrations were standardized within sex and combined for analyses.
Genotyping
DNA was isolated from buccal cells obtained during a cheek swab and mouthwash procedure and polymerase chain reaction procedures were used to genotype the 5-HTTLPR. Details are available in our prior work (Beevers et al., 2009). The 5-HTTLPR allele frequency distribution was SS: n = 6 (24%), SL: n = 5 (20%), LL: = 14 (56%). Genotype distribution for Caucasian subjects used in final analyses was in Hardy-Weinberg equilibrium, (X2 = 3.12, p = .08); however, this was not the case for the full sample, (X2 = 7.68, p = .006). Nevertheless, race did not moderate our focal genotype × hormone effect, t (19)=-.313, p=.76.
Results: Study 1
Preliminary regression analyses identified four cases associated with failures of the standard least squares regression assumptions, leaving N = 25 subjects in the primary analysis. Regression analyses indicated no significant variability attributable to SS versus SL genotype groups on cortisol change (t(18)=.649, p=.52), allowing for the creation of an S carrier group. Moreover, the p-value for the primary interaction when separating the S carrier groups by race was p = .039 for Caucasians compared to p = .040 for all subjects. For demographics, see Table 1. Consistent with prior research, (Popma et al., 2007; Mehta et al., 2008; Mehta & Josephs, 2010), baseline (time 1) testosterone and cortisol concentrations were modestly positively correlated with men and women combined: r(43) = .351, p < . 05.
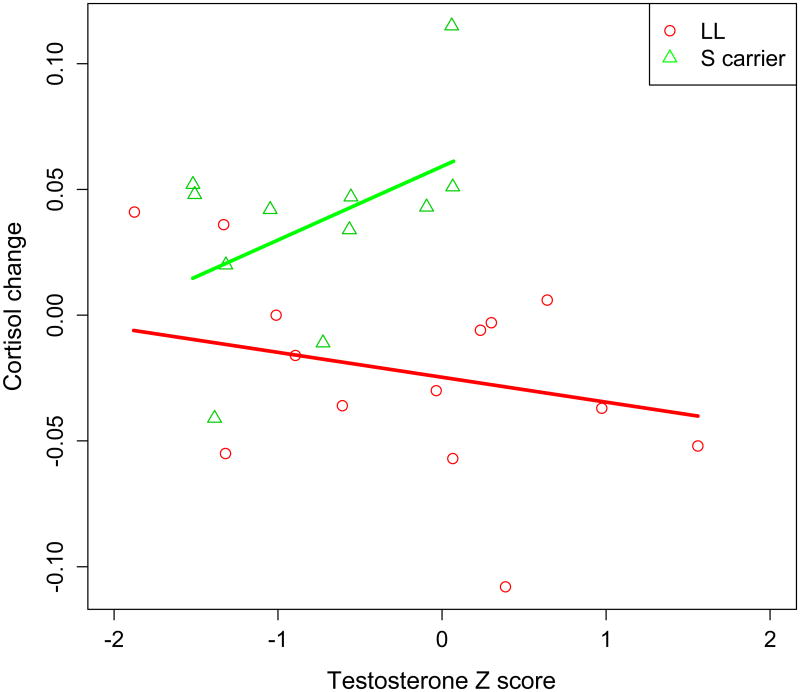
Ordinary least squares linear regression used time 2 cortisol as the dependent variable and testosterone z score, genotype, and the testosterone z score by genotype interaction as predictor variables. Time1 cortisol and BDI-II scores were included as covariates. In addition to a main effect of genotype status showing that S carriers were associated with higher cortisol change than L homozygotes (p<.001), analysis revealed a significant testosterone by genotype interaction, t(19) = 2.21, p = .04, effect size r = 0.45 (see Fig 1). Higher testosterone was associated with higher cortisol stress response among S carriers, whereas higher testosterone was associated with lower cortisol stress response among L homozygotes.
Figure 1.
Interaction of testosterone (standardized) by 5-HTTLPR on cortisol change in response to a social exclusion threat. Higher tesosterone is associated with higher cortisol stress response among S carriers (green line), whereas higher testosterone is associated with lower cortisol stress response among L homozygotes (red line).
Methods: Study 2
Sixty-three students (55% female) with the same inclusion criteria as Study 1 (see Table 1 for demographic information) participated to fulfill a course requirement. This study took approximately 90 minutes to complete and was conducted between 1300h and 1600h. Procedures in Study 2 were identical to Study 1 with the following exceptions:
After an initial 20-minute resting period, participants provided the first of two 3 mL saliva samples collected via passive drool (time 1). Participants then completed the Computerized Mirror-Tracing Persistence Task (MTPT-C). The second saliva sample was collected twenty-two minutes after completing the MTPT-C (time 2). Next, participants were debriefed and asked to sign a post-hoc consent form to include their data (100% of participants agreed).
Assessments
Computerized Mirror-tracing Persistence Task (MTPT-C)
The MTPT-C is an impossible task that involves moving a dot along the outline of geometric shapes presented on a computer monitor using the computer mouse (Daughters et al., 2005). Moving the computer mouse produces the inverse movement of the dot on the monitor. When the dot moves off the geometric shape or does not move for more than two seconds a loud buzz emits and the dot returns to the beginning of the shape (Bornovalova et al., 2008, Ellis, Fischer, & Beevers, 2010).
Hormone assays
Saliva from Studies 1 and 2 were analyzed simultaneously, and both studies shared immunoassay kits. Thus, the assay procedures and coefficients of variance in Study 2 were identical to Study 1.
Genotyping
Procedures were identical to Study 1. The 5-HTTLPR genotype frequency distribution was SS: n = 14 (24.6%), SL: n = 28 (49.1%), LL: n = 15 (26.3%). Genotype distribution for the sample used in final analyses was in Hardy-Weinberg equilibrium, (X2 = 0.021, p = 0.884).
Results: Study 2
Starting with N=63 subjects, preliminary data screening identified two cases with anomalous testosterone levels (> 3sd) and four cases associated with failures of the standard least squares regression assumptions, leaving N = 57 subjects in the primary analysis. Regression analyses indicated no significant variability attributable to SS versus SL genotype groups on cortisol change (t(50)=.435 p=.665). Furthermore, separating the S genotype groups made virtually no difference on the statistical significance of our primary outcome (t(50)=2.40, p = .020, compared to a p-value of .018, see next paragraph), so an S carrier group was used for analyses. Further, given the mixed-race composition of our sample, we examined whether the genotype by testosterone interaction was moderated by race (dichotomized into Caucasian and non-Caucasian groups). This 3-way interaction was non-significant, t (51)=-575, p=.568, indicating that the interactive influence of 5-HTTLPR genotype and testosterone on cortisol change was not significantly different for Caucasians and non-Caucasians. Replicating Study 1, Time 1 testosterone and cortisol concentrations were modestly positively correlated with men and women combined: r(65) = .448, p < .001.
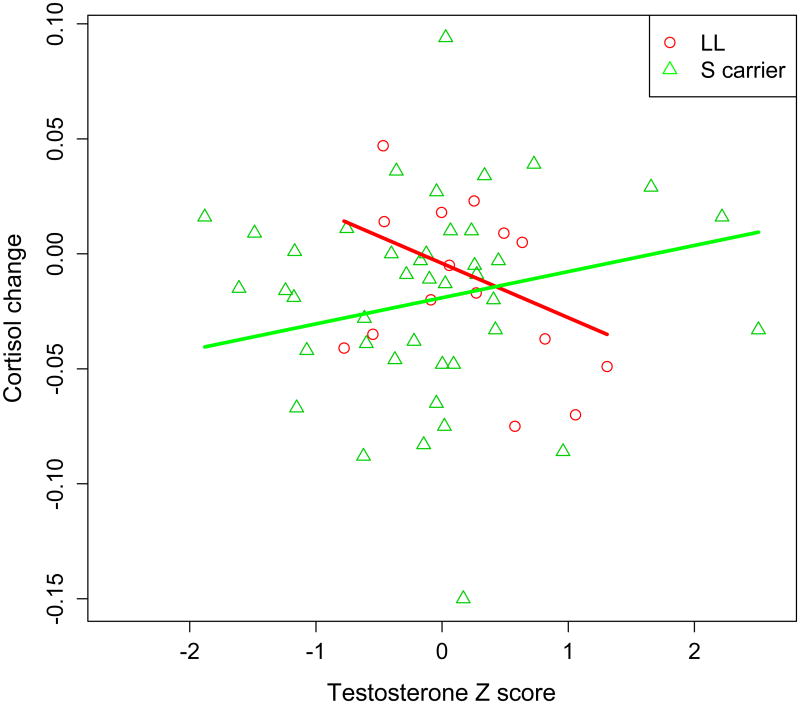
Ordinary least squares linear regression used time 2 cortisol as the dependent variable and testosterone z score, genotype, and the testosterone z score by genotype interaction as predictor variables (time1 cortisol and BDI-II scores were included as covariates). In addition to a trend showing that S carriers were marginally associated with higher cortisol change than L homozygotes (p=.127), regression analyses revealed a significant testosterone by genotype interaction, t(51) = 2.437, p = .018, effect size r = 0.32 (see Fig 2).
Figure 2.
Interaction of testosterone (standardized) by 5-HTTLPR on cortisol change in response to a cognitive/perceptual threat. Higher tesosterone is associated with higher cortisol stress response among S carriers (green line), whereas higher testosterone is associated with lower cortisol stress response among L homozygotes (red line).
Higher endogenous testosterone was associated with higher cortisol stress response among S carriers, whereas higher endogenous testosterone was associated with lower cortisol stress response among L homozygotes.
Methods: Study 3
Participants were 137 U.S. soldiers with no prior exposure to a war zone environment scheduled for deployment to Iraq from Fort Hood (see Table 1 for demographic information). They were all unpaid volunteers from a larger, longitudinal study examining risk factors for combat-related PTSD. Participants were free of endocrine disorders and were not taking antidepressants, stimulants, or steroid hormones. This study took approximately 90 minutes to complete and was conducted between 1400h and 1700h to minimize hormone variability due to diurnal hormone cycles.
Participants first provided demographic information and completed questionnaires, including the CES-D (Radloff, 1977). After a 20-minute rest period, participants provided the first of two 3 mL saliva samples collected via passive drool (time 1). Participants then underwent the CO2 challenge. The second saliva sample was collected thirty minutes after completion of the CO2 challenge (time 2).
Assessments
CO2 inhalation
Participants underwent a single inhalation of medical grade mixture of 35% carbon dioxide (CO2) and 65% oxygen (O2). Subjects were instructed to exhale completely and take in a full vital capacity breath of the CO2/O2 gas mixture and to hold the gas in their lungs for 5 full sec. The subject then breathed normally as possible until the effects subsided (approximately 30 sec.). Heightened fearful responding to single inhalations of 35% CO2 has been demonstrated among patient and non-clinical samples (Fyer et al., 1987).
Hormone assays
Saliva was frozen immediately after the second sample was taken. A the completion of the study, saliva samples were packed in dry ice and shipped to Salimetrics (State College, PA, USA) for analysis. All cortisol and T samples were assayed in duplicate. T intra-assay coefficient of variance (CV) for cortisol (3.5% CV) and T (4.6% CV) and the inter assay CV for cortisol (5.1% CV) and T (9.9% CV) were within the acceptable tolerance. T concentrations were standardized within sex and combined for analyses.
Genotyping
DNA was isolated from saliva obtained with the Oragene™ DNA self-collection kit using a triplex PCR protocol followed by double restriction endonuclease digesti (Wendland et al., 2006). The 5-HTTLPR genotype frequency distribution was SS: n = 24 (19.2%), SL: n = 69 (54.4%), LL: n = 33 (26.4%). Genotype frequencies did not deviate from Hardy-Weinberg equilibrium, (X2 = 1.09, p = 0.29).
Results: Study 3
One hundred thirty seven participants were included in initial analysis. Preliminary dat screening identified six cases with anomalous testosterone scores (> 3sd) and six cases associated with failures of the standard least squares regression assumptions, leaving N = 125 participants in the primary analysis. The procedure used in Studies 1 and 2 determined that SS and SL groups did not differ on the primary outcome measure (t(118)=1.46, p=,15), nor did separating the S genotype groups make a difference on the statistical significance of our prima outcome (t(118)=2.277, p=.025), compared to a p-value of .019, see below, allowing SS and S variants to be combined into a single S carrier group and compared to L homozygotes. Furthe the 3-way interaction between 5-HTTLPR group, testosterone, and race (Caucasian, non-Caucasian) was not significant, t (119)=.332, p=.741. Failing to replicate Studies 1 and 2, time testosterone and cortisol concentrations were not correlated: r(156) = .012, p = .886, perhaps due to a restriction in the range of participants' time 1 testosterone values (see Figure 3).
Figure 3.
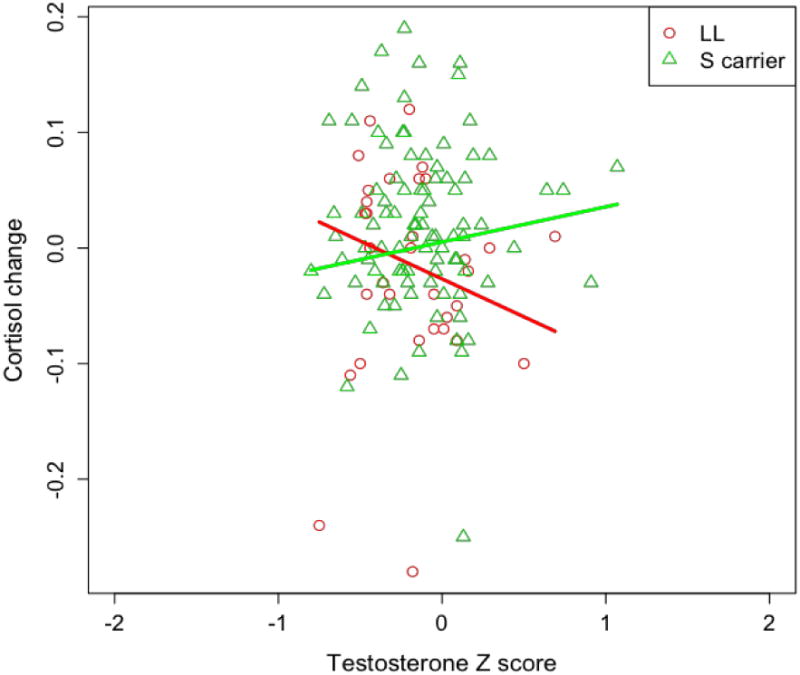
Interaction of testosterone (standardized) by 5-HTTLPR on cortisol change in response to a physical threat. Higher tesosterone is associated with higher cortisol stress response among S carriers (green line), whereas higher testosterone is associated with lower cortisol stress response among L homozygotes (red line).
Diagnostic checks continued to reveal problems with normality (excessive tail density) that were not traceable to a small number of specific cases. Accordingly, we used least absolute deviations regression, a robust regression technique well-suited to such situations (Koenker, 2009). Regression analyses used time 2 cortisol as the dependent variable and testosterone z score, genotype, and the testosterone z score by 5-HTTLPR interaction as predictor variables. Time 1 cortisol and CES-D scores were included as covariates. In addition to statistically significant main effects of time 1 cortisol and genotype, showing that S carriers were associated with a higher cortisol response than L homozygotes (p=.048), regression analysis revealed a significant testosterone by 5-HTTLPR interaction, t(119) = 2.339, p=.021, effect size r = 0.21. This interaction is shown in Figure 3 and documents that higher testosterone was associated with higher cortisol stress response among S carriers whereas higher endogenous testosterone was associated with lower cortisol stress response among L homozygotes.
Samples were also genotyped for a functional single nucleotide polymorphism rs25531 located within the 5-HTTLPR (Wendland et al., 2006). The 5-HTTLPR (S/LG carriers, LALA) × testosterone interaction continued to significantly predict change in cortisol, t(119) = 2.339, p=.021. Thus, it is likely that the absence of tri-allelic classification in Studies 1 and 2 are not significant limitations of the current work.
Combining effect sizes across the three studies
Two of the cardinal guiding principles of science are replication and generalization. We touch on generalization in the discussion, and focus now on replication, a topic that has generated quite a bit of attention lately in the genetic association literature (Risch et al., 2009; Caspi et al., 2010; Karg et al., 2011). To assess the magnitude of an effect, and its potential for replication, statistical combination of effect sizes, standard practice as applied across k independent studies, is equally applicable when comparing multiple variants of a manipulation within a study (Hedges & Olkin, 1985; Rosenthal & Rubin, 1986; Gleser & Olkin, 1994; Nakagawa & Cuthill, 2007).
Following standard statistical procedures (Gleser & Olkin, 1994; Rosenthal & Rosnow, 2008), we determined the statistical significance of the upward sloping simple effect (the relationship between testosterone and cortisol stress response among S carriers) from each of the three individual studies, and then combined each effect to assess the composite result. Using the Rosenthal and Rosnow technique for combining effects across studies Rosenthal & Rosnow, 2008), we converted each simple effect into a z-score, and then combined z-scores. The z-score from Study 1 was z=1.37; from Study 2, z=1.88; and from Study 3, z=1.405. The weighted (by residual df) average effect size for the simple effect of testosterone on cortisol stress response among S carriers was r = 0.217, combined z = 2.69, p = .0036. Further, our set of three studies is mathematically equivalent to a single study with N = 154. That is, for an effect size of r = .217, a predictor (with one degree of freedom) reaches significance of p = .0036 when residual degrees of freedom equal 150 (N-1, less 3 degrees of freedom for the three predictors in this analysis). Thus, the simple effect of endogenous testosterone on cortisol stress response among S carriers observed across the three studies had a small-to-medium-sized effect, accounted for 4.7% of the variance, was equivalent to a single study with 154 participants, and was highly unlikely (p=.0036) to be a Type I error.
For the second analysis, we determined the statistical significance of the downward sloping simple effect (the relationship between testosterone and cortisol stress response among LL carriers) from each of the three individual studies, and then combined each effect to assess the composite result. As with the S carriers, we converted each simple effect into a z-score, and then combined z-scores. The z-score from Study 1 was z=0.91; from Study 2, z=2.41; and from Study 3, z=1.75. The weighted (by residual df) average effect size for the simple effect of testosterone on a downward sloping cortisol stress response among LL carriers was r = 0.397, combined z = 2.93, p = .0034 (two-tailed). Further, our set of three studies is mathematically equivalent to a single study with N = 55. That is, for an average effect size of r = .397, a predictor (with one degree of freedom) reaches significance of p = .0034 at a residual degrees of freedom of 51 (N-1, less 3 degrees of freedom for the three predictors in this analysis). Thus, the simple effect of endogenous testosterone on downward sloping cortisol stress response among LL carriers observed across the three studies had a medium-large sized effect, accounted for 15.8% of the variance, was equivalent to a single study with 55 participants, and was highly unlikely (p=.0034) to be a Type I error.
For the third and final analysis, we assessed the status of the testosterone × genotype interaction on cortisol stress response by combining the interaction findings from the three studies. The weighted (by residual df) average effect size for the genotype × T interaction was r = 0.266, combined z = 3.905, p = .00009. Further, our set of three studies is mathematically equivalent to a single study with N = 215. That is, for an effect size of r = .266, a predictor (with one degree of freedom) reaches significance of p = .00009 when residual degrees of freedom equal 209 (N-1, less 5 degrees of freedom for the five predictors in our primary analyses). Thus, the 5-HTTLPR × T interaction observed across three studies had a medium-sized effect on cortisol change, accounted for 7% of the variance, was equivalent to a study with 215 participants, and was highly unlikely to be a Type I error.
Discussion
The primary result across three studies was that 5-HTTLPR S carriers higher in testosterone showed elevated cortisol responses to threat, despite the diverse nature of the threat. At first blush, the threats used in the current studies appear to diverge radically from those used in the 5-HTTLPR literature. However, as mentioned earlier, this appearance may be more illusory than real. Although the manipulations used in Studies 2 and 3 were not explicitly “social”, they are equivalent to the non-social status manipulations that have been used successfully in the testosterone literature to evoke defensive and/or stressful reactions among those higher in testosterone (Newman, Kosson, & Patterson, 1992; van Honk et al., 1999; 2001, Josephs et al., 2003; 2006; Wirth & Schultheiss, 2007; Mehta et al., 2008; Liening, Josephs, & Ristvedt, 2011). Furthermore, on the surface, the three manipulations are very different from each other, and we view this as a major strength of this paper. That diverse manipulations produced equivalent patterns of results argues for a robust effect, and against an artifactual explanation for both the primary testosterone by 5-HTTLPR interaction and for the simple main effect of testosterone on cortisol stress response among S carriers.
Results are also consistent with evidence that the 5-HTTLPR influences sensitivity to emotionally provocative stimuli (Hariri & Holmes, 2006), including stimuli that are known to evoke heightened responses among individuals who are higher in testosterone (van Honk et al., 1999; 2001, Josephs et al., 2006, Wirth & Schultheiss, 2007). By demonstrating that 5-HTTLPR S carriers with higher levels of testosterone are susceptible to both social and non-social status threats, a previously under-examined role for status threats in the 5-HTTLPR literature emerges (and is consistent with the finding obtained by (Kalin et al., 2008). Of course, many biologists would argue that few behaviors are more important to reproductive success and health, including mental health (Malatynska & Knapp, 2005) than those relating to rank and status (Cant & Johnstone, 2000; Sapolsky, 2005; Rivers & Josephs, 2010) So perhaps it is not surprising that threats to status, whether social or non-social, evoke a stress response in those sensitive to threat. To the degree that an exaggerated stress response contributes to the onset of psychiatric problems, such as major depressive disorder (Gotlib et al., 2008), high testosterone, 5-HTTLPR S carriers may be particularly vulnerable to depression following events that threaten an individual's social status, such as divorce, demotion, chronic physical illness, or unemployment (Booth et al., 1999; Rivers & Josephs 2010).
We were surprised to find that subjects who were homozygous for the 5-HTTLPR L allele showed a reliable, inverse relationship between testosterone and cortisol change, given the near-exclusive focus in the literature on the S allele. Lending support to these findings, Canli and his colleagues report divergent responses of S and L carriers at multiple levels of representation (Canli et al., 2006, Mueller et al., 2011). At the endophenotypic level, perfusion imaging revealed that life stress correlated positively with resting activity in the amygdala and hippocampus in S carriers but negatively in L carriers (Canli et al., 2006). At the verbal level, this same group found that life stress correlated positively with trait rumination in S carriers but negatively in L carriers. At the physiologic level, (Mueller et al., 2011) found an interaction between the 5-HTTLPR genotype and life stress on acute cortisol change, in which the cortisol stress response was positively correlated with life stress in S carriers but negatively correlated with life stress in L carriers.
This negative relationship between testosterone and cortisol change in L carriers may also have important neuropsychiatric significance. A recent review noted a remarkable series of parallels in the literatures on psychopathic individuals and L homozygotes (Glenn, 2011). Across nearly thirty studies, the two literatures showed strong parallels between findings, supporting the conclusion that “the long/long genotype may be a potential risk factor for the development of psychopathic traits” (Glenn, 2011).
Mirroring these parallels, another review found strong convergence between high endogenous testosterone levels and psychopathy, from impulsivity to juvenile delinquency to overall criminality (Glenn, 2009). High levels of testosterone also reduce sensitivity to punishment and increase sensitivity to reward, (van Honk et al., 2004) a characteristic that has been shown in psychopaths (Newman et al., 1992). Taken together, these data suggest that the interaction between testosterone and the 5-HTTLPR genotype may also contribute to the development of psychopathic behavior, although clearly additional research to confirm this possibility is needed.
The current set of studies suffered from several weaknesses. The range of testosterone scores among S carriers in Study 1 was restricted (see Fig 1), limiting conclusions regarding higher testosterone levels and cortisol change. In Study 1, the genotype distribution deviated from Hardy-Weinberg equilibrium, the sample size was very small, and effect size was atypically large (although this last finding is typical of initially reported effects, and is thought to be a function of statistical regression). Finally, Study 2 found only marginal support for a genotype main effect (significant genotype main effects were observed in Studies 1 & 3).
Ironically, the weaknesses and differences between studies suggest a phenomenon that may be quite robust to violations in genotype distribution, type of threat, restriction of range issues, and population demographics (Studies 1 and 2 used a college sample whereas Study 3 used a military sample).
As with any genetic association study, population stratification is a potential concern. This confound is unlikely as analyses indicated the 5-HTTLPR × testosterone interaction was not significantly different for Caucasian and non-Caucasian groups in any study. Nevertheless, genomic control of potential population stratification is preferred.
Of additional concern is the apparent paradox that emerges when contrasting studies linking testosterone to reduced fear responses (Archer, 1976; Coe et al., 1982; Vandenheede, 1993; Boissy & Bouissou, 1994; King et al., 2005; van Honk et al., 2005; Hermans et al., 2006) to studies linking testosterone levels to an increased level of stress hormones and heightened HPA reactivity (Schoech, Ketterson, & Nolan, 1999). On its face, it may seem inconsistent to suggest that testosterone can reduce fear and increase stress. However, there is good reason to suspect that the fear-reducing properties of testosterone may give way to behaviors that underlie testosterone's link to an increase in basal stress levels and HPA axis sensitivity. For example, increases in T during mating season are associated with increases in the frequency of aggressive interactions (Hegner & Wingfield, 1987), more time spent engaging in territorial defense, an overall increase in physical activity with a concurrent decrease in resting activity, and an increase in the size of an individual's territory or home range (Chandler et al., 1994). These activities, which are consistent with the profile of a confident and generally fearless individual, are generally also thought to be stressful (Schoech et al., 1999), and in fact, evidence from both field and lab studies supports this latter conclusion (see supplementary material).
A significant number of striking parallels between two literatures inspired us to conduct three studies, which served to replicate and extend existing research into the biological basis of stress reactivity. Prior to these studies, it was known the 5-HTTLPR genotype and variation in testosterone levels were both linked to an exaggerated stress response to threat. Although the precise mechanism underlying this synergy is yet to be discovered, it now appears that testosterone and the 5-HTTLPR may operate in concert to produce exaggerated stress responses to threats to status, both social and non-social.
Supplementary Material
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Archer J. Testosterone and fear behavior in male chicks. Physiology & Behavior. 1976;17(4):561–564. doi: 10.1016/0031-9384(76)90151-7. [DOI] [PubMed] [Google Scholar]
- Beck AT, Epstein N, Brown G, Steer RA. An inventory for measuring clinical anxiety: Psychometric properties. Journal of Consulting and Clinical Psychology. 1988;56(6):893–897. doi: 10.1037//0022-006x.56.6.893. [DOI] [PubMed] [Google Scholar]
- Beevers CG, Wells TT, Ellis AJ, McGeary JE. Association of the serotonin transporter gene promoter region 5-HTTLPR polymorphism with biased attention for emotional stimuli. Journal of Abnormal Psychology. 2009;118(3):670–681. doi: 10.1037/a0016198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belsky J, Jonassaint C, Pluess M, Stanton M, Brummett B, Williams R. Vulnerability genes or plasticity genes? Mol Psychiatry. 2009;14(8):746–754. doi: 10.1038/mp.2009.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boissy A, Bouissou MF. Effects of Androgen Treatment on Behavioral and Physiological Responses of Heifers to Fear-Eliciting Situations. Hormones and Behavior. 1994;1(28):66–83. doi: 10.1006/hbeh.1994.1006. [DOI] [PubMed] [Google Scholar]
- Booth A, Johnson DR, Granger DA. Testosterone and Men's Depression: The Role of Social Behavior. Journal of Health and Social Behavior. 1999;40(2):130–140. [PubMed] [Google Scholar]
- Bornovalova MA, Gratz KL, Daughters SB, Nick B, Delany-Brumsey A, Lynch TR, Kosson D, et al. A multimodal assessment of the relationship between emotion dysregulation and borderline personality disorder among inner-city substance users in residential treatment. Journal of Psychiatric Research. 2008;42(9):717–726. doi: 10.1016/j.jpsychires.2007.07.014. [DOI] [PubMed] [Google Scholar]
- Canli T, Qiu M, Omura K, Congdon E, Haas BW, Amin Z, Herrmann MJ, et al. Neural Correlates of Epigenesis. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(43):16033–16038. doi: 10.1073/pnas.0601674103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cant MA, Johnstone RA. Power Struggles, Dominance Testing, and Reproductive Skew. The American Naturalist. 2000;155(3):406–417. doi: 10.1086/303328. [DOI] [PubMed] [Google Scholar]
- Caspi A, Hariri AR, Holmes A, Uher R, Moffitt TE. Genetic Sensitivity to the Environment: The Case of the Serotonin Transporter Gene and Its Implications for Studying Complex Diseases and Traits. Am J Psychiatry. 2010;167(5):509–527. doi: 10.1176/appi.ajp.2010.09101452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler CR, Ketterson ED, Nolan V, Ziegenfus C. Effects of testosterone on spatial activity in free-ranging male dark-eyed juncos, Junco hyemalis. Animal Behaviour. 1994;47(6):1445–1455. [Google Scholar]
- Chiao JY, Blizinsky KD. Culture–gene coevolution of individualism–collectivism and the serotonin transporter gene. Proceedings of the Royal Society B: Biological Sciences. 2010;277(1681):529–537. doi: 10.1098/rspb.2009.1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coe CL, Franklin D, Smith ER, Levine S. Hormonal responses accompanying fear and agitation in the squirrel monkey. Physiology & Behavior. 1982;29(6):1051–1057. doi: 10.1016/0031-9384(82)90297-9. [DOI] [PubMed] [Google Scholar]
- Daughters SB, Lejuez CW, Bornovalova MA, Kahler CW, Strong DR, Brown RA. Distress tolerance as a predictor of early treatment dropout in a residential substance abuse treatment facility. Journal of Abnormal Psychology. 2005;114(4):729–734. doi: 10.1037/0021-843X.114.4.729. [DOI] [PubMed] [Google Scholar]
- Derntl B, Windischberger C, Robinson S, Kryspin-Exner I, Gur RC, Moser E, Habel U. Amygdala activity to fear and anger in healthy young males is associated with testosterone. Psychoneuroendocrinology. 2009;34(5):687–693. doi: 10.1016/j.psyneuen.2008.11.007. [DOI] [PubMed] [Google Scholar]
- Ellis AJ, Fischer KM, Beevers CG. Is dysphoria about being red and blue? Potentiation of anger and reduced distress tolerance among dysphoric individuals. Cognition and Emotion. 2010;24(4):596–608. [Google Scholar]
- Fyer MR, Uy J, Martinez J, Goetz R, Klein DF, Fyer A, Liebowitz MR, et al. CO2 challenge of patients with panic disorder. The American Journal of Psychiatry. 1987;144(8):1080–1082. doi: 10.1176/ajp.144.8.1080. [DOI] [PubMed] [Google Scholar]
- Glenn AL. The Handbook of Neuropsychiatric Biomarkers, Endophenotypes, and Genes. Springer Science + Business Media B.V; 2009. Neuroendocrine Markers of Psychopathy; pp. 59–70. [Google Scholar]
- Glenn AL. The other allele: Exploring the long allele of the serotonin transporter gene as a potential risk factor for psychopathy: A review of the parallels in findings. Neuroscience & Biobehavioral Reviews. 2011;35(3):612–620. doi: 10.1016/j.neubiorev.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleser LJ, Olkin I. The Handbook of research synthesis. Russell Sage Foundation; 1994. Stochastically dependent effect sizes; pp. 340–398. [Google Scholar]
- Gotlib IH, Joormann J, Minor KL, Hallmayer J. HPA-Axis Reactivity: A Mechanism Underlying the Associations Among 5-HTTLPR, Stress, and Depression. Biological psychiatry. 2008;63(9):847–851. doi: 10.1016/j.biopsych.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariri AR, Holmes A. Genetics of emotional regulation: the role of the serotonin transporter in neural function. Trends in Cognitive Sciences. 2006;10(4):182–191. doi: 10.1016/j.tics.2006.02.011. [DOI] [PubMed] [Google Scholar]
- Hedges L, Olkin I. Statistical Methods for Meta-Analysis. Academic Press; 1985. [Google Scholar]
- Hegner RE, Wingfield JC. Effects of experimental manipulation of testosterone levels on parental investment and breeding success in male house sparrows. The Auk. 1987;104(3):462–469. [Google Scholar]
- Hermans EJ, Putman P, Baas JM, Koppeschaar HP, van Honk J. A Single Administration of Testosterone Reduces Fear-Potentiated Startle in Humans. Biological Psychiatry. 2006;59(9):872–874. doi: 10.1016/j.biopsych.2005.11.015. [DOI] [PubMed] [Google Scholar]
- Josephs RA, Newman ML, Brown RP, Beer JM. Status, Testosterone, and Human Intellectual Performance. Psychological Science. 2003;14(2):158–163. doi: 10.1111/1467-9280.t01-1-01435. [DOI] [PubMed] [Google Scholar]
- Josephs RA, Sellers JG, Newman ML, Mehta PH. The Mismatch Effect: When Testosterone and Status Are at Odds. Journal of Personality and Social Psychology. 2006;90(6):999–1013. doi: 10.1037/0022-3514.90.6.999. [DOI] [PubMed] [Google Scholar]
- Kalin NH, Shelton SE, Fox AS, Rogers J, Oakes TR, Davidson RJ. The serotonin transporter genotype is associated with intermediate brain phenotypes that depend on the context of eliciting stressor. Mol Psychiatry. 2008;13(11):1021–1027. doi: 10.1038/mp.2008.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karg K, Burmeister M, Shedden K, Sen S. The Serotonin Transporter Promoter Variant 5-HTTLPR , Stress, and Depression Meta-analysis Revisited: Evidence of Genetic Moderation. Arch Gen Psychiatry. 2011;68(5):444–454. doi: 10.1001/archgenpsychiatry.2010.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemper TD. Social structure and testosterone: explorations of the socio-bio-social chain. Rutgers University Press; 1990. [Google Scholar]
- King JA, De Oliveira WL, Patel N. Deficits in testosterone facilitate enhanced fear response. Psychoneuroendocrinology. 2005;30(4):333–340. doi: 10.1016/j.psyneuen.2004.09.005. [DOI] [PubMed] [Google Scholar]
- Koenker R. quantreg: Quantile Regression. R package version 4.27 2009 [Google Scholar]
- Liening SH, Josephs RA, Ristvedt S. Early detection behaviors among men: Testosterone, 5-HTTLPR, anxiety, and the dismissal of threatening medical information; Paper presented at the annual meeting of the Society for Personality and Social Psychology; San Antonio, TX. 2011. [Google Scholar]
- Malatynska E, Knapp RJ. Dominant-submissive behavior as models of mania and depression. Neuroscience & Biobehavioral Reviews. 2005;29(4-5):715–737. doi: 10.1016/j.neubiorev.2005.03.014. [DOI] [PubMed] [Google Scholar]
- Manuck SB, Marsland AL, Flory JD, Gorka A, Ferrell RE, Hariri AR. Salivary testosterone and a trinucleotide CAG length polymorphism in the androgen receptor gene predict amygdala reactivity in men. Psychoneuroendocrinology. 2010;35(1):94–104. doi: 10.1016/j.psyneuen.2009.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta PH, Beer J. Neural Mechanisms of the Testosterone–Aggression Relation: The Role of Orbitofrontal Cortex. Journal of Cognitive Neuroscience. 2011;22(10):2357–2368. doi: 10.1162/jocn.2009.21389. [DOI] [PubMed] [Google Scholar]
- Mehta PH, Jones AC, Josephs RA. The social endocrinology of dominance: Basal testosterone predicts cortisol changes and behavior following victory and defeat. Journal of Personality and Social Psychology. 2008;94(6):1078–1093. doi: 10.1037/0022-3514.94.6.1078. [DOI] [PubMed] [Google Scholar]
- Mehta PH, Josephs RA. Testosterone and Cortisol Jointly Regulate Dominance: Evidence for a Dual-Hormone Hypothesis. Hormones & Behavior. 2010;58:898–906. doi: 10.1016/j.yhbeh.2010.08.020. [DOI] [PubMed] [Google Scholar]
- Menard CS, Harlan RE. Up-regulation of androgen receptor immunoreactivity in the rat brain by androgenic-anabolic steroids. Brain Research. 1993;62(1-2):226–236. doi: 10.1016/0006-8993(93)90823-6. [DOI] [PubMed] [Google Scholar]
- Mueller A, Armbruster D, Moser DA, Canli T, Lesch KP, Brocke B, Kirschbaum C. Interaction of serotonin transporter gene-linked polymorphic region and stressful life events predicts cortisol stress response. Neuropsychopharmacology. 2011;36(7):1332–1339. doi: 10.1038/npp.2011.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munafò MR, Brown SM, Hariri AR. Serotonin transporter 5-HTTLPR genotype and amygdala activation: a meta-analysis. Biological Psychiatry. 2008;63(9):852–857. doi: 10.1016/j.biopsych.2007.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa S, Cuthill IC. Effect size, confidence interval and statistical significance: a practical guide for biologists. Biological Reviews of the Cambridge Philosophical Society. 2007;82(4):591–605. doi: 10.1111/j.1469-185X.2007.00027.x. [DOI] [PubMed] [Google Scholar]
- Newman JP, Kosson DS, Patterson CM. Delay of Gratification in Psychopathic and Nonpsychopathic Offenders. Journal of Abnormal Psychology. 1992;101(4):630–636. doi: 10.1037//0021-843x.101.4.630. [DOI] [PubMed] [Google Scholar]
- Newman ML, Sellers JG, Josephs RA. Testosterone, cognition, and social status. Hormones and Behavior. 2005;47(2):205–211. doi: 10.1016/j.yhbeh.2004.09.008. [DOI] [PubMed] [Google Scholar]
- Pezawas L, Meyer-Lindenberg A, Drabant EM, Verchinski BA, Munoz KE, Kolachana BS, Egan MF, et al. 5-HTTLPR polymorphism impacts human cingulate-amygdala interactions: a genetic susceptibility mechanism for depression. Nature Neuroscience. 2005;8(6):828–834. doi: 10.1038/nn1463. [DOI] [PubMed] [Google Scholar]
- Popma A, Vermeiren R, Geluk C, Rinne T, van den Brink W, Knol D, Jansen L, vanEngeland H, Doreleijers T. Cortisol moderates the relationship between testosterone and aggression in delinquent male adolescents. Biological Psychiatry. 2007;61:405–411. doi: 10.1016/j.biopsych.2006.06.006. [DOI] [PubMed] [Google Scholar]
- Radloff LS. The CES-D Scale. Applied Psychological Measurement. 1977;1(3):385–401. [Google Scholar]
- Risch N, Herrell R, Lehner T, Liang KY, Eaves L, Hoh J, Griem A, et al. Interaction Between the Serotonin Transporter Gene 5-HTTLPR , Stressful Life Events, and Risk of Depression. JAMA: The Journal of the American Medical Association. 2009;301(23):2462–2471. doi: 10.1001/jama.2009.878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivers JJ, Josephs RA. The Social Psychology of Power. New York: Guilford Press; 2010. Dominance and Health: The Role of Social Rank in Physiology and Illness; pp. 87–112. [Google Scholar]
- Rosenthal R, Rosnow R. Essentials of behavioral research: Methods and data analysis. 3. New York: McGraw Hill; 2008. [Google Scholar]
- Rosenthal R, Rubin DB. Meta-Analytic Procedures for Combining Studies With Multiple Effect Sizes. Psychological Bulletin. 1986;99(3):400–406. [Google Scholar]
- Sapolsky RM. The Influence of Social Hierarchy on Primate Health. Science. 2005;308(5722):648–652. doi: 10.1126/science.1106477. [DOI] [PubMed] [Google Scholar]
- Schoech SJ, Ketterson ED, Nolan V. Exogenous Testosterone and the Adrenocortical Response in Dark-Eyed Juncos. The Auk. 1999;116(1):64–72. [Google Scholar]
- de Souza Silva MA, Mattern C, Topic B, Buddenberg TE, Huston JP. Dopaminergic and serotonergic activity in neostriatum and nucleus accumbens enhanced by intranasal administration of testosterone. European Neuropsychopharmacology. 2009;19(1):53–63. doi: 10.1016/j.euroneuro.2008.08.003. [DOI] [PubMed] [Google Scholar]
- Twenge JM, Baumeister RF, Tice DM, Stucke TS. If you can't join them, beat them: Effects of social exclusion on aggressive behavior. Journal of Personality and Social Psychology. 2001;81(6):1058–1069. doi: 10.1037//0022-3514.81.6.1058. [DOI] [PubMed] [Google Scholar]
- Vandenheede M. Effect of Androgen Treatment on Fear Reactions in Ewes. Hormones and Behavior. 1993;27(4):435–448. doi: 10.1006/hbeh.1993.1032. [DOI] [PubMed] [Google Scholar]
- van Honk J, Peper JS, Schutter DJLG. Testosterone Reduces Unconscious Fear but Not Consciously Experienced Anxiety: Implications for the Disorders of Fear and Anxiety. Biological Psychiatry. 2005;58(3):218–225. doi: 10.1016/j.biopsych.2005.04.003. [DOI] [PubMed] [Google Scholar]
- van Honk J, Schutter DJLG, Hermans EJ, Putman P, Tuiten A, Koppeschaar H. Testosterone shifts the balance between sensitivity for punishment and reward in healthy young women. Psychoneuroendocrinology. 2004;29(7):937–943. doi: 10.1016/j.psyneuen.2003.08.007. [DOI] [PubMed] [Google Scholar]
- van Honk J, Tuiten A, Hermans E, Putnam P, Koppeschaar H, Thijssen J, Verbaten R, et al. A single administration of testosterone induces cardiac accelerative responses to angry faces in healthy young women. Behavioral Neuroscience. 2001;115(1):238–242. doi: 10.1037/0735-7044.115.1.238. [DOI] [PubMed] [Google Scholar]
- van Honk J, Tuiten A, Verbaten R, van den Hout M, Koppeschaar H, Thijssen J, de Haan E. Correlations among Salivary Testosterone, Mood, and Selective Attention to Threat in Humans. Hormones and Behavior. 1999;36(1):17–24. doi: 10.1006/hbeh.1999.1521. [DOI] [PubMed] [Google Scholar]
- van Wingen GA, Zylicz SA, Pieters S, Mattern C, Verkes RJ, Buitelaar JK, Fernandez G. Testosterone Increases Amygdala Reactivity in Middle-Aged Women to a Young Adulthood Level. Neuropsychopharmacology. 2008;34(3):539–547. doi: 10.1038/npp.2008.2. [DOI] [PubMed] [Google Scholar]
- van Wingen G, Mattern C, Verkes RJ, Buitelaar J, Fernández G. Testosterone reduces amygdala-orbitofrontal cortex coupling. Psychoneuroendocrinology. 2010;35(1):105–113. doi: 10.1016/j.psyneuen.2009.09.007. [DOI] [PubMed] [Google Scholar]
- Way BM, Taylor SE. The Serotonin Transporter Promoter Polymorphism Is Associated with Cortisol Response to Psychosocial Stress. Biological Psychiatry. 2010;67(5):487–492. doi: 10.1016/j.biopsych.2009.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendland JR, Martin BJ, Kruse MR, Lesch KP, Murphy DL. Simultaneous genotyping of four functional loci of human SLC6A4, with a reappraisal of 5-HTTLPR and rs25531. Mol Psychiatry. 2006;11(3):224–226. doi: 10.1038/sj.mp.4001789. [DOI] [PubMed] [Google Scholar]
- Williams KD. Ostracism. Annual Review of Psychology. 2007;58:425–452. doi: 10.1146/annurev.psych.58.110405.085641. [DOI] [PubMed] [Google Scholar]
- Wirth MM, Schultheiss OC. Basal testosterone moderates responses to anger faces in humans. Physiology & Behavior. 2007;90(2-3):496–505. doi: 10.1016/j.physbeh.2006.10.016. [DOI] [PubMed] [Google Scholar]
- Zyphur MJ, Narayanan J, Koh G, Koh D. Testosterone-status mismatch lowers collective efficacy in groups: Evidence from a slope-as-predictor multilevel structural equation model. Organizational Behavior and Human Decision Processes. 2009;110:70–79. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.