Abstract
Adults with parental history of type 2 diabetes have high metabolic morbidity, which is exacerbated by physical inactivity. Self-reported sleep <6 h/day is associated with increased incidence of obesity and diabetes, which may be mediated in part by sleep-loss-related reduction in physical activity. We examined the relationship between habitual sleep curtailment and physical activity in adults with parental history of type 2 diabetes. Forty-eight young urban adults with parental history of type 2 diabetes (27F/21M; mean [SD] age 26 [4] y; BMI 23.8 [2.5] kg/m2) each completed 13 [2] days of sleep and physical activity monitoring by wrist actigraphy and waist accelerometry while following their usual lifestyle at home. Laboratory polysomnography was used to screen for sleep disorders. The primary outcome of the study was the comparison of total daily activity counts between participants with habitual sleep <6 vs. ≥6 h/night. Secondary measures included daily time spent sedentary and in light, moderate, and vigorous physical activity. Short sleepers had no sleep abnormalities and showed signs of increased sleep pressure consistent with a behavioral pattern of habitual sleep curtailment. Compared to participants who slept ≥6 h/night, short sleepers had 27% fewer daily activity counts (P=0.042), spent less time in moderate-plus-vigorous physical activity (−43 min/day; P=0.010), and remained more sedentary (+69 min/day; P=0.026). Our results indicate that young urban adults with parental history of type 2 diabetes who habitually curtail their sleep have less daily physical activity and more sedentary living, which may enhance their metabolic risk.
INTRODUCTION
Adults with parental history of type 2 diabetes are themselves at high risk for developing the disease, particularly in the setting of physical inactivity and excessive weight gain. Prevention of obesity in first degree relatives of diabetic patients is accompanied by a reduction in diabetes risk by nearly 40% (1) and twin studies, which control for varying genetic susceptibility to obesity, suggest that physical activity has the largest influence on total and visceral adiposity in comparison to a number of other environmental factors (2). The Diabetes Prevention Program further demonstrated that while weight loss was the main factor for reducing the incidence of diabetes in high-risk individuals, higher physical activity played two important roles: 1) it helped maintain achieved weight loss; and 2) among participants who did not meet weight loss goals, reaching the target for increased activity resulted in an independent reduction in diabetes incidence by 44% (3). Thus, the identification of behavioral factors related to the maintenance of adequate physical activity in susceptible populations could result in improved strategies for metabolic risk reduction.
Today, many Americans sleep less than 6 hours per night (4) and self-reported short sleep duration has been associated with increased incidence of obesity and diabetes (5, 6). Since individuals with inadequate sleep commonly report excessive daytime sleepiness and decreased physical functioning (7), it has been hypothesized that reduced physical activity is one of the factors which mediate the association of short sleep with metabolic morbidity. Limited by the reliability and precision of questionnaires based on subjective recall (8, 9), cross-sectional analyses of the relationship between self-reported sleep duration and physical activity in adults have given inconsistent results showing either positive (10–12), negative (13), or no significant association (14–16). Moreover, in addition to the well-established preventive role of moderate- and vigorous-intensity physical activity, recent data suggest that the amount of daytime sedentary time and other body movement can also affect the metabolic health of at-risk individuals (17–19). However, despite the increasing availability of portable accelerometers which allow continuous monitoring of sleep-wake and physical activity patterns in free-living individuals, objective measurements to determine whether habitual short sleep is accompanied by reduced amount and intensity of physical activity and more sedentary behavior in adults at risk for type 2 diabetes are not yet available. This study examined the relation of habitual sleep duration with objectively-measured daily body movement, sedentary behavior, and time spent in light, moderate, and vigorous physical activity in adults with parental history of type 2 diabetes.
METHODS AND PROCEDURES
Men and women between the ages of 21 and 40 y with a body mass index (BMI) between 19 and 27 kg/m2, who lived in the greater Chicago area and had at least one parent with type 2 diabetes were recruited through local advertisements. Volunteers who passed a brief telephone interview were invited for screening in our Clinical Research Center. Body weight and height were measured upon arrival in the morning using a calibrated medical scale (Scale-Tronix, Wheaton, IL) and stationary Harpenden stadiometer (Holtain, Crymych, Wales) with subjects in the fasting state and dressed in light clothing without shoes. Volunteers were excluded from participation for the following reasons: presence of any acute or chronic medical condition; self-reported sleep problems (Pittsburgh Sleep Quality Index score >7), scheduled night-shift work during the past 6 months, frequent travel across time zones (crossing ≥ 2 time zones ≥ 2 times per month or anytime during the previous 4 weeks), or habitual daytime naps (>1 per week); pregnancy or childbirth during the past year; depressed mood (Center for Epidemiologic Studies of Depression score ≥16 confirmed by clinical interview); smoking, excessive intake of alcohol (>14 drinks/week for men; >7 for women), or use of any prescription medications or illegal drugs, or any over-the-counter medicines or supplements that can affect sleep, physical activity and energy homeostasis; abnormal results on physical examination and laboratory testing (complete blood counts, comprehensive metabolic and thyroid function panels, and 12-lead ECG). The study protocol was approved by the Institutional Review Board of the University of Chicago. Research volunteers gave written informed consent and were paid for their participation.
Habitual sleep and physical activity monitoring
Participants were asked to complete 14 consecutive days of sleep and activity monitoring while following their usual lifestyle at home. A small bi-axial accelerometer equipped with an event marker (Actiwatch-64, Mini-Mitter Respironics, Bend, OR) was attached to a wrist band on the non-dominant arm of each subject and actigraphy data were collected continuously in 1-minute epochs to assess usual sleep duration under free-living conditions (20). Participants were asked to press the event-marking button each night before going to sleep and again when they got out of bed each morning. In order to record the total count and intensity distribution of body movement, participants were fitted with a portable multi-axial activity monitor (Actical, Mini-Mitter Respironics, Bend, OR) attached to an elastic waist band over the iliac crest. Subjects were instructed to keep daily sleep logs and to wear the Actiwatch and Actical devices around the clock for the full 14-day monitoring period except when bathing or showering. They were told that the recordings from these devices will indicate when they slept and how regular were their activity-rest cycles. To be included in the analysis, participants were required to have worn the Actiwatch for at least 7 nights and the Actical for at least 6 valid days, where a valid day contained at least 23 h of recorded data. A total of 59 subjects were enrolled in the study, 6 self-discontinued their participation before completing the study (new job, relocating, or family medical emergency) and 5 did not wear the devices regularly to accumulate enough valid days and were excluded. The remaining 48 participants completed an average of 13 [SD 2] days of home monitoring.
Polysomnography
At the end of the study protocol each participant was asked to undergo one night of laboratory polysomnography (Neurofax-1100 EEG Acquisition System, Nihon-Kohden) including electroencephalography, electrooculography, electromyography, airflow (oronasal thermocouples and nasal pressure recording), thoracic and abdominal respiratory effort (piezoelectric belts), pulse oximetry, and electrocardiography monitoring to exclude the presence of primary sleep pathology, sleep disordered breathing (respiratory disturbance index >10 or apnea index >2) and sleep movement disorders. Sleep recordings were obtained in 43 subjects (90%) who kept their appointments. Sleep was scheduled between 23:00-00:30 and 7:30–9:00 based on self-reported sleep habits and time-in-bed was fixed to 8.5 h. Records were scored in 30-second epochs of wake, stage 1, 2, 3, 4, and rapid-eye-movement sleep. Respiratory events, periodic leg movements, and arousals were scored according to current clinical guidelines (21). Total sleep time was calculated as the sum of all epochs scored as sleep. Sleep efficiency was calculated as the percent of time in bed that was scored as sleep. Sleep onset latency was defined as the time between lights-off and the first epoch of stage 1 sleep.
Data analysis and statistics
Wrist actigraphy and waist accelerometry data were downloaded after each period of home monitoring. Nighttime sleep was scored using Actiware Sleep version 3.4 and a sensitivity setting of 40, facilitated by individual going-to-sleep and morning wake-up time stamps or sleep diary entries (when Actiwatch event markers were missing) (20). The habitual sleep duration of each study participant was calculated as the average number of minutes scored as sleep across all recorded nights. Actical data were analyzed using version 2.12 of the software provided with the device, which translated accelerations recorded in 1-minute epochs into categorical measures of time spent in light-, moderate-, and vigorous-intensity physical activity based on an algorithm with estimated intensity cutoffs of <3.0 METS, ≥3 to <6.0 METS, and ≥6.0 METS (22). The output included the total number of activity counts and the minutes of sedentary time and light-, moderate-, and vigorous-intensity physical activity for each day when the device was worn. These results were averaged across all recorded days to obtain individual measures of habitual physical activity (total activity counts per 24 h) and its distribution into categories of sedentary, light-, moderate-, and vigorous-intensity physical activity (minutes for each category per 24 h). To account for the contribution of sleep duration to total sedentary time, we subtracted the habitual sleep time (Actiwatch data) from the 24-hour sedentary time of each participant (Actical data) to calculate the amount of habitual sedentary time when he or she was awake.
The question “How many hours of actual sleep did you get at night during the past month?” was used to measure self-reported sleep duration. The global sleep disturbance score of the Pittsburgh Sleep Quality Index was used as a measure of subjective sleep quality (23). The Epworth Sleepiness Scale was used to measure subjective daytime sleepiness (24).
All statistical analyses were conducted with SPSS version 18.0 (SPSS Inc., Chicago, IL). First, we used multiple linear regression analysis to examine the association of habitual sleep duration with the number of counts of total daily activity in the entire study sample. Objectively-measured sleep time at home, age, BMI, gender, race/ethnicity, and years of education (as a surrogate measure of socioeconomic status) were entered in the initial model as independent variables followed by backward stepwise regression analysis to identify the predictors of total daily activity as our main outcome measure.
Next, since sleeping <6 h per night has been associated with increased risk of diabetes and obesity (5, 6), study participants were classified according to their objectively-measured sleep times at home as either 1) short sleepers with average sleep time <6 h/day or 2) reference sleepers with average sleep ≥6 h/day. Since 20 of the 48 study participants had objectively-measured habitual sleep duration <6 h per night, 20 of the remaining 28 reference sleepers were selected to match as closely as possible the age, BMI, gender, race/ethnicity, and level of education of the short sleepers in order to serve as controls in our further analysis (Table 1). The primary outcome variable of the study (total daily activity counts) and several secondary measures of activity intensity and sedentary behavior were compared using analysis of variance with sleep group (short vs. reference) as a between-subject factor. Time spent in vigorous physical activity (minutes in 24 h) was not normally distributed (Kolmogorov-Smirnoff test P<0.05) and was square root transformed prior to this analysis. No correction for multiple comparisons of secondary endpoints was made given the exploratory nature of these analyses.
Table 1.
Descriptive characteristics of the participants and their sleep
| Participant Characteristics | All | Sleep ≥6 h/d | Sleep < 6 h/d | P |
|---|---|---|---|---|
| Number of participants (n) | 48 | 20 | 20 | |
| Age (y) | 26 ± 4 | 26 ± 3 | 28 ± 5 | 0.267 |
| Body mass index (BMI, kg/m2) | 23.8 ± 2.5 | 23.3 ± 3.0 | 23.9 ± 2.0 | 0.459 |
| Gender distribution (female/male) | 27/21 | 12/8 | 12/8 | 1.000 |
| African/Asian/Hispanic race/ethnicity | 13/6/4 | 6/3/1 | 7/3/3 | 0.523 |
| Level of education (y) | 17 ± 2 | 17 ± 2 | 17 ± 2 | 0.495 |
| Depressed mood score (CES-D) | 7 ± 6 | 5 ± 4 | 7 ± 7 | 0.364 |
| Fasting blood glucose (mg/dL) | 86 ± 7 | 84 ± 6 | 87 ± 7 | 0.155 |
| Free thyroxine index | 8.8 ± 1.5 | 8.7 ± 1.6 | 8.7 ± 1.5 | 0.960 |
| Self-Reported Measures of Sleep | ||||
| Self-reported sleep duration (min/day) | 451 ± 62 | 454 ± 58 | 425 ± 57 | 0.121 |
| Epworth Sleepiness Scale score | 5.9 ± 3.5 | 4.4 ± 2.7 | 7.4 ± 4.0 | 0.010 |
| Pittsburgh Sleep Quality Index score | 2.7 ± 1.5 | 2.6 ± 1.5 | 3.1 ± 1.7 | 0.260 |
| Home Sleep Monitoring | ||||
| Length of monitoring (days) | 13 ± 2 | 13 ± 2 | 13 ± 2 | |
| Sleep duration (min/day) | 371 ± 54 | 399 ± 28 | 319 ± 26 | <0.001 |
| Sleep onset time (h:min) | 01:07 ± 1:20 | 00:33 ± 1:03 | 01:43 ± 1:09 | 0.002 |
| Wake-up time (h:min) | 08:19 ± 1:18 | 08:09 ± 1:02 | 08:04 ± 1:17 | 0.814 |
| Laboratory Polysomnography | ||||
| Number of participants (n) | 43 | 18 | 17 | |
| Lights-off time (h:min) | 00:02 ± 0:32 | 00:00 ± 0:42 | 00:01 ± 0:27 | 0.958 |
| Lights-on time (h:min) | 08:32 ± 0:34 | 08:31 ± 0:39 | 08:28 ± 0:34 | 0.799 |
| Sleep onset latency (min) | 25 ± 29 | 16 ± 16 | 19 ± 25 | 0.631 |
| Latency to rapid-eye-movement sleep (min) | 104 ± 51 | 110 ± 55 | 85 ± 41 | 0.145 |
| Sleep efficiency (%) | 88 ± 8 | 88 ± 7 | 90 ± 9 | 0.384 |
| Arousal index (events/h) | 14 ± 7 | 15 ± 8 | 11 ± 4 | 0.080 |
| Wake after sleep onset (min) | 41 ± 30 | 47 ± 31 | 34 ± 31 | 0.201 |
| Stage 1 sleep (min) | 31 ± 15 | 30 ± 12 | 32 ± 17 | 0.598 |
| Stage 2 sleep (min) | 264 ± 37 | 266 ± 36 | 266 ± 44 | 0.966 |
| Slow wave sleep (stages 3+4, min) | 51 ± 29 | 52 ± 29 | 51 ± 30 | 0.953 |
| Rapid eye movement sleep (min) | 97 ± 31 | 99 ± 25 | 104 ± 38 | 0.626 |
| Total sleep time (min) | 443 ± 48 | 446 ± 41 | 454 ± 58 | 0.644 |
| Respiratory disturbance index (events/h) | 2 ± 3 | 2 ± 3 | 3 ± 3 | 0.865 |
Data are mean ± SD. P values reflect comparisons of participants with habitual sleep duration <6 vs. ≥6 h/d using a Chi-square test for categorical and one-way analysis of variance for continuous variables. CES-D: Center for Epidemiologic Studies Depression Scale. All participants had normal thyroid stimulating hormone (TSH) concentrations in screening fasting blood samples. The normal reference range for the reported free thyroxine index is 6.0 to 10.5.
RESULTS
Multiple linear regression analysis to explore the predictors of free-living physical activity as a main outcome variable among all 48 study participants (Table 2) showed a significant positive association between habitual sleep duration and the total amount of daily body movement (39778 more daily activity counts for each 1 h of sleep; 95% CI 4204 to 75352; P = 0.029). In this analysis, habitual sleep duration was the single strongest predictor of total daily body movement among the other independent variables including age, BMI, gender, level of education (as a surrogate of socioeconomic status), and race/ethnicity (dichotomized as Caucasian vs. Non-Caucasian; alternative analyses using binary coding variables for African American, Asian and Hispanic race/ethnicity produced similar results – data not shown).
Table 2.
Association between habitual sleep duration and free-living physical activity
| Total daily movement (activity counts) | ||||
|---|---|---|---|---|
| Model 1 (all predictors entered) | B | 95% CI | Beta | P |
| Age (y) | −51 | −8961; 8859 | −0.002 | 0.991 |
| BMI (kg/m2) | −2875 | −17182; 11433 | −0.064 | 0.687 |
| Gender (male vs. female) | −31566 | −100875; 37748 | −0.140 | 0.363 |
| Caucasian race/ethnicity | 10861 | −70667; 92389 | 0.049 | 0.789 |
| Level of education (y) | −4840 | −26316; 16635 | −0.070 | 0.651 |
| Habitual sleep duration (h) | 38943 | −6135; 84022 | 0.308 | 0.089 |
| Model 2 (two main predictors) | ||||
| Gender (male vs. female) | −36710 | −100282; 26863 | −0.163 | 0.251 |
| Habitual sleep duration (h) | 37353 | 1645; 73060 | 0.296 | 0.041 |
| Model 3 (single best predictor) | ||||
| Habitual sleep duration (h) | 39778 | 4204; 75352 | 0.315 | 0.029 |
Model 1 shows results from a multiple linear regression analysis in which age, body mass index (BMI), gender, race/ethnicity (Caucasian vs. Non-Caucasian), years of education (as a surrogate of socioeconomic status), and habitual sleep duration by wrist actigraphy were entered together as independent variables to explore the predictors of free-living physical activity (daily count of total body movement by waist acceleromentry) as a main outcome variable. Model 2 (P = 0.049, R = 0.354, R2 = 0.125) shows the two strongest predictors of total daily movement in our study sample (n=48) derived using backward stepwise regression and Model 1 as an initial starting point. Model 3 (P = 0.029, R = 0.315, R2 = 0.099) shows the single best predictor of total daily movement in our study sample (n=48) derived using backward stepwise regression and Model 1 as an initial starting point. B: unstandardized regression coefficients; 95% CI: 95% confidence interval for B; Beta: standardized regression coefficients.
The 20 short sleepers in this study obtained 1 h 20 min less sleep during an average night at home (P<0.001) compared to a group of 20 reference sleepers matched by age, BMI, gender, race/ethnicity, and level of education (Table 1). There was no significant difference in morning wake-up time between the two groups, however, short sleepers stayed up over an hour later on an average night (P=0.002; Table 1). Self-reported sleep exceeded objectively-measured sleep duration in both groups (Table 1), but short sleepers overestimated their usual sleep duration considerably more than the participants in the reference group (average over-reporting: 105 ± 62 vs. 56 ± 54 min/day; P=0.011). If self-reported sleep duration was used instead of objectively-measured sleep time by wrist actigraphy, only 3 of the subjects in the short-sleep group (15%) would have been classified as short sleepers, whereas 19 of the subjects in the reference group (95%) would have been correctly classified as having habitual sleep duration ≥6 h/day (P<0.001, chi-square test). Both groups had good subjective sleep quality (Table 1), however, short sleepers reported significantly more daytime sleepiness (P=0.010). When time-in-bed was fixed to 8.5 h in the laboratory, the measured quantity and quality of sleep of the short sleepers was comparable to that of the reference sleep group (Table 1); indeed, short sleepers tended to have a better consolidated sleep with fewer arousals (P=0.073).
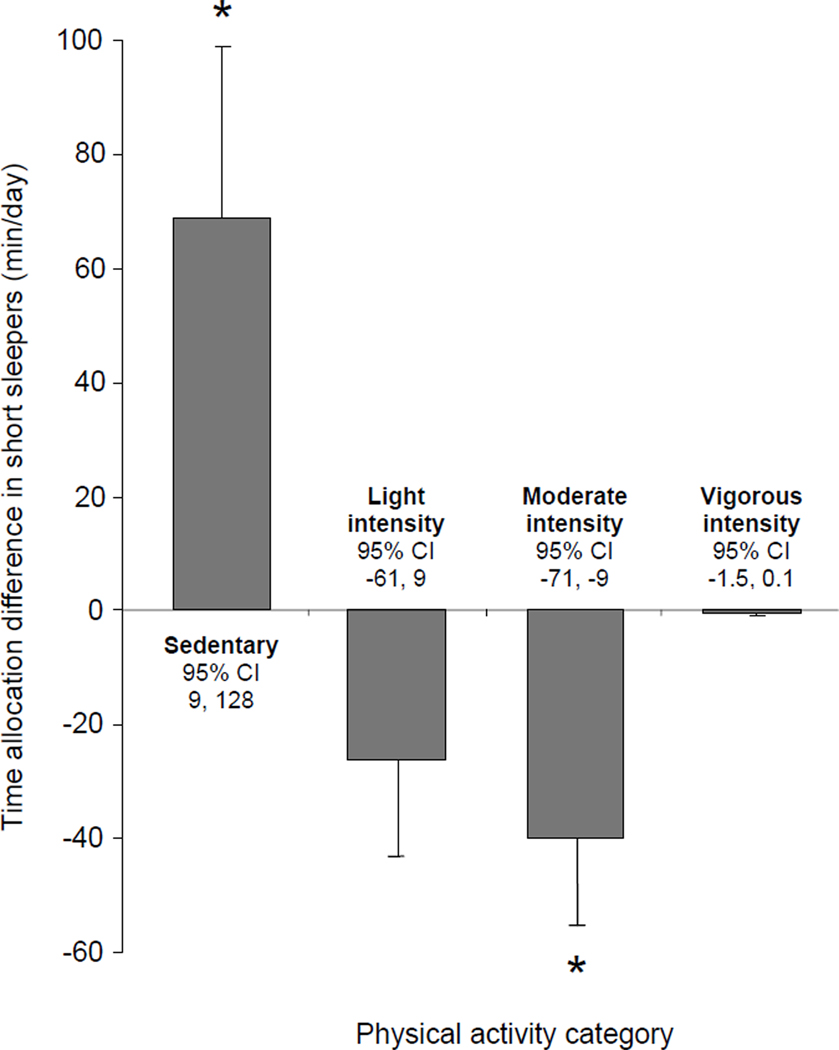
Total daily movement and its distribution among categories of sedentary, moderate, and moderate-plus-vigorous physical activity differed significantly between the two sleep groups (Table 3 and Figure 1). Compared to participants with habitual sleep duration ≥6 h/day, short sleepers had 27% less total daily movement (−65530 counts/day; 95%CI: −2578 to −128483; P=0.042), spent less time engaged in moderate-plus-vigorous physical activity (−43 min/day; 95%CI: −11 to −75; P=0.010), and remained more sedentary (+69 min/day; 95%CI: 9 to 128; P=0.026) (Table 3 and Figure 1).
Table 3.
Amount and intensity distribution of daily physical activity
| Sleep ≥ 6 h/d | Sleep < 6 h/d | P | |
|---|---|---|---|
| Primary Outcome Measure | |||
| Total movement (activity counts/day) | 247239 ± 120864 | 181709 ± 68793 | 0.042 |
| Secondary Endpoints | |||
| Light-intensity physical activity | |||
| (min/day) | 234 ± 62 | 208 ± 45 | 0.140 |
| (% of daily wake time) | 22 ± 6 | 19 ± 4 | 0.020 |
| Moderate-intensity physical activity | |||
| (min/day) | 155 ± 58 | 115 ± 35 | 0.012 |
| (% of daily wake time) | 15 ± 6 | 10 ± 3 | 0.003 |
| Vigorous-intensity physical activity | |||
| (min/day) | 6 ± 7 | 3 ± 4 | 0.106 |
| (% of daily wake time) | 0.5 ± 0.7 | 0.2 ± 0.3 | 0.076 |
| Moderate-plus-vigorous activity | |||
| (min/day) | 161 ± 61 | 118 ± 36 | 0.010 |
| (% of daily wake time) | 15 ± 6 | 11 ± 3 | 0.002 |
| Sedentary time while awake | |||
| (min while awake/day) | 647 ± 113 | 793 ± 70 | <0.001 |
| (% of daily wake time) | 62 ± 11 | 71 ± 6 | 0.003 |
| Total sedentary time including sleep | |||
| (min/day) | 1045 ± 111 | 1114 ± 71 | 0.026 |
Data are mean ± SD. P values reflect univariate comparisons of physical activity measures between the two sleep groups (n=20 each).
Figure 1.
Mean ± SE differences in daily time allocation to sedentary, light-, moderate- and vigorous-intensity physical activities in study participants with habitual sleep < 6 h/day (N=20) compared to a similar group of subjects with average sleep ≥6 h/day (N=20). CI: confidence interval; Asterisk: P<0.03.
DISCUSSION
Physical activity is a complex behavior which plays an important role in diabetes prevention and can be influenced by a number of biological, psychosocial, and environmental factors. We tested the hypothesis that free-living urban adults with parental history of type 2 diabetes who habitually sleep short hours will have reduced daily physical activity. Indeed, multiple linear regression analysis of the data from our entire study sample showed a significant positive association between objectively-measured habitual sleep duration and the total amount of daily body movement (Table 2). In addition, compared to a similar group of participants with average sleep duration ≥6 h/d (Table 1), those who slept <6 h/d had significantly lower amounts of total body movement, spent less time engaged in moderate-plus-vigorous physical activity, and were more sedentary despite having more daily waking time available to them (Figure 1 and Table 3). These findings are in agreement with the hypothesis that sleeping short hours could be associated with reduced everyday physical activity, which may contribute in part to the relationship of chronic sleep insufficiency with increased incidence of obesity and diabetes (5, 6).
Based on wrist actigraphy data, nearly half of the participants at the Chicago site of the CARDIA study had habitual sleep duration <6 h/day (4). Consistent with these observations, 40% of the consecutively enrolled young adults with parental history of type 2 diabetes in our convenience sample had objectively-measured short sleep (Table 1). Evaluation of these short sleepers by laboratory polysomnography revealed that they were able to sleep as much as the subjects in the reference group during one night of inpatient monitoring and did not suffer from any sleep disorders that could diminish the quantity and quality of their sleep (Table 1). Instead, short sleepers had significantly higher subjective daytime sleepiness and a tendency towards more consolidated sleep with fewer arousals during overnight polysomnography consistent with the presence of a behavioral pattern of habitual sleep curtailment and chronic sleep debt.
So far, large cross-sectional analyses of the relationship between habitual sleep duration and physical activity in adults have used self-reported data which have limited reliability (8, 9) and can lead to conflicting results showing either positive (10–12), negative (13), or no significant association (14–16). Few studies have examined the effects of experimental sleep deprivation on objectively-measured physical activity. Schmid et al. studied healthy young men who were exposed to one night of experimental sleep restriction and found a decline in movement counts with a shift from higher intensity towards lower intensity daytime activities (25). Brondel et al. imposed overnight sleep restriction followed by structured exposure to physical activity and laboratory meals during the first half of the day and free-living activity in the afternoon and evening, and reported opposite results (26). Finally, Roehrs et al. found that overnight sleep deprivation resulted in a higher percentage of inactivity time during the next day (27), whereas Bosy-Westphal et al. did not detect effects of sleep restriction on daytime activity assessed by heart rate monitoring and pedometry (28). Unfortunately, all of these experiments involved acute sleep restriction, which may have different effects when compared to the continuous impact of chronic sleep curtailment on human physical activity. Nevertheless, the reduction in objectively-measured activity of free-living adults who curtail their sleep in our study (Figure 1) resembles the effects of experimental sleep restriction described by Roehrs et al. (27) and Schmid et al. (25). While this similarity raises the possibility that chronic sleep curtailment may result in reduced amount and intensity of everyday physical activity, the observational nature of our data does not allow such inference. Indeed, causality could also flow in opposite direction whereby adoption of a sedentary lifestyle (e.g. one involving increased TV watching or use of other information, entertainment, and social media outlets) could lead to secondary sacrifice of sleep and physical activity (29).
Irrespective of the underlying causal mechanisms, habitual sleep curtailment in our group of young urban adults at risk for developing type 2 diabetes was associated with 27% lower levels of total body movement and nearly 45 min less daily moderate-plus-vigorous physical activity (Figure 1 and Table 3). If confirmed by larger population-based studies, these findings could have important public health implications. Higher amounts of total body movement have been associated with improved insulin sensitivity, better glucose tolerance, and reduced incidence of type 2 diabetes (17, 18, 29, 30). In addition, moderate and vigorous-intensity physical activity has a well-established role in the prevention of type 2 diabetes (3, 31, 32). For example, walking for exercise at least 2.5 h vs. less than 1 h per week in the Finnish Diabetes Prevention Study was associated with a more than 60% lower risk of incident diabetes (31). Furthermore, current guidelines recommend at least 30 minutes of moderate-intensity physical activity at least 5 days a week or 30 minutes of vigorous-intensity physical activity at least 3 times a week in order to reduce cardiometabolic risk in susceptible individuals (32). Thus, future efforts to define the mechanisms which contribute to the association of chronic sleep insufficiency with chronic metabolic morbidity should include objective measurements of the amount and intensity distribution of habitual physical activity.
The amount of daily sedentary time, itself, has been associated with insulin resistance, abnormal glucose metabolism, and increased metabolic risk (19, 30). Our short sleepers had longer waking hours at their disposal, but devoted the entire waking time gained by sleep curtailment to various sedentary behaviors. When we examined the total amount of 24-hour sedentary time (including the amount of daily sleep which was longer in the reference group), short sleepers still spent more than 1 extra hour each day engaged in sedentary behaviors (Figure 1). Data from the American Time Use Survey suggest that short sleepers may trade sleep in exchange for commuting, work, socializing, watching television, or using other media (33). The types of sedentary behaviors that are favored by young urban adults at risk for type 2 diabetes who curtail their sleep remain to be explored. Additional experimental work is also needed to determine whether chronic sleep insufficiency may increase the propensity of individuals with pre-existing risk for obesity and type 2 diabetes to adopt increasingly sedentary lifestyles.
Our study has several strengths and limitations. We collected proof-of-concept data from a carefully screened sample of healthy individuals at risk for type 2 diabetes, while avoiding the confounding effects of obesity and poor general or emotional health. The use of laboratory polysomnography and objective monitoring of habitual sleep and free-living physical activity also allowed us to exclude the presence of sleep pathology and avoid assessments based on imprecise self-reports of these behaviors (see Results for an example of systemic bias in self-reported sleep duration). Finally, it was important to study a population with high risk for type 2 diabetes in order to inform future behavioral and primary prevention research focused on sleep and metabolic risk reduction. Despite its strengths, this study included a relatively small number of participants whose free-living behavior was monitored during a single period of time. Although our objective sleep data were similar to those of population-based reports (4), study participants were not randomly selected and results may not be entirely representative of the relationship between sleep and physical activity in this high-risk group. Finally, while wrist actigraphy offers a valuable non-invasive approach for monitoring the habitual pattern of nighttime rest in free-living individuals, it does not provide direct measures of polygraphically documented sleep. Nevertheless, this methodology is well-validated in normal sleepers and provides surrogate estimates of nighttime sleep that correlate strongly with corresponding polysomnography-based assessments (20).
In conclusion, our findings suggest that a large number of healthy young adults with increased risk for type 2 diabetes habitually curtail their sleep. Compared to participants with ≥6 h of daily sleep, those who slept less were more sedentary and had reduced amounts of total body movement and time spent in moderate-and-vigorous physical activity. Larger studies are warranted to explore the hypothesis that reduced physical activity may contribute to the association of chronic sleep insufficiency with incident obesity and type 2 diabetes.
Acknowledgements
This work was supported by NIH grants R01-HL089637, CTSA-RR024999, and P60-DK020595. We thank Luis Alcantar in the Department of Medicine at the University of Chicago and the staff of the University of Chicago Clinical Research Center for their excellent technical assistance.
Footnotes
Disclosure Statement
The authors have no conflict of interest
REFERENCES
- 1.Sargeant LA, Wareham NJ, Khaw KT. Family history of diabetes identifies a group at increased risk for the metabolic consequences of obesity and physical inactivity in EPIC-Norfolk: a population-based study. The European Prospective Investigation into Cancer. International Journal of Obesity. 2000;24:1333–1339. doi: 10.1038/sj.ijo.0801383. [DOI] [PubMed] [Google Scholar]
- 2.Samaras K, Kelly PJ, Chiano MN, Spector TD, Campbell LV. Genetic and environmental influences on total-body and central abdominal fat: the effect of physical activity in female twins. Annals of Internal Medicine. 1999;130:873–882. doi: 10.7326/0003-4819-130-11-199906010-00002. [DOI] [PubMed] [Google Scholar]
- 3.Hamman RF, Wing RR, Edelstein SL, et al. Effect of weight loss with lifestyle intervention on risk of diabetes. Diabetes Care. 2006;29:2102–2107. doi: 10.2337/dc06-0560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lauderdale DS, Knutson KL, Yan LL, et al. Objectively measured sleep characteristics among early-middle-aged adults: the CARDIA study. Am J Epidemiol. 2006;164:5–16. doi: 10.1093/aje/kwj199. [DOI] [PubMed] [Google Scholar]
- 5.Cappuccio FP, Taggart FM, Kandala NB, et al. Meta-analysis of short sleep duration and obesity in children and adults. SLEEP. 2008;31:619–626. doi: 10.1093/sleep/31.5.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cappuccio FP, D'Elia L, Strazzullo P, Miller MA. Quantity and quality of sleep and incidence of type 2 diabetes: a systematic review and meta-analysis. Diabetes Care. 2010;33:414–420. doi: 10.2337/dc09-1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weaver TE, Laizner AM, Evans LK, et al. An instrument to measure functional status outcomes for disorders of excessive sleepiness. SLEEP. 1997;20:835–843. [PubMed] [Google Scholar]
- 8.Lauderdale DS, Knutson KL, Yan LL, Liu K, Rathouz PJ. Self-reported and measured sleep duration: how similar are they? Epidemiology. 2008;19:838–845. doi: 10.1097/EDE.0b013e318187a7b0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shephard RJ. Limits to the measurement of habitual physical activity by questionnaires. Br J Sports Med. 2003;37:197–206. doi: 10.1136/bjsm.37.3.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu X, Uchiyama M, Kim K, et al. Sleep loss and daytime sleepiness in the general adult population of Japan. Psychiatry Res. 2000;93:1–11. doi: 10.1016/s0165-1781(99)00119-5. [DOI] [PubMed] [Google Scholar]
- 11.Ohida T, Kamal AM, Uchiyama M, et al. The influence of lifestyle and health status factors on sleep loss among the Japanese general population. SLEEP. 2001;24:333–338. doi: 10.1093/sleep/24.3.333. [DOI] [PubMed] [Google Scholar]
- 12.Patel SR, Blackwell T, Redline S, et al. The association between sleep duration and obesity in older adults. Int J Obes. 2008;32:1825–1834. doi: 10.1038/ijo.2008.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chaput JP, Despres JP, Bouchard C, Tremblay A. The association between sleep duration and weight gain in adults: a 6-year prospective study from the Quebec Family Study. SLEEP. 2008;31:517–523. doi: 10.1093/sleep/31.4.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Imaki M, Hatanaka Y, Ogawa Y, Yoshida Y, Tanada S. An epidemiological study on relationship between the hours of sleep and life style factors in Japanese factory workers. Journal of Physiological Anthropology & Applied Human Science. 2002;21:115–120. doi: 10.2114/jpa.21.115. [DOI] [PubMed] [Google Scholar]
- 15.Youngstedt SD, Perlis ML, O'Brien PM, et al. No association of sleep with total daily physical activity in normal sleepers. Physiol Behav. 2003;78:395–401. doi: 10.1016/s0031-9384(03)00004-0. [DOI] [PubMed] [Google Scholar]
- 16.Patel SR, Malhotra A, White DP, Gottlieb DJ, Hu FB. Association between reduced sleep and weight gain in women. Am J Epidemiol. 2006;164:947–954. doi: 10.1093/aje/kwj280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ekelund U, Griffin SJ, Wareham NJ. Physical activity and metabolic risk in individuals with a family history of type 2 diabetes. Diabetes Care. 2007;30:337–342. doi: 10.2337/dc06-1883. [DOI] [PubMed] [Google Scholar]
- 18.Balkau B, Mhamdi L, Oppert JM, et al. Physical activity and insulin sensitivity: the RISC study. Diabetes. 2008;57:2613–2618. doi: 10.2337/db07-1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Helmerhorst HJ, Wijndaele K, Brage S, Wareham NJ, Ekelund U. Objectively measured sedentary time may predict insulin resistance independent of moderate- and vigorous-intensity physical activity. Diabetes. 2009;58:1776–1779. doi: 10.2337/db08-1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ancoli-Israel S, Cole R, Alessi C, Chambers M, Moorcroft W, Pollak CP. The role of actigraphy in the study of sleep and circadian rhythms. SLEEP. 2003;26:342–392. doi: 10.1093/sleep/26.3.342. [DOI] [PubMed] [Google Scholar]
- 21.Iber C, Ancoli-Israel S, Chesson ALJ, Quan SF. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. 2007 [Google Scholar]
- 22.Heil DP. Predicting activity energy expenditure using the Actical activity monitor. Res Q Exerc Sport. 2006;77:64–80. doi: 10.1080/02701367.2006.10599333. [DOI] [PubMed] [Google Scholar]
- 23.Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Research. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 24.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. SLEEP. 1991;14:540–545. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 25.Schmid SM, Hallschmid M, Jauch-Chara K, et al. Short-term sleep loss decreases physical activity under free-living conditions but does not increase food intake under time-deprived laboratory conditions in healthy men. Am J Clin Nutr. 2009;90:1476–1482. doi: 10.3945/ajcn.2009.27984. [DOI] [PubMed] [Google Scholar]
- 26.Brondel L, Romer MA, Nougues PM, Touyarou P, Davenne D. Acute partial sleep deprivation increases food intake in healthy men. Am J Clin Nutr. 2010;91:1550–1559. doi: 10.3945/ajcn.2009.28523. [DOI] [PubMed] [Google Scholar]
- 27.Roehrs T, Turner L, Roth T. Effects of sleep loss on waking actigraphy. SLEEP. 2000;23:793–797. [PubMed] [Google Scholar]
- 28.Bosy-Westphal A, Hinrichs S, Jauch-Chara K, et al. Influence of partial sleep deprivation on energy balance and insulin sensitivity in healthy women. Obes Facts. 2008;1:266–273. doi: 10.1159/000158874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hu FB, Li TY, Colditz GA, Willett WC, Manson JE. Television watching and other sedentary behaviors in relation to risk of obesity and type 2 diabetes mellitus in women. JAMA. 2003;289:1785–1791. doi: 10.1001/jama.289.14.1785. [DOI] [PubMed] [Google Scholar]
- 30.Healy GN, Wijndaele K, Dunstan DW, et al. Objectively measured sedentary time, physical activity, and metabolic risk: the Australian Diabetes, Obesity and Lifestyle Study (AusDiab) Diabetes Care. 2008;31:369–371. doi: 10.2337/dc07-1795. [DOI] [PubMed] [Google Scholar]
- 31.Laaksonen DE, Lindstrom J, Lakka TA, et al. Physical activity in the prevention of type 2 diabetes: the Finnish diabetes prevention study. Diabetes. 2005;54:158–165. doi: 10.2337/diabetes.54.1.158. [DOI] [PubMed] [Google Scholar]
- 32.Sigal RJ, Kenny GP, Wasserman DH, Castaneda-Sceppa C, White RD. Physical activity/exercise and type 2 diabetes: a consensus statement from the American Diabetes Association. Diabetes Care. 2006;29:1433–1438. doi: 10.2337/dc06-9910. [DOI] [PubMed] [Google Scholar]
- 33.Basner M, Dinges DF. Dubious bargain: trading sleep for Leno and Letterman. SLEEP. 2009;32:747–752. doi: 10.1093/sleep/32.6.747. [DOI] [PMC free article] [PubMed] [Google Scholar]