Abstract
Melanoma cells driven by mutant B-RAF are highly resistant to chemotherapeutic treatments. Recent Phase 1 results with PLX4032/RG7204/Vemurafenib, which selectively inhibits B-RAF/MEK/ERK1/2 signaling in mutant B-RAF cells, has given encouragement to this struggling field. Nearly all patients in the phase 1–3 studies saw at least some response and the overall response rates were in between 81 and 48%. However, despite initial tumor shrinkage, most responders in the trial experienced tumor relapse over time. These findings indicate that both intrinsic and acquired resistance may affect the clinical efficacy of PLX4032. It is critical to optimize PLX4032 activity to improve response rates and understand why some patients with the B-RAF mutation do not respond. We have previously shown that the stemness factor, Forkhead box D3 (FOXD3), is up-regulated following inhibition of B-RAF-MEK signaling in mutant B-RAF melanoma cells. Here, we show that up-regulation of FOXD3 following treatment with PLX4032 and PLX4720 (the non-clinical tool compound for PLX4032) confers resistance to cell death. Small interfering RNA (siRNA)-mediated knockdown of FOXD3 significantly enhanced the cell death response after PLX4032/4720 treatment in mutant B-RAF melanoma cell lines. Additionally, up-regulation of FOXD3 after PLX4720 treatment was attenuated in non-adherent conditions and correlated with enhanced cell death. Ectopic expression of FOXD3 in non-adherent cells significantly reduced cell death in response to PLX4720 treatment. Together, these data indicate that up-regulation of FOXD3 is an adaptive response to RAF inhibitors that promotes a state of drug resistance.
Keywords: RAF inhibitor, ERK1/2, FOXD3, chemotherapeutic resistance
Introduction
Melanoma is the deadliest form of skin cancer and the number of new cases of melanoma increases every year. Advanced melanoma is associated with resistance to conventional chemotherapies, and the 5-year survival rate for patients with metastatic melanoma remains low. In 50–60% of melanomas, mutations in the B-RAF serine-threonine kinase activate the MEK-ERK1/2 pathway (Davies et al., 2002). The most common mutation in B-RAF is a V600E substitution in the activation domain that causes its kinase function to remain constitutively active (Davies et al., 2002). Overwhelming evidence shows that B-RAFV600E is a driver mutation that promotes melanoma growth and survival (Dhomen and Marais, 2007). However, the presence of B-RAF mutations in the vast majority of benign nevi indicates that B-RAFV600E is not sufficient for malignant progression (Michaloglou et al., 2005; Pollock et al., 2003).
PLX4032/RG7204 (now known as Vemurafenib) was identified as a potent and selective chemical inhibitor of mutant B-RAF signaling (Bollag et al., 2010). Recent phase 1–3 trials with PLX4032 demonstrated that the majority of melanoma patients, selected for mutant B-RAF positivity, show tumor regression (Chapman et al., 2011; Flaherty et al., 2010; Ribas et al., 2011). The phase 3 trial in previously untreated patients additionally showed improved overall survival and progression free survival (Chapman et al., 2011). However, the tumor regression of most patients was only temporary and the length of tumor-free survival while on PLX4032 averaged around seven months (Flaherty et al., 2010; Smalley and Sondak, 2010). Additionally, 19–52 % of patients in the phase 1–3 trials did not show tumor regression by RECIST criteria (Chapman et al., 2011; Flaherty et al., 2010; Ribas et al., 2011). These results indicate that in order to improve the current clinical treatment of mutant B-RAF melanomas, intrinsic and acquired resistance should be addressed.
Previous data has associated the presence of stem cell-like subpopulations with chemotherapeutic resistance of many cancers (Dean et al., 2005; Li et al., 2008). FOXD3 (formerly Genesis/HFH2) is a member of the Forkhead box (FOX) transcription factor family (Sutton et al., 1996). FOXD3 is important for maintaining the pluripotency and self-renewal of embryonic stem cells (Hanna et al., 2002; Liu and Labosky, 2008), perhaps in part through regulation of Nanog and Oct4 (Pan et al., 2006). In the developing neural crest, Foxd3 is required for the maintenance of cells and regulates lineage specification (Mundell and Labosky, 2011; Teng et al., 2008; Thomas and Erickson, 2008). Previous work from our laboratory has shown that FOXD3 expression is up-regulated following inhibition of mutant B-RAF-MEK signaling in mutant B-RAF melanoma cells (Abel and Aplin, 2010). Here, we show that the up-regulation of FOXD3 after treatment with PLX4032 or its non-clinical grade analog, PLX4720, provides resistance to cell death in mutant B-RAF melanoma cells.
Results
FOXD3 is basally expressed in a subset of mutant B-RAF melanomas
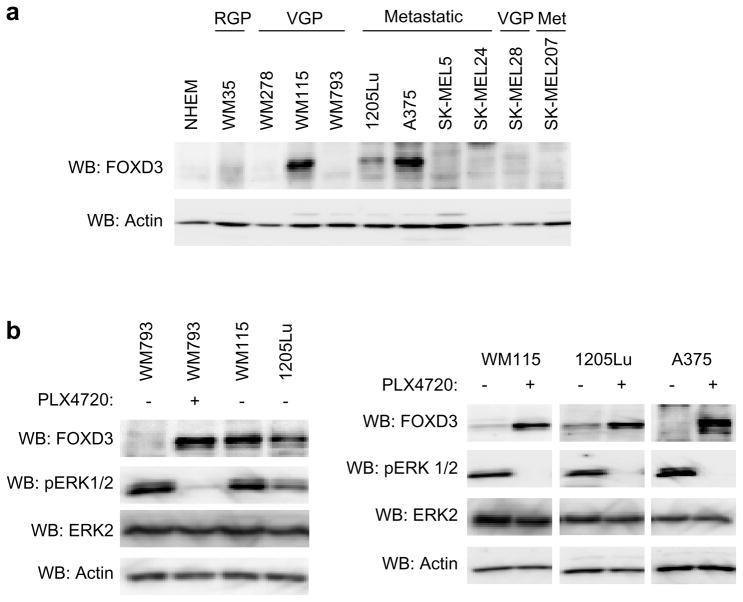
We have previously shown that FOXD3 is dramatically up-regulated following inhibition of the B-RAF-MEK signaling pathway in mutant B-RAF melanoma cells (Abel and Aplin, 2010). FOXD3 up-regulation occurred at the mRNA level following B-RAF depletion/inhibition in multiple cell lines (Abel and Aplin, 2010 and data not shown). While basal FOXD3 expression was poorly detectable in the majority of melanoma cell lines, FOXD3 was detected in a subset, specifically: WM115, 1205Lu and A375 cells (Figure 1a). Basal FOXD3 expression in these cells was comparable to the PLX4720-induced level in low expressing lines such as WM793 cells (Figure 1b, left panel). We analyzed FOXD3 induction in WM115, 1205Lu and A375 cells following inhibition of mutant B-RAF signaling using PLX4720, the non-clinical sister compound of PLX4032. PLX4720 acts in a manner that is indistinguishable from PLX4032 (Bollag et al., 2010; Joseph et al., 2010; Tsai et al., 2008). In WM115, 1205Lu and A375 cells, PLX4720 treatment induced additional expression of FOXD3 (Figure 1b, right panel). In summary, a subset of mutant B-RAF melanoma cells basally express FOXD3, which is further induced following B-RAF inhibition.
Figure 1.
Basal and PLX4720-induced FOXD3 expression in melanoma cell lines. (a) Total cell lysates from a panel of mutant B-RAF melanoma cell lines were analyzed by Western blotting using antibodies to FOXD3 and actin (loading control). (b) WM793 cells (low basal FOXD3) were treated with DMSO (−) or 5 μM PLX4720 for 24 hrs. WM793 cell lysates were analyzed alongside WM115 and 1205Lu cell lysates from basal conditions for FOXD3, phosphoERK1/2, ERK2, and actin. WM115, 1205Lu and A375 cells were treated with DMSO (−) or 5 μM PLX4720 for 24 hrs. Cell lysates were similarly analyzed by Western blotting.
FOXD3 provides resistance to the RAF inhibitors, PLX4720 and PLX4032
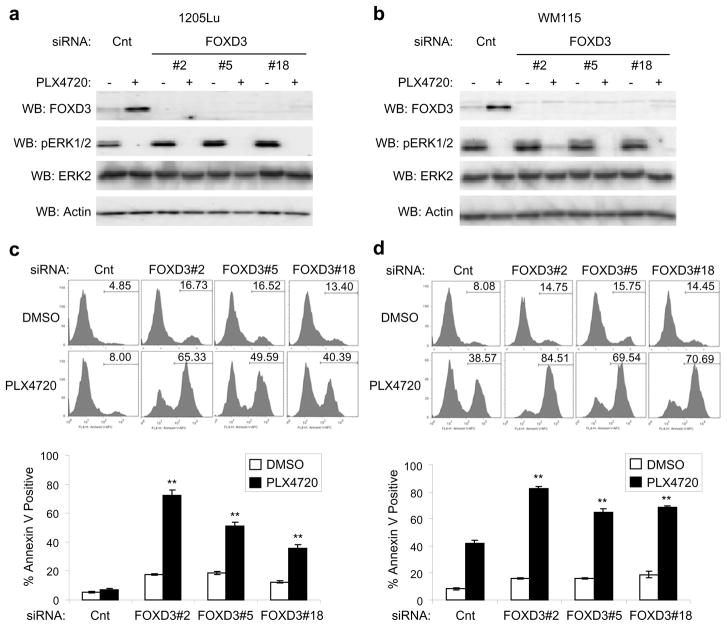
Since FOXD3 is a marker for embryonic stem cells (Sutton et al., 1996) and due to links between cancer stem cells and drug resistance (Dean et al., 2005; Li et al., 2008), we postulated that FOXD3 expression may protect melanoma cells from chemotherapeutics. We focused on RAF inhibitors, as the clinical benefit elicited by PLX4032 is being hampered by acquired resistance (Bollag et al., 2010; Flaherty et al., 2010; Smalley and Sondak, 2010) and FOXD3 is up-regulated in response to B-RAF inhibition. To investigate the role of FOXD3 in PLX4720 resistance, we efficiently knocked down FOXD3 in mutant B-RAF melanoma cell lines, 1205Lu and WM115 (Figures 2a and b). PhosphoERK1/2 inhibition by PLX4720 was equivalent between control and FOXD3 knockdowns suggesting no dramatic alterations in drug efflux following FOXD3 knockdown. Knockdown of basal FOXD3 expression only slightly enhanced cell death in 1205Lu and WM115 cells; however, knockdown of induced FOXD3 rendered both cell lines dramatically susceptible to PLX4720 treatment based on increased Annexin V staining (Figures 2c and d). This effect was observed with multiple independent siRNAs to FOXD3, arguing against off-target effects. Increased cleaved caspase 3 was also observed in FOXD3-deficient cells treated with PLX4720 (Supplementary Figure 1). Similar to PLX4720, FOXD3 knockdown also enhanced cell death after treatment with the clinical grade compound, PLX4032 (Supplementary Figures 2a and b). Surprisingly, FOXD3 knockdown only marginally enhanced the cytotoxic actions of a distinct RAF inhibitor, GDC0879 (Hoeflich et al., 2009) and the MEK inhibitor, U0126, despite increased expression of FOXD3. (Supplementary Figures 2c and d). No enhancement of annexin V staining was observed following FOXD3 knockdown in combination with the topoisomerase II inhibitor, etoposide. Thus, the effects of FOXD3 cannot be generalized to other chemotherapeutics. In summary, FOXD3 depletion renders melanoma cells sensitive to PLX4720/4032-induced cell death.
Figure 2.
Knockdown of FOXD3 increases cell death after acute treatment with PLX4720. (a) 1205Lu cells were transfected with non-targeting control siRNA or three distinct FOXD3 siRNA sequences (#2, #5, and #18). After 72 hrs, fresh media containing either DMSO or 5 μM PLX4720 was added for an additional 24 hrs. Cell lysates were analyzed by Western blotting, as indicated. (b) Same as (a) except with WM115 cells. (c) Same as (a), except that cells were harvested and stained with Annexin V-APC for cell death analysis after 48 hrs of PLX4720 treatment. Representative traces are shown. X axis, fluorescence intensity; Y axis, cell counts, with percent of Annexin V-APC staining-positive cells indicated. Quantitation of data from three independent experiments is represented by the mean percentage of cells staining positive for Annexin V-APC. Error bars represent standard error. **p value<0.01 comparing PLX4720-treated, non-targeting control knockdown cells to PLX4720-treated FOXD3 knockdown cells based on unpaired Student t-test. (d) Same as (c), except that WM115 cells were analyzed.
FOXD3 expression is sufficient to promote resistance to PLX4720-induced cell death
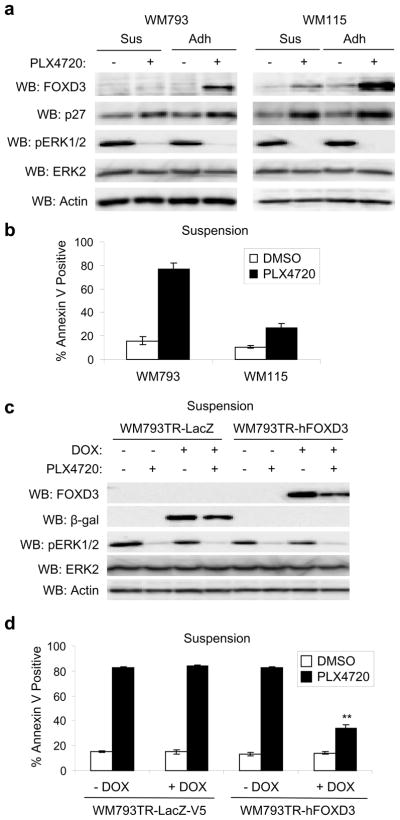
Low FOXD3 expressing lines, such as WM793 and WM278 cells, are partially sensitive to cell death in adherent cultures but dramatically susceptible to PLX4720 in suspension and 3-D collagen (Shao and Aplin, 2010). We observed that non-adherent WM793 cells treated with PLX4720 failed to efficiently induce FOXD3 expression, in the same conditions that a distinct B-RAF effector, such as p27Kip1, still showed equivalent increases (Figure 3a, left panels). WM115 cells, which are more resistant to PLX4720-induced cell death compared to WM793 cells (Figure 3b), displayed adhesion regulation of FOXD3 expression but importantly retained detectable expression of FOXD3 in PLX4720-treated non-adherent cultures (Figure 3a, right panels).
Figure 3.
FOXD3 expression protects cells from anoikis after PLX4720 treatment. (a) WM793 and WM115 cells were maintained on plates coated with 2% Bactoagar (Sus) or allowed to adhere to uncoated plates (Adh) −/+ 5 μM PLX4720. After 24 hrs, cells were lysed in sample buffer and analyzed by Western blotting, as indicated. (b) Same as (a) except that cells were harvested and stained with Annexin V-APC for cell death analysis after 48 hrs of PLX4720 treatment. Quantitation of data from three independent experiments is represented by the mean percentage of cells staining positive for Annexin V-APC. Error bars represent standard error. (c) WM793TR cells harboring doxycycline-inducible β-galactosidase (β-gal; LacZ) or FOXD3 were induced with 100 ng/mL doxycycline for 72 hrs. Cells were then detached and maintained on 2% Bactoagar −/+ 5 μM PLX4720 for a further 24 hrs. Cells were lysed and lysates analyzed by Western blotting. (d) Same as (c), except that cells were harvested and stained with Annexin V-APC after 48 hrs. Quantitation of data from three independent experiments is represented by the mean percentage of cells staining positive for Annexin V-APC. Error bars represent standard error. **p value<0.01 comparing PLX4720-treated cells in the absence and presence of doxycycline based on unpaired Student t-test.
Next, we tested whether FOXD3 expression was sufficient to promote resistance to PLX4720. In these experiments, we utilized WM793TR-FOXD3 cells, in which FOXD3 expression could be induced in PLX4720-treated non-adherent cells (Figure 3c). As in parental cells, PLX4720 treatment enhanced annexin V staining in non-induced WM793TR-FOXD3 cells and in both non-induced and induced WM793TR-LacZ control cells. Importantly, expression of FOXD3 significantly protected against PLX4720-induced cell death in non-adherent conditions (Figure 3d). Expression of FOXD3 did not alter the ability of PLX4720 to inhibit phosphoERK1/2, again arguing against alterations of drug efflux (Figure 3c). These data suggest that FOXD3 expression provides protection against acute cell death caused by PLX4720 treatment.
PLX4720 causes increased mitochondrial membrane depolarization in FOXD3-deficient cells
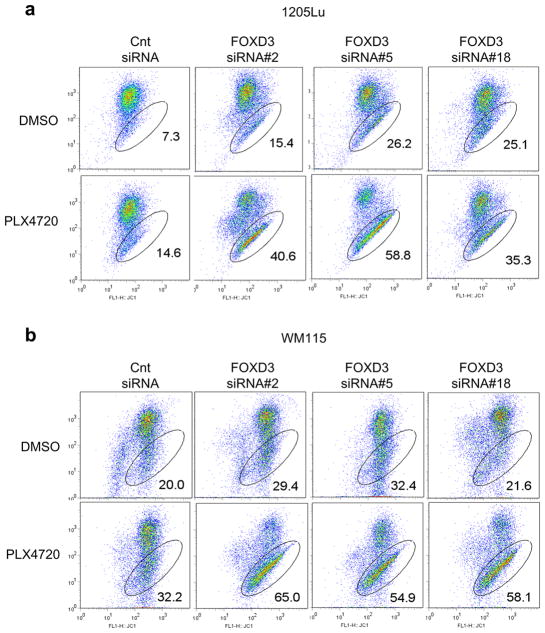
Previous data has shown that melanoma cells undergo cell death after inhibition of ERK1/2 signaling that is dependent on mitochondrial membrane depolarization (Wang et al., 2007). Therefore, we determined whether FOXD3-deficient cells showed changes in mitochondrial membrane stability after PLX4720 treatment. Using multiple, independent siRNA sequences, knockdown of FOXD3 caused a dramatic increase in mitochondrial membrane depolarization after PLX4720 treatment in both 1205Lu and WM115 cells (Figures 4a and b). Additionally, ectopic expression of FOXD3 decreased mitochondrial membrane depolarization after PLX4720 treatment (Supplementary Figures 3a and b). This demonstrates that cell death of FOXD3-deficient cells in response to PLX4720 treatment is accompanied by a decrease in mitochondrial membrane integrity.
Figure 4.
Knockdown of FOXD3 increases mitochondrial membrane depolarization after PLX4720 treatment. (a) 1205Lu cells were transfected for 72 hrs with the indicated siRNAs and then treated with DMSO or PLX4720 (5 μM) for an additional 24 hrs. Cells were then harvested and stained with JC-1 for analysis of mitochondrial membrane depolarization. Scatter plots are representative of three independent experiments. X axis, JC-1; Y axis, R-Phycoerythrin, with percent of cells showing mitochondrial membrane depolarization indicated. (b) Same as (a), except WM115 cells were analyzed.
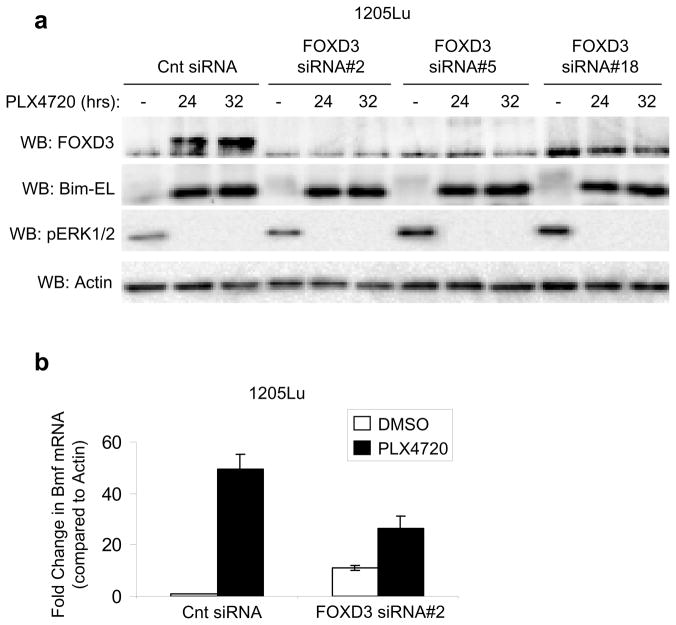
FOXD3 does not alter the expression of Bim-EL or Bmf after PLX4720 treatment
Cell death after ERK1/2 inhibition is dependent on changes in the expression of Bcl-2 family member proteins such as increased Bim-EL and Bmf expression and decreased Mcl-1 expression (Shao and Aplin, 2010; Wang et al., 2007). Increased mitochondrial membrane depolarization in FOXD3-deficient cells did not correlate with increased PLX4720-induced changes in the expression of Bim-EL and Bmf (Figures 5a and b), consistent with the notion that FOXD3 depletion doesn’t potentiate inhibition of MEK-ERK1/2 signaling. Further analysis did not identify any consistent difference in the expression of other Bcl-2 family members (Mcl-1, Bcl-2, Bcl-XL, survivin, puma, noxa, Bid, Bnip3 or Bip) between FOXD3-expressing cells and FOXD3-deficient cells after PLX4720 treatment (data not shown). Additionally, ectopic expression of Mcl-1 was unable to fully rescue the increased cell death of FOXD3-deficient cells treated with PLX4720 (Supplementary Figures 4a and b). In summary, FOXD3 provides resistance to mitochondrial membrane depolarization independent of changes in Bim-EL, Bmf or Mcl-1 expression.
Figure 5.
FOXD3 does not alter expression of Bim-EL and Bmf. (a) 1205Lu cells were transfected with the indicated siRNAs. After 72 hrs, fresh media containing either DMSO or 5 μM PLX4720 was added for indicated time points. Cell lysates were analyzed by Western blotting. (b) 1205Lu cells were transfected with control or FOXD3 siRNA#2 for 72 hrs and then treated with DMSO or PLX4720 (5 μM) for an additional 24 hrs. Cells were harvested for total RNA isolation and qRT-PCR analysis for Bmf and actin (control). Quantitation of data from three independent experiments is represented as the mean relative mRNA level of Bmf in each condition.
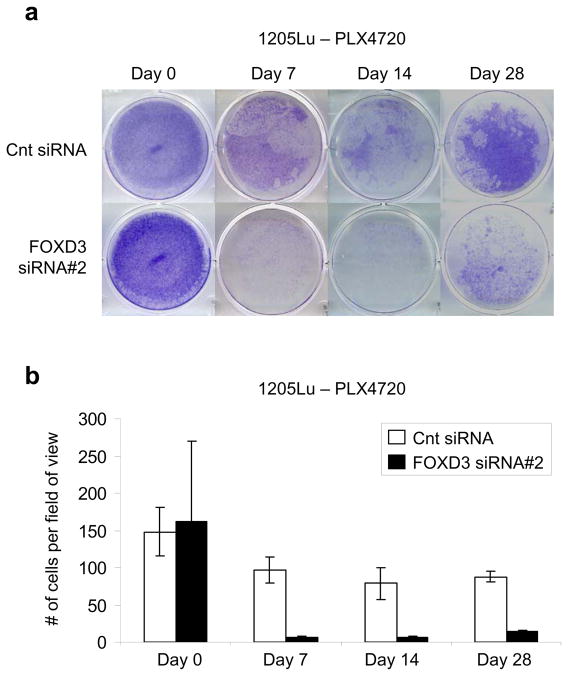
FOXD3-deficient cells have a decreased ability to develop long-term resistance to PLX4720
Our data show that FOXD3-deficient cells display high levels of cell death after short-term exposure (48 hr) to PLX4720 (Figures 2c and d). Since most patients in the PLX4032 clinical trials develop resistance after long-term exposure, we determined the effect of FOXD3 knockdown in the presence of chronic exposure to PLX4720. Control siRNA transfected cells showed initial survival against PLX4720 that was maintained through 28 days of treatment. However, cells that were initially depleted of FOXD3 had a decreased ability to establish PLX4720-resistant colonies (Figures 6a and b). This demonstrates that preventing FOXD3 up-regulation decreases long-term resistance to PLX4720.
Figure 6.
Depletion of FOXD3 impairs long-term resistance to PLX4720. (a) 1205Lu cells were transfected with the indicated siRNAs. After 72 hrs, a representative plate of each siRNA was stained with crystal violet (day 0). The remaining plates were treated with 5 μM PLX4720. Media was changed every 48 hrs and fresh PLX4720 was added. Remaining plates were stained with crystal violet after 7, 14 and 28 days. (b) Quantitation of data from two independent experiments is represented by the mean percentage of cells per field of view. Error bars represent standard deviation.
Discussion
It is hypothesized that subpopulations of tumor cells, termed cancer stem cells, may have inherent chemotherapeutic resistance (Dean et al., 2005; Li et al., 2008). Our data indicate that the stemness factor, FOXD3, promotes melanoma cell resistance to a clinically relevant RAF inhibitor. FOXD3 is up-regulated following inhibition of the B-RAF/MEK/ERK1/2 pathway selectively in mutant B-RAF melanoma cell types (Abel and Aplin, 2010). Thus, FOXD3 up-regulation may be an adaptive response to B-RAF inhibition. Melanoma cells are well known for their plasticity (Hendrix et al., 2007). Recently, Sharma et al. have suggested that tumor cells have the potential to convert to a transient, drug-tolerant state that allows subpopulations of cells to maintain viability after a potentially lethal stimulus (Sharma et al., 2010). Notably the transient nature of this tolerant state is predicted to result in additional tumor cell death following further rounds of treatment with intervening “drug holidays”. In their studies, drug tolerance was mediated by enhanced signaling via IGF-1R and by enhanced expression of the histone demethylase, JARID1a (Sharma et al., 2010). FOXD3 may play a role in opposing the formation of active chromatin structures in pluripotent cells (Liber et al., 2010). Furthermore, FOXD3 up-regulation was reversible following removal of PLX4720 (data not shown), similar to the drug tolerant state in the Sharma et al. study. Together these studies indicate that an adaptive chromatin regulation response to targeted therapies that may contribute ultimately to the acquisition of a resistant state.
The acquisition of a drug tolerant state is thought to provide a time window for secondary genetic events that provide permanent resistance. Recent studies have uncovered some of the mechanisms associated with acquired resistance to PLX4032 (Aplin et al., 2011). In one study, secondary mutations in N-RAS were detected in two relapsing metastases from the same patient (Nazarian et al., 2010). Supporting evidence in cultured cell systems demonstrated that expression of N-RAS in mutant B-RAF melanoma cells was sufficient to nullify the inhibitory effect of PLX4032 on the ERK1/2 pathway and cell growth (Nazarian et al., 2010). Studies from the Garraway group indicate that up-regulation of the MAP3K, Cot1, and acquired mutation in MEK1 provide other routes to promote re-activation of the ERK1/2 pathway in relapsing tumors (Johannessen et al., 2010; Wagle et al., 2011). ERK1/2-independent resistance mechanisms have also been proposed. PDGFRβ was up-regulated in relapsing tumors from four out of eleven PLX4032-treated patients and PDGFRβ knockdown induced cell cycle arrest and/or apoptosis in cell lines that have acquired resistance to PLX4032 in culture (Nazarian et al., 2010). PLX4032 resistant lines with up-regulated expression of PDGFRβ were resistant to MEK inhibitors. An additional study from the Herlyn group has implicated up-regulation of IGF-1R and activation of the Akt pathway in acquired resistance to PLX4032 (Villanueva et al., 2010). These data are consistent with our previous findings that constitutive Akt3 activity counteracts PLX4720-induced apoptosis in melanoma cells (Shao and Aplin, 2010). Together, these data indicate that FOXD3 may promote a transient resistant state that is superseded by permanent resistant mechanisms such as secondary mutations in N-RAS or MEK1, up-regulation of MAP3Ks and up-regulation of receptor tyrosine kinases.
Loss of ERK1/2 signaling in melanoma cells leads to up-regulation of pro-apoptotic BH3-only domain proteins including Bim-EL and Bmf (Shao and Aplin, 2010). However, Bim-EL and Bmf up-regulation in FOXD3-deficient cells following PLX4720 treatment is comparable to the up-regulation observed in control cells, consistent with FOXD3-mediated effects being independent of the ERK1/2 activation status and FOXD3 depletion not affecting drug efflux. Therefore, FOXD3 up-regulation after treatment with RAF inhibitors may permit the survival of melanoma cells despite strong pro-apoptotic signaling. FOXD3 knockdown rendered cells highly sensitive to PLX4720 and PLX4032 but surprisingly less sensitive to the RAF inhibitor GDC0879, the MEK inhibitor U1026, and B-RAF depletion (Supplementary figure 2 and data not shown). It is currently unclear if this is due to a more selective nature of PLX4720/4032 towards mutant B-RAF or whether PLX4720/4032 are targeting additional kinases. With regard to the latter, PLX4720 and PLX4032 inhibit with several kinases, for example protein tyrosine kinase 6 (PTK6, BRK), at nanomolar IC50 values in vitro (Bollag G et al. 2010 and Tsai et al. 2008). Thus, it is possible that additional PLX4720/4032 target inhibition may cooperate with ERK1/2-dependent increases in BH3-only proteins to promote pro-apoptotic effects.
Regardless of the mechanism of FOXD3 action, our studies indicate that quantifying FOXD3 basal expression and PLX4032-induced up-regulation of FOXD3 in patients may be a correlate for disease-free survival benefit with this drug. We also show that up-regulation of FOXD3 following treatment with PLX4720 is attenuated following loss of adhesion to the extracellular matrix. Adhesion-dependent FOXD3 up-regulation correlates with enhanced cell death susceptibility following B-RAF inhibition. It is therefore possible that blockade of signals from the extracellular matrix through treatment with integrin inhibitors may offer a benefit as a combination therapy with PLX4032.
Materials and Methods
Cell culture
Human melanoma cell lines, WM793, WM115, and 1205Lu, were kindly donated by Dr. Meenhard Herlyn (Wistar Institute, Philadelphia, PA). A375 cells were purchased from the American Type Culture Collection. WM793TR-FOXD3 cells have been reported previously (Abel and Aplin, 2010) and FOXD3 expression was induced by the addition of 100 ng/ml doxycycline to the medium. All cells were cultured, as previously described (Abel and Aplin, 2010). The B-RAF mutational status of all cell lines has been verified by DNA sequencing. For cell suspension assays, cells were replated onto dishes coated with bactoagar (2%). Cells were then processed for Western blot analysis or cell death assays after the indicated time.
Western blotting
Western blotting was done as previously described (Boisvert-Adamo and Aplin, 2006). The following antibodies were utilized: anti-phosphoERK1/2 (Thr202/Tyr204, #4377; Cell Signaling Technology, Beverley, MA); anti-actin (#A2066, Sigma-Aldrich, St. Louis, MO); anti-FOXD3 (Poly6317, BioLegend, San Diego, CA); anti-ERK2 (DV-154, Santa-Cruz Biotech, Santa Cruz, CA); anti-β-galactosidase (Z378A, Promega, Madison, WI); anti-p27Kip1 (#610241, BD Biosciences, San Jose, CA); and anti-Bim-EL (ADI-AAP-330, Enzo Life Sciences, Plymouth Meeting, PA). Signal was detected using peroxidase-conjugated secondary antibody followed by development using chemiluminescence substrate (Pierce, Rockford, IL) and a Versadoc Imaging system equipped with Quantity-One software (Bio-Rad, Hercules, CA).
siRNA transfections
Cells were transfected with siRNAs at a final concentration of 25 nmol/L using Lipofectamine RNAiMAX (Invitrogen, Carlsbad, CA). Non-targeting control (UGGUUUACAUGUCGACUAA), FOXD3 #2 (ACGACGGGCUGGAAGAGAA), FOXD3 #5 (AGACGGCGCUCAUGAUGCA), and FOXD3 #18 (GCAAUAGGGACGCGCCAAU) siRNAs were purchased from Dharmacon (Lafayette, CO).
Cell death assays
Analysis of Annexin V staining (BD Biosciences, San Jose, CA) was performed as previously described (Shao and Aplin, 2010). Staining was measured by flow cytometry on the FACS Calibur (BD Biosciences), and data were analyzed using Flowjo software (Three Star, Inc., Ashland, OR).
Mitochondrial membrane depolarization assays
Adherent cells and non-adherent cells were collected and washed with PBS. Cells were then resuspended in PBS at a concentration of 1 × 106 cells/mL and stained with 2 μM JC-1 at 37°C, 5% CO2 for 15 minutes. Cells were then washed once with PBS and analyzed by flow cytometry on the FACS Calibur (BD Biosciences). Data were analyzed using Flowjo software (Three Star, Inc.).
Quantitative reverse transcription-PCR
Quantitative reverse transcription-PCR (qRT-PCR) was performed as previously described (Shao and Aplin, 2010). The following primers were used: Bmf - forward, 5′-gaggtacagattgccgaaag-3′; Bmf - reverse, 5′-ttcaaagcaaggttgtgca-3′; Actin – forward, 5′-tacctcatgaagatcctcacc-3′; Actin – reverse, 5′-tttcgtggatgccacaggac-3′. Relative mRNA levels were calculated using the comparative Ct (ΔCt) method. Quantitation of mRNA levels relative to actin represents data from three independent experiments.
Long-term survival assay
Cells were transfected with the indicated siRNAs. After 72 hours, a representative plate was washed with PBS and stained for 25 minutes with crystal violet dissolved in buffered formalin solution (day 0). Stain was then washed gently with water and allowed to dry. Other plates were continuously treated with PLX4720 (5 μM) and stained after indicated time points. Images were taken of each plate, and cells were counted in five representative fields of view (20x).
Supplementary Material
Acknowledgments
We thank Dr. Gideon Bollag (Plexxikon, Berkeley, CA) for providing PLX4720, Dr. Meenhard Herlyn (Wistar Institute, Philadelphia, PA) for WM melanoma cell lines, and Dr. Fred Kaplan for critical comments on this manuscript. This work was supported by National Institutes of Health (GM067893, CA125103), American Cancer Society (RSG-08-03-01-CSM), and a Joanna M. Nicolay Melanoma Foundation scholarship. The Kimmel Cancer Center is supported by National Cancer Institute Support Grant 1P30CA56036.
Footnotes
Disclosure of Potential Conflicts of Interest
The authors declare no conflict of interest.
References
- Abel EV, Aplin AE. FOXD3 is a mutant B-RAF-regulated inhibitor of G1/S progression in melanoma cells. Cancer Res. 2010;70:2891–2900. doi: 10.1158/0008-5472.CAN-09-3139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aplin AE, Kaplan FM, Shao Y. Mechanisms of Resistance to RAF Inhibitors in Melanoma. J Invest Dermatol. 2011 doi: 10.1038/jid.2011.147. Epub May 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boisvert-Adamo K, Aplin AE. B-RAF and PI-3 kinase signaling protect melanoma cells from anoikis. Oncogene. 2006;25:4848–4856. doi: 10.1038/sj.onc.1209493. [DOI] [PubMed] [Google Scholar]
- Bollag G, Hirth P, Tsai J, Zhang J, Ibrahim PN, Cho H, et al. Clinical efficacy of a RAF inhibitor needs broad target blockade in BRAF-mutant melanoma. Nature. 2010;467:596–599. doi: 10.1038/nature09454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman PB, Hauschild A, Robert C, Haanen JB, Ascierto P, Larkin J, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364:2507–2516. doi: 10.1056/NEJMoa1103782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–954. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- Dean M, Fojo T, Bates S. Tumour stem cells and drug resistance. Nature Rev Cancer. 2005;5:275–284. doi: 10.1038/nrc1590. [DOI] [PubMed] [Google Scholar]
- Dhomen N, Marais R. New insight into BRAF mutations in cancer. Curr Opin Genet Dev. 2007;17:31–39. doi: 10.1016/j.gde.2006.12.005. [DOI] [PubMed] [Google Scholar]
- Flaherty KT, Puzanov I, Kim KB, Ribas A, McArthur GA, Sosman JA, et al. Inhibition of mutated, activated BRAF in metastatic melanoma. N Engl J Med. 2010;363:809–819. doi: 10.1056/NEJMoa1002011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanna LA, Foreman RK, Tarasenko IA, Kessler DS, Labosky PA. Requirement for Foxd3 in maintaining pluripotent cells of the early mouse embryo. Genes Dev. 2002;16:2650–2661. doi: 10.1101/gad.1020502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrix MJC, Seftor EA, Seftor REB, Kasemeier-Kulesa J, Kulesa PM, Postovit L-M. Reprogramming metastatic tumour cells with embryonic microenvironments. Nat Rev Cancer. 2007;7:246–255. doi: 10.1038/nrc2108. [DOI] [PubMed] [Google Scholar]
- Hoeflich KP, Herter S, Tien J, Wong L, Berry L, Chan J, et al. Antitumor efficacy of the novel RAF inhibitor GDC-0879 is predicted by BRAFV600E mutational status and sustained extracellular signal-regulated kinase/mitogen-activated protein kinase pathway suppression. Cancer Res. 2009;69:3042–3051. doi: 10.1158/0008-5472.CAN-08-3563. [DOI] [PubMed] [Google Scholar]
- Johannessen CM, Boehm JS, Kim SY, Thomas SR, Wardwell L, Johnson LA, et al. COT drives resistance to RAF inhibition through MAP kinase pathway reactivation. Nature. 2010;468:968–972. doi: 10.1038/nature09627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph EW, Pratilas CA, Poulikakos PI, Tadi M, Wang W, Taylor BS, et al. The RAF inhibitor PLX4032 inhibits ERK signaling and tumor cell proliferation in a V600E BRAF-selective manner. Proc Natl Acad Sci USA. 2010;107:14903–14908. doi: 10.1073/pnas.1008990107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Lewis MT, Huang J, Gutierrez C, Osborne CK, Wu M-F, et al. Intrinsic resistance of tumorigenic breast cancer cells to chemotherapy. J Natl Cancer Inst. 2008;100:672–679. doi: 10.1093/jnci/djn123. [DOI] [PubMed] [Google Scholar]
- Liber D, Domaschenz R, Holmqvist P-H, Mazzarella L, Georgiou A, Leleu M, et al. Epigenetic priming of a pre-B cell-specific enhancer through binding of Sox2 and Foxd3 at the ESC stage. Cell Stem Cell. 2010;7:114–126. doi: 10.1016/j.stem.2010.05.020. [DOI] [PubMed] [Google Scholar]
- Liu Y, Labosky PA. Regulation of embryonic stem cell self-renewal and pluripotency by Foxd3. Stem Cells. 2008;26:2475–2484. doi: 10.1634/stemcells.2008-0269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaloglou C, Vredeveld LC, Soengas MS, Denoyelle C, Kuilman T, van der Horst CM, et al. BRAFE600-associated senescence-like cell cycle arrest of human naevi. Nature. 2005;436:720–724. doi: 10.1038/nature03890. [DOI] [PubMed] [Google Scholar]
- Mundell NA, Labosky PA. Neural crest stem cell multipotency requires Foxd3 to maintain neural potential and repress mesenchymal fates. Development. 2011;138:641–652. doi: 10.1242/dev.054718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazarian R, Shi H, Wang Q, Kong X, Koya RC, Lee H, et al. Melanomas acquire resistance to B-RAF(V600E) inhibition by RTK or N-RAS upregulation. Nature. 2010;468:973–977. doi: 10.1038/nature09626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan G, Li J, Zhou Y, Zheng H, Pei D. A negative feedback loop of transcription factors that controls stem cell pluripotency and self-renewal. Faseb J. 2006;20:1730–1732. doi: 10.1096/fj.05-5543fje. [DOI] [PubMed] [Google Scholar]
- Pollock PM, Harper UL, Hansen KS, Yudt LM, Stark M, Robbins CM, et al. High frequency of BRAF mutations in nevi. Nat Genet. 2003;33:19–20. doi: 10.1038/ng1054. [DOI] [PubMed] [Google Scholar]
- Ribas A, Kim K, LM S, Gonzalez R, Pavlick A, Weber J, et al. BRIM-2: an open-label, multicenter phase II study of vemurafenib in previously treated patients with BRAFV600E mutation-positive melanoma. J Clin Oncol. 2011;29(Suppl):8509. [Google Scholar]
- Shao Y, Aplin A. Akt3-mediated resistance to apoptosis in B-RAF targeted melanoma cells. Cancer Res. 2010;70:6670–6681. doi: 10.1158/0008-5472.CAN-09-4471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma S, Lee D, Li B, Quinlan M, Takahashi F, Maheswaran S, et al. A chromatin-mediated reversible drug-tolerant state in cancer cell subpopulations. Cell. 2010;141:69–80. doi: 10.1016/j.cell.2010.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smalley KSM, Sondak VK. Melanoma - an unlikely poster child for personalized cancer therapy. N Engl J Med. 2010;363:876–878. doi: 10.1056/NEJMe1005370. [DOI] [PubMed] [Google Scholar]
- Sutton J, Costa R, Klug M, Field L, Xu D, Largaespada DA, et al. Genesis, a winged helix transcriptional repressor with expression restricted to embryonic stem cells. J Biol Chem. 1996;271:23126–23133. doi: 10.1074/jbc.271.38.23126. [DOI] [PubMed] [Google Scholar]
- Teng L, Mundell NA, Frist AY, Wang Q, Labosky PA. Requirement for Foxd3 in the maintenance of neural crest progenitors. Development. 2008;135:1615–1624. doi: 10.1242/dev.012179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas AJ, Erickson CA. The making of a melanocyte: the specification of melanoblasts from the neural crest. Pigment Cell Melanoma Res. 2008;21:598–610. doi: 10.1111/j.1755-148X.2008.00506.x. [DOI] [PubMed] [Google Scholar]
- Tsai J, Lee JT, Wang W, Zhang J, Cho H, Mamo S, et al. Discovery of a selective inhibitor of oncogenic B-Raf kinase with potent antimelanoma activity. Proc Natl Acad Sci USA. 2008;105:3041–3046. doi: 10.1073/pnas.0711741105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villanueva J, Vultur A, Lee JT, Somasundaram R, Fukunaga-Kalabis M, Cipolla AK, et al. Acquired resistance to BRAF inhibitors mediated by a RAF kinase switch in melanoma can be overcome by cotargeting MEK and IGF-1R/PI3K. Cancer Cell. 2010;18:683–695. doi: 10.1016/j.ccr.2010.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagle N, Emery C, Berger MF, Davis MJ, Sawyer A, Pochanard P, et al. Dissecting Therapeutic Resistance to RAF Inhibition in Melanoma by Tumor Genomic Profiling. J Clin Oncol. 2011;29:3085–3096. doi: 10.1200/JCO.2010.33.2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YF, Jiang CC, Kiejda KA, Gillespie S, Zhang XD, Hersey P. Apoptosis induction in human melanoma cells by inhibition of MEK is caspase-independent and mediated by the Bcl-2 family members PUMA, Bim, and Mcl-1. Clin Cancer Res. 2007;13:4934–4942. doi: 10.1158/1078-0432.CCR-07-0665. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.