Abstract
This paper evaluates the use of oligovalent amyloid-binding molecules as potential agents that can reduce the enhancement of HIV-1 infection in cells by SEVI fibrils. These naturally occurring amyloid fibrils found in semen have been implicated as mediators that can facilitate the attachment and internalization of HIV-1 virions to immune cells. Molecules that are capable of reducing the role of SEVI in HIV-1 infection may, therefore, represent a novel strategy to reduce the rate of sexual transmission of HIV-1 in humans. Here, we evaluated a set of synthetic, oligovalent derivatives of BTA (a known amyloid-binding molecule) for their capability to bind cooperatively to aggregated amyloid peptides and to neutralize the effects of SEVI in HIV-1 infection. We demonstrate that these BTA derivatives exhibit a general trend of increased binding to aggregated amyloids as a function of increasing valence number of the oligomer. Importantly, we find that oligomers of BTA show improved capability to reduce SEVI-mediated infection of HIV-1 in cells compared to a BTA monomer, with the pentamer exhibiting a 65-fold improvement in efficacy compared to a previously reported monomeric BTA derivative. These results, thus, support the use of amyloid-targeting molecules as potential supplements for microbicides to curb the spread of HIV-1 through sexual contact.
A recent study reported that an abundant peptide found in semen, PAP248–286, forms aggregated amyloid species (called semen-derived enhancer of virus infection or SEVI) that can potentially increase infection of HIV-1 in cells by up to 400,000-fold.1 Although the molecular mechanism of SEVI-mediated transmission of HIV-1 remains poorly understood, evidence suggests that SEVI binds to both HIV-1 virions and cell membranes, and facilitates viral infection.1–4 Methods to suppress the effects of such natural enhancers of HIV-1 transmission may, therefore, significantly reduce the global spread of HIV through sexual contact among high-risk populations.
We recently reported that targeting SEVI fibrils with the amyloid-binding molecule BTA-EG6, a hexa(ethylene glycol) derivative of benzothiazole aniline (BTA), reduced SEVI- and semen-mediated enhancement of HIV infection in T cells at a concentration corresponding to 50 percent inhibition (IC50) of 13 µM.5 We hypothesized that BTA-EG6 forms a protein-resistive coating on SEVI,5 effectively blocking the interaction of these fibrils with HIV-1 virions and cells. A key characteristic of this approach to reducing HIV transmission compared to more traditional microbicide candidates is that we target a naturally abundant mediator (i.e., SEVI) in the cellular attachment of HIV, rather than targeting the virions themselves. Although BTA-EG6 was not toxic to cervical cells and did not exhibit any pro-inflammatory activity at a concentration that was 10 times the IC50 for efficacy,5 strategies to create more potent compounds would be desirable for further development of SEVI-neutralizing agents as supplements to current microbicide candidates.6–8
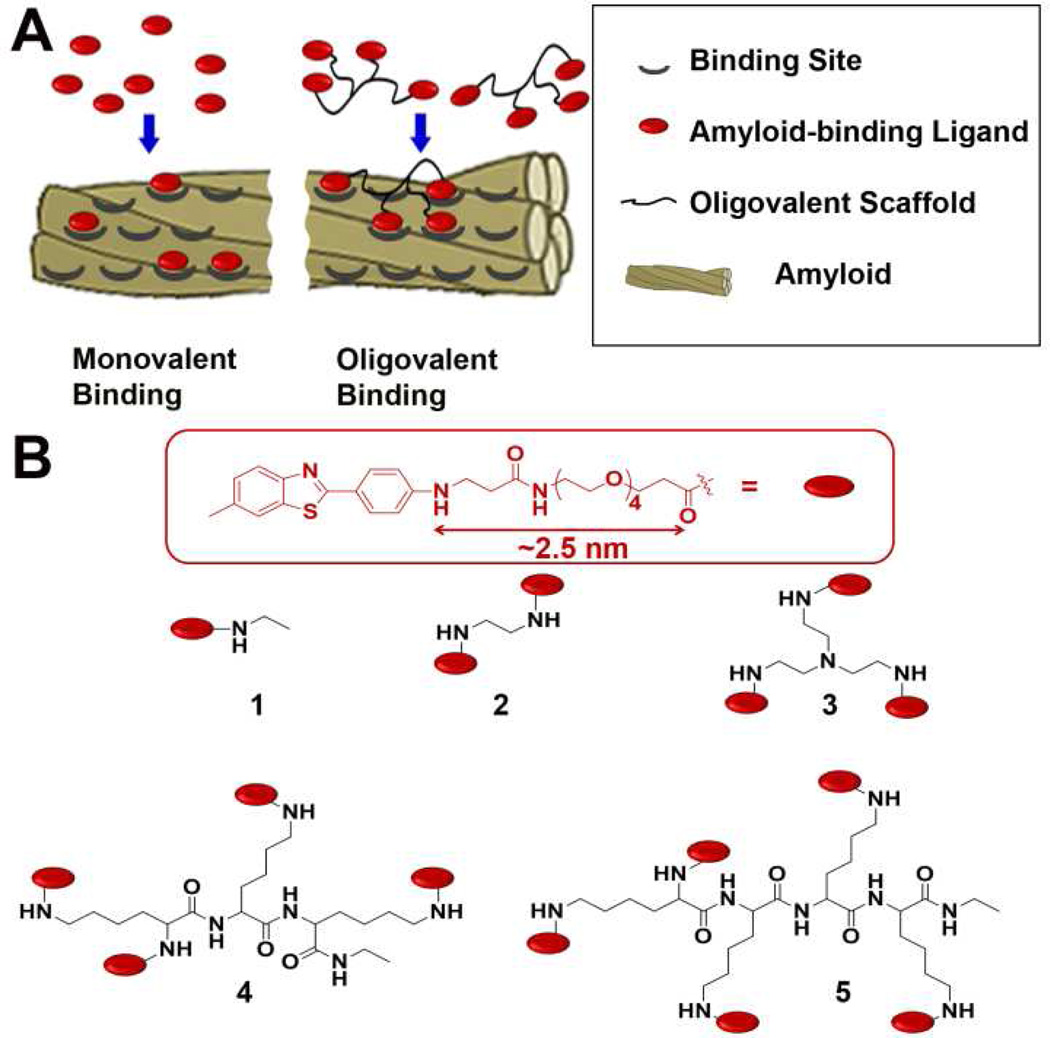
Here, we explore whether oligomers of BTA exhibit improved capability to reduce SEVI-enhanced infection of HIV in cells compared to a BTA monomer. Multivalent binding—that is, the multiple, simultaneous binding of two or more ligands and receptors—is ubiquitous in nature,9 and has been explored as a strategy to increase the affinity of small molecules to multivalent targets.10–24 Amyloid fibrils formed from the self-assembly of peptides putatively display multiple, identical, and periodically spaced binding sites for small molecules along the fibrillar surface,25–29 and, thus, represent an excellent biological target for multivalent ligand design (Figure 1).
Figure 1.
Structures of monovalent and oligovalent amyloid-binding molecules. A) Cartoon depicting the monovalent (left) or oligovalent (right) binding of molecules to amyloid fibrils. B) Chemical structures of monovalent (1) and oligovalent (2–5) derivatives of benzothiazole aniline (BTA). A rudimentary estimate of the length (in fully extended conformation) of the flexible group attached to BTA was calculated using Chem-Bio3D Ultra 12.0 software.
Lockhart et al.25 and Wu et al.29 had previously reported that BTA derivatives associate with amyloid fibrils derived from Alzheimer’s-related amyloid-β (Aβ) peptides, with data suggesting a spacing between adjacent binding sites of ~2 nm. Since little information is available regarding the interaction of small molecules with SEVI, we designed and synthesized BTA oligomers 2–5 (Figure 1) based on what was known about the binding of BTA to aggregated Aβ(1–42) peptides. We designed the BTA monomer 1 to carry a tetra(ethylene glycol) group terminated with a carboxyl moiety, which we subsequently used to generate oligovalent BTA derivatives 2–5 by reaction with commercial oligo-amine spacers using standard amidation chemistry (see supporting information for details). Although oligo(ethylene glycols) are quite flexible (which theoretically diminishes the potential gain in conformational entropy for oligovalent binding9,30–33), we incorporated them into the design of compounds 2–5 because of their known minimal interaction with proteins34,35 and for their water solubilizing properties.36,37 We estimated that the flexible spacer on each BTA monomer could span a length of ~2.5 nm when modeled in fully extended conformation (Figure 1), suggesting that the BTA units on oligomers 2–5 could easily span the expected ~2 nm distance between binding sites on Aβ fibrils. 38 Additionally, dimers of Thioflavin T (ThT, a BTA-based histological amyloid staining agent), where the ThT moieties were linked by 2–5 ethylene glycol units, have recently been reported to associate with Aβ fibrils with increased affinity compared to ThT alone.39 These studies suggest that BTA oligomers 2–5 could also bind oligovalently to amyloid targets.
Table 1 lists the apparent Kd values for compounds 1–5 to fibrils formed from Aβ(1–42) peptides, as estimated using a known fluorescence binding assay.5,26,40,41 As expected, BTA dimer 2 bound more strongly than monomer 1 to Aβ(1–42) fibrils, albeit with only a modest 8-fold lower Kd value. The flexibility of the oligo(ethylene glycol) groups presumably attenuates the degree of cooperative binding of the two BTA units in 2 to the fibrillar surface. Surprisingly, BTA trimer 3 and tetramer 4 bound only with similar Kd values to Aβ(1–42) fibrils compared to dimer 2. One possible explanation for this result is that the structures of 3 and 4 make it preferable for these molecules to bind divalently to the amyloid surface. Alternatively, it may also be possible that 3 and 4 bind to amyloid fibrils with a valency greater than 2, but incur significant loss in expected binding energy due to partial docking of the BTA units to their respective binding sites. For BTA pentamer 5, the measured Kd value was an additional 10-fold lower than tetramer 4 and was 117-fold lower than the Kd value for monomer 1. If we assume that all five BTA moieties in 5 participate in binding to the Aβ fibrils, we can approximate a Kd value per BTA moiety of 10 nM. Although the effects of multivalent binding to Aβ fibrils are modest for compounds 2–5, there appears to be a general trend of improved binding from monomer to pentamer (most notably from monomer to dimer and from tetramer to pentamer) within this series of compounds.
Table 1.
Table of apparent Kd values obtained for compounds 1–5 for binding to fibrils formed from synthetic Aβ(1–42) or SEVI fibrils. These values were estimated using a previously reported fluorescence binding assay.26
| Compound # | Kd (nM) to Aβ(1–42) fibrils |
Kd (nM) to SEVI fibrils |
|---|---|---|
| 1 | 235 ± 75 | 236 ± 90 |
| 2 | 29 ± 4 | 69 ± 1 |
| 3 | 26 ± 6 | 40 ± 6 |
| 4 | 20 ± 5 | 59 ± 6 |
| 5 | 2.0 ± 0.4 | 0.4 ± 0.2 |
When we examined the apparent Kd values of compounds 1–5 to SEVI fibrils, we found a similar trend for increased binding of the oligomeric BTA compounds as we observed for their binding to Aβ(1–42) fibrils (Table 1). We, again, observed the greatest improvement in binding as a function of increasing valence number in the oligomer when we compared the monomer to dimer and the tetramer to pentamer. We found that BTA pentamer 5 had a Kd value of 0.4 nM for binding to aggregated SEVI peptides (or 2 nM per BTA moiety if we assume that all five BTA moieties in 5 participate in binding to the SEVI fibrils), which was 590-fold lower than the Kd value of monomer 1.
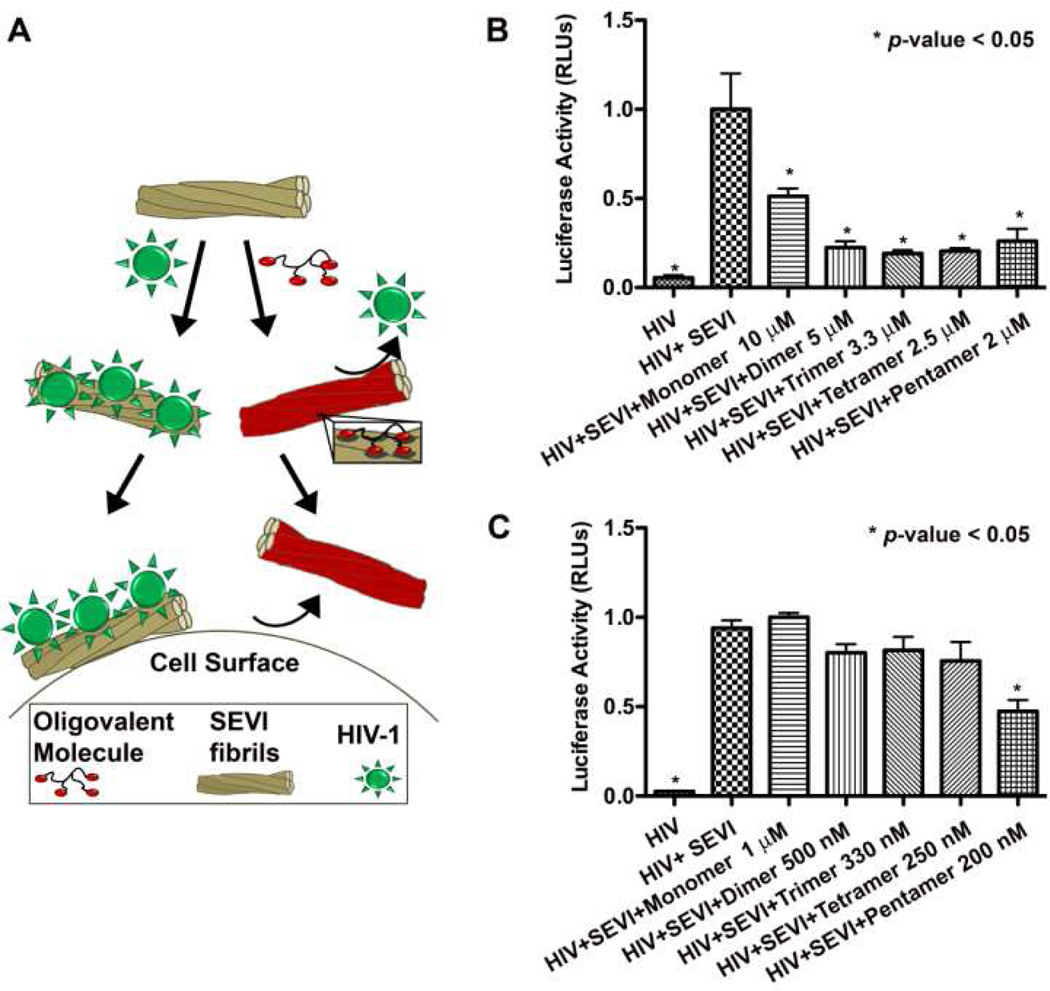
In order to investigate whether the improved binding of BTA oligomers 2–5 to SEVI fibrils compared to monomer 1 would translate into improved efficacy for blocking SEVI-mediated HIV-1 infection (Figure 2A), we evaluated compounds 1–5 for their capability to inhibit SEVI-enhanced infection of HIV-1IIIB in TZM-bl cells. TZM-bl cells are a HeLaderived cell line that express high levels of the CD4 receptor, CCR5 and CXCR4 co-receptors, and contain the HIV-1 LTR-driven luciferase cassette.42,43 Since HIV-1 LTR is a weak transcriptional regulator in the absence of its cognate, Tat, the expression levels of luciferase in these cells are directly proportional to the extent of HIV-1 infection. In these HIV-1 infectivity experiments, we chose concentrations of compounds 1–5 that maintained a 10 µM or 1 µM concentration of the BTA moiety in all samples of monomer and oligomers (e.g., since there are 2 BTA moieties in dimer 2, we used a 5 µM concentration of dimer compared to a 10 µM concentration of monomer to afford the same total concentration of BTA). We expected that this experimental design would highlight any multivalent enhancement of efficacy from the oligomers compared to monomer. Figure 2B shows that all of the oligomers were more effective at reducing SEVI-mediated enhancement of HIV-1 infection compared to monomer 1. As a control, compounds 1–5, at all concentrations tested, did not have any significant effect on HIV infection in these cells in the absence of SEVI (see Figure S3 in the supporting information). We found that 5 µM dimer, 3.3 µM trimer, 2.5 µM tetramer, or 2 µM pentamer all reduced SEVI-mediated HIV-1 infectivity almost completely to the level of infection observed in the absence of SEVI (Figure 2B). This level of activity from all oligomers 2–5 is superior to the activity of monomer 1 and to the activity reported previously for BTA-EG6 (which required a concentration of 44 µM to nearly completely neutralize the effects of SEVI on HIV-1 infection5). Figure 2C shows that BTA pentamer 5, which exhibited the lowest Kd value for binding to SEVI fibrils, reduced SEVI-mediated HIV-1 infectivity by approximately 50% at a concentration of 200 nM (i.e., indicating an IC50 of ~ 200 nM for pentamer 5, or an IC50 of 1 µM per BTA moiety assuming that all five BTA moieties in 5 participate in binding to the SEVI fibrils). This level of activity from pentamer 5 is 65-fold more effective than the previously reported BTA-EG6 (which exhibited an IC50 of 13 µM for reducing SEVI-enhanced infection of HIV-15). We attribute at least part of the increased efficacy of oligomers 2–5 with respect to the monomer 1 to the capability of the oligomers to bind multivalently to SEVI fibrils.
Figure 2.
Inhibition of SEVI-mediated enhancement of HIV-1 infection by compounds 1–5. A) Schematic illustration showing the proposed coating of SEVI fibrils with amyloid-binding oligomers. These coatings prevent the direct interaction of HIV-1 with SEVI fibrils and, thus, prevent SEVI-mediated enhancement of viral infection in cells. B) Graph showing the reduction of SEVI-mediated enhancement of HIV-1IIIB infection in TZM-bl cells in the presence of compounds 1–5 (10 µM total BTA moiety), as estimated by cellular luciferase expression levels. C) Graph showing the reduction of SEVI-mediated enhancement of HIV-1IIIB infection in TZM-bl cells in the presence of 1–5 (1 µM total BTA moiety), as estimated by cellular luciferase expression levels. RLUs = relative luciferase units. All data are presented as normalized values relative to the luciferase expression in cells that were exposed to HIV-1IIIB+SEVI. A p-value of < 0.05 was considered statistically significantly different compared to cells treated with HIV-1IIIB+SEVI as determined by 1-way ANOVA with Tukey’s post test.
We have, thus, demonstrated proof-of-principle that multivalent display of amyloid-binding groups results in improved binding to both Aβ and SEVI fibrils. We showed that oligomers of BTA were significantly more effective in attenuating SEVI-mediated HIV-1 infectivity than their monomeric counterpart. These studies provide further support that amyloid-targeting agents can form a bio-resistive coating on aggregated amyloids and inhibit deleterious interactions of these naturally occurring biomaterials with other biomolecules.5,27,28,44 They also further support that amyloid-targeting agents may have important utility as prophylactic supplements for microbicides to reduce sexual transmission of HIV. Efforts to use polymeric9,16,45 and more rigid water-soluble46 and biocompatible scaffolds47–49 to generate multivalent amyloid-targeting agents with improved efficacy for reducing SEVI-mediated infection of HIV are currently underway.
Supplementary Material
ACKNOWLEDGMENT
This work was supported, in part by NIH grants R21AI094511, T32GM007356 (to J.S.O.), T32DA007232 (to J. S. O.), a California HIV/AIDS Research Program grant (ID10-SD-034), and by the University of Rochester Developmental Center for AIDS Research (NIH P30AI078498). We would also like to acknowledge the NIH for support of the Mass Spectrometry facility at UCSD (1S10RR025636-01A1)
ABBREVIATIONS
- HIV-1
human immunodeficiency virus-1
- SEVI
semen-derived enhancer of virus infection
- BTA
benzothiazole aniline
- ThT
Thioflavin T
- LTR
long terminal repeat
Footnotes
ASSOCIATED CONTENT
Details for the synthesis and characterization of compounds 1–5, experimental protocol for the fluorescence-based binding and HIV infection assay. Complete ref 1. This information is available free of charge via the Internet at http://pubs.acs.org.
REFERENCES
- 1.Munch J, et al. Cell. 2007;131:1059. doi: 10.1016/j.cell.2007.10.014. [DOI] [PubMed] [Google Scholar]
- 2.Roan NR, Munch J, Arhel N, Mothes W, Neidleman J, Kobayashi A, Smith-McCune K, Kirchhoff F, Greene WC. J. Virol. 2009;83:73. doi: 10.1128/JVI.01366-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nanga RPR, Brender JR, Vivekanandan S, Popovych N, Ramamoorthy A. J. Am. Chem. Soc. 2009;131:17972. doi: 10.1021/ja908170s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brender JR, Hartman K, Gottler LM, Cavitt ME, Youngstrom DW, Ramamoorthy A. Biophys. J. 2009;97:2474. doi: 10.1016/j.bpj.2009.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Olsen JS, Brown C, Capule CC, Rubinshtein M, Doran TM, Srivastava RK, Feng CY, Nilsson BL, Yang J, Dewhurst S. J. Biol. Chem. 2010;285:35488. doi: 10.1074/jbc.M110.163659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kirchhoff F, Munch J. Future Virol. 2011;6:183. [Google Scholar]
- 7.Roan NR, Sowinski S, Munch J, Kirchhoff F, Greene WC. J. Biol. Chem. 2010;285:1861. doi: 10.1074/jbc.M109.066167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hauber I, Hohenberg H, Holstermann B, Hunstein W, Hauber J. Proc. Natl. Acad. Sci. USA. 2009;106:9033. doi: 10.1073/pnas.0811827106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mammen M, Choi SK, Whitesides GM. Angew. Chem. Intl. Ed. 1998;37:2755. doi: 10.1002/(SICI)1521-3773(19981102)37:20<2754::AID-ANIE2754>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 10.Rao JH, Lahiri J, Isaacs L, Weis RM, Whitesides GM. Science. 1998;280:708. doi: 10.1126/science.280.5364.708. [DOI] [PubMed] [Google Scholar]
- 11.Logsdon LA, Schardon CL, Ramalingam V, Kwee SK, Urbach AR. J. Am. Chem. Soc. 2011;133:17087. doi: 10.1021/ja207825y. [DOI] [PubMed] [Google Scholar]
- 12.Breslow R, Zhang BL. J. Am. Chem. Soc. 1996;118:8495. [Google Scholar]
- 13.Zhang BL, Breslow R. J. Am. Chem. Soc. 1993;115:9353. [Google Scholar]
- 14.Kanai M, Mortell KH, Kiessling LL. J. Am. Chem. Soc. 1997;119:9931. [Google Scholar]
- 15.Mann DA, Kanai M, Maly DJ, Kiessling LL. J. Am. Chem. Soc. 1998;120:10575. [Google Scholar]
- 16.Mortell KH, Weatherman RV, Kiessling LL. J. Am. Chem. Soc. 1996;118:2297. [Google Scholar]
- 17.Courtney AH, Puffer EB, Pontrello JK, Yang ZQ, Kiessling LL. Proc. Natl. Acad. Sci. USA. 2009;106:2500. doi: 10.1073/pnas.0807207106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reczek JJ, Kennedy AA, Halbert BT, Urbach AR. J. Am. Chem. Soc. 2009;131:2408. doi: 10.1021/ja808936y. [DOI] [PubMed] [Google Scholar]
- 19.Krishnamurthy VMELA, Whitesides GM. Weinheim, Germany: Wiley-VCH Verlag GmbH & Co.; 2006. p. 11. [Google Scholar]
- 20.Cheng YY, Zhao LB, Li YW, Xu TW. Chem. Soc. Rev. 2011;40:2673. doi: 10.1039/c0cs00097c. [DOI] [PubMed] [Google Scholar]
- 21.Wilson AJ. Chem. Soc. Rev. 2009;38:3289. doi: 10.1039/b807197g. [DOI] [PubMed] [Google Scholar]
- 22.Jayaraman N. Chem. Soc. Rev. 2009;38:3463. doi: 10.1039/b815961k. [DOI] [PubMed] [Google Scholar]
- 23.Badjic JD, Nelson A, Cantrill SJ, Turnbull WB, Stoddart JF. Acc. Chem. Res. 2005;38:723. doi: 10.1021/ar040223k. [DOI] [PubMed] [Google Scholar]
- 24.Takahashi T, Mihara H. Acc. Chem. Res. 2008;41:1309–1318. doi: 10.1021/ar8000475. [DOI] [PubMed] [Google Scholar]
- 25.Lockhart A, Ye L, Judd DB, Merritt AT, Lowe PN, Morgenstern JL, Hong GZ, Gee AD, Brown J. J. Biol. Chem. 2005;280:7677. doi: 10.1074/jbc.M412056200. [DOI] [PubMed] [Google Scholar]
- 26.LeVine H., III Amyloid. 2005;12:5. doi: 10.1080/13506120500032295. [DOI] [PubMed] [Google Scholar]
- 27.Inbar P, Yang J. Bioorg. Med. Chem. Lett. 2006;16:1076. doi: 10.1016/j.bmcl.2005.10.067. [DOI] [PubMed] [Google Scholar]
- 28.Inbar P, Li CQ, Takayama SA, Bautista MR, Yang J. Chembiochem. 2006;7:1563. doi: 10.1002/cbic.200600119. [DOI] [PubMed] [Google Scholar]
- 29.Wu C, Wang ZX, Lei HX, Duan Y, Bowers MT, Shea JE. J. Mol. Biol. 2008;384:718. doi: 10.1016/j.jmb.2008.09.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lundquist JJ, Toone EJ. Chem. Rev. 2002;102:555. doi: 10.1021/cr000418f. [DOI] [PubMed] [Google Scholar]
- 31.Kitov PI, Sadowska JM, Mulvey G, Armstrong GD, Ling H, Pannu NS, Read RJ, Bundle DR. Nature. 2000;403:669. doi: 10.1038/35001095. [DOI] [PubMed] [Google Scholar]
- 32.Merritt EA, Zhang ZS, Pickens JC, Ahn M, Hol WGJ, Fan EK. J. Am. Chem. Soc. 2002;124:8818. doi: 10.1021/ja0202560. [DOI] [PubMed] [Google Scholar]
- 33.Page MI, Jencks WP. Proc. Natl. Acad. Sci. USA. 1971;68:1678. doi: 10.1073/pnas.68.8.1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chapman RG, Ostuni E, Liang MN, Meluleni G, Kim E, Yan L, Pier G, Warren HS, Whitesides GM. Langmuir. 2001;17:1225. [Google Scholar]
- 35.Siegers C, Biesalski M, Haag R. Chem. Eur. J. 2004;10:2831. doi: 10.1002/chem.200306073. [DOI] [PubMed] [Google Scholar]
- 36.Zalipsky S. Bioconj. Chem. 1995;6:150. doi: 10.1021/bc00032a002. [DOI] [PubMed] [Google Scholar]
- 37.Flexible linkers have often been used in the design of multivalent compounds, with demonstrated success. Moreover, unlike rigid linkers, flexible linkers can adopt several conformations with ease and without strain. The incorporation of flexible linkers in the design of multivalent compounds can thus optimize the binding of tethered ligands to multiple binding sites. See Ref. 19 for additional details.
- 38.We also synthesized BTA oligomers comprising shorter spacers (unpublished results), but the poor water solubility of these oligomers made them unsuitable for further analysis in cellular assays.
- 39.Qin L, Vastl J, Gao J. Mol. Biosyst. 2010;6:1791. doi: 10.1039/c005255h. [DOI] [PubMed] [Google Scholar]
- 40.Chang WM, Dakanali M, Capule CC, Sigurdson CJ, Yang J, T'heodorakis EA. ACS Chem. Neurosci. 2011;2:249. doi: 10.1021/cn200018v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sutharsan J, Dakanali M, Capule CC, Haidekker MA, Yang J, Theodorakis EA. Chemmedchem. 2010;5:56. doi: 10.1002/cmdc.200900440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Platt EJ, Wehrly K, Kuhmann SE, Chesebro B, Kabat D. J. Virol. 1998;72:2855. doi: 10.1128/jvi.72.4.2855-2864.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.The CXCR4 co-receptor is endogenously expressed in HeLa cells. See Ref. 42 for additional details.
- 44.Habib LK, Lee MTC, Yang J. J. Biol. Chem. 2010;285:38933. doi: 10.1074/jbc.M110.132860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kolonko EM, Kiessling LL. J. Am. Chem. Soc. 2008;130:5626. doi: 10.1021/ja8001716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Semetey V, Moustakas D, Whitesides GM. Angew. Chem. Intl. Ed. 2006;45:588. doi: 10.1002/anie.200502991. [DOI] [PubMed] [Google Scholar]
- 47.Yin YHAD. Chemical Biology, Wiley-VCH, 2007. 2007:250. [Google Scholar]
- 48.Rosenzweig BA, Ross NT, Adler MJ, Hamilton AD. J. Am. Chem. Soc. 2010;132:6749. doi: 10.1021/ja100485n. [DOI] [PubMed] [Google Scholar]
- 49.Rosenzweig BA, Ross NT, Tagore DM, Jayawickramarajah J, Saraogi I, Hamilton AD. J. Am. Chem. Soc. 2009;131:5020. doi: 10.1021/ja809219p. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.