Abstract
There is recent and widespread interest in the damage associated molecular pattern molecules S100A8 and S100A9 in cardiovascular science. These proteins have a number of interesting features and functions. For example, S100A8/A9 have both intracellular and extracellular actions, they are abundantly expressed in inflammatory and autoimmune states primarily by myeloid cells, but also by other vascular cells, and they modulate inflammatory processes, in part through toll-like receptor 4 (TLR4) and the receptor for advanced glycation end products (RAGE). S100A8/A9 also have anti-inflammatory and immune regulatory actions. Furthermore, increased plasma levels of S100A8/A9 predict cardiovascular events in humans, and deletion of these proteins protects Apoe−/− mice from atherosclerosis. Understanding the roles of S100A8 and S100A9 in vascular cell types, and the mechanisms whereby these proteins mediate their biological effects may offer new therapeutic strategies to prevent, treat, and predict cardiovascular diseases.
Introduction
S100A8 (calgranulin A or migration inhibitory factor-related protein 8; MRP-8) and its binding partner S100A9 (calgranulin B or MRP-14) are members of the S100 calcium-binding family of proteins, which are increased in a number of inflammatory and autoimmune states.1 S100A8 and S100A9 form a heterocomplex, termed S100A8/A9 or calprotectin, but the two proteins may also have distinct functions and are regulated in part by different mechanisms.2 The role of S100A8 and S100A9 in biology and disease is complex.3–7 S100A8/A9 are generally viewed as inflammatory, but further studies have revealed both anti-inflammatory and immune regulatory actions.2,6–7 The ability of S100A8/A9 to modulate inflammatory processes appears to be both context- and cell type-specific, suggesting an intricate network of regulation. Another layer of complexity surrounding the actions of S100A8/A9 is that these proteins have both intracellular and extracellular functions. The intracellular functions include calcium- and arachidonic acid binding, and regulation of microtubuli.8–9 Released S100A8/A9 exert extracellular functions, some of which are mediated by toll-like receptor 4 (TLR4),10 the receptor for advanced glycation end products (RAGE),11 or other receptors.12 S100A8/A9 are released from damaged/dying or activated cells through an atypical pathway that appears to require protein kinase C13 and RAGE,14 and S100A8 and S100A9 are therefore included in the group of proteins termed damage associated molecular pattern (DAMP) molecules.6
Recently, S100A8/A9 were found to be of significance in cardiovascular disease both in humans and mice. In this review, we discuss S100A8/A9 as mediators of biological effects in the cardiovascular system with a special focus on atherosclerosis and cardiac dysfunction, and S100A8/A9 as markers of cardiovascular events.
What cardiovascular cell types express and release S100A8/A9?
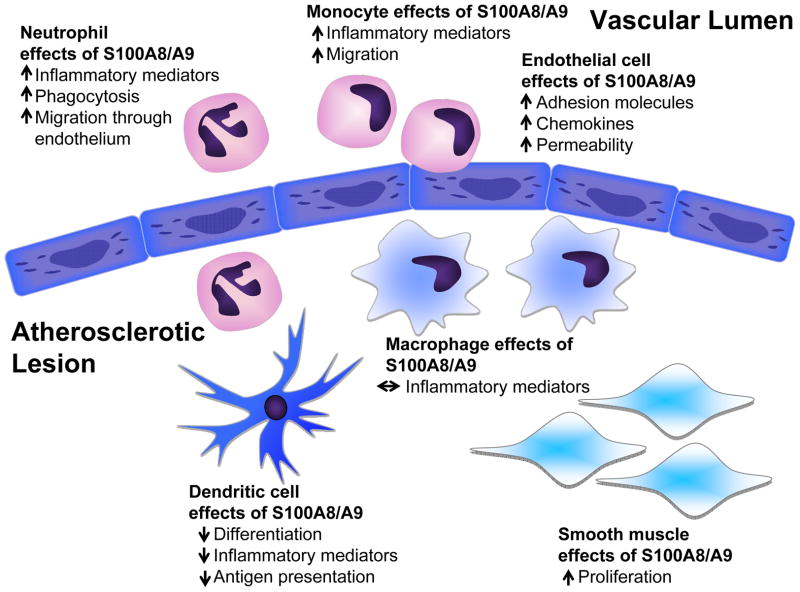
Many cell types influence cardiovascular disease progression. Understanding which cells express and release S100A8/A9 is one of the first steps in elucidating the cardiovascular effects of S100A8/A9. Constitutive S100A8/A9 expression is believed to be limited to neutrophils and monocytes. This expression is tightly regulated throughout hematopoietic development and is lost during macrophage maturation. However, recent reports have highlighted the maintained expression of S100A8/A9 in some mature myeloid cell populations, and the induction of S100A8/A9 expression in non-myeloid cardiovascular cell types, as discussed below. These cell populations play distinct roles in cardiovascular biology and disease, and are affected by S100A8/A9 in different ways (Figure 1).
Figure 1. Schematic representation of the potential effects of S100A8/A9 in atherosclerosis.
In neutrophils, S100A8/A9 promote expression of inflammatory mediators, phagocytosis, and migration through the vascular endothelium. Likewise, in monocytes, S100A8/A9 stimulate migration and an inflammatory phenotype. Endothelial cells express S100A8/A9 following exposure to inflammatory stimuli, and when added exogenously, these proteins promote adhesion molecule expression, chemokine expression, and permeability of the endothelial layer, all of which may promote atherogenesis. Macrophages express and release significantly less S100A8/A9 than do DCs. S100A8/A9 suppress DC differentiation, antigen presentation, and release of inflammatory mediators. S100A8/A9 promote proliferation of VSMCs, which might contribute to fibrous cap formation. Thus, S100A8/A9 affect the major cell types involved in atherosclerosis, and these proteins have cell type-selective effects. The relative contribution of these cell types during different stages of lesion progression is likely to govern the overall effect of S100A8/A9 inhibition.
Expression of S100A8/A9 in hematopoietic development, neutrophils and monocytes
S100A8 and S100A9 are predominantly expressed in, and released from, myeloid cells upon cellular activation.1,15–16 An early study of S100A9 gene expression in human cells demonstrated the tight regulation of S100A9 gene expression in a differentiation- and lineage-specific manner within the hematopoietic system.17 Primitive, uncommitted CD34+CD38−CD33− and early myeloid CD34+CD33+ progenitor cells lack expression of S100A9. During monocyte maturation, up-regulation of S100A9 correlates with the expression of CD11b and CD14. Within the neutrophilic pathway, S100A9 is only detectable in CD15+ cells, in which it slightly precedes and then correlates with CD11b expression. Mature CD15+CD16+CD33+ neutrophils express S100A9, indicating that S100A9 expression is initiated during maturation of promyelocytes towards myelocytes and then maintained up to the level of mature neutrophils. Similar patterns of expression are observed in murine myeloid development.18–19 Other lineage hematopoietic cells, including the CD19+ B-lineage, CD3+ T-lineage and GlyA-positive erythroid cells, are S100A9 negative,17 although recent reports describe S100A8 and S100A9 gene expression in a highly purified B-cell population20 and in platelets.21
S100A8 and S100A9 constitute approximately 40% of the cytosolic protein fraction in circulating human blood neutrophils and 1% in monocytes.16 Although expression is downregulated during maturation of peripheral blood monocytes to tissue macrophages, expression can be found in macrophages in inflamed tissues.22–24
Expression of S100A8/A9 in other myeloid-derived cell populations
S100A8/A9 are expressed in dendritic cells (DCs) and myeloid-derived suppressor cells (MDSCs). Although S100A8/A9 expression diminishes during differentiation into DCs25 some expression is maintained in DCs.26 Moreover, gene expression of S100A8 and S100A9 is significantly upregulated in mature human DCs after IL-10 treatment.27 In contrast to macrophages, DCs constitutively release S100A8/A9.26 MDSCs also synthesize and secrete S100A8/A9, which in turn activate RAGE and promote MDSC migration.28 These cells accumulate in tumor-bearing hosts and in response to inflammation, where they inhibit T and NK cell activation and DC differentiation. Another myeloid-derived cell population termed fibrocytes express S100A8/A9.29 Human fibrocytes are believed to be derived from circulating CD14+ monocytes, express typical macrophage markers, and to play a role in tissue repair and fibrosis.29 The roles of MDSCs and fibrocytes in cardiovascular disease are unknown.
Expression of S100A8/A9 in non-myeloid cells
The hallmark of S100A8 and S100A9 expression in non-myeloid cells is their gene induction in response to stress. There is little S100A8/A9 in endothelial cells and vascular smooth muscle cells (VSMCs) under normal conditions. Expression in endothelial cells can be induced after activation with LPS, IL-1β, or TNF-α,26,30 or after exposure to elevated glucose levels in vitro or diabetes in vivo.31 No detectable release of S100A8/A9 has been found from cultured endothelial cells.26,30 In VSMCs, S100A9 expression is induced by the gram-negative bacterium Porphyromonas gingivalis,32 and S100A8/A9 expression is stimulated by LPS in cardiomyocytes.11 Together, these studies show that S100A8/A9 expression can be induced in non-myeloid cardiovascular cells through inflammatory stimuli likely to be present in atherosclerotic lesions and other cardiovascular pathologies.
S100A8/A9 release from intact non-myeloid cells is significantly lower than that from myeloid cells, suggesting that the biological effects of S100A8/A9 expressed by these cells might be largely intracellular, unless membrane integrity is compromised. Accordingly, Croce et al. demonstrated that the effects of S100A8/A9 on VSMC proliferation are mediated by intracellular actions.33
S100A8/A9 play important roles in atherosclerosis and vascular injury
Human studies
Monocytes play important roles in all stages of atherosclerosis in humans and mice.34 It was recently shown that the human CD14+CD16− monocyte population expresses more S100A8 than does the CD14+CD16+ monocyte population, similar to the elevated expression of S100A8 in mouse Ly-6C+ monocytes, as compared to Ly-6Clo monocytes.35 In mice, the Ly-6C+ monocyte population preferentially infiltrates lesions of atherosclerosis,36–37 but the role of different monocyte populations in human atherosclerosis is not well understood. Within human lesions, S100A9 immunoreactivity is associated with macrophages, microvessels and calcified areas.38 A subsequent study demonstrated that the percentage of S100A8/A9-positive macrophages is higher in rupture-prone lesions, as compared to stable ones.39 Furthermore, increased serum levels and expression of S100A8/A9 were observed in infiltrated neutrophils in atherosclerotic plaques of patients with unstable angina.40
The interest in S100A8/A9 in relation to cardiovascular disease increased markedly when Healy et al.21 demonstrated that plasma levels of S100A9 among apparently healthy women predict the risk of future nonfatal myocardial infarction, nonfatal stroke, and cardiovascular death. S100A8/A9 was subsequently found to be an early marker for detection of acute coronary syndromes,41 and the risk of a recurrent cardiovascular event was increased with each increasing quartile of S100A8/A9 in the PROVE IT-TIMI 22 trial.42 Together, these studies demonstrate that plasma levels of S100A8/A8 appear to be a marker of cardiovascular risk in humans.
Mouse studies
Monocyte populations that infiltrate atherosclerotic lesions in Apoe−/− mice express S100A9.43 Studies in mice have also demonstrated that S100A8/A9 are upregulated in macrophages overexpressing urokinase plasminogen activator concomitant with increased plaque rupture in LDL receptor (LDLR)-deficient (Ldlr−/−) mice,44 and that S100A8 gene expression is reduced in macrophages from regressing lesions.45 Together, these studies suggest that S100A8/A9 are upregulated in activated macrophages in vivo. Interestingly, a connection to diabetic vascular disease was established with the findings that macrophages from diabetic Ldlr−/− mice express and secrete higher levels of S100A9 as compared to macrophages from non-diabetic mice,46 and that elevated glucose levels result in increased expression of S100A8 in isolated macrophages.45 These results are corroborated by findings of increased S100A9 immunoreactivity in macrophage-rich lesions in these diabetic mice46 and in diabetic Apoe−/− mice.47
Mouse models have been used to investigate whether S100A8/A9 play causative roles in atherosclerosis. An interesting study on S100a9−/−;Apoe−/− mice demonstrates that this is indeed the case.33 These double knockout mice had an approximate 30% reduction in en face aortic lesion area in response to a high-fat diet, as compared to Apoe−/− controls. This reduction was not due to differences in plasma lipids, but to a reduced accumulation of lesion macrophages.33 The same study showed that both neointimal thickening following femoral artery injury, and lesions in a model of thrombohemorrhagic vasculitis are reduced in S100a9−/− mice.33 Interestingly, accumulation of both monocytes and neutrophils was reduced in S100a9−/− mice, suggesting that S100A8/A9 promote accumulation of both cell types at sites of vascular injury and atherosclerosis.
Based on these findings and the concept that a majority of S100A8/A9 is derived from myeloid cells, we hypothesized that the protective effects of S100A9-deficiency on atherosclerosis would be mimicked by S100A9-deficiency specifically in bone marrow-derived cells. We therefore undertook a study in which Ldlr−/− mice were transplanted with bone marrow from S100a9−/− mice or wildtype littermate controls and then fed a high-fat diet for 20 weeks.26 Surprisingly, neither atherosclerosis nor macrophage accumulation in lesions was affected by bone marrow S100A9-deficiency. Lesion neutrophils were not abundant in this study.26 The lack of effect of bone marrow S100A9-deficiency on atherosclerosis might be due to the production and secretion of S100A8/A9 at sufficiently high levels from non-bone marrow-derived cells, that other DAMPs or their receptors compensate for the loss of bone marrow-derived S100A8/A9, or that intracellular S100A8/A9 levels in non-myeloid cell types play a more important role than previously recognized. The latter possibility is supported by data describing S100A8/A9 expression in both VSMCs and endothelial cells, and “pro-atherosclerotic” effects of S100A8/A9 in these cell types.32,33,48 However, we cannot exclude the possibility that S100A8/A9 might be relatively more important mediators in atherosclerosis in Apoe−/− mice as compared to Ldlr−/− mice.
A more significant question is whether S100A8/A9 promote atherosclerosis and cardiovascular disease in humans. New tools, such as S100A8/A9 neutralizing antibodies or specific inhibitors of S100A8/A9 secretion, will have to be developed and proved safe before this important question can be addressed. S100A8 and S100A9 polymorphism studies49 might also shed additional light onto the roles of these proteins in cardiovascular disease in humans.
S100A8/A9 and cardiac dysfunction
S100A8/9 have important functions in the injured heart, especially in contributing to cardiovascular dysfunction as a result of sepsis. S100a9−/− mice are largely protected from endotoxin-induced cardiomyocyte dysfunction, measured as reduced ejection fraction.11 The effects of S100A8/A9 appear to be mediated by altered calcium flux following RAGE activation, since both cardiac S100A8 and S100A9 were found to co-immunoprecipitate with RAGE following LPS injection, and RAGE blockade abolished the decreased calcium flux by S100A8 or S100A9.11 On the other hand, in a rat model of experimental autoimmune myocarditis, treatment with recombinant human S100A8/A9 results in improved left ventricular ejection fraction, reduced infiltration of immune cells, and reduced levels of cytokines, as compared to saline-injected controls.50 Thus, the role of S100A8/A9 in the heart might depend on the stimuli and the contribution of RAGE or other receptors. The role of S100A8/A9 in the human heart is unknown.
Is S100A8/A9 pro- or anti-inflammatory in cells contributing to cardiovascular disease?
As discussed above, S100A8/A9 can activate TLR4 and RAGE, indicating a pro-inflammatory role for extracellular S100A8/A9. However, anti-inflammatory effects of these S100A proteins have also been described, which might be mediated by oxidation and/or S-nitrosylation of the S100A proteins,7 arachidonic acid binding,8 or other effects. Furthermore, it is becoming increasingly clear that S100A8/A9 have different functions in different cell types involved in atherosclerosis (Figure 1). For example, S100A8/A9 promote an inflammatory phenotype in neutrophils, while suppressing an inflammatory phenotype in DCs.26 We thus proposed that the overall function of S100A8/A9 on atherosclerosis depends on the relative levels of cell types involved in the disease process.26 What evidence supports cell type-specific effects of S100A8/A9 in vascular cells?
Neutrophils
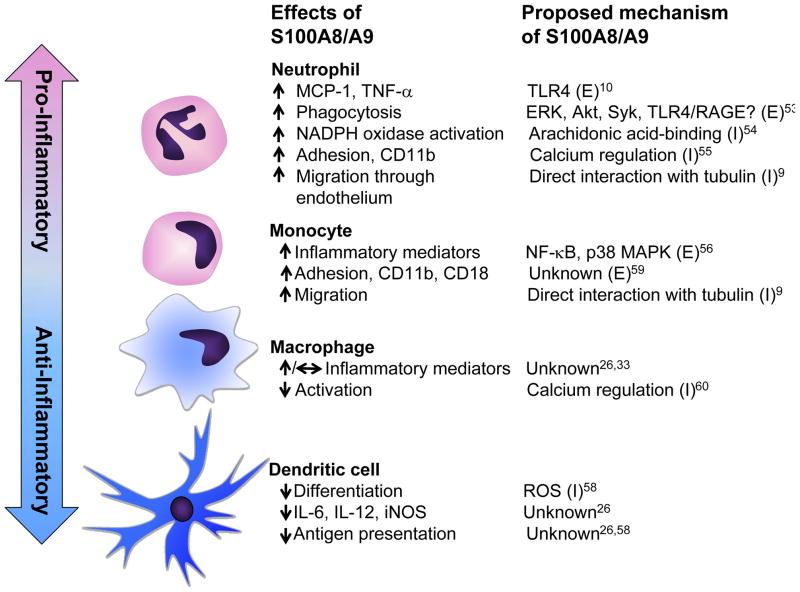
Neutrophils are present in lesions of atherosclerosis, although at relatively levels lower than those of monocytes/macrophages.26,51–52 S100A8/A9 are abundant in neutrophils, and not surprisingly, many of the effects of S100A8/A9 have been described in neutrophils, in which S100A8/A9 are pro-inflammatory, at least in part through extracellular activation of TLR4 (Figure 2).10,26 The ability of S100A9 to promote phagocytosis in neutrophils has also been attributed to extracellular activation of TLR4, RAGE, or another receptor.53 Accordingly, neutrophils from S100a9−/− mice show decreased activation of NADPH oxidase, an enzyme involved in pathogen killing following phagocytosis, as compared to wild-type mice.54 This effect, however, appears to be mediated by intracellular S100A8/A9 activating NADPH oxidase through their intracellular arachidonic acid-binding activity (Figure 2).54 Furthermore, intracellular S100A8/A9 contribute to neutrophil CD11b surface expression, neutrophil adhesion, and migration into inflamed tissues through calcium and microtubule regulation.9,55 Thus, neutrophil S100A8/A9 mediate phagocytosis and inflammatory effects by both extracellular and intracellular pathways.
Figure 2. S100A8/9 differentially modify phenotypic states of neutrophils, monocytes/macrophages, and dendritic cells through extracellular and intracellular mechanisms.
The effects of S100A8/A9 on processes involved in inflammation, and the proposed signaling pathways are shown for different myeloid-derived cells. (E), extracellular effects; (I), intracellular effects of S100A8/A9
Monocytes/macrophages
S100A8/A9 levels in monocytes are ~40-fold lower than those of neutrophils.16 Extracellular S100A8/A9 enhance the inflammatory cytokine production by human monocytes,56 and monocytes from S100a9−/− mice exhibit a reduced ability to migrate toward chemokines.33 Furthermore, mouse S100a9−/− peritoneal macrophages isolated 48 h after thioglycollate-injection have an impaired ability to release cytokines following LPS-stimulation.33 However, in mature peritoneal macrophages isolated 5 days after thioglycollate-injection, S100A9-deficiency does not affect cytokine release in response to LPS.26 It is well-known that as monocytes differentiate into macrophages, S100A8/A9 expression is markedly downregulated.23–24,57 Therefore, it is possible that the distinct responses to loss of endogenous S100A8/A9 between monocyte/early macrophages and mature macrophages are due to differences in S100A8/A8 expression. Another interesting possibility, based on forced S100A9 overexpression studies, is that S100A9 might delay myeloid differentiation,58 possibly explaining the different effects of S100A8/A9-deficiency in monocytes and different states of macrophage differentiation. The mechanisms whereby S100A8/A9 promote an inflammatory phenotype of monocytes/macrophages involve activation of the NF-κB and p38 MAPK pathways.56 It is tempting to speculate that this is due, at least in part, to activation of TLR4 and/or RAGE. S100A8/A9-mediated stimulation of monocyte adhesion has also been attributed to extracellular effects.59 On the other hand, S100A9 released in macrophages from phagocytosed apoptotic neutrophils has been proposed to inhibit macrophage activation through S100A9’s calcium-binding activity.60 These findings suggest that, like in neutrophils, S100A8/A9 have both extracellular and intracellular effects in monocytes/macrophages (Figure 2), and that the significance of endogenous S100A8/A9 depends on the expression levels in different monocyte/macrophage maturation and activation states.
Dendritic cells
DCs contribute significantly to atherosclerosis, at least in mice.61–62 DC differentiation is blocked by S100A9 overexpression, and it has been shown that S100A8/A9-overexpressing DCs have a lower ability to stimulate the proliferation of allogeneic T cells than do control DCs.58 The same study58 showed that overexpression of S100A9 in hematopoietic cells in mice results in accumulation of myeloid progenitors at the expense of DC and macrophage differentiation, and suggested that the effects of S100A9 were due to increased reactive oxygen species. Consistent with these results, S100a9−/− DCs promote a higher T cell proliferation compared to wild-type DC in mixed lymphocyte reactions.26 In addition, S100a9−/− DCs exhibit increased release of cytokines following stimulation with TLR2 or TLR4 ligands, as compared to DCs from wildtype littermates,26 and express more DC cell surface markers, (CD205, IA) and co-stimulatory molecules (CD40, CD86) compared to wild-type cells (unpublished data, 2011). Because TLR4 and RAGE activation is thought to promote DC maturation,63–64 S100A8/A9 might act through mechanisms distinct from TLR4 and RAGE to suppress inflammatory effects in DCs, perhaps indirectly by suppressing DC differentiation.
Endothelial cells
Exogenous S100A8/A9 increase expression of adhesion molecules, such as VCAM-1 and ICAM-1, and chemokines, as well as permeability in endothelial cells.48 Together, these effects could promote infiltration of immune cells into inflamed tissues.
S100A8/A9 are part of a larger network of proteins with cardiovascular effects
S100A8 and S100A9 belong to a multigenic family of S100 proteins that are differentially expressed in a wide variety of cell types.65 The functional diversity of S100 proteins is achieved by their specific cell- and tissue-expression patterns, structural variations, different metal ion binding properties, as well as their ability to form homo-, hetero- and oligomeric assemblies.66 Some S100 proteins, in particular S100A8, S100A9, and S100A12, are released from cells as a result of cellular activation. In the extracellular milieu, they function as ligands of pattern-recognition receptors, such as TLR410 and RAGE,11 and possibly other receptors, as discussed above. Both TLR467 and RAGE68–69 have been found to promote atherosclerosis in mice, although the effect of TLR4-deficiency is not consistently observed.70–71
Beside S100A8 and S100A9, S100A12, S100B, and S100A4 are implicated in the pathogenesis of atherosclerosis. S100A12 has been described as an endogenous RAGE ligand with pro-inflammatory functions in humans72–73, but is not expressed in the mouse.74 Elevated S100A12 levels are found in ruptured coronary artery plaques in patients suffering sudden cardiac death75 and in sera from patients with coronary artery disease.76 The latter study did not detect increased cytokine production in human monocytes or macrophages stimulated with human S100A12,76 suggesting that S100A12 actions on inflammation might be as complex as those of S100A8/A9. However, a recent study demonstrated that expression of human S100A12 in VSMCs results in increased atherosclerosis and more calcification of lesions in Apoe−/− mice.77 The S100A12 transgene also elicited the expression of genes involved in osteogenesis by cellular pathways that were dependent on RAGE and oxidative stress signaling. These findings suggest a causative involvement of S100A12 in atherosclerosis and vascular calcification.77
Another S100 protein, S100A4, exerts growth-promoting effects in VSMCs78 and, therefore, might play a role in atherogenesis. In humans, S100A4 is barely detectable in coronary artery media, but is markedly expressed in VSMCs of atheromatous and restenotic coronary artery lesions.78 There is no direct evidence available for S100A4/RAGE interaction.
Besides its abundance in astrocytes, S100B is expressed in cells outside the brain, including in DCs and VSMCs.79 S100B exerts effects on both endothelial cells and VSMCs that might have an impact on atherosclerosis. These effects are mainly RAGE-dependent.80 In human endothelial cells, S100B up-regulates the gene expression of monocyte chemoattractant protein 1 (MCP-1), and RAGE.81 In VSMCs, S100B has been found to stimulate IL-6 and MCP-1 production and cell migration.82
Very recently, elevated serum levels of S100B, S100A6 and S100P were found to be associated with the acute coronary syndrome, and serum levels and myocardial expression of these proteins were related to infarct size.83 Thus, the group of S100 protein family members with potential roles in cardiovascular biology and disease is rapidly expanding.
Importantly, these S100 proteins, other DAMPs, and their receptors are likely to impact each other and to synergize in vivo. As an example, activation of RAGE results in increased expression and release of S100A8 and S100A9,14 and AGEs exacerbate the pro-inflammatory effects of S100A8/A9,84 contributing to a likely positive feedback loop in states of inflammation. Similarly, in the setting of diabetes, DAMPs, RAGE, and TLR4 are all upregulated in monocytes/macrophages,47,85 potentially contributing to a “perfect storm” of activation of this network of proteins.
Many questions remain unanswered
We have reviewed the evidence that S100A8/A9 are both biomarkers and mediators of cardiovascular disease. However, more research is needed to elucidate the complex effects of S100A8 and S100A9. For example, to what extent are the biological effects of S100A8/A9 mediated by intracellular versus extracellular actions?; why and how do S100A8/A9 mediate pro-inflammatory and anti-inflammatory effects in different cell types and disease states?; what is the relative contribution of S100A8/A9 versus other DAMPs?; which are the cell types that contribute to S100A8/A9 expression and secretion in different disease states; and to what extent do S100A8 and S100A9 contribute to cardiovascular disease in humans? Answers to the questions above are needed before S100A8/A9 can be considered as therapeutic targets.
Acknowledgments
Acknowledgments: None
Sources of funding: These studies was supported in part by NIH grants HL062887, HL092969 (project 2), HL097365 to (KEB) and “Interdisziplinäres Zentrum für Klinische Forschung”, University of Muenster, project Ker3/086/04, and “Deutsche Forschungsgemeinschaft”, projects KE 820/6-1 and KE 820/2-4 (CK). MMA was supported by a Cardiovascular Postdoctoral Training Grant (T32 HL07828).
Footnotes
Disclosures: None
References
- 1.Nacken W, Roth J, Sorg C, Kerkhoff C. S100A9: a myeloid S100 representative as a prominent player in innate immunity. Microsc Res Tech. 2003;60:569–580. doi: 10.1002/jemt.10299. [DOI] [PubMed] [Google Scholar]
- 2.Perera C, McNeil HP, Geczy CL. S100 Calgranulins in inflammatory arthritis. Immunol Cell Biol. 2010;88:41–49. doi: 10.1038/icb.2009.88. [DOI] [PubMed] [Google Scholar]
- 3.Ehrchen JM, Sunderkötter C, Foell D, Vogl T, Roth J. The endogenous Toll-like receptor 4 agonist S100A8/S100A9 (calprotectin) as innate amplifier of infection, autoimmunity, and cancer. J Leukoc Biol. 2009;86:557–566. doi: 10.1189/jlb.1008647. [DOI] [PubMed] [Google Scholar]
- 4.Ghavami S, Chitayat S, Hashemi M, Eshraghi M, Chazin WJ, Halayko AJ, Kerkhoff C. S100A8/A9: a Janus-faced molecule in cancer therapy and tumorgenesis. Eur J Pharmacol. 2009;625:73–83. doi: 10.1016/j.ejphar.2009.08.044. [DOI] [PubMed] [Google Scholar]
- 5.Croce K. S100A8/A9 complex: more than just a biomarker of cardiovascular risk? Circ J. 2010;74:626–627. doi: 10.1253/circj.cj-10-0192. [DOI] [PubMed] [Google Scholar]
- 6.Lim SY, Raftery MJ, Geczy CL. Oxidative modifications of DAMPs suppress inflammation: The Case for S100A8 and S100A9. Antioxid Redox Signal. 2011 Apr 11; doi: 10.1089/ars.2010.3641. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 7.Goyette J, Geczy CL. Inflammation-associated S100 proteins: new mechanisms that regulate function. Amino Acids. 2011;41:821–42. doi: 10.1007/s00726-010-0528-0. [DOI] [PubMed] [Google Scholar]
- 8.Kerkhoff C, Klempt M, Kaever V, Sorg C. The two calcium-binding proteins S100A8 and S100A9 are involved in the metabolism of arachidonic acid in human neutrophils. J Biol Chem. 1999;274:32672–32679. doi: 10.1074/jbc.274.46.32672. [DOI] [PubMed] [Google Scholar]
- 9.Vogl T, Ludwig S, Goebeler M, Strey A, Thorey IS, Reichelt R, Foell D, Gerke V, Manitz MP, Nacken W, Werner S, Sorg C, Roth J. MRP8 and MRP14 control microtubule reorganization during transendothelial migration of phagocytes. Blood. 2004;104:4260–4268. doi: 10.1182/blood-2004-02-0446. [DOI] [PubMed] [Google Scholar]
- 10.Vogl T, Tenbrock K, Ludwig S, Leukert N, Ehrhardt C, van Zoelen MA, Nacken W, Foell D, van der Poll T, Sorg C, Roth J. Mrp8 and Mrp14 are endogenous activators of Toll-like receptor 4, promoting lethal, endotoxin-induced shock. Nat Med. 2007;13:1042–1049. doi: 10.1038/nm1638. [DOI] [PubMed] [Google Scholar]
- 11.Boyd JH, Kan B, Roberts H, Wang Y, Walley KR. S100A8 and S100A9 mediate endotoxin-induced cardiomyocyte dysfunction via the receptor for advanced glycation end products. Circ Res. 2008;102:1239–1246. doi: 10.1161/CIRCRESAHA.107.167544. [DOI] [PubMed] [Google Scholar]
- 12.Kerkhoff C, Sorg C, Tandon NN, Nacken W. Interaction of S100A8/S100A9-arachidonic acid complexes with the scavenger receptor CD36 may facilitate fatty acid uptake by endothelial cells. Biochemistry. 2001;40:241–248. doi: 10.1021/bi001791k. [DOI] [PubMed] [Google Scholar]
- 13.Rammes A, Roth J, Goebeler M, Klempt M, Hartmann M, Sorg C. Myeloid-related protein (MRP) 8 and MRP14, calcium-binding proteins of the S100 family, are secreted by activated monocytes via a novel, tubulin-dependent pathway. J Biol Chem. 1997;272:9496–9502. doi: 10.1074/jbc.272.14.9496. [DOI] [PubMed] [Google Scholar]
- 14.Eggers K, Sikora K, Lorenz M, Taubert T, Moobed M, Baumann G, Stangl K, Stangl V. RAGE-dependent regulation of calcium-binding proteins S100A8 and S100A9 in human THP-1. Exp Clin Endocrinol Diabetes. 2011;119:353–357. doi: 10.1055/s-0030-1268426. [DOI] [PubMed] [Google Scholar]
- 15.Kerkhoff C, Klempt M, Sorg C. Novel insights into structure and function of MRP8 (S100A8) and MRP14 (S100A9) Biochim Biophys Acta. 1998;1448:200–211. doi: 10.1016/s0167-4889(98)00144-x. [DOI] [PubMed] [Google Scholar]
- 16.Edgeworth J, Gorman M, Bennett R, Freemont P, Hogg N. Identification of p8,14 as a highly abundant heterodimeric calcium binding protein complex of myeloid cells. J Biol Chem. 1991;266:7706–7713. [PubMed] [Google Scholar]
- 17.Kerkhoff C, Hofmann HA, Vormoor J, Melkonyan H, Roth J, Sorg C, Klempt M. Binding of two nuclear complexes to a novel regulatory element within the human S100A9 promoter drives the S100A9 gene expression. J Biol Chem. 2002;277:41879–41887. doi: 10.1074/jbc.M207990200. [DOI] [PubMed] [Google Scholar]
- 18.Goebeler M, Roth J, Henseleit U, Sunderkötter C, Sorg C. Expression and complex assembly of calcium-binding proteins MRP8 and MRP14 during differentiation of murine myelomonocytic cells. J Leukoc Biol. 1993;53:11–18. doi: 10.1002/jlb.53.1.11. [DOI] [PubMed] [Google Scholar]
- 19.Henkel GW, McKercher SR, Maki RA. Identification of three genes up-regulated in PU.1 rescued monocytic precursor cells. Int Immunol. 2002;14:723–732. doi: 10.1093/intimm/dxf040. [DOI] [PubMed] [Google Scholar]
- 20.Husson H, Carideo EG, Neuberg D, Schultze J, Munoz O, Marks PW, Donovan JW, Chillemi AC, O’Connell P, Freedman AS. Gene expression profiling of follicular lymphoma and normal germinal center B cells using cDNA arrays. Blood. 2002;99:282–289. doi: 10.1182/blood.v99.1.282. [DOI] [PubMed] [Google Scholar]
- 21.Healy AM, Pickard MD, Pradhan AD, Wang Y, Chen Z, Croce K, Sakuma M, Shi C, Zago AC, Garasic J, Damokosh AI, Dowie TL, Poisson L, Lillie J, Libby P, Ridker PM, Simon DI. Platelet expression profiling and clinical validation of myeloid-related protein-14 as a novel determinant of cardiovascular events. Circulation. 2006;113:2278–2284. doi: 10.1161/CIRCULATIONAHA.105.607333. [DOI] [PubMed] [Google Scholar]
- 22.Odink K, Cerletti N, Brüggen J, Clerc RG, Tarcsay L, Zwadlo G, Gerhards G, Schlegel R, Sorg C. Two calcium-binding proteins in infiltrate macrophages of rheumatoid arthritis. Nature. 1987;330:80–82. doi: 10.1038/330080a0. [DOI] [PubMed] [Google Scholar]
- 23.Lagasse E, Clerc RG. Cloning and expression of two human genes encoding calcium-binding proteins that are regulated during myeloid differentiation. Mol Cell Biol. 1988;8:2402–2410. doi: 10.1128/mcb.8.6.2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zwadlo G, Brüggen J, Gerhards G, Schlegel R, Sorg C. Two calcium-binding proteins associated with specific stages of myeloid cell differentiation are expressed by subsets of macrophages in inflammatory tissues. Clin Exp Immunol. 1988;72:510–515. [PMC free article] [PubMed] [Google Scholar]
- 25.Le Naour F, Hohenkirk L, Grolleau A, Misek DE, Lescure P, Geiger JD, Hanash S, Beretta L. Profiling changes in gene expression during differentiation and maturation of monocyte-derived dendritic cells using both oligonucleotide microarrays and proteomics. J Biol Chem. 2001;276:17920–17931. doi: 10.1074/jbc.M100156200. [DOI] [PubMed] [Google Scholar]
- 26.Averill MM, Barnhart S, Becker L, Li X, Heinecke JW, Leboeuf RC, Hamerman JA, Sorg C, Kerkhoff C, Bornfeldt KE. S100A9 differentially modifies phenotypic states of neutrophils, macrophages, and dendritic cells: implications for atherosclerosis and adipose tissue inflammation. Circulation. 2011;123:1216–1226. doi: 10.1161/CIRCULATIONAHA.110.985523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kumar A, Steinkasserer A, Berchtold S. Interleukin-10 influences the expression of mrp8 and mrp14 in human dendritic cells. Int Arch Allergy Immunol. 2003;132:40–47. doi: 10.1159/000073263. [DOI] [PubMed] [Google Scholar]
- 28.Sinha P, Okoro C, Foell D, Freeze HH, Ostrand-Rosenberg S, Srikrishna G. Proinflammatory S100 proteins regulate the accumulation of myeloid-derived suppressor cells. J Immunol. 2008;181:4666–4675. doi: 10.4049/jimmunol.181.7.4666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pilling D, Fan T, Huang D, Kaul B, Gomer RH. Identification of markers that distinguish monocyte-derived fibrocytes from monocytes, macrophages, and fibroblasts. PLoS One. 2009;4:e7475. doi: 10.1371/journal.pone.0007475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yen T, Harrison CA, Devery JM, Leong S, Iismaa SE, Yoshimura T, Geczy CL. Induction of the S100 chemotactic protein, CP-10, in murine microvascular endothelial cells by proinflammatory stimuli. Blood. 1997;90:4812–4821. [PubMed] [Google Scholar]
- 31.Yao D, Brownlee M. Hyperglycemia-induced reactive oxygen species increase expression of the receptor for advanced glycation end products (RAGE) and RAGE ligands. Diabetes. 2010;59:249–255. doi: 10.2337/db09-0801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Inaba H, Hokamura K, Nakano K, Nomura R, Katayama K, Nakajima A, Yoshioka H, Taniguchi K, Kamisaki Y, Ooshima T, Umemura K, Murad F, Wada K, Amano A. Upregulation of S100 calcium-binding protein A9 is required for induction of smooth muscle cell proliferation by a periodontal pathogen. FEBS Lett. 2009;583:128–134. doi: 10.1016/j.febslet.2008.11.036. [DOI] [PubMed] [Google Scholar]
- 33.Croce K, Gao H, Wang Y, Mooroka T, Sakuma M, Shi C, Sukhova GK, Packard RR, Hogg N, Libby P, Simon DI. Myeloid-related protein-8/14 is critical for the biological response to vascular injury. Circulation. 2009;120:427–436. doi: 10.1161/CIRCULATIONAHA.108.814582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moore KJ, Tabas I. Macrophages in the pathogenesis of atherosclerosis. Cell. 2011;145:341–355. doi: 10.1016/j.cell.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ingersoll MA, Spanbroek R, Lottaz C, Gautier EL, Frankenberger M, Hoffmann R, Lang R, Haniffa M, Collin M, Tacke F, Habenicht AJ, Ziegler-Heitbrock L, Randolph GJ. Comparison of gene expression profiles between human and mouse monocyte subsets. Blood. 2010;115:e10–19. doi: 10.1182/blood-2009-07-235028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tacke F, Alvarez D, Kaplan TJ, Jakubzick C, Spanbroek R, Llodra J, Garin A, Liu J, Mack M, van Rooijen N, Lira SA, Habenicht AJ, Randolph GJ. Monocyte subsets differentially employ CCR2, CCR5, and CX3CR1 to accumulate within atherosclerotic plaques. J Clin Invest. 2007;117:185–194. doi: 10.1172/JCI28549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Swirski FK, Libby P, Aikawa E, Alcaide P, Luscinskas FW, Weissleder R, Pittet MJ. Ly-6Chi monocytes dominate hypercholesterolemia-associated monocytosis and give rise to macrophages in atheromata. J Clin Invest. 2007;117:195–205. doi: 10.1172/JCI29950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McCormick MM, Rahimi F, Bobryshev YV, Gaus K, Zreiqat H, Cai H, Lord RS, Geczy CL. S100A8 and S100A9 in human arterial wall. Implications for atherogenesis. J Biol Chem. 2005;280:41521–41529. doi: 10.1074/jbc.M509442200. [DOI] [PubMed] [Google Scholar]
- 39.Ionita MG, Vink A, Dijke IE, Laman JD, Peeters W, van der Kraak PH, Moll FL, de Vries JP, Pasterkamp G, de Kleijn DP. High levels of myeloid-related protein 14 in human atherosclerotic plaques correlate with the characteristics of rupture-prone lesions. Arterioscler Thromb Vasc Biol. 2009;29:1220–1227. doi: 10.1161/ATVBAHA.109.190314. [DOI] [PubMed] [Google Scholar]
- 40.Miyamoto S, Ueda M, Ikemoto M, Naruko T, Itoh A, Tamaki S, Nohara R, Terasaki F, Sasayama S, Fujita M. Increased serum levels and expression of S100A8/A9 complex in infiltrated neutrophils in atherosclerotic plaque of unstable angina. Heart. 2008;94:1002–1007. doi: 10.1136/hrt.2007.121640. [DOI] [PubMed] [Google Scholar]
- 41.Altwegg LA, Neidhart M, Hersberger M, Müller S, Eberli FR, Corti R, Roffi M, Sütsch G, Gay S, von Eckardstein A, Wischnewsky MB, Lüscher TF, Maier W. Myeloid-related protein 8/14 complex is released by monocytes and granulocytes at the site of coronary occlusion: a novel, early, and sensitive marker of acute coronary syndromes. Eur Heart J. 2007;28:941–948. doi: 10.1093/eurheartj/ehm078. [DOI] [PubMed] [Google Scholar]
- 42.Morrow DA, Wang Y, Croce K, Sakuma M, Sabatine MS, Gao H, Pradhan AD, Healy AM, Buros J, McCabe CH, Libby P, Cannon CP, Braunwald E, Simon DI. Myeloid-related protein 8/14 and the risk of cardiovascular death or myocardial infarction after an acute coronary syndrome in the Pravastatin or Atorvastatin Evaluation and Infection Therapy: Thrombolysis in Myocardial Infarction (PROVE IT-TIMI 22) trial. Am Heart J. 2008;155:49–55. doi: 10.1016/j.ahj.2007.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Eue I, Langer C, Eckardstein A, Sorg C. Myeloid related protein (MRP) 14 expressing monocytes infiltrate atherosclerotic lesions of ApoE null mice. Atherosclerosis. 2000;151:593–597. doi: 10.1016/s0021-9150(00)00476-7. [DOI] [PubMed] [Google Scholar]
- 44.Farris SD, Hu JH, Krishnan R, Emery I, Chu T, Du L, Kremen M, Dichek HL, Gold E, Ramsey SA, Dichek DA. Mechanisms of urokinase plasminogen activator (uPA)-mediated atherosclerosis: role of the uPA receptor and S100A8/A9 proteins. J Biol Chem. 2011;286:22665–22677. doi: 10.1074/jbc.M110.202135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Parathath S, Grauer L, Huang LS, Sanson M, Distel E, Goldberg IJ, Fisher EA. Diabetes adversely affects macrophages during atherosclerotic plaque regression in mice. Diabetes. 2011;60:1759–1769. doi: 10.2337/db10-0778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Johansson F, Kramer F, Barnhart S, Kanter JE, Vaisar T, Merrill RD, Geng L, Oka K, Chan L, Chait A, Heinecke JW, Bornfeldt KE. Type 1 diabetes promotes disruption of advanced atherosclerotic lesions in LDL receptor-deficient mice. Proc Natl Acad Sci U S A. 2008;105:2082–2087. doi: 10.1073/pnas.0709958105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Soro-Paavonen A, Watson AM, Li J, Paavonen K, Koitka A, Calkin AC, Barit D, Coughlan MT, Drew BG, Lancaster GI, Thomas M, Forbes JM, Nawroth PP, Bierhaus A, Cooper ME, Jandeleit-Dahm KA. Receptor for advanced glycation end products (RAGE) deficiency attenuates the development of atherosclerosis in diabetes. Diabetes. 2008;57:2461–2469. doi: 10.2337/db07-1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Viemann D, Strey A, Janning A, Jurk K, Klimmek K, Vogl T, Hirono K, Ichida F, Foell D, Kehrel B, Gerke V, Sorg C, Roth J. Myeloid-related proteins 8 and 14 induce a specific inflammatory response in human microvascular endothelial cells. Blood. 2005;105:2955–2962. doi: 10.1182/blood-2004-07-2520. [DOI] [PubMed] [Google Scholar]
- 49.Li Q, Meng H, Zhang L, Xu L, Chen Z, Shi D, Feng X, Zhu X, Zhao H, Cao C. Correlation between single nucleotide polymorphisms in a calprotectin subunit gene and risk of periodontitis in a Chinese population. Ann Hum Genet. 2007;71:312–324. doi: 10.1111/j.1469-1809.2006.00326.x. [DOI] [PubMed] [Google Scholar]
- 50.Otsuka K, Terasaki F, Ikemoto M, Fujita S, Tsukada B, Katashima T, Kanzaki Y, Sohmiya K, Kono T, Toko H, Fujita M, Kitaura Y. Suppression of inflammation in rat autoimmune myocarditis by S100A8/A9 through modulation of the proinflammatory cytokine network. Eur J Heart Fail. 2009;11:229–237. doi: 10.1093/eurjhf/hfn049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.van Leeuwen M, Gijbels MJ, Duijvestijn A, Smook M, van de Gaar MJ, Heeringa P, de Winther MP, Tervaert JW. Accumulation of myeloperoxidase-positive neutrophils in atherosclerotic lesions in LDLR−/− mice. Arterioscler Thromb Vasc Biol. 2008;28:84–89. doi: 10.1161/ATVBAHA.107.154807. [DOI] [PubMed] [Google Scholar]
- 52.Rotzius P, Thams S, Soehnlein O, Kenne E, Tseng CN, Björkström NK, Malmberg KJ, Lindbom L, Eriksson EE. Distinct infiltration of neutrophils in lesion shoulders in ApoE−/− mice. Am J Pathol. 2010;177:493–500. doi: 10.2353/ajpath.2010.090480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Simard JC, Simon MM, Tessier PA, Girard D. Damage-associated molecular pattern S100A9 increases bactericidal activity of human neutrophils by enhancing phagocytosis. J Immunol. 2011;186:3622–3631. doi: 10.4049/jimmunol.1002956. [DOI] [PubMed] [Google Scholar]
- 54.Kerkhoff C, Nacken W, Benedyk M, Dagher MC, Sopalla C, Doussiere J. The arachidonic acid-binding protein S100A8/A9 promotes NADPH oxidase activation by interaction with p67phox and Rac-2. FASEB J. 2005;19:467–469. doi: 10.1096/fj.04-2377fje. [DOI] [PubMed] [Google Scholar]
- 55.Manitz MP, Horst B, Seeliger S, Strey A, Skryabin BV, Gunzer M, Frings W, Schönlau F, Roth J, Sorg C, Nacken W. Loss of S100A9 (MRP14) results in reduced interleukin-8-induced CD11b surface expression, a polarized microfilament system, and diminished responsiveness to chemoattractants in vitro. Mol Cell Biol. 2003;23:1034–1043. doi: 10.1128/MCB.23.3.1034-1043.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sunahori K, Yamamura M, Yamana J, Takasugi K, Kawashima M, Yamamoto H, Chazin WJ, Nakatani Y, Yui S, Makino H. The S100A8/A9 heterodimer amplifies proinflammatory cytokine production by macrophages via activation of nuclear factor kappa B and p38 mitogen-activated protein kinase in rheumatoid arthritis. Arthritis Res Ther. 2006;8:R69. doi: 10.1186/ar1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hogg N, Allen C, Edgeworth J. Monoclonal antibody 5.5 reacts with p8,14, a myeloid molecule associated with some vascular endothelium. Eur J Immunol. 1989;19:1053–1061. doi: 10.1002/eji.1830190615. [DOI] [PubMed] [Google Scholar]
- 58.Cheng P, Corzo CA, Luetteke N, Yu B, Nagaraj S, Bui MM, Ortiz M, Nacken W, Sorg C, Vogl T, Roth J, Gabrilovich DI. Inhibition of dendritic cell differentiation and accumulation of myeloid-derived suppressor cells in cancer is regulated by S100A9 protein. J Exp Med. 2008;205:2235–2249. doi: 10.1084/jem.20080132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bouma G, Lam-Tse WK, Wierenga-Wolf AF, Drexhage HA, Versnel MA. Increased serum levels of MRP-8/14 in type 1 diabetes induce an increased expression of CD11b and an enhanced adhesion of circulating monocytes to fibronectin. Diabetes. 2004;53:1979–1986. doi: 10.2337/diabetes.53.8.1979. [DOI] [PubMed] [Google Scholar]
- 60.De Lorenzo BH, Godoy LC, Novaes e Brito RR, Pagano RL, Amorim-Dias MA, Grosso DM, Lopes JD, Mariano M. Macrophage suppression following phagocytosis of apoptotic neutrophils is mediated by the S100A9 calcium-binding protein. Immunobiology. 2010;215:341–347. doi: 10.1016/j.imbio.2009.05.013. [DOI] [PubMed] [Google Scholar]
- 61.Paulson KE, Zhu SN, Chen M, Nurmohamed S, Jongstra-Bilen J, Cybulsky MI. Resident intimal dendritic cells accumulate lipid and contribute to the initiation of atherosclerosis. Circ Res. 2010;106:383–390. doi: 10.1161/CIRCRESAHA.109.210781. [DOI] [PubMed] [Google Scholar]
- 62.Weber C, Meiler S, Döring Y, Koch M, Drechsler M, Megens RT, Rowinska Z, Bidzhekov K, Fecher C, Ribechini E, van Zandvoort MA, Binder CJ, Jelinek I, Hristov M, Boon L, Jung S, Korn T, Lutz MB, Förster I, Zenke M, Hieronymus T, Junt T, Zernecke A. CCL17-expressing dendritic cells drive atherosclerosis by restraining regulatory T cell homeostasis in mice. J Clin Invest. 2011:121. doi: 10.1172/JCI44925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dumitriu IE, Baruah P, Valentinis B, Voll RE, Herrmann M, Nawroth PP, Arnold B, Bianchi ME, Manfredi AA, Rovere-Querini P. Release of high mobility group box 1 by dendritic cells controls T cell activation via the receptor for advanced glycation end products. J Immunol. 2005;174:7506–7515. doi: 10.4049/jimmunol.174.12.7506. [DOI] [PubMed] [Google Scholar]
- 64.Kaisho T, Takeuchi O, Kawai T, Hoshino K, Akira S. Endotoxin-induced maturation of MyD88-deficient dendritic cells. J Immunol. 2001;166:5688–5694. doi: 10.4049/jimmunol.166.9.5688. [DOI] [PubMed] [Google Scholar]
- 65.Leclerc E, Fritz G, Vetter SW, Heizmann CW. Binding of S100 proteins to RAGE: an update. Biochim Biophys Acta. 2009;1793:993–1007. doi: 10.1016/j.bbamcr.2008.11.016. [DOI] [PubMed] [Google Scholar]
- 66.Fritz G, Botelho HM, Morozova-Roche LA, Gomes CM. Natural and amyloid self-assembly of S100 proteins: structural basis of functional diversity. FEBS J. 2010;277:4578–4590. doi: 10.1111/j.1742-4658.2010.07887.x. [DOI] [PubMed] [Google Scholar]
- 67.Michelsen KS, Wong MH, Shah PK, Zhang W, Yano J, Doherty TM, Akira S, Rajavashisth TB, Arditi M. Lack of toll-like receptor 4 or myeloid differentiation factor 88 reduces atherosclerosis and alters plaque phenotype in mice deficient in apolipoprotein E. Proc Natl Acad Sci U S A. 2004;101:10679–10684. doi: 10.1073/pnas.0403249101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Harja E, Bu DX, Hudson BI, Chang JS, Shen X, Hallam K, Kalea AZ, Lu Y, Rosario RH, Oruganti S, Nikolla Z, Belov D, Lalla E, Ramasamy R, Yan SF, Schmidt AM. Vascular and inflammatory stresses mediate atherosclerosis via RAGE and its ligands in apoE−/− mice. J Clin Invest. 2008;118:183–194. doi: 10.1172/JCI32703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Barlovic DP, Soro-Paavonen A, Jandeleit-Dahm KA. RAGE biology, atherosclerosis and diabetes. Clin Sci (Lond) 2011;121:43–55. doi: 10.1042/CS20100501. [DOI] [PubMed] [Google Scholar]
- 70.Wright SD, Burton C, Hernandez M, Hassing H, Montenegro J, Mundt S, Patel S, Card DJ, Hermanowski-Vosatka A, Bergstrom JD, Sparrow CP, Detmers PA, Chao YS. Infectious agents are not necessary for murine atherogenesis. J Exp Med. 2000;191:1437–42. doi: 10.1084/jem.191.8.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Coenen KR, Gruen ML, Lee-Young RS, Puglisi MJ, Wasserman DH, Hasty AH. Impact of macrophage toll-like receptor 4 deficiency on macrophage infiltration into adipose tissue and the artery wall in mice. Diabetologia. 2009;52:318–328. doi: 10.1007/s00125-008-1221-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hofmann MA, Drury S, Fu C, Qu W, Taguchi A, Lu Y, Avila C, Kambham N, Bierhaus A, Nawroth P, Neurath MF, Slattery T, Beach D, McClary J, Nagashima M, Morser J, Stern D, Schmidt AM. RAGE mediates a novel proinflammatory axis: a central cell surface receptor for S100/calgranulin polypeptides. Cell. 1999;97:889–901. doi: 10.1016/s0092-8674(00)80801-6. [DOI] [PubMed] [Google Scholar]
- 73.Yang Z, Tao T, Raftery MJ, Youssef P, Di Girolamo N, Geczy CL. Proinflammatory properties of the human S100 protein S100A12. J Leukoc Biol. 2001;69:986–994. [PubMed] [Google Scholar]
- 74.Fuellen G, Foell D, Nacken W, Sorg C, Kerkhoff C. Absence of S100A12 in mouse: implications for RAGE-S100A12 interaction. Trends Immunol. 2003;24:622–624. doi: 10.1016/j.it.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 75.Burke AP, Kolodgie FD, Zieske A, Fowler DR, Weber DK, Varghese PJ, Farb A, Virmani R. Morphologic findings of coronary atherosclerotic plaques in diabetics: a postmortem study. Arterioscler Thromb Vasc Biol. 2004;24:1266–1271. doi: 10.1161/01.ATV.0000131783.74034.97. [DOI] [PubMed] [Google Scholar]
- 76.Goyette J, Yan WX, Yamen E, Chung YM, Lim SY, Hsu K, Rahimi F, Di Girolamo N, Song C, Jessup W, Kockx M, Bobryshev YV, Freedman SB, Geczy CL. Pleiotropic roles of S100A12 in coronary atherosclerotic plaque formation and rupture. J Immunol. 2009;183:593–603. doi: 10.4049/jimmunol.0900373. [DOI] [PubMed] [Google Scholar]
- 77.Hofmann Bowman MA, Gawdzik J, Bukhari U, Husain AN, Toth PT, Kim G, Earley J, McNally EM. S100A12 in vascular smooth muscle accelerates vascular calcification in apolipoprotein E-null mice by activating an osteogenic gene regulatory program. Arterioscler Thromb Vasc Biol. 2011;31:337–344. doi: 10.1161/ATVBAHA.110.217745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Brisset AC, Hao H, Camenzind E, Bacchetta M, Geinoz A, Sanchez JC, Chaponnier C, Gabbiani G, Bochaton-Piallat ML. Intimal smooth muscle cells of porcine and human coronary artery express S100A4, a marker of the rhomboid phenotype in vitro. Circ Res. 2007;100:1055–1062. doi: 10.1161/01.RES.0000262654.84810.6c. [DOI] [PubMed] [Google Scholar]
- 79.Donato R. S100: a multigenic family of calcium-modulated proteins of the EF-hand type with intracellular and extracellular functional roles. Int J Biochem Cell Biol. 2001;33:637–668. doi: 10.1016/s1357-2725(01)00046-2. [DOI] [PubMed] [Google Scholar]
- 80.Tsoporis JN, Izhar S, Leong-Poi H, Desjardins JF, Huttunen HJ, Parker TG. S100B interaction with the receptor for advanced glycation end products (RAGE): a novel receptor-mediated mechanism for myocyte apoptosis postinfarction. Circ Res. 2010;106:93–101. doi: 10.1161/CIRCRESAHA.109.195834. [DOI] [PubMed] [Google Scholar]
- 81.Feng L, Matsumoto C, Schwartz A, Schmidt AM, Stern DM, Pile-Spellman J. Chronic vascular inflammation in patients with type 2 diabetes: endothelial biopsy and RT-PCR analysis. Diabetes Care. 2005;28:379–384. doi: 10.2337/diacare.28.2.379. [DOI] [PubMed] [Google Scholar]
- 82.Reddy MA, Li SL, Sahar S, Kim YS, Xu ZG, Lanting L, Natarajan R. Key role of Src kinase in S100B-induced activation of the receptor for advanced glycation end products in vascular smooth muscle cells. J Biol Chem. 2006;281:13685–13693. doi: 10.1074/jbc.M511425200. [DOI] [PubMed] [Google Scholar]
- 83.Cai XY, Lu L, Wang YN, Jin C, Zhang RY, Zhang Q, Chen QJ, Shen WF. Association of increased S100B, S100A6 and S100P in serum levels with acute coronary syndrome and also with the severity of myocardial infarction in cardiac tissue of rat models with ischemia-reperfusion injury. Atherosclerosis. 2011;217:536–542. doi: 10.1016/j.atherosclerosis.2011.05.023. [DOI] [PubMed] [Google Scholar]
- 84.Ehlermann P, Eggers K, Bierhaus A, Most P, Weichenhan D, Greten J, Nawroth PP, Katus HA, Remppis A. Increased proinflammatory endothelial response to S100A8/A9 after preactivation through advanced glycation end products. Cardiovasc Diabetol. 2006;5:6. doi: 10.1186/1475-2840-5-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Devaraj S, Dasu MR, Rockwood J, Winter W, Griffen SC, Jialal I. Increased toll-like receptor (TLR) 2 and TLR4 expression in monocytes from patients with type 1 diabetes: further evidence of a proinflammatory state. J Clin Endocrinol Metab. 2008;93:578–583. doi: 10.1210/jc.2007-2185. [DOI] [PMC free article] [PubMed] [Google Scholar]