Abstract
Deficits in episodic memory are associated with deficits in the ability to imagine future experiences (i.e., mental time travel). We show that K.C., a person with episodic amnesia and an inability to imagine future experiences, nonetheless systematically discounts the value of future rewards, and his discounting is within the range of controls in terms of both rate and consistency. Because K.C. is neither able to imagine personal uses for the rewards nor provide a rationale for selecting larger future rewards over smaller current rewards, the current study demonstrates a dissociation between imagining and making decisions involving the future. Thus, although those capable of mental time travel may use it in making decisions about future rewards, the present results demonstrate that it is not required for such decisions.
Much of the richness in human mental life derives from mental time travel, the ability to remember our personal pasts and imagine our personal futures. These abilities are entwined: People with hippocampal damage and corresponding episodic memory impairment also have an impaired ability to imagine the future (Klein et al., 2002; Kwan et al., 2010; Rosenbaum et al., 2005; Tulving, 1985; see Addis et al., 2007 for neuroimaging evidence). Some propose that hippocampally mediated episodic memory is part of a system that subserves future-oriented decision-making in general (Bar, 2009, 2010; Buckner, 2010; Gupta et al., 2009; Suddendorf and Corballis, 2007; Szpunar, 2010). A novel way to test this supposition is to examine how neurocognitive memory disorders, such as amnesia, affect such decision-making. The ability to choose between smaller, immediate rewards and larger, later rewards is a fundamental aspect of future-oriented decision-making, and one that is plausibly influenced by the ability to imagine one’s possible future (Atance and O’Neil, 2001; Atance and Jackson, 2009; Benoit et al., 2011; Boyer, 2008; Liberman and Trope, 2008; Peters and Büchel, 2010; Tulving, 2002).
Imagining future episodes requires one to both orient oneself to a future time and construct a narrative of an event at that time. A recent study of patients with hippocampal amnesia revealed a deficit in event construction, although the task did not require temporal orientation (Hassabis et al., 2007). It remains to be determined whether such individuals are able to perform a task that requires temporal orientation but not necessarily event construction. The distinction between temporal orientation and event construction could be important because the two might be independent processes. Indeed, an imaging study with healthy individuals suggests that the hippocampus may be selectively activated for the latter but not the former (Nyberg et al., 2010).
The well-documented tendency to discount the value of future rewards requires future-orientation but need not involve event construction, although some researchers believe it plays an important role (Berns et al., 2007; Boyer, 2009; Luhmann, 2009). Thus, it is of considerable interest how someone with extensive bilateral hippocampal damage, who is unable to construct details of future events, performs on a task that requires only valuation of future rewards. Is such an individual also unable to find value in future rewards or alternatively, does he systematically discount the value of future rewards as a function of the delay until their receipt, as do non-amnesic individuals? To answer this question, we tested K.C., a well-characterized amnesic individual. K.C. sustained bilateral hippocampal damage in a 1981 motorcycle accident and as a result, is unable to recall any past personal events or to imagine any future personal event (Rosenbaum et al., 2005).
Berns et al. (2007) have emphasized the role of anticipation of future events in making choices whose consequences play out over time. Different predictions emerge, however, depending on what aspects of future events are anticipated. For example, Boyer (2008) has hypothesized that the capacity to imagine future rewards has the evolutionary function of counteracting the tendency to discount future rewards. If so, then when K.C. is given a choice between a smaller, immediate reward and a larger, delayed reward, he (unlike the controls) should devalue the future and exhibit a selective bias toward only choosing the immediate reward given that he is unable to imagine the future. Alternatively, Luhmann and colleagues (Luhmann et al., 2008; Luhmann, 2009) have argued that people imagine the wait period itself and that the anticipated unpleasantness of waiting for a delayed reward biases subjects toward immediate rewards. According to this view, K.C. (unlike controls) should not exhibit a bias toward choosing a smaller, immediate reward and instead, should always choose the larger amount. Thus, based on either Boyer’s hypothesis or that of Luhmann, one would expect K.C. to show a non-temporal strategy in his decision-making. That is, he should either always choose the smaller, immediate reward according to the first account, or always choose the larger, later reward according to the second account; in either case, there should be no systematic effect of delay on K.C.’s choices.
K.C. was 58 years old at the time of testing. He is right-handed and has 15 years of formal education. His MRI scans reveal extensive volume loss in medial temporal lobe structures, most notably the hippocampal formation and surrounding parahippocampal gyrus, bilaterally. Additional affected areas include the septal area, posterior thalamus, and caudate nucleus, bilaterally, as well as his left amygdala, mammillary bodies, and anterior thalamus.
K.C.’s injury left him with a unique neuropsychological profile. A formal test of mental time travel using a modified version of the Autobiographical Interview with Galton-Crovtiz cuing (Addis et al., 2008; Kwan et al., 2010) revealed that his performance was at floor, with a total of only three episodic details generated for five past events and none generated for five future events. K.C.’s performance represents a striking deficit, and differs considerably from what is observed even in patients with probable Alzheimer’s disease (Addis et al., 2009).
Despite such severe impairment in episodic thought and construction, K.C. has retained facts about himself and the world. He also functions well in many other cognitive domains, including correct assessment of other people’s current mental states (Rosenbaum et al., 2007). Detailed neuropsychological assessment shows that this pattern of preserved semantic and impaired episodic memory abilities has remained stable since the time of his accident. K.C. continues to demonstrate average IQ and relatively preserved cognitive functioning outside of his episodic memory impairment and a conservative response bias with no evidence of confabulation (Rosenbaum et al., 2005, 2009).
For the present study, K.C. and 18 healthy, male, right-handed controls completed a computerized version of an established measure of delay discounting (Green and Myerson, 2004). Even though the discounting task on which K.C. was tested involved hypothetical rewards, it has been shown that healthy individuals’ choices regarding hypothetical rewards are highly correlated with their choices regarding real rewards (e.g., Johnson & Bickel, 2002; Madden et al., 2003). Controls were matched for age (M = 56.6, S.D. = 6.24) and education (M = 16.19, S.D. =2.28), as well as for other factors known to influence delay discounting, including history of gambling and use of alcohol, cigarettes, and recreational drugs. For the delay discounting task, participants made a series of choices between hypothetical monetary offers – a smaller, immediate amount and a larger, future amount. They were told that the task assesses their preferences and that there are no correct or incorrect choices. Participants were tested on multiple occasions in order to assess the consistency of K.C.’s performance relative to controls.
For each of two future amounts ($100 and $2000), participants made six choices for each of seven delays (1 week, 1 month, 3 months, 6 months, 1 year, 3 years, and 10 years) presented in random order. The first choice at each delay was between the delayed amount and an immediate amount that was equal to half of the delayed amount (e.g., $100 in 1 month or $50 now). For each of the subsequent choices at that delay, the amount of the immediate reward was adjusted based on the participant’s previous choice. If the participant chose the immediate reward, then its amount was decreased on the following trial; if the participant chose the larger, delayed reward, then the amount of the immediate reward on the next trial was increased. The size of the adjustment to the immediate reward after the first choice was half of the smaller amount. Subsequently, the size of the adjustment to the immediate reward decreased with each successive choice and was always equal to half of the previous adjustment, rounded to the nearest dollar. This iterative procedure converged rapidly on an estimate of the amount of an immediate reward corresponding to the subjective value of the delayed reward (Estle et al., 2006).
The 18 control participants completed the discounting task 3 times: twice on the first testing day (approximately one hour apart) and a third time approximately 1 week later. Participants committed to 2 sessions but were unaware that they would be retested on the same task. Upon each retest, participants were instructed to make their choices based on their current preferences and not to try to replicate their previous performance. K.C. was tested 6 times over the span of approximately 1 month and was never able to explicitly recall previous testing sessions.
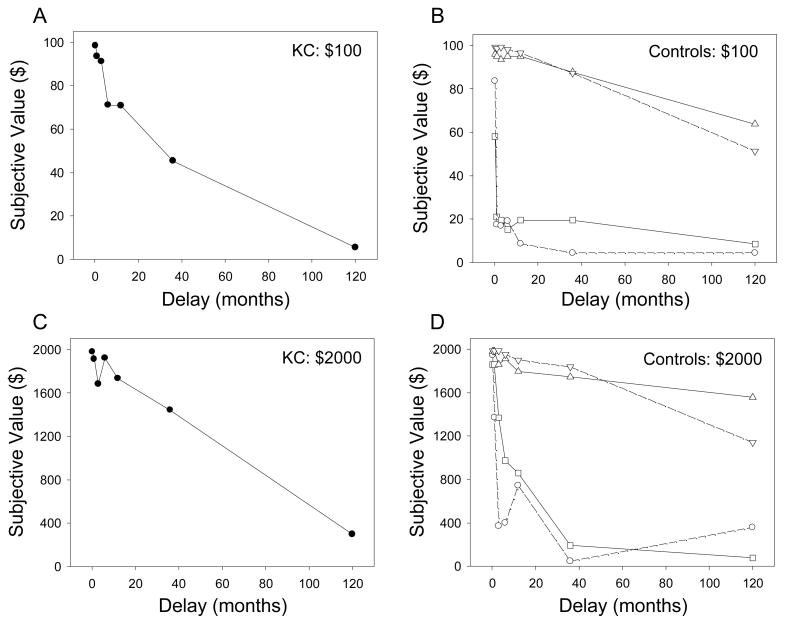
As may be seen in Figure 1, the subjective value that K.C. placed on a future reward decreased systematically with the delay until the receipt of that reward. K.C. exhibited clear discounting of both the $100 and $2000 delayed amounts (see left panels) and although his rate of discounting is relatively steep at the longest delay, it is still within the range of the controls (see right panels).
Figure 1.
Discounting of $100 and $2000 delayed rewards. (A) Mean subjective values at each delay to the $100 reward for K.C. (B) Mean subjective values at each delay to the $100 reward for the two control participants who showed the steepest and the two who showed the shallowest discounting. (C) Mean subjective values at each delay to the $2000 reward for K.C. (D) Mean subjective values at each delay to the $2000 reward for the two control participants who showed the steepest and the two who showed the shallowest discounting.
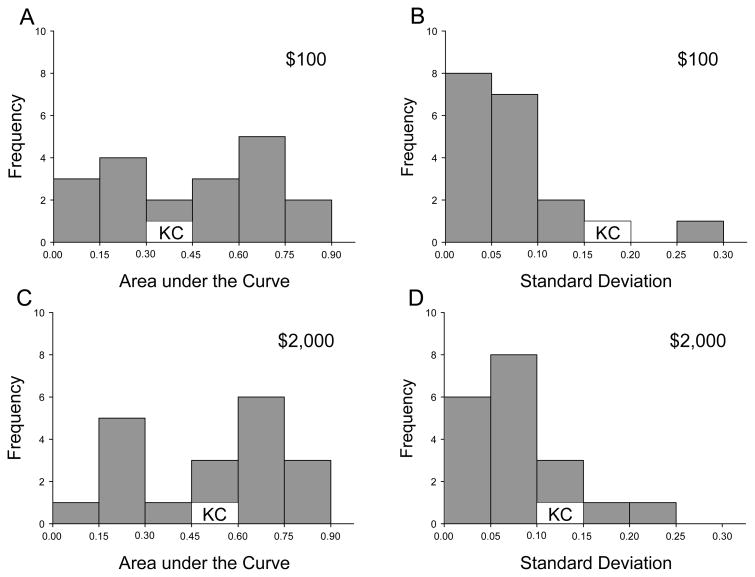
In addition to examining each participant’s discounting curve (i.e., subjective value as a function of delay), we calculated the areas under the curve (AuCs) for each testing session for each participant. The AuCs measure represents the area under the observed subjective values, and provides a single, theoretically neutral measure of the degree of discounting. Both subjective value and delay are normalized for purposes of calculating the AuCs measure (Myerson et al., 2001) which, as a result, ranges between 0.0 (maximally steep discounting) and 1.0 (no discounting). As may be seen in Figure 2, which shows the frequency distributions of the mean AuCs for K.C. and the controls (see left panels), K.C.’s AuCs for both the $100 and $2,000 rewards are close to the median values for the controls.
Figure 2.
Frequency histograms of the mean Areas under the Curve (AuCs) and their within-subject standard deviations (SDs) for the $100 and $2000 delayed rewards. (A) Frequencies of AuCs for the $100 reward. (B) Frequencies of within-subject SDs for the $100 reward. (C) Frequencies of AuCs for the $2,000 reward. (D) Frequencies of SDs for the $2,000 reward. AuCs and SDs for K.C. are indicated.
It is important to note that K.C. showed a magnitude effect (i.e., shallower discounting of the larger delayed amount), a standard finding in the human delay discounting literature (Green and Myerson, 2004): The mean areas under K.C.’s normalized discounting curves were .371 (SD=.177) for the smaller amount and .553 (SD=0.103) for the larger amount. The corresponding overall means (and SDs) for the control group were .437 (.079) and .502 (.081).
We also compared the variability of K.C.’s discounting across repeated testing sessions with that of the controls. As can be seen in the right-hand panels of Figure 2, K.C.’s intra-subject variability, although relatively high for the $100 amount, was within the range for the controls for both reward amounts. A modified t-test designed to compare performance of individual patients with a small control group (Crawford and Howell, 1998) failed to reveal a significant difference between K.C.’s variability and that of controls for either the $100 amount, t(17) = 1.533, p = .144, or the $2000 amount t(17) = .397, p =.696. Crawford and Howell’s modified t-test also provides estimates of the percentage of the normal population falling below a patient’s score. K.C.’s performance variability was at the 92nd percentile and 65th percentile for the $100 and $2000 amounts, respectively.
Taken together, these findings demonstrate that K.C., a person with extensive hippocampal damage and resulting episodic amnesia, still values future rewards despite being unable to construct the details of either past or future events. The results contribute to two separate literatures: First, the finding that K.C. can perform a task that requires future orientation but not event construction supports theoretical accounts of amnesia as primarily a deficit in construction (Hassabis et al, 2007; Rosenbaum et al., 2009; Schacter & Addis, 2009). Second, the finding that K.C. systematically discounted the value of future rewards is inconsistent with the predictions of accounts that emphasize the critical role of imagining future events in making choices when the decisions involve future outcomes (Boyer, 2008; Luhmann et al., 2008). K.C. did discount delayed rewards relatively steeply, however, and this may reflect the damage to his hippocampus. Indeed, non-human animals with hippocampal lesions tend to be more impulsive than controls (Cheung & Cardinal, 2005; Mariano et al., 2009). Nevertheless, it is unclear whether animals and humans engage in the same underlying processes when valuing future rewards. Although animal and human discounting appear similar in certain regards, animals differ from humans in ways that may affect discounting behavior: Animals, unlike K.C. and other humans, do not show a magnitude effect (Freeman et al., 2009; Green et al., 2004), and it is has been argued that, like K.C., animals are incapable of mental time travel (Tulving, 2002, but see Clayton et al., 2003).
An interview with K.C. after he performed the discounting task provided further evidence for a dissociation between the ability to value future rewards and the ability to imagine experiencing future rewards. K.C. reported a “blank” state of mind when asked to construct ways in which he might use future rewards that he had chosen over immediate rewards. When asked about his overall strategy, he reported relying on a gut feeling to choose “the best deal,” whereas controls reported relying on both episodic (e.g., “I thought about how I might spend the money in my retirement”) and non-episodic (e.g., “I estimated accumulated interest”) future-oriented constructions.
In contrast to K.C.’s strategy, which may rely on his relatively intact semantic memory (i.e., knowledge about the world and one’s self) (Rosenbaum et al., 2005), the decision-making strategies of healthy individuals, like those of the controls in the present study, often involve future-oriented imagery (Buckner, 2010; Liberman and Trope, 2008; Luhmann et al., 2008; Suddendorf and Corballis, 2007). Peters and Büchel (2010) recently reported that individualized episodic cues reduced the rate at which participants discounted future rewards, and this effect was predicted by the degree of self-reported imagery during decision making. Importantly, the effects of episodic cues were also predicted by the degree of coupling between activity in the hippocampus/amygdala and anterior cingulate cortex. Thus, the role of hippocampal activity in the relation between episodic thinking and the valuation of future rewards is a rich area for further research, even though the current study shows that the valuation of future rewards can occur independently of intact hippocampal function. Interestingly, recent patient research points to a critical role for the medial orbitofrontal cortex (Sellitto et al., 2010), though the role of this region in mental time travel is uncertain.
The present results suggest that in the absence of episodic memory, decision-making about future events can be based on semantic memory. These findings support the distinction between imagining and knowing about the future. Continued research with K.C. is needed to determine whether other aspects of future-oriented behavior can also proceed in the absence of mental time travel, or whether discounting is a unique exception. What is clear is that an amnesic person with hippocampal damage who shows a clear distinction between episodic and semantic memory can provide a unique resource for investigating the mechanisms underlying future-oriented decision-making.
Acknowledgments
This research was supported by Canadian Institutes of Health Research grant (MOP 93535) and a New Investigator Award to RSR. Preparation of the manuscript also was supported by the Centre for Programs at Washington University in St. Louis, and by NIH grant MH055308 to LG and JM. We thank M. Binns, N. Carson and B. Graham for technical assistance and M. Moscovitch, K.K. Szpunar, and E. Tulving for useful comments.
References
- Addis DR, Sacchetti DC, Ally BA, Budson AE, Schacter DL. Episodic simulation of future events is impaired in mild Alzheimer’s disease. Neuropsychologia. 2009;47:2660–2671. doi: 10.1016/j.neuropsychologia.2009.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Addis DR, Wong AT, Schacter DL. Remembering the past and imaging the future: Common and distinct neural substrates during event construction and elaboration. Neuropsychologia. 2007;45:1363–1377. doi: 10.1016/j.neuropsychologia.2006.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Addis DR, Wong AT, Schacter DL. Age-related changes in the episodic simulation of future events. Psychological Science. 2008;19:33–41. doi: 10.1111/j.1467-9280.2008.02043.x. [DOI] [PubMed] [Google Scholar]
- Atance CM, Jackson LK. The development and coherence of future-oriented behaviors during the preschool years. Journal of Experimental Child Psychology. 2009;102:379–391. doi: 10.1016/j.jecp.2009.01.001. [DOI] [PubMed] [Google Scholar]
- Atance CM, O’Neil DK. Episodic future thinking. Trends in Cognitive Sciences. 2001;5:533–539. doi: 10.1016/s1364-6613(00)01804-0. [DOI] [PubMed] [Google Scholar]
- Bar M. Predictions: a universal principle in the operation of the human brain. Introduction. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences. 2009;364:1181–1182. doi: 10.1098/rstb.2008.0321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar M. Wait for the second marshmallow? Future-oriented thinking and delayed reward discounting in the brain. Neuron. 2010;66:4–5. doi: 10.1016/j.neuron.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benoit RG, Gilbert SJ, Burgess PW. A neural mechanism mediating the impact of episodic prospection on farsighted decisions. Journal of Neuroscience. 2011;31:6771–6779. doi: 10.1523/JNEUROSCI.6559-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berns GS, Laibson D, Loewenstein GL. Intertemporal choice: Toward an integrative framework. Trends in Cognitive Sciences. 2007;11:482–488. doi: 10.1016/j.tics.2007.08.011. [DOI] [PubMed] [Google Scholar]
- Boyer P. Evolutionary economics of mental time travel? Trends in Cognitive Sciences. 2008;12:219–224. doi: 10.1016/j.tics.2008.03.003. [DOI] [PubMed] [Google Scholar]
- Buckner RL. The role of the hippocampus in prediction and imagination. Annual Review of Psychology. 2010;61:27–48. doi: 10.1146/annurev.psych.60.110707.163508. [DOI] [PubMed] [Google Scholar]
- Cheung THC, Cardinal RN. Hippocampal lesions facilitate instrumental learning with delayed reinforcement but induce impulsive choice in rats. BMC Neuroscience. 2005;6:36. doi: 10.1186/1471-2202-6-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton NS, Bussey TJ, Dickinson A. Can animals recall the past and plan for the future? Nature Reviews Neuroscience. 2003;4:685–691. doi: 10.1038/nrn1180. [DOI] [PubMed] [Google Scholar]
- Crawford JR, Howell DC. Comparing an individual’s test score against norms derived from small samples. The Clinical Neuropsychologist. 1998;12:482–486. [Google Scholar]
- Estle SJ, Green L, Myerson J, Holt DD. Differential effects of amount on temporal and probability discounting of gains and losses. Memory and Cognition. 2006;34:914–928. doi: 10.3758/bf03193437. [DOI] [PubMed] [Google Scholar]
- Freeman KB, Green L, Myerson J, Woolverton WL. Delay discounting of saccharin in rhesus monkeys. Behavioural Processes. 2009;82:214–218. doi: 10.1016/j.beproc.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green L, Myerson J. A discounting framework for choice with delayed and probabilistic rewards. Psychological Bulletin. 2004;130:769–792. doi: 10.1037/0033-2909.130.5.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green L, Myerson J, Holt DD, Slevin JR, Estle SJ. Discounting of delayed food rewards in pigeons and rats: Is there a magnitude effect? Journal of the Experimental Analysis of Behavior. 2004;81:39–50. doi: 10.1901/jeab.2004.81-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta R, Duff MC, Denburg NL, Cohen NJ, Bechara A, Tranel D. Declarative memory is critical for sustained advantageous complex decision-making. Neuropsychologia. 2009;47:1686–1693. doi: 10.1016/j.neuropsychologia.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassabis D, Kumaran D, Vann SD, Maguire EA. Patients with hippocampal amnesia cannot imagine new experiences. Proceedings of the National Academy of Sciences. 2007;104:1726–1731. doi: 10.1073/pnas.0610561104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MW, Bickel WK. Within-subject comparison of real and hypothetical money rewards in delay discounting. Journal of the Experimental Analysis of Behavior. 2002;77:129–146. doi: 10.1901/jeab.2002.77-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein SB, Loftus J, Kihlstrom JF. Memory and temporal experience: The effects of episodic memory loss on an amnesic patient’s ability to remember the past and imagine the future. Social Cognition. 2002;20:353–379. [Google Scholar]
- Kwan D, Carson N, Addis DR, Rosenbaum RS. Deficits in past remembering extend to future imagining in a case of developmental amnesia. Neuropsychologia. 2010;48:3179–3186. doi: 10.1016/j.neuropsychologia.2010.06.011. [DOI] [PubMed] [Google Scholar]
- Liberman N, Trope Y. The psychology of transcending the here and now. Science. 2009;322:1201–1205. doi: 10.1126/science.1161958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luhmann CC. Temporal decision-making: Insights from cognitive neuroscience. Frontiers in Behavioral Neuroscience. 2009;3:1–9. doi: 10.3389/neuro.08.039.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luhmann CC, Chun M, Yi DY, Lee D, Wang XJ. Neural dissociation between delay and uncertainty intertemporal choice. Journal of Neuroscience. 2008;28:14459–14466. doi: 10.1523/JNEUROSCI.5058-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden GJ, Begotka AM, Raiff BR, Kastern LL. Delay discounting of real and hypothetical rewards. Experimental and Clinical Psychopharmacology. 2003;11:139–145. doi: 10.1037/1064-1297.11.2.139. [DOI] [PubMed] [Google Scholar]
- Mariano TY, Bannerman DM, McHugh SB, Preston TJ, Rudebeck PH, Rudebeck SR, Campbell TG. Impulsive choice in hippocampal but not orbitofrontal cortex-lesioned rats on a nonspatial decision-making maze task. European Journal of Neuroscience. 2009;30:472–484. doi: 10.1111/j.1460-9568.2009.06837.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myerson J, Green L, Warusawitharana M. Area under the curve as a measure of discounting. Journal of the Experimental Analysis of Behavior. 2001;76:235–243. doi: 10.1901/jeab.2001.76-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyberg L, Kim ASN, Habib R, Levine B, Tulving E. Consciousness of subjective time in the brain. Proceedings of the National Academy of Sciences. 2010;107:22356–22359. doi: 10.1073/pnas.1016823108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters J, Büchel C. Episodic future thinking reduces reward delay discounting through an enhancement of prefrontal-mediotemporal interactions. Neuron. 2010;66:138–148. doi: 10.1016/j.neuron.2010.03.026. [DOI] [PubMed] [Google Scholar]
- Rosenbaum RS, Gilboa A, Levine B, Winocur G, Moscovitch M. Amnesia as an impairment of detail generation and binding: Evidence from personal, fictional, and semantic narratives in K.C. Neuropsychologia. 2009;47:2181–2187. doi: 10.1016/j.neuropsychologia.2008.11.028. [DOI] [PubMed] [Google Scholar]
- Rosenbaum RS, Kohler S, Schacter DL, Moscovitch M, Westmacott R, Black SE, Gao F, Tulving E. The case of K.C.: Contributions of a memory-impaired person to memory theory. Neuropsychologia. 2005;43:989–1021. doi: 10.1016/j.neuropsychologia.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Rosenbaum RS, Stuss DT, Levine B, Tulving E. Theory of mind is independent of episodic memory. Science. 2007;318:1257. doi: 10.1126/science.1148763. [DOI] [PubMed] [Google Scholar]
- Schacter DL, Addis DR. On the nature of medial temporal lobe contributions to the constructive simulation of future events. Philosophical Transactions of the Royal Society B-Biological Sciences. 2009;364:1245–1253. doi: 10.1098/rstb.2008.0308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellitto M, Ciaramelli E, di Pellegrino G. Myopic discounting of future rewards after medial orbitofrontal damage in humans. Journal of Neuroscience. 2010;30:16429–16436. doi: 10.1523/JNEUROSCI.2516-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suddendorf T, Corballis MC. The evolution of foresight: What is mental time travel, and is it unique to humans? Behavioral and Brain Sciences. 2007;30:299–313. doi: 10.1017/S0140525X07001975. [DOI] [PubMed] [Google Scholar]
- Szpunar KK. Episodic future thought: an emerging concept. Perspectives on Psychological Science. 2010;5:142–162. doi: 10.1177/1745691610362350. [DOI] [PubMed] [Google Scholar]
- Tulving E. Memory and consciousness. Canadian Psychology. 1985;26:1–12. [Google Scholar]
- Tulving E. Episodic memory: From the mind to the brain. Annual Review of Psychology. 2002;53:1–25. doi: 10.1146/annurev.psych.53.100901.135114. [DOI] [PubMed] [Google Scholar]