Abstract
Eradication of HIV reservoirs in the brain necessitates penetration of antiviral agents across the blood-brain barrier (BBB), a process limited by drug efflux proteins such as P-glycoprotein (P-gp) at the membrane of brain capillary endothelial cells. We present an innovative chemical strategy toward the goal of therapeutic brain penetration of the P-gp substrate and anti-viral agent abacavir, in conjunction with a traceless tether. Dimeric prodrugs of abacavir were designed to have two functions: inhibit P-gp efflux at the BBB and revert to monomeric therapeutic within cellular reducing environments. The prodrug dimers are potent P-gp inhibitors in cell culture and in a brain capillary model of the BBB. Significantly, these agents demonstrate anti-HIV activity in two T-cell-based HIV assays, a result that is linked to cellular reversion of the prodrug to abacavir. This strategy represents a platform technology that may be applied to other therapies with limited brain penetration due to P-glycoprotein.
INTRODUCTION
Although viral loads have been dramatically reduced in HIV patients using highly active antiretroviral therapy (HAART), a significant hurdle to the total eradication of HIV is the presence of reservoirs of the virus.1,2 These HIV reservoirs are found in locations such as the central nervous system, macrophages and lymphocytes and occur, in large part, due to the limited ability of antiretroviral therapies to enter these sites.3,4 In the brain, for instance, the failure of many HAART agents to block the accumulation of reservoirs of the virus is largely a result of limited penetration across the blood-brain barrier.5 This lack of penetration is due to a number of physiochemical properties of the drugs and also to the presence of drug transporters at the BBB. These transporters include multidrug resistance proteins (MDRs) of the ATP-binding cassette (ABC) family that are localized to the apical membrane of brain capillary endothelial cells.5–7 Of these ABC transporters, P-glycoprotein (P-gp) has been our focus as it is expressed at particularly high levels in brain capillaries and is currently implicated in the transport of more HAART compounds than other MDRs.5 P-gp is also expressed at other reservoirs of HIV besides the BBB, including macrophages and lymphocytes.8,9
P-gp is an ATP-dependent, integral membrane protein that transports a large variety of hydrophobic agents out of the plasma membrane into the extracellular milieu.10,11 One proposed functional model is that P-gp may reduce intracellular drug concentrations by acting as a "hydrophobic vacuum cleaner", effectively increasing drug efflux by recognition and removal of compounds from the membrane before they reach the cytosol to elicit their effects.12,13 In vitro studies have demonstrated that the HIV protease inhibitors (PIs) saquinavir, amprenavir, nelfinavir, ritonavir, and indinavir, and the HIV reverse transcriptase inhibitor (RTI) abacavir are substrates of P-gp. 5,14–20 The results of these in vitro studies have been confirmed in vivo. For example, significantly increased levels of abacavir and the PIs listed above (20-fold, and 7.4- to 36.3-fold, respectively) have been found in the brains of dosed P-gp-null mice versus dosed wild-type mice.16,18,21 Thus, P-gp actively limits the brain penetration of antiretroviral drugs used to treat HIV-infected patients. Therefore, therapies that include inhibition of P-gp represent promising treatment strategies for HIV patients and their use may ultimately lead to eradication of the cellular and anatomical reservoirs of HIV.
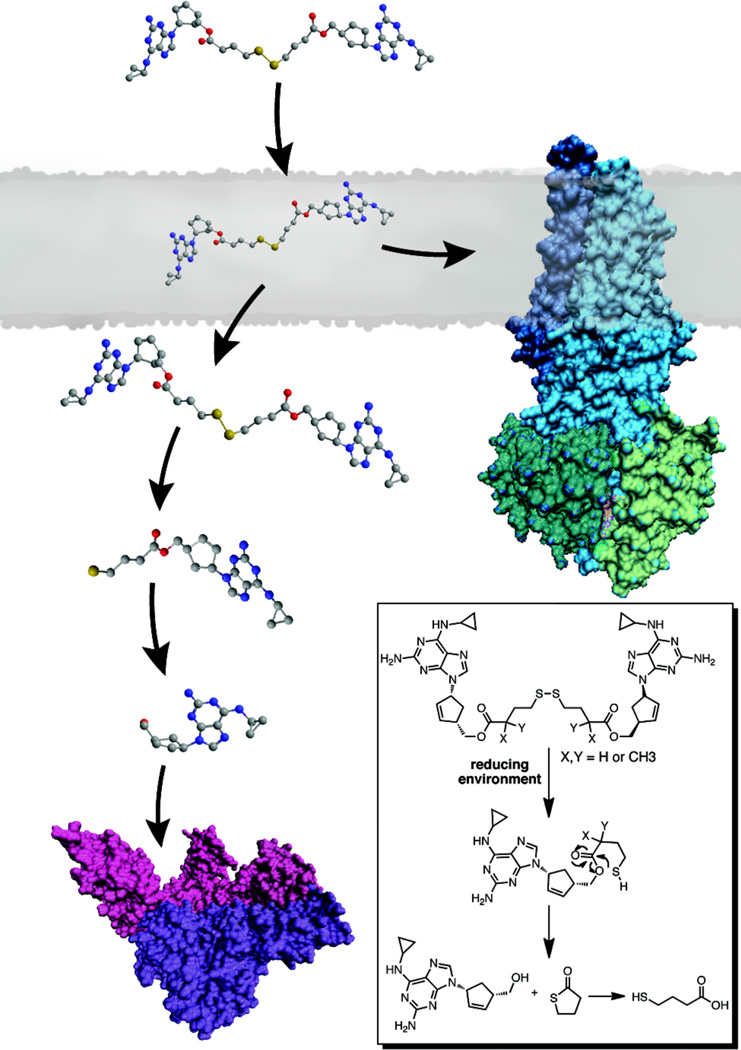
Numerous studies have pointed to the existence of at least two spatially distinct substrate binding sites within the transmembrane domain of P-gp that function in transport or regulation of transport.22–26 Furthermore, in the recently solved crystal structure of mouse P-gp, the drug binding site appears to be formed by the contacts between transmembrane helices leading to a large and fluid internal cavity that is able to accommodate the binding of multiple molecules.27 To exploit this multiplicity of binding sites within the transporter region of P-gp, we have developed an approach to convert P-gp substrates into potent, dimeric P-gp inhibitors.28,29 Dimerization of P-gp substrates should increase the affinity for drug binding sites, thus lowering the off rate, resulting in inhibition rather than efflux. In this study, we describe a novel design, using an HIV therapeutic, in which a dimeric P-gp inhibitor also functions as a prodrug with the potential to revert back to the corresponding monomeric drug in the reducing environment of the cell (Figure 1 and inset). This reversion is an important feature of our design in that the P-gp inhibitor is also a prodrug of the therapeutic agent itself. Herein we disclose a set of dimeric prodrug agents based on the HIV RTI abacavir, that were designed to function in two ways: (1) as inhibitors of P-gp, the major drug efflux protein at the BBB, by occupying multiple binding sites within the transporter and (2) as prodrug dimers that upon entry into cells would revert to their monomeric forms in the reducing environment of the cytosol, thus delivering the therapy (Figure 1).
Figure 1.
Design of P-gp inhibitors: dimeric prodrugs containing a traceless linker. These novel dimeric prodrug P-gp inhibitors also revert to the monomeric drugs within the reducing environment of the cell (inset) using a traceless linker strategy.
RESULTS
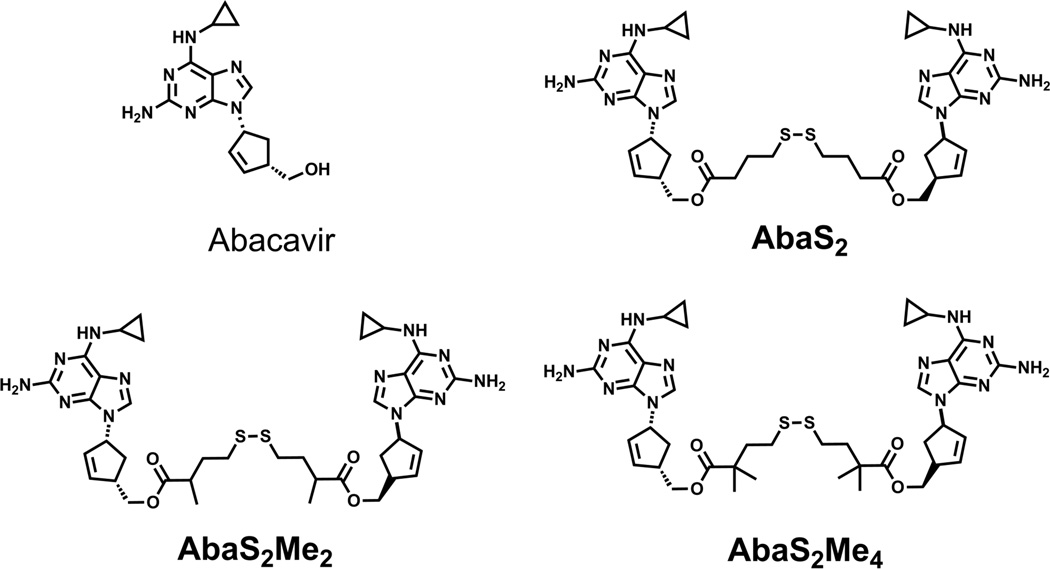
Our dimeric prodrug strategy requires a tethering group that would be removed completely from the monomers once inside the cell – hence a “traceless” tether. The addition of a disulfide within the tether is a key component to the designed breakdown of the dimeric agents. Under reducing conditions, such as those that exist in the cytosol, the disulfide linkage within the tether will reduce and the remaining tether would rearrange to regenerate two molecules of the monomeric therapeutic agent (Figure 1, inset). Although a similar strategy has been previously used to link a single therapeutic agent to cell delivery agents such as folate and cell penetrating peptides,30–32 we now describe a dual therapy strategy targeting P-gp. Specifically for abacavir, the primary hydroxyl groups of two monomers are linked via ester linkages containing a central disulfide unit (AbaS2, Figure 2) and evaluated in terms of P-gp inhibition, rate of reductive monomer release and stability of the ester linkages in human plasma. The role of additional methyl units adjacent to the ester carbonyl in the tethers was also evaluated for stability (AbaS2Me2 and AbaS2Me4, Figure 2).
Figure 2.
Structures of abacavir and abacavir prodrug homodimers: AbaS2, AbaS2Me2 and AbaS2Me4.
Briefly, the dimers AbaS2, AbaS2Me2 and AbaS2Me4 were synthesized by treating PyBOP activated bis-carboxylic acids with abacavir in the presence of DIEA and DMAP. The abacavir dimers were purified to homogeneity by reverse phase HPLC and structures were confirmed by 1H NMR, 13C NMR and MALDI-TOF mass spectrometry (See Supporting Information); AbaS2Me2 was obtained as a mixture of stereoisomers that was used without further separation.
We evaluated the potency of the abacavir dimers in a P-gp overexpressing T lymphoblastoid cell line (12D7-MDR).33 Inhibition of P-gp-mediated transport of the fluorescent substrates calcein-AM and NBD-abacavir (NBD-Aba)34 was measured as the increase in cellular fluorescence by flow cytometery. All dimers demonstrated potent inhibition of P-gp mediated efflux of fluorescent substrates (Table 1), whereas monomeric abacavir was only minimally effective at a concentration of 500 µM. Likewise the tether alone or the reduced product of the tether showed no inhibition up to a concentration of 200 µM. Methylation improved the activity of the AbaS2Me2 and AbaS2Me4 as compared to AbaS2, with AbaS2Me4 being more than 700-fold more potent than the starting monomeric abacavir at inhibiting P-gp-mediated efflux from cells.
Table 1.
Inhibition of P-gp-mediated efflux in 12D7 T-cells and P-gp substrate binding competition in vitro
| Compound | IC50 (µM) 12D7-MDR (Calcein-AM) |
IC50 (µM) 12D7-MDR (NBD-Aba) |
IC50 [125I]IAAP competition |
|---|---|---|---|
| abacavir | > 500 | > 500 | >100 µM |
| AbaS2 | 4.8 ± 0.1 | 4.9 ± 0.4 | 170 ± 42 nM |
| AbaS2Me2 | 2.4 ± 0.1 | 2.1 ± 0.2 | 62 ± 24 nM |
| AbaS2Me4 | 0.6 ± 0.1 | 0.7 ± 0.1 | 65 ± 28 nM |
To elucidate the mechanism of efflux inhibition by abacavir dimers, we probed their ability to compete for transporter binding sites with a known photoactive substrate of P-gp, [125I]iodoarylazidoprazosin (125I-IAAP). Crude Sf9 membranes expressing P-gp were incubated with 125I-IAAP and the abacavir dimers followed by photo-crosslinking.35 Covalent modification of P-gp by 125I-IAAP was monitored by autoradiography of the resulting SDS-PAGE gels as a function of increasing concentrations of AbaS2, AbaS2Me2 and AbaS2Me4. All dimers competed for 125I-IAAP binding sites on P-gp in a concentration-dependent manner with IC50 values in the nanomolar range (Table 1), with AbaS2Me4 being 1500-fold more potent than monomeric abacavir. These data further support abacavir dimers as inhibitors of P-gp mediated efflux through interaction with the substrate binding domains.
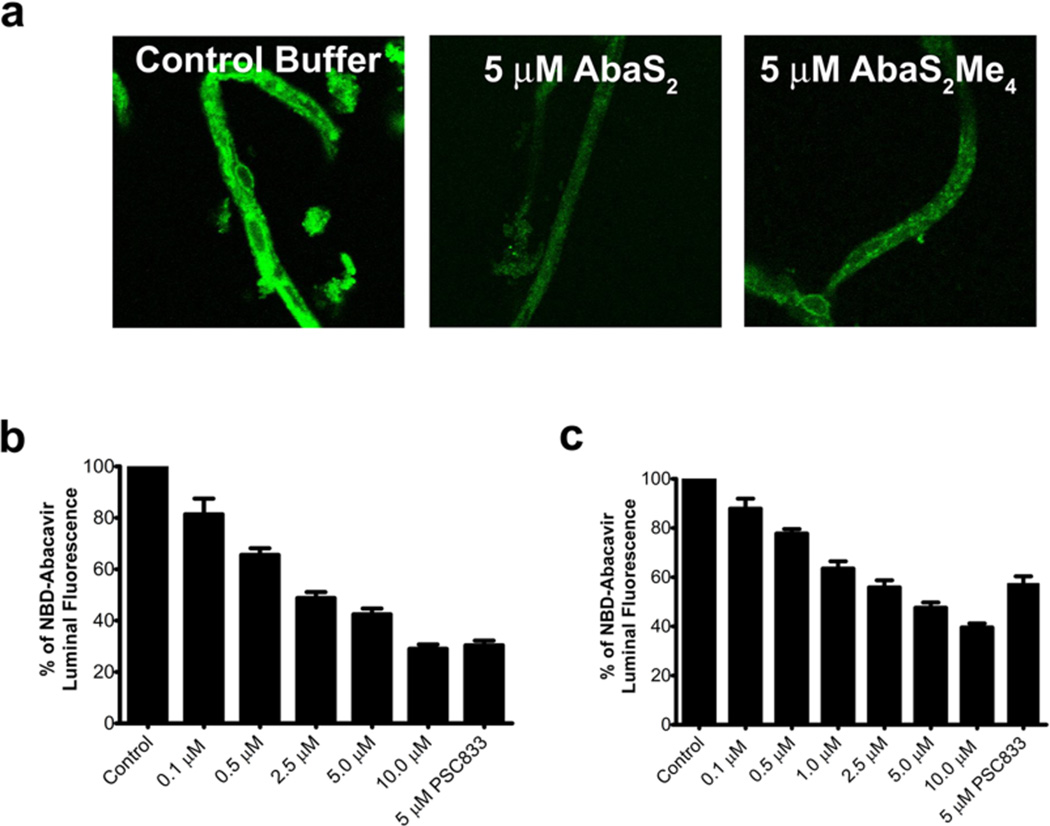
Abacavir and other HIV therapeutics are actively effluxed by P-gp at the BBB. Given that our ultimate goal is to increase brain penetration of therapeutics by inhibiting P-gp at the blood-brain-barrier, we employed isolated rat brain capillaries that express endogenous levels of P-gp.36 These experiments were designed to test the ability of abacavir dimers to interact with P-gp present at the BBB. When exposed to a fluorescent P-gp substrate, these capillaries concentrate fluorescence within the lumen by a process that is blocked by P-gp inhibitors.36,37 This robust assay has been used previously to demonstrate transport and inhibition of P-gp with established P-gp inhibitors as well as transporter modulation.7 For the assay, we incubated freshly isolated capillaries with a fluorescent substrate, NBD-abacavir, in the absence (control) and presence of AbaS2 or AbaS2Me4; the potent P-gp inhibitor, PSC833, served as a positive control. Accumulation of NBD-abacavir within the capillaries was visualized by confocal microscopy (Figure 3). Control capillaries showed intense NBD-abacavir fluorescence within the lumens, whereas exposure to 5 µM AbaS2 or AbaS2Me4 substantially reduced luminal fluorescence (Figure 3a). Quantitation of luminal fluorescence showed concentration-dependent reductions with increasing concentrations of AbaS2 or AbaS2Me4 (Figure 3b and Figure 3c). Thus, the abacavir dimers are capable of inhibiting P-gp transport in a BBB model with endogenous levels P-gp expression.
Figure 3.
P-gp efflux is inhibited by prodrug abacavir homodimers in rat brain capillaries. (a) Rat brain capillaries were incubated in the presence or absence of 5 µM AbaS2 or AbaS2Me4 in PBS (pH 7.4), followed by the addition of 2 µM NBD-abacavir in PBS (pH 7.4). Analysis was made by confocal scanning microscopy at 40X magnification. (b and c) Concentration dependent inhibition of P-gp transport of NBD-abacavir in rat brain capillaries by (b) AbaS2 and (c) AbaS2Me4 using 5 µM PSC833 as the positive control.
The abacavir prodrug dimers were designed first to inhibit P-gp at the cell membrane and then release therapeutic abacavir within the intracellular reducing environment. The ester linkages of the dimers, therefore, must have sufficient stability to withstand esterases within blood, but still be sufficiently reactive through the reduced tether mechanism in the cytosol (Figure 1, inset). AbaS2, AbaS2Me2 and AbaS2Me4 were evaluated for their stability to plasma esterases and reducing conditions using human plasma and dithiothreitol (DTT), respectively. Each dimer was incubated with either human plasma (55%) or DTT (10 mM) at 37 °C, and the loss of dimer and appearance of monomer were monitored by reverse phase HPLC. In plasma, the unhindered abacavir dimer AbaS2 displayed a relatively rapid reversion to monomer with a half life (T1/2) of 1.6 hrs (Table 2). The addition of methyl groups to the abacavir dimer tethers affected susceptibility to plasma esterases; two methyl groups (AbaS2Me2) increased stability 4-fold, whereas the addition of four methyl groups (AbaS2Me4) strikingly resulted in less than 10% ester hydrolysis after 100 hrs. Under reducing conditions AbaS2 reverted to monomer with a T1/2 of 8.8 hrs (Table 2). Although ester hydrolysis was reduced by methylation, the reductive pathway to monomer production was much less affected. AbaS2Me2 displayed a similar rate of breakdown to monomer as AbaS2, whereas only a 1.5-fold decrease in monomer release was observed for AbaS2Me4 as compared to AbaS2Me2. These data are significant as they demonstrate that that it is possible to slow or halt the breakdown of dimeric agents in human plasma, while still allowing release of monomer through the reductive pathway within cells.
Table 2.
Reversion of abacavir prodrug dimers to monomeric abacavir in vitro
| Compound | t1/2 (hr) Human plasmaa |
t1/2 (hr) DTTb |
|---|---|---|
| abacavir | NA | NA |
| AbaS2 | 1.6 ± 0.1 | 8.8 ± 0.4 |
| AbaS2Me2 | 7.1 ± 1.6 | 11.7 ± 0.3 |
| AbaS2Me4 | > 100 | 17.2 ± 0.1 |
Concentration of compounds was 60 µM in assay
Concentration of compounds was 70 µM in assay
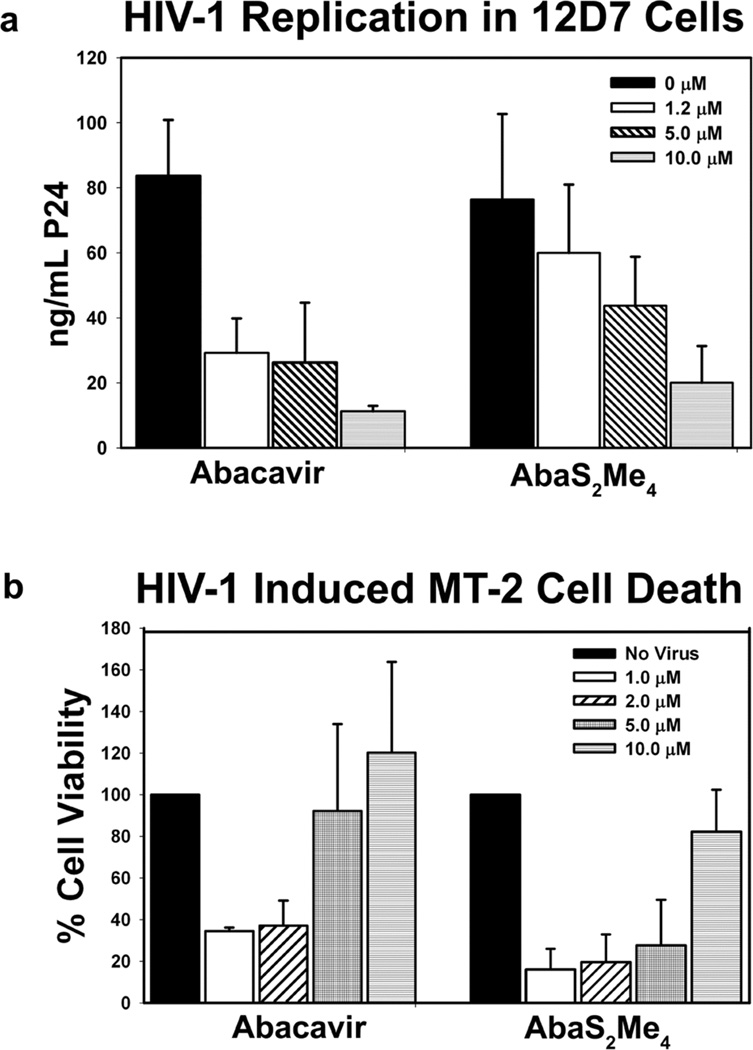
We hypothesize that antiviral activity will only be afforded by the breakdown of AbaS2Me4 to the active RTI abacavir, as AbaS2Me4 did not demonstrate intrinsic RTI activity (See Supporting Information). To ascertain that an abacavir prodrug dimer would have anti-HIV activity due to reversion to monomeric therapeutic within the reducing environment of cells, we employed two cell-based assays of anti-HIV activity: HIV titer in 12D7 cells and HIV-induced toxicity in MT-2 cells (Figure 4).38 The 12D7 and MT-2 cells were infected with HIV-1LAI using a previously described protocol. Antiretroviral activity was assayed by monitoring the production of HIV p24 by ELISA in the cell-free culture medium of infected cells exposed to varying concentrations of AbaS2Me4 and monomeric abacavir after 12 – 14 days.39 In addition, an MTT assay was employed to determine the ability of AbaS2Me4 to protect MT-2 cells from the cytopathic effect of HIV-1LAI after seven days. We confirmed that AbaS2Me4 was stable in the culture medium used for the above antiviral cell-based experiments. In both assays AbaS2Me4 demonstrated a dose-responsive increase in antiviral activity within 2.5- to 4-fold of the abacavir monomer activity (Figure 4). Together these data indicate that the observed cellular antiviral activity is due to reversion of the dimer into therapeutic abacavir within the reducing environment of the cell.
Figure 4.
(a) Inhibition of HIV-1 replication in the presence or absence of increasing concentrations of abacavir or AbaS2Me4. IC50 values of approximately 0.6 and 2.5 µM were obtained for abacavir and AbaS2Me4, respectively (b) Inhibition of HIV-induced cell death with increasing concentrations of abacavir or AbaS2Me4. IC50 values of approximately 2.5 and 6.5 µM were obtained for abacavir and AbaS2Me4, respectively.
DISCUSSION
Eradication of HIV reservoirs in the brain will necessitate the penetration of antiviral agents across the BBB, through processes that either evade P-gp40–43, or block its activity directly. Herein we have focused on this latter strategy with dimerized P-gp substrates that were designed to have two functions: to inhibit P-gp efflux at the BBB, and to act as prodrugs and revert to the functional, monomeric therapeutic within the reducing environment of the cytosol. The HIV RTI abacavir was chosen for these experiments as in vivo experiments have clearly demonstrated the role of P-gp in limiting the entry of abacavir into the brain.18 Prodrug abacavir dimers were synthesized, therefore, using a “traceless tether” that was designed to respond to a reducing environment and regenerate abacavir through molecular rearrangement. Additional methyl groups were added to the tether in an effort to tune the rate of breakdown of the prodrugs under a variety of conditions.
We have demonstrated that the prodrug abacavir dimers are potent P-gp inhibitors both in cell culture and, notably, in a brain capillary model of the BBB. The significantly reduced susceptibility of the prodrugs to plasma esterases with increased methylation state of the dimeric tethers, combined with only small changes in reductive monomer release, clearly demonstrate that we can engineer the rate of release of monomer from the prodrugs. Importantly, the abacavir dimer AbaS2Me4 demonstrated anti-HIV activity in two separate T-cell-based HIV assays, while this agent was itself inactive in an in vitro RT assay. These data strongly suggest that the observed cellular antiviral activity of AbaS2Me4 is linked to the reversion of this prodrug dimer to the RT-active monomeric abacavir.
Dimeric prodrugs of antiretroviral agents, such as those described herein, have interesting potential for use in conjunction with HAART, as a number of these therapies are substrates of P-gp. Co-administration of the dimeric prodrugs with monomeric drugs would allow for accumulation of the therapeutic agent within the brain via two pathways: enhanced entry of monomeric drug through inhibition of P-gp at the BBB, and breakdown of the dimeric P-gp inhibitors within endothelial cells at the BBB to provide additional monomeric therapeutic. This approach precludes the need for additional P-gp inhibitors with HAART and, as such, lowers the risk of possible drug toxicities.44 Since combination therapy is the hallmark of HAART treatment due to drug resistance found with single therapy regimes, this dimeric prodrug design provides the opportunity to crosslink different antiviral agents that are P-gp substrates, such as abacavir and saquinavir, into heterodimeric prodrug inhibitors. This overall strategy represents a platform technology that may be readily applied to other therapies with limited brain penetration due to P-gp efflux activity, from the anticancer agents topotecan and taxol to the anti-schizophrenia drugs quetiapine and paliperidone.
Supplementary Material
ACKNOWLEDGMENT
We acknowledge the National Institutes of Health and the Purdue Research Foundation for support of this work.
Footnotes
ASSOCIATED CONTENT
Supporting Information. Experimental procedures, synthetic schemes, characterization and reverse transcriptase data. This material is available free of charge via the Internet at http://pubs.acs.org.
REFERENCES
- 1.Pierson T, McArthur J, Siliciano RF. Annu Rev Immunol. 2000;18:665. doi: 10.1146/annurev.immunol.18.1.665. [DOI] [PubMed] [Google Scholar]
- 2.Lambotte O, Deiva K, Tardieu M. Brain Pathol. 2003;13:95. doi: 10.1111/j.1750-3639.2003.tb00010.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kandanearatchi A, Williams B, Everall IP. Brain Pathol. 2003;13:104. doi: 10.1111/j.1750-3639.2003.tb00011.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Groothuis DR, Levy RM. J Neurovirol. 1997;3:387. doi: 10.3109/13550289709031185. [DOI] [PubMed] [Google Scholar]
- 5.Varatharajan L, Thomas SA. Antiviral Res. 2009;82:A99. doi: 10.1016/j.antiviral.2008.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Loscher W, Potschka H. Nat Rev Neurosci. 2005;6:591. doi: 10.1038/nrn1728. [DOI] [PubMed] [Google Scholar]
- 7.Miller DS. Trends Pharmacol Sci. 2010;31:246. doi: 10.1016/j.tips.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chaudhary PM, Mechetner EB, Roninson IB. Blood. 1992;80:2735. [PubMed] [Google Scholar]
- 9.Neyfakh AA, Serpinskaya AS, Chervonsky AV, Apasov SG, Kazarov AR. Exp Cell Res. 1989;185:496. doi: 10.1016/0014-4827(89)90318-2. [DOI] [PubMed] [Google Scholar]
- 10.Gottesman MM, Hrycyna CA, Schoenlein PV, Germann UA, Pastan I. Annu Rev Genet. 1995;29:607. doi: 10.1146/annurev.ge.29.120195.003135. [DOI] [PubMed] [Google Scholar]
- 11.Hrycyna CA. Semin Cell Dev Biol. 2001;12:247. doi: 10.1006/scdb.2000.0250. [DOI] [PubMed] [Google Scholar]
- 12.Raviv Y, Pollard HB, Bruggemann EP, Pastan I, Gottesman MM. J Biol Chem. 1990;265:3975. [PubMed] [Google Scholar]
- 13.Shapiro AB, Corder AB, Ling V. Eur J Biochem. 1997;250:115. doi: 10.1111/j.1432-1033.1997.00115.x. [DOI] [PubMed] [Google Scholar]
- 14.Kim AE, Dintaman JM, Waddell DS, Silverman JA. J Pharmacol Exp Ther. 1998;286:1439. [PubMed] [Google Scholar]
- 15.Lee CG, Gottesman MM, Cardarelli CO, Ramachandra M, Jeang KT, Ambudkar SV, Pastan I, Dey S. Biochemistry. 1998;37:3594. doi: 10.1021/bi972709x. [DOI] [PubMed] [Google Scholar]
- 16.Kim RB, Fromm MF, Wandel C, Leake B, Wood AJ, Roden DM, Wilkinson GR. J Clin Invest. 1998;101:289. doi: 10.1172/JCI1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Choo EF, Leake B, Wandel C, Imamura H, Wood AJ, Wilkinson GR, Kim RB. Drug Metab Dispos. 2000;28:655. [PubMed] [Google Scholar]
- 18.Shaik N, Giri N, Pan G, Elmquist WF. Drug Metab Dispos. 2007;35:2076. doi: 10.1124/dmd.107.017723. [DOI] [PubMed] [Google Scholar]
- 19.Park S, Sinko PJ. J Pharmacol Exp Ther. 2005;312:1249. doi: 10.1124/jpet.104.076216. [DOI] [PubMed] [Google Scholar]
- 20.Polli JW, Jarrett JL, Studenberg SD, Humphreys JE, Dennis SW, Brouwer KR, Woolley JL. Pharm Res. 1999;16:1206. doi: 10.1023/a:1018941328702. [DOI] [PubMed] [Google Scholar]
- 21.Gimenez F, Fernandez C, Mabondzo A. J Acquir Immune Defic Syndr. 2004;36:649. doi: 10.1097/00126334-200406010-00001. [DOI] [PubMed] [Google Scholar]
- 22.Bruggemann EP, Currier SJ, Gottesman MM, Pastan I. J Biol Chem. 1992;267:21020. [PubMed] [Google Scholar]
- 23.Loo TW, Bartlett MC, Clarke DM. J Biol Chem. 2003;278:39706. doi: 10.1074/jbc.M308559200. [DOI] [PubMed] [Google Scholar]
- 24.Martin C, Berridge G, Higgins CF, Mistry P, Charlton P, Callaghan R. Mol Pharmacol. 2000;58:624. doi: 10.1124/mol.58.3.624. [DOI] [PubMed] [Google Scholar]
- 25.Dey S, Ramachandra M, Pastan I, Gottesman MM, Ambudkar SV. Proc Natl Acad Sci U S A. 1997;94:10594. doi: 10.1073/pnas.94.20.10594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shapiro AB, Ling V. Eur J Biochem. 1997;250:130. doi: 10.1111/j.1432-1033.1997.00130.x. [DOI] [PubMed] [Google Scholar]
- 27.Aller SG, Yu J, Ward A, Weng Y, Chittaboina S, Zhuo R, Harrell PM, Trinh YT, Zhang Q, Urbatsch IL, Chang G. Science. 2009;323:1718. doi: 10.1126/science.1168750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pires MM, Hrycyna CA, Chmielewski J. Biochemistry. 2006;45:11695. doi: 10.1021/bi0608109. [DOI] [PubMed] [Google Scholar]
- 29.Sauna ZE, Andrus MB, Turner TM, Ambudkar SV. Biochemistry. 2004;43:2262. doi: 10.1021/bi035965k. [DOI] [PubMed] [Google Scholar]
- 30.El Alaoui A, Schmidt F, Amessou M, Sarr M, Decaudin D, Florent JC, Johannes L. Angew Chem Int Ed Engl. 2007;46:6469. doi: 10.1002/anie.200701270. [DOI] [PubMed] [Google Scholar]
- 31.Jones LR, Goun EA, Shinde R, Rothbard JB, Contag CH, Wender PA. J Am Chem Soc. 2006;128:6526. doi: 10.1021/ja0586283. [DOI] [PubMed] [Google Scholar]
- 32.Henne WA, Doorneweerd DD, Hilgenbrink AR, Kularatne SA, Low PS. Bioorg Med Chem Lett. 2006;16:5350. doi: 10.1016/j.bmcl.2006.07.076. [DOI] [PubMed] [Google Scholar]
- 33.Lee CG, Pastan I, Gottesman MM. Methods Enzymol. 1998;292:557. doi: 10.1016/s0076-6879(98)92044-4. [DOI] [PubMed] [Google Scholar]
- 34.Namanja HA, Hrycyna CA, Chmielewski J. 2011 manuscript in preparation. [Google Scholar]
- 35.Hrycyna CA, Ramachandra M, Pastan I, Gottesman MM. Methods Enzymol. 1998;292:456. doi: 10.1016/s0076-6879(98)92035-3. [DOI] [PubMed] [Google Scholar]
- 36.Miller DS, Nobmann SN, Gutmann H, Toeroek M, Drewe J, Fricker G. Mol Pharmacol. 2000;58:1357. doi: 10.1124/mol.58.6.1357. [DOI] [PubMed] [Google Scholar]
- 37.Hartz AM, Bauer B, Fricker G, Miller DS. Mol Pharmacol. 2004;66:387. doi: 10.1124/mol.104.001503. [DOI] [PubMed] [Google Scholar]
- 38.Richman DD, Kornbluth RS, Carson DA. J. Exper. Med. 1987;166:1144. doi: 10.1084/jem.166.4.1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Davis DA, Brown CA, Singer KE, Wang V, Kaufman J, Stahl SJ, Wingfield P, Maeda K, Harada S, Yoshimura K, Kosalaraksa P, Mitsuya H, Yarchoan R. Antiviral Res. 2006;72:89. doi: 10.1016/j.antiviral.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 40.Wong HL, Chattopadhyay N, Wu XY, Bendayan R. Adv Drug Deliv Rev. 2010;62:503. doi: 10.1016/j.addr.2009.11.020. [DOI] [PubMed] [Google Scholar]
- 41.Regina A, Demeule M, Che C, Lavallee I, Poirier J, Gabathuler R, Beliveau R, Castaigne JP. Br J Pharmacol. 2008;155:185. doi: 10.1038/bjp.2008.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huwyler J, Wu D, Pardridge WM. Proc Natl Acad Sci U S A. 1996;93:14164. doi: 10.1073/pnas.93.24.14164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rao KS, Ghorpade A, Labhasetwar V. Expert Opin Drug Deliv. 2009;6:771. doi: 10.1517/17425240903081705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bissett D, Kerr DJ, Cassidy J, Meredith P, Traugott U, Kaye SB. Br J Cancer. 1991;64:1168. doi: 10.1038/bjc.1991.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.