Abstract
As the threshold nucleated cell dose for single unit umbilical cord blood (UCB) in adults has not to date been firmly established, we prospectively compared single vs. 2-unit UCB transplantation after reduced intensity conditioning (RIC) in adult patients with hematologic malignancies. Study design specified one UCB unit if the cryopreserved total nucleated cell (TNC) dose was ≥2.5×107/kg recipient weight, otherwise 2-units matched at minimum 4/6 HLA loci to the patient and 3/6 to each other were infused. Twenty-seven patients received 1 unit; 23 patients received 2 units. Median time to absolute neutrophil count (ANC) >500/μL was 24 days (95% CI 22–28 days), 25 days for 1-unit and 23 days for 2-units (p=0.99). At day 100, ANC >500/μL was 88.4% and 91.3% in the 1 and 2-unit groups (p=0.99), respectively. Three-year event free survival (EFS) was 28.6% and 39.1% in the 1 and 2-unit groups (p=0.71), respectively. Infusion of 2 units was associated with significantly lower relapse risk, 30.4% vs. 59.3% (p=0.045). Infused cell doses (TNC, CD3+, CD34+, CD56+CD3neg) did not impact engraftment, overall survival (OS), or EFS. Taken together, single unit UCB transplantation with threshold cell dose ≥2.5×107/kg recipient weight after RIC is a viable option for adults, although infusion of 2 units confers a lower relapse incidence.
Keywords: hematopoietic cell transplantation, acute myeloid leukemia, umbilical cord blood
Introduction
Experience with unrelated allogeneic umbilical cord blood (UCB) transplantation in the non-myeloablative setting has been limited previously due to concerns of recipient rejection of a single unit graft with low nucleated cell content for most adult patients. Two-unit UCB graft infusion has been explored in an attempt to overcome these graft cell dose limitations for adult patients in both the myeloablative, and non-myeloablative setting.
Potential UCB immunologic and stem cell homing mechanisms underlying engraftment of the dominant unit in patients treated with two-units are not fully understood. The minimum threshold for cryopreserved total nucleated cell (TNC) doses to allow engraftment of single UCB grafts in adult hematology patients has not been clearly determined in the reduced intensity conditioning (RIC) setting; and is potentially influenced by HLA and Killer Inhibitory Receptor disparity, ABO mismatch, among other factors. Current recommendations for UCB TNC threshold cell doses for adult patients in the myeloablative setting is ≥2.5×107/kg recipient weight, rendering favorable outcomes similar to that observed in patients receiving standard bone marrow and mobilized peripheral blood progenitor cell grafts from adult donors.
Several retrospective series have observed low relapse rates in hematologic malignancy patients with two-unit compared to single UCB graft infusion. Interpretation of these data is challenging however due to variations in the preparatory regimens used, patient selection differences including marked age variation, and modifications in supportive care including graft vs host disease (GvHD) prophylaxis and anti-thymocyte globulin (ATG) administration during the course of these studies; factors that may contribute to the benefits otherwise attributed specifically to the infusion of one vs. two UCB grafts.
The purpose of this study was to test the feasibility, safety, and efficacy of infusing two UCB units into adult patients treated uniformly with the same RIC, GvHD prophylaxis, and supportive care regimen; and determining whether rates and kinetics of engraftment, acute GvHD incidence, relapse rates, and survival may differ from the infusion of one UCB unit. We also examined the influence, if any, of UCB graft infused TNC, CD34+ hematopoietic progenitors, natural killer cells, and T-cell doses on procedure outcomes including: overall survival (OS), allogeneic engraftment, and event free survival (EFS). We outline herein the analyses of this clinical trial.
Methods
Transplant Protocol and Patient Eligibility
The Institutional Review Board (IRB) of Case Western Reserve University/University Hospitals Case Medical Center approved the clinical protocol: registered at http://clinicaltrials.gov (NCT00054236). Written informed consent was obtained from all patients. Eligibility criteria included the following: 1) histologic confirmation of a hematologic malignancy with high risk features such as early relapse, high risk cytogenetic abnormalities, or failure of standard treatments; 2) patients unable to tolerate fully myeloablative conditioning either due to advanced age (>55 years), extensive prior treatment, or co-morbid diseases such as suboptimal visceral organ function, or recent life-threatening infection; 3) lack of available 5/6 or fully HLA-matched related donor; 4) Karnofsky performance status ≥70%, and adequate organ function (creatinine clearance >40 ml/min; AST/ALT <4× upper limit of normal; total serum bilirubin <2 mg/dl; cardiac ejection fraction >40%; FVC, FEV1, and DLCO >60% of predicted for age on pulmonary function testing). Patients were not eligible if they had uncontrolled infection at time of enrollment, had active CNS disease, chronic myeloid leukemia in blast crisis without complete response to re-induction chemotherapy, acute leukemia in refractory relapse, extensive bone marrow fibrosis, or were seropositive for HIV.
Conditioning Regimen, Graft Selection, and Supportive Care
RIC consisted of fludarabine 35 mg/m2/day intravenous (iv) over 30 minutes daily for 5 days on days T−8 to T−4 prior to UCB infusion (day 0), cyclophosphamide 1 gm/m2/day iv over 2 hours on days T−3 and T−2 with mesna 1 gm/m2 each day, horse ATG (Pharmacia-Upjohn, Kalamazoo, MI) 30 mg/kg/day iv on days T−3 and T−2, and total body irradiation 200 cGy on day T−1. Preparative regimen dosing was based on actual weight unless the actual weight was ≥125% of the ideal weight. In these patients, the adjusted body weight [ideal weight + 25% (difference actual – ideal weight)] was used.
Trial design specified selection of UCB grafts to be matched at 4/6 HLA loci or better to the recipient and minimum 3/6 match to each other. HLA DNA typing specified the match at the antigen level for HLA-A and HLA-B loci, and allele level at DRB-1 loci. Blanks were interpreted as homozygous for that locus and disparities were scored accordingly. The algorithm for unit selection specified: 1) the best HLA matched unit, with 2) the highest cell dose. Selection criteria for one UCB unit required a cryopreserved nucleated cell dose ≥2.5×107/kg recipient weight. Selection criteria for two UCB units required a combined cell dose ≥3.0×107/kg (≥1.5×107/kg each unit). The total UCB nucleated cell dose (cryopreserved and infused) was calculated on the patient's actual body weight. All UCB grafts were obtained from FACT or AABB accredited banks. A backup graft source was identified for all patients enrolled on the study. For those patients who had received prior hematopoietic cell transplants, HLA typing was re-verified by short tandem repeat (STR) analyses to determine if the patient's blood genotype was their own HLA typing or the typing of donor of the antecedent transplant. The graft was then selected to be a 4/6 or better to the verified HLA typing.
Supportive care included administration of granulocyte colony-stimulating factor (Amgen, Thousand Oaks, CA) at a dose of 5 μg/kg/day subcutaneously starting day T+7 after UCB infusion until absolute neutrophil count (ANC) >2,500/μl was attained for 3 consecutive days. GvHD prophylaxis consisted of 1) cyclosporine A (Sandimmune, Novartis, East Hannover, NJ) starting at 3 mg/kg/day iv or orally in two divided doses day T−2 to T+60 then tapered in the absence of GvHD and discontinued by day T+100, and 2) mycophenolate mofetil (Cellcept, Hoffmann-La Roche Ltd, Basel, Switzerland) iv or orally 45 mg/kg/day (in 3 divided doses) day T+1 to T+30. All patients received antimicrobial, fungal, and viral prophylaxis as previously described.
Chimerism
Recipient chimerism studies were performed on peripheral blood mononuclear cell samples weekly × 4, monthly for the first 6 months, and at 9, 12, 14, 16, 18, and 24 months after transplant. Analyses were performed using either quantitative polymerase chain reaction (PCR) for STR regions for sex-matched grafts and two unit grafts or fluorescence in situ hybridization (FISH) for the Y chromosome in patients receiving a single sex mismatched graft.
UCB Graft Infusion and Flow Cytometric Analyses
UCB grafts were thawed and washed according to standard procedures and infused within 60 minutes of thawing. After thaw, an aliquot was obtained for the following tests: viable nucleated cell count (by trypan blue dye exclusion), enumeration of CD34+ and lymphocyte populations by flow cytometry, colony forming unit methylcellulose assays, and bacterial and fungal cultures. A 0.5 mL aliquot of each infused graft was analyzed by flow cytometry for hematopoietic progenitors (CD34+) and lymphocyte populations (CD3+, CD56+CD3neg, and CD8negCD4+CD25+). Approximately 25,000–30,000 total events were analyzed on a BD FACS Calibur (Franklin Lakes, NJ) for each UCB unit aliquot. UCB graft post-thaw aliquot CD34+ and lymphocyte FACS percentages were multiplied by enumerated infused viable nucleated cells and divided by actual patient weight to calculate the cell dose/kg for each cell population of interest infused in each consecutive study patient.
Statistical Analysis
Study Endpoints included: hematopoietic recovery, donor engraftment failure, severe grade III/IV acute GvHD, grade 3 or 4 infusion related toxicity, and day +100 overall survival. Interim analysis was performed following the accrual of every 6 patients to each cohort (1-unit vs 2-unit) to monitor the safety of the trial. Stopping rules included: 1) grade 3 or 4 infusion related toxicity >20%, 2) hematopoietic recovery failure rate >15%, 3) engraftment failure rate >10%, 4) grade III/IV acute GvHD rate >20%, and 5) day +100 OS <60% in either group. Statistical comparisons between the 1-unit and 2-unit groups for GvHD, EFS, and OS were a planned as part of the trial design.
Secondary study endpoints included OS and EFS, incidence and rate of allogeneic donor engraftment and graft failure, kinetics of neutrophil and platelet recovery, incidence of acute and chronic GvHD, treatment related mortality (TRM), and disease relapse. OS was measured from the date of transplantation (T=0) to the date of death and censored at the date of last follow-up for survivors. EFS was measured from date of transplantation to the date of death or relapse and censored at the date of last follow up for survivors without relapse. OS and EFS distribution, hematopoietic recovery (ANC >500/μL, platelets >20,000/μL), and engraftment (chimerism >60%) were estimated by Kaplan-Meier methods and the differences in survival and engraftment between patients who received 1 vs. 2-units were examined by log rank test. TRM was defined as death without relapse and the cause of death related to the transplantation. The association between number of UCB units and TRM was examined by chi-square test/Fisher exact test.
Patient and graft characteristics examined by descriptive analysis included: age, actual weight, gender, number of UCB units infused, underlying disease, acute GvHD, chronic GvHD, engraftment parameters (chimerism), hematopoietic recovery (ANC and platelet recovery), and UCB graft cell dose variables (TNC, CD3+, CD56+CD3neg, CD34+ total and CD8negCD4+CD25+). The differences in cell dose variables (TNC, CD3+, CD34+, CD56+CD3neg, and CD8negCD4+CD25+) between 1-unit and 2-unit UCB groups were examined by T-test/Kruskal-Wallis test. Neutrophil recovery was defined by an ANC >500 cells/μL for three consecutive days and platelet recovery was defined as the first day of seven consecutive days of >20,000 platelets/μL, unsupported by transfusions. The cumulative incidences of neutrophil and platelet recovery were calculated to account for competing risks. Primary engraftment failure was defined as failure to restore donor hematopoiesis by day 42. Secondary engraftment failure was defined as a permanent loss of engraftment previously documented by donor chimerism on two separate occasions.
Effects of graft cell subpopulations including CD34+ progenitors, CD3+ T-cells, CD56+CD3neg natural killer cells, and TNC populations on EFS, OS, and time to engraftment were evaluated by Cox proportional hazard model. For those patients receiving two UCB units the graft characteristics including cell populations of the predominating unit were used to examine effects, if any, on EFS, OS, and engraftment. The effects of the number of UCB units infused and infused cell dose variables (TNC, CD3+, CD34+, and CD56+CD3neg) on relapse were examined by multivariate logistic regression. Patients were evaluated daily for acute GvHD while hospitalized with data entry of highest stage each week and at subsequent weekly outpatient visits thereafter. Using binary distribution with confidence intervals by Wilson's method, acute GvHD and chronic GvHD incidences were estimated. The association between number of UCB units and acute GvHD was examined by univariate logistic regression.
Results
Patient and Graft Characteristics
All consecutive eligible study patients were included in the analyses. During the time period 9/2003 to 1/2010, 50 eligible patients were consecutively consented and enrolled on protocol, including 17 females (34%) and 33 males (66%) with a median age of 53.5 (range 21–71) years (Table 1). The median patient weight was 84.3 (range 52–122) kg. The weight difference between the two groups was statistically significant (p=0.02). Acute myeloid leukemia (AML), including therapy-related myeloid neoplasm (t-MN), secondary AML (2nd AML), and de novo AML, comprised the majority of study patients. Study patients were heavily pre-treated, including 26 patients who received 29 prior hematopoietic cell transplants; 17 patients with 18 prior autologous transplants, and 10 patients with 11 prior allogeneic transplants.
TABLE 1.
| Patient Characteristics | All | 1-Unit | 2-Unit | p value |
|---|---|---|---|---|
| Patients, no. (%) | 50 | 27 (54) | 23 (46) | |
| Age (y), median (range) | 53.5 (21–71) | 50 (25–71) | 54 (21–69) | 0.46* |
| Male, no. (%) | 33 (66) | 16 (59) | 17 (74) | 0.28** |
| Weight (kg), median (range) | 84.3 (52–122) | 77.8 (55–101) | 90 (52–122) | 0.02* |
| Acute Myeloid Leukemia, no. (%) | 31 (62) | 19 (70) | 12 (52) | 0.19** |
| 1st or 2nd Complete Remission | 23 (46) | 14 (52) | 9 (39) | 0.50*** |
| > 2nd Complete Remission | 3 | 1 | 2 | |
| Relapse/persistent disease | 5 | 4 | 1 | |
| Myelodysplastic Syndrome, no. | 3 | 1 | 2 | 0.59*** |
| Relapse/persistent disease | 3 | 1 | 2 | |
| Acute Lymphoid Leukemia, no. | 3 | 2 | 1 | >0.99*** |
| 1st or 2nd Complete Remission | 1 | 1 | 0 | >0.99*** |
| > 2nd Complete Remission | 2 | 1 | 1 | |
| Non-Hodgkin's Lymphoma, no. | 7 | 3 | 4 | 0.69*** |
| 1st or 2nd Complete Remission | 3 | 1 | 2 | >0.99*** |
| > 2nd Complete Remission | 1 | 1 | 0 | |
| Partial Remission | 2 | 1 | 1 | |
| Relapse | 1 | 0 | 1 | |
| Other^, no. | 6 | 2 | 4 | 0.40*** |
| No. of Prior Therapies | ||||
| ≤ 2 Prior Chemotherapy Regimens | 33 (66) | 18 (67) | 15 (65) | 0.91** |
| > 2 Prior Chemotherapy Regimens | 17 (34) | 9 (33) | 8 (35) | |
| No. of Prior Therapies for AML | ||||
| ≤ 2 Prior Chemotherapy Regimens | 23 (74) | 14 (74) | 9 (75) | >0.99*** |
| > 2 Prior Chemotherapy Regimens | 8 (26) | 5 (26) | 3 (25) | |
| Patients with Prior Transplants, no. (%) | 26 (52) | 11 (41) | 15 (65) | 0.08** |
Includes: Chronic Myeloid Leukemia, Hodgkins Lymphoma, Multiple Myeloma, Prolymphocytic Leukemia, Chronic Lymphocytic Leukemia
p-value from T test
p-values from Chi-Square test
p values from Fisher's Exact test
Twenty-seven patients were transplanted with 1 UCB unit (54%) and 23 patients received 2 units (46%) (Table 2). A total of 40 (54.8%) of the 73 transplanted units were matched at 4/6 HLA loci to the patient. In the case of patients who received 2 units, the units were matched at least 4/6 to the patient and a minimum 3/6 to each other (3/6 n=4, 4/6 n=8, 5/6 n=7, 6/6 n=4). Forty-six patients demonstrating donor engraftment (four patients had no dominant unit) were evaluated for infused graft TNC dose, CD3+, CD34+, and CD56+CD3neg cell doses. Patients who were transplanted with one UCB unit received higher numbers of CD34+ cells (p = 0.0008), CD56+CD3neg cells (p = 0.01), and TNC (p < 0.0001) per actual body weight compared to the predominate unit in patients receiving a 2-unit UCB transplant (Table 2). There was no difference in the number of infused CD3+ graft T-cells between the two groups (p = 0.43).
TABLE 2.
| Graft Characteristics | p value | |
|---|---|---|
| Number of UCB units | ||
| One UCB unit, no. (%) | 27 (54) | |
| Two UCB units, no. (%) | 23 (46) | |
| Collected cell dose (no. patients) ^ | ||
| Total nucleated cell dose × 107/kg | ||
| One UCB unit (26), median (range) | 2.78 (1.98 – 6.22) | |
| Two UCB units (23), median (range) | 3.75 (2.12 – 7.62) | 0.0004* |
| Predominant unit (19), median (range) | 1.89 (1.22 – 4.25) | <.0001* |
| CD34+ cell dose × 105/kg | ||
| One UCB unit (26), median (range) | 0.95 (0.30 – 2.43) | |
| Two UCB units (20), median (range) | 1.08 (0.49 – 4.97) | 0.278* |
| Predominant unit (17), median (range) | 0.64 (0.27 – 3.93) | 0.033* |
| Infused cell dose | ||
| Total nucleated cell dose × 107/kg | ||
| One UCB unit, median (range) | 2.58 (1.73 – 5.48) | |
| Two UCB units, median (range) | 3.42 (2.01 – 6.91) | 0.0002* |
| Predominant unit, median (range) | 1.71 (0.97 – 3.60) | <.0001* |
| CD34+cell dose × 105/kg | ||
| One UCB unit, median (range) | 1.72 (0.21 – 5.39) | |
| Two UCB units, median (range) | 1.45 (0.67 – 4.67) | 0.661* |
| Predominant unit, median (range) | 0.75 (0.05 – 3.87) | 0.0008* |
| CD3+ cell dose × 106/kg | ||
| One UCB unit, median (range) | 5.72 (2.95 – 12.89) | |
| Two UCB units, median (range) | 11.09 (6.15 – 16.43) | <.0001** |
| Predominant unit, median (range) | 5.75 (2.90 – 8.18) | 0.281** |
| CD56+CD3neg × 106/kg | ||
| One UCB unit, median (range) | 3.04 (0.01 – 5.79) | |
| Two UCB units, median (range) | 3.86 (2.29 – 8.16) | 0.021** |
| Predominant unit, median (range) | 1.91 (1.06 – 4.24) | 0.007** |
| CD4+CD25+ × 105/kg (no. patients)^ | ||
| One UCB unit (20), median (range) | 6.22 (0 – 12.90) | |
| Two UCB units (22), median (range) | 8.66 (4.49 – 17.47) | 0.005** |
| Predominant unit (18), median (range) | 4.91 (2.10 – 10.16) | 0.301** |
| HLA | ||
| One UCB unit, no. | ||
| 6/6 | 0 | |
| 5/6 | 6 | |
| 4/6 | 17 | |
| 3/6 | 4 | |
| Two UCB units, no. | ||
| 6/6 + 6/6 | 1 | |
| 6/6 + 5/6 | 1 | |
| 5/6 + 5/6 | 9 | |
| 5/6 + 4/6 | 1 | |
| 4/6 + 4/6 | 11 |
Missing data
p-values from Kruskal-Wallis test
p-values from T test
Allogeneic Engraftment and Neutrophil Recovery
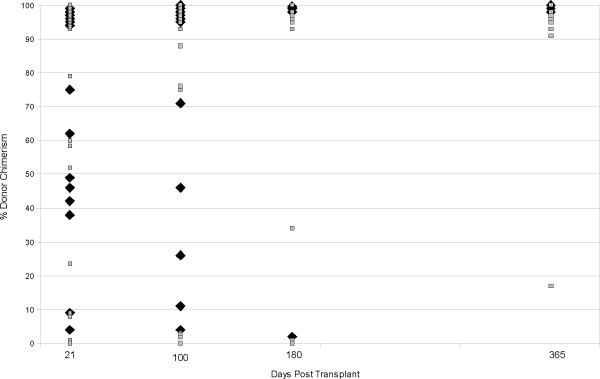
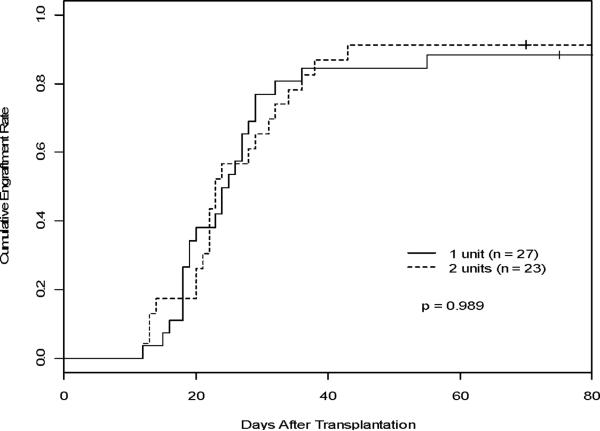
The majority of study patients (36 patients) demonstrated predominant UCB donor engraftment by chimerism analysis on or before day T+42 (Figure 1) (Table 3). All patients demonstrating predominant donor chimerism (>60%) at early time points went on to attain full donor chimerism (Figure 1). Cumulative engraftment rate by donor chimerism at 100 days was 73.8% (95% CI: 0.61–0.85). No differences were seen in the cumulative rates of attaining donor chimerism at day 100 between the two groups; 72.4% in the 1-unit group and 75.2% in the 2-unit group (p = 0.95) (Figure 2A). Median time to attain donor chimerism >60% was 21 days (95% CI: 17 days-28 days). Twelve patients (25%) had donor engraftment failure by chimerism, 7 in the 1-unit group and 5 in the 2-unit group. Engraftment by chimerism was not evaluable in 2 patients, 1 patient in each group, due to early death (T+16 and T+27). All engrafting patients in the 2-unit group showed early predominance of one UCB unit with no stable mixed chimerism identified. The 4 patients in the 2-unit group who did not have a dominant unit identified by chimerism demonstrated primary graft failure. Late engraftment (>42 days) was seen in 1 patient in the 2-unit group. Of the 36 patients who achieved predominant donor chimerism, three patients demonstrated temporary decreases to <60% donor chimerism, but regained predominant donor chimerism at a later date. Secondary graft failure occurred in 2 patients in the 1-unit group attributed to treatment for CMV infection and an unknown etiology.
Figure 1. Donor Chimerism.
Percentage donor chimerism obtained by day of transplantation. Gray squares represent 1-unit transplant recipients and black diamonds represent the dominant unit in the 2-unit transplant recipients.
TABLE 3.
| Patient Outcomes | 1-Unit | 2-Unit | p value |
|---|---|---|---|
| Donor Chimerism > 60%, no. (%) | 19 (73)a | 17 (77)^ | 0.95* |
| Primary Graft Failure, no. (%) | 7 (27)a | 5 (23)^ | 0.74** |
| Engraftment > 42 days, no. | 0 | 1 | |
| Autologous Recovery, no. | 4 | 3 | |
| Second UCB Transplant, no. | 3 | 1 | |
| Acute Myeloid Leukemia, no. | 3 | 1 | |
| 1st or 2nd Complete Remission, no. | 3 | 1 | |
| Secondary Graft Failure, no. (%) | 2 (8)a | 0 | 0.49*** |
| Autologous Recovery, no. | 0 | 0 | |
| Second UCB Transplant, no. | 1 | 0 | |
| Neutrophils > 500/μL, no. (%) | 23 (85) | 21 (91) | 0.99* |
| 3 Year Overall Survival, % (CI) | 35.9% (0.18 – 0.54) | 39.1% (0.20 – 0.58) | 0.86** |
| 3 Year Event-Free Survival, % (CI) | 28.6% (0.13 – 0.46) | 39.1% (0.20 – 0.58) | 0.71** |
| Relapse, no. (%) | 16 (59.3%) | 7 (30.4%) | 0.045**** |
| Disease | |||
| Acute Myeloid Leukemia, no. | 10 | 4 | >0.99*** |
| Acute Lymphoid Leukemia, no. | 2 | 0 | |
| Non-Hodgkin's Lymphoma, no. | 2 | 1 | |
| Disease Status at Transplant | |||
| 1st or 2nd Complete Remission, no. | 9 | 3 | 0.64*** |
| > 2nd Complete Remission, no. | 2 | 0 | |
| Partial Remission, no. | 3 | 2 | |
| Relapse/persistent disease, no. | 2 | 2 | |
| Time to Relapse | |||
| Relapse < 100 days, no. | 6 | 4 | 0.46*** |
| Relapse 100 – 180 days, no. | 2 | 2 | |
| Relapse 180 – 365 days, no. | 5 | 1 | |
| Relapse > 1 year, no. | 3 | 0 | |
| Infectious Complications | |||
| Fungemia | 0 | 0 | |
| Gram + bacteremias, no. infections/no. patients | 21/10 | 18/13 | 0.17** |
| Gram − bacteremias, no. infections/no. patients | 11/11 | 20/12 | 0.42** |
| CMV reactivation, no. patients | 10 | 8 | 0.87** |
| EBV reactivation, no.patients | 4 | 7 | 0.31*** |
| Acute GvHD, Grade III–IV, % (CI)# | 19.2% (0.09 – 0.38) | 17.4% (0.07 – 0.37) | 0.87**** |
| Chronic GvHD, % (CI)† | 21.7% (0.10 – 0.42) | 26.3% (0.12 – 0.49) | 0.73**** |
| Transplant Related Mortality < 100 days, no. (%) | 0 | 3 (13.0%) | 0.48*** |
| Transplant Related Mortality > 100 days, no. (%) | 3 (11.1%) | 4 (17.4%) |
Percentages listed are that of patients surviving to engraftment (n=26)
Percentages listed are that of patients surviving to engraftment (n=22)
One patient died early from relapsed disease in the 1-unit group
Percentages listed are that of patients surviving to day 100 (n=23 for 1-unit group n=19 for 2-unit group)
p-values from Log-Rank test
p-values from Chi-Square test
p-values from Fisher's Exact test
p-values from Univariate Logistic Regression
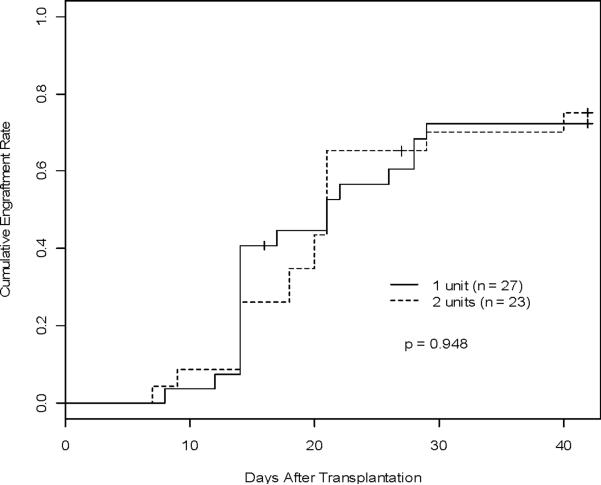
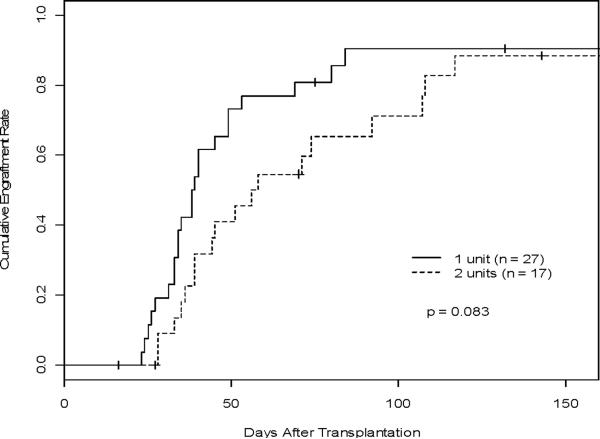
Figure 2. Engraftment of ANC >500.
A) Comparison of the cumulative engraftment rate (predominant chimerism as defined >60%) between the 1-unit and 2-unit groups estimated, accounting for competing risks. B) Cumulative incidences of platelet recovery between the 1-unit and 2-unit groups estimated, accounting for competing risks. C) Cumulative incidence of neutrophil recovery (ANC >500) estimated, accounting for competing risks in the one vs two unit groups.
At day 100, 89.8% (95% CI: 0.80–0.96) of patients on this study recovered either autologous or donor ANC >500/μL for at least 3 consecutive days, 88.4% in the 1-unit group and 91.3% in the 2-unit group (Figure 2B). There was no significant difference in time to ANC >500/μL recovery between the two groups (p = 0.99). Median time to neutrophil recovery was 24 days (95% CI: 22 days-28 days) (mean 27.7 days), 25 days in the 1-unit group and 23 days in the 2-unit group (mean 27.9 days and 26.3 days, respectively). Failure of neutrophil recovery occurred in 6 patients (12.0%), 4 patients in the 1-unit group (1 early death), and 2 patients in the 2-unit group. The median time to platelet recovery was 45 days (95% CI: 38 days-56 days) (mean 58.1 days), 38.5 days in the 1-unit group and 57 days in the 2-unit group (mean 46.2 days and 68.2 days, respectively). The rate of platelet engraftment for both the 1-unit and 2-unit groups was 81.3% at 100 days (95% CI: 0.69–0.91). No differences were noted comparing the 1-unit group (90.4%) and the 2-unit group (71.1%) (p = 0.08) at 100 days (Figure 2C). Nine patients (18.0%) failed to engraft their platelets, 4 in the 1-unit group (1 early death) and 5 in the 2-unit group (1 early death). Forty-six patients were included in correlation analyses of graft cell doses and engraftment by predominant donor chimerism or ANC >500/μL. There was no significant association of day to attain predominant chimerism or ANC >500/μL with infused UCB graft TNC (p=0.84, p=0.06), CD3+ (p=0.62, p=0.68), CD56+CD3neg (p=0.93, p=0.41), or CD34+ (p=0.95, p=0.10) cells using the dominant unit in 2-unit recipients.
Survival and TRM
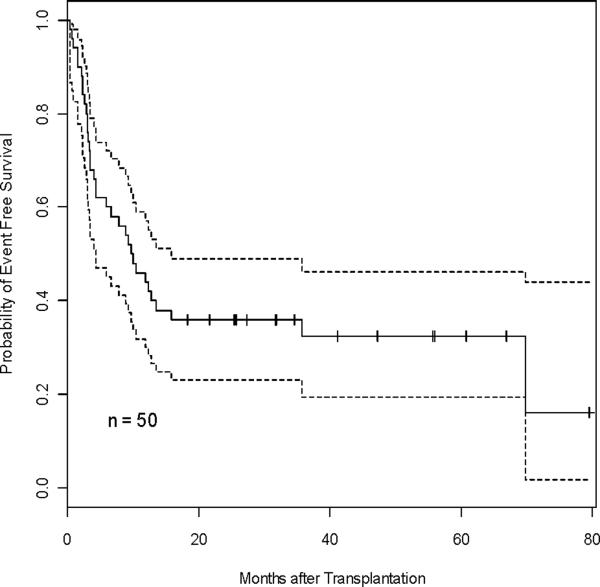
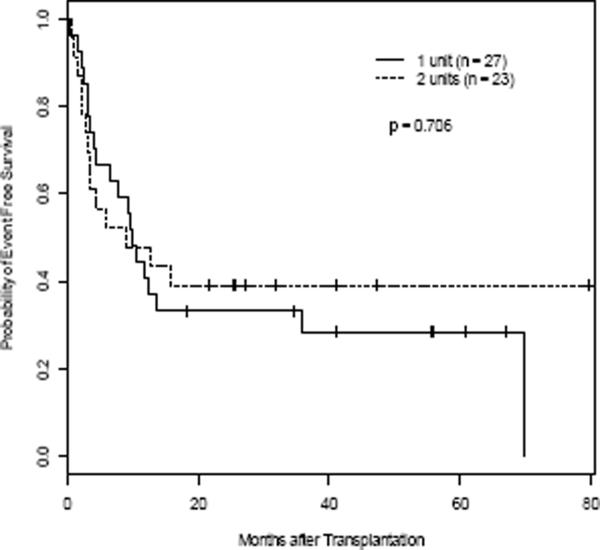
The Kaplan-Meier estimates for OS and EFS are depicted in Figure 3A–D. OS at 3 months was 88.9% in the 1-unit group and 87.0% in the 2-unit group. The three-year OS and EFS for all patients were 37.6% and 32.4%. Median OS and EFS were 14.3 months and 9.9 months. Three-year OS and EFS were 35.9% and 28.6% for the 1-unit group and 39.1% and 39.1% for the 2-unit group, respectively (Table 3). OS and EFS at 60 months was 26.2% and 28.6% for the 1-unit group, and 39.1% and 39.1% for the 2-unit group. There was no difference between the two groups in OS and EFS (p=0.86 and p=0.71). For OS, the hazard of dying for patients infused with 2-units was 11% higher than those infused with 1-unit after adjustment for the effects of age, gender, and disease type, although the difference was not significant (p=0.76). Similarly, the hazard ratio for EFS comparing 2-unit to 1-unit was 1.26 (p=0.52) after controlling the effects of age, gender, and disease type. There was no correlation in 46 evaluable patients between the UCB graft TNC (p=0.84, p=0.62), CD56+CD3neg (p=0.16, p=0.17), CD34+ (p=0.50, p=0.37), or CD3+ (p=0.38, p=0.23) cells infused, and OS or EFS (using the dominant unit in the 2-unit recipients). The median follow up for the entire group was 14.3 months (range 0.5 months – 80 months). For those patients surviving (n = 16), the median follow up was 38 months (range 18 – 80 months).
Figure 3. OS and EFS.
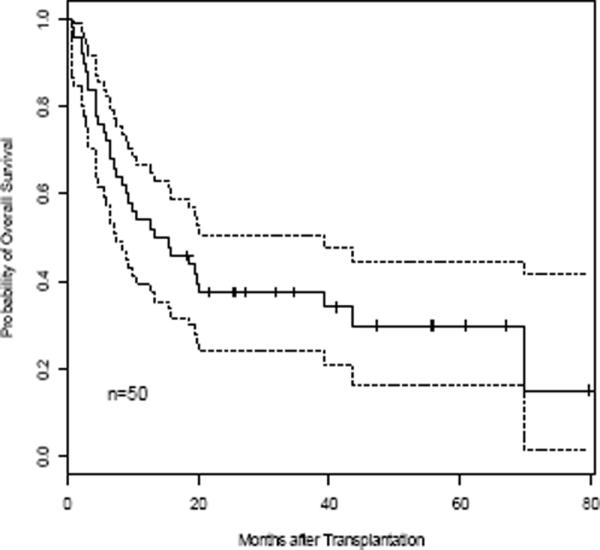
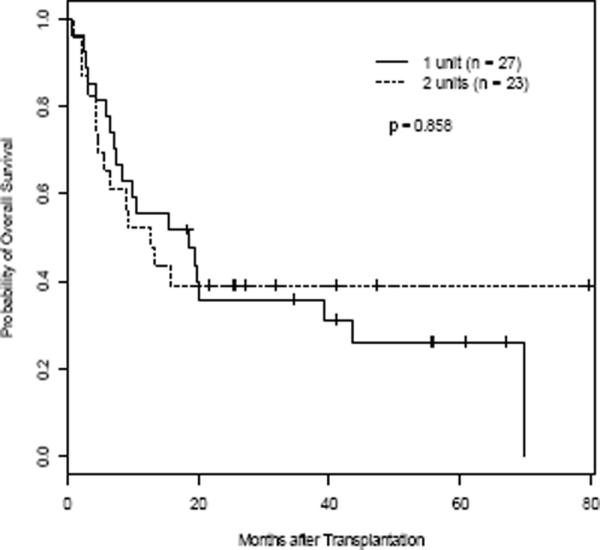
Kaplan-Meier estimate for OS (A, C) and EFS (B, D) for all patients (A, B), and according to number of units transplanted for all pts (C, D), respectively. Hashed line indicates 95% CI (A & B).
Early (< 100 days) TRM was not seen in the 1-unit group. Late TRM (> 100 days) occurred in three patients (11.1%) with two of the three patients dying from transplant-related causes after day 180 (Table 3). In the 2-unit group, 7 of 23 patients (30.4%) died from non-relapse causes, three before day 100, and one patient each on day 132 and day 270. The remaining two patients died at >1 year from complications from related to transplantation (Table 4). There was no association between number of UCB units transplanted and overall TRM (p=0.16). No grade 3 or grade 4 UCB infusion related toxicities were seen in either the 1-unit group or the 2-unit group.
TABLE 4.
| Causes of Death | 1-Unit | 2-Unit | p value |
|---|---|---|---|
| Early (Less than 100 days after transplantation) | |||
| Disease Relapse | 4 | 1* | 0.14** |
| EBV Associated Post Transplant Lymphoma | 0 | 0 | |
| GvHD | 0 | 0 | |
| Infection | 0 | 0 | |
| Organ Failure | 0 | 3 | |
| Other | 0 | 0 | |
| Late (More than 100 days after transplantation) | |||
| Disease Relapse | 6 | 0.14** | |
| EBV Associated Post Transplant Lymphoma | 1 | 0 | |
| GvHD | 0 | 1 | |
| Infection | 4^ | 3 | |
| Organ Failure | 2^ | 0 | |
| Other | 1 | 0 | |
| Unknown | 4 | 0 |
Patient also had EBV associated post transplant lymphoma and organ failure
Includes 3 patients who relapsed then died after a second transplant
p-values from Fisher's Exact test
Relapse and Graft vs. Host Disease Incidence
Sixteen (59.3%) patients in the 1-unit group relapsed, compared to only seven (30.4%) patients in the 2-unit group (p=0.045) (Table 3). The number of UCB units infused was a significant predictor for relapse by univariate analysis. The odds of relapsing for patients with 1-unit were 5.5 times higher than that for those receiving 2-units (p=0.013) after adjustment for confounders by multivariate analysis. None of the UCB graft cell dose variables (TNC, CD3+, CD34+, and CD56+CD3neg) were significant predictors of relapse for either the 1-unit or 2-unit groups. Of four patients who had received HLA 3/6 matched single units (match criteria of high resolution class II DRB1 and low resolution class I molecular typing), two are alive without disease recurrence, one patient each had progressive disease and autologous recovery followed by relapsed disease.
The rate of grade II–IV acute GvHD was 44.9% (95% CI: 0.32–0.59) in the two groups and the rate of grade III acute GvHD was 18.4% overall (95% CI: 0.10–0.31). Grade III acute GvHD was observed in 5 of 26 (19.2%) patients in the 1-unit group and 4 of 23 (17.4%) in the 2-unit group (Table 3). No patient demonstrated grade IV acute GvHD. All patients were followed for one year for chronic GvHD evaluation. Chronic GvHD was observed in 5 of 23 (4 limited, 1 extensive) (21.7%) evaluable 1-unit patients, and five (3 limited, 2 extensive) (26.3%) of 19 evaluable 2-unit patients. The overall rate of chronic GvHD for all study patients was 23.8% (95% CI: 0.13–0.39). There was no significant association between the number of UCB units infused with occurrence of either acute GvHD (p=0.87) nor chronic GvHD (p=0.73).
Discussion
To our knowledge, this study is the first prospective investigation directly comparing a single UCB unit versus two-unit approach in adult hematologic malignancy patients treated with uniform RIC and supportive care including acute GvHD prophylaxis. Published work to date has included retrospective and more recently prospective studies supporting the administration of two units in adult patients in the myeloablative and RIC setting. Our study design used the availability of a single UCB unit of specified HLA match and nucleated cryopreserved cell dose (≥2.5×107/kg) as biologic assignment to one vs. two-unit UCB transplantation. Minimum required combined TNC dose of ≥3.0×107/kg was similar to previously reported requirements for two-unit recipients. However, as the threshold cell dose in the RIC setting has not been established, we chose a target dose of ≥2.5×107/kg above which patients were eligible for single unit transplantation, based on previous reports identifying safety in the myeloablative setting. The target cell dose selected is lower than minimum threshold cell doses previously reported by other investigators in two-unit studies in adults treated with non-myeloablative conditioning. Furthermore, this lower threshold cell dose allowed a larger proportion of study patients to proceed to transplant with a single unit graft. These observations may allow an adult patient lacking two UCB units of specified HLA match and cell content to proceed to transplantation with anticipated survival similar to that of 2 unit recipients.
Brunstein et al. reported the largest single institution series to date including 110 patients receiving UCB grafts after non-myeloablative conditioning with 85% of patients receiving a double unit UCB graft. A total of 35% of patients in this study were treated with ATG to reduce risk of graft rejection, which correlated with reduced risk of acute GvHD and higher TRM. Important challenges to analyses of clinical trials published to date include variance in patient selection, a potential lack of uniformity in patient characteristics in those receiving one vs. two-units with a large proportion of single unit recipients being pediatric patients, as well as changes over time in conditioning and GvHD prophylaxis including ATG administration.
The patients enrolled in this study are reflective of an adult hematology practice including the predominance of myeloid leukemia over lymphoid malignancy, and are comparable to other reports in terms of age and weight range. Of note, most patients included in reports on RIC outcomes with conventional adult donor and UCB grafts, had either no or prior autologous hematopoietic cell transplantation. Our study patient population had extremely high-risk (t-MN, 2nd AML) disease and was heavily pre-treated, including >50% of patients receiving prior autologous or allogeneic transplantation, implicating a possible higher risk of relapse- and non-relapse mortality. Despite this high risk, heavily pretreated patient population, EFS at three years was 28.6% in the 1-unit group and 39.1% in the 2-unit group and five-year EFS was 28.6% in the 1-unit group and 39.1% in the 2-unit group. These data support the use of RIC and UCB allogeneic transplantation as safe and effective treatment in these patients with advanced disease and extensive prior therapy. Additionally, these data compare favorably to previous single institution trials and larger retrospective series. Recent data published by the National Marrow Donor Program summarize transplant outcomes in adults treated with RIC and conventional adult-derived graft sources and show a 5-year OS of 23%.
A major concern for UCB transplant safety and efficacy for adult patients is the limited TNC and CD34+ progenitor cell content in the graft, generally a log lower than adult-derived grafts. Several studies have shown neutrophil engraftment after UCB transplantation correlating with graft TNC dose, CD34+ cell dose, CD3+cell dose and CD8+ cell dose. However, the influence of these graft cell populations is not seen consistently across all trials. CD34+ UCB graft cell dose has also been observed to correlate with EFS, OS, and lower TRM in some studies. From these studies the threshold of TNC dosing for UCB transplantation in the RIC setting has not been firmly established. We observed no differences in cumulative rates of attaining predominant donor chimerism at day 100 in this study, which were 72.4% in the 1-unit group compared to 75.2% observed in the 2-unit group. No differences were seen between the two groups in rates and kinetics of neutrophil and platelet engraftment. Additionally, we did not detect any statistically significant influence of TNC, CD34+, nor CD3+ UCB graft infused cell dose on engraftment or survival. This observation is consistent with the study by Brunstein et al, but differs from that reported in single and double unit UCB transplantation in the myeloablative setting.
Infusion of two UCB units did however significantly impact the relapse risk in this high risk patient population with 1-unit recipients having a relapse risk significantly higher than 2-unit recipients, suggesting strong graft-vs. malignancy effect of 2-unit UCB infusion as reported in prior retrospective studies. Sixteen (59.3%) patients in the 1-unit group in this study notably relapsed, compared to only 7 (30.4%) patients in the 2-unit group (p=0.045) in this study cohort of predominantly high risk or recurrent AML patients. Other investigators including Verneris et al. noted that relapse was significantly lower for early stage (1st or 2nd complete remission) patients who received two UCB units (RR 0.5, p<0.03) in 177 acute leukemia patients treated with myeloablative conditioning. The benefit of stronger graft vs. lymphoma effect has also been reported by the Eurocord-Netcord and lymphoma working party of the European group for Blood and Marrow Transplantation in 104 adult patients treated with 1 or 2-unit UCB after RIC with lower risk of relapse observed in recipients of double-unit UCB (p=0.03). Consistently, one UCB unit predominates in transplant recipients receiving two or more UCB units usually by 4–6 weeks after transplant. Infusion of the nonengrafting unit may augment UCB engraftment via immune activation and/or inhibition of recipient-mediated immune rejection. Since RIC transplantation depends on “allogeneic effect” to eliminate malignancy, each UCB unit represents an intact immune system with potential donor-recipient and donor-donor interactions that may render additional benefits of two-unit infusion, including enhanced graft vs. malignancy effects.
UCB transplantation, despite frequent HLA-mismatch, carries a surprisingly low risk for acute and chronic GvHD ; this risk could be conceivably higher in 2-unit recipients as previously reported. The combined rate of grade II–IV acute GvHD in this study was 44.9% in the two groups. Grade III acute GvHD rate was only 18.4%, and no patient demonstrated grade IV disease. The incidence of chronic GvHD was only 23.8%. We observed no significant association between the number of UCB units infused and occurrence of either acute or chronic GvHD. These observations may be attributable in part to administration of ATG to all study patients in this series.
This single institution feasibility study suggests that double UCB transplantation elicits stronger graft vs. malignancy effects in high risk adult patients with primarily myeloid leukemia. Nevertheless, single unit UCB transplantation at threshold nucleated cell dose exceeding 2.5×107/kg recipient weight remains a valid treatment option with equivalent survival rates for patients lacking two UCB units of specified HLA match and cell doses previously reported. While similar results have been reported in prior retrospective analyses by other groups, the data in this prospective study strengthens these observations: e.g. lower relapse risk after the infusion of two umbilical cord blood units, and similar survival outcomes in those transplanted with an adequate single unit or two units if one adequate unit is not available. There are also some differences: graft-versus-host disease may not be increased in recipients of two units, and graft cell dose may not be a risk factor for OS or EFS comparing the two groups. Whether limited patient numbers and/or length of follow up may underlie these differences, there are no prospective studies reported to date with UCB directly comparing single vs. double unit infusion in the setting of RIC. Given the small numbers of patients, prolonged accrual time, and the heterogeneity of the patient population in this study, definitive conclusions should be taken cautiously. Further studies are needed in larger multi-institutional prospective trials: 1) to more firmly establish the minimum safe threshold dose for single unit UCB in adult patients treated with RIC, and 2) to identify key parameters for graft selection in the two UCB unit setting that may contribute to enhanced graft vs. malignancy effects confirmed in this prospective single institution study.
Acknowledgements
The authors would like to thank all members of the Seidman Cancer Center for their excellence in patient care. This work was supported by: RO1-AI47289-01 (MJL), the Stem Cell Facility of the Case Comprehensive Cancer Center 5P30CA043703 (ClinicalTrials.gov identifier: NCT00003335), the Abraham J. and Phyllis Katz Foundation (MJL), and the Dr. Donald and Ruth Weber Goodman Philanthropic Fund (MJL).
Footnotes
Conflict of Interest Disclosure: All authors declare no competing financial interest.
References
- 1.Chao NJ, Koh LP, Long GD, Gasparetto C, Horwitz M, Morris A, et al. Adult recipients of umbilical cord blood transplants after nonmyeloablative preparative regimens. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2004;10(8):569–75. doi: 10.1016/j.bbmt.2004.05.001. Epub 2004/07/30. [DOI] [PubMed] [Google Scholar]
- 2.Komatsu T, Narimatsu H, Yoshimi A, Kurita N, Kusakabe M, Hori A, et al. Successful engraftment of mismatched unrelated cord blood transplantation following reduced intensity preparative regimen using fludarabine and busulfan. Annals of hematology. 2007;86(1):49–54. doi: 10.1007/s00277-006-0190-5. Epub 2006/10/13. [DOI] [PubMed] [Google Scholar]
- 3.Narimatsu H, Watanabe M, Kohno A, Sugimoto K, Kuwatsuka Y, Uchida T, et al. High incidence of graft failure in unrelated cord blood transplantation using a reduced-intensity preparative regimen consisting of fludarabine and melphalan. Bone marrow transplantation. 2008;41(8):753–6. doi: 10.1038/sj.bmt.1705978. Epub 2008/01/16. [DOI] [PubMed] [Google Scholar]
- 4.Uchida N, Wake A, Takagi S, Yamamoto H, Kato D, Matsuhashi Y, et al. Umbilical cord blood transplantation after reduced-intensity conditioning for elderly patients with hematologic diseases. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2008;14(5):583–90. doi: 10.1016/j.bbmt.2008.03.003. Epub 2008/04/16. [DOI] [PubMed] [Google Scholar]
- 5.Kanda J, Rizzieri DA, Gasparetto C, Long GD, Chute JP, Sullivan KM, et al. Adult dual umbilical cord blood transplantation using myeloablative total body irradiation (1350 cGy) and fludarabine conditioning. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2011;17(6):867–74. doi: 10.1016/j.bbmt.2010.09.009. Epub 2010/09/28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barker JN, Weisdorf DJ, DeFor TE, Blazar BR, McGlave PB, Miller JS, et al. Transplantation of 2 partially HLA-matched umbilical cord blood units to enhance engraftment in adults with hematologic malignancy. Blood. 2005;105(3):1343–7. doi: 10.1182/blood-2004-07-2717. Epub 2004/10/07. [DOI] [PubMed] [Google Scholar]
- 7.Lekakis L, Giralt S, Couriel D, Shpall EJ, Hosing C, Khouri IF, et al. Phase II study of unrelated cord blood transplantation for adults with high-risk hematologic malignancies. Bone marrow transplantation. 2006;38(6):421–6. doi: 10.1038/sj.bmt.1705467. Epub 2006/08/08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brunstein CG, Fuchs EJ, Carter SL, Karanes C, Costa LJ, Wu J, et al. Alternative donor transplantation after reduced intensity conditioning: results of parallel phase 2 trials using partially HLA-mismatched related bone marrow or unrelated double umbilical cord blood grafts. Blood. 2011;118(2):282–8. doi: 10.1182/blood-2011-03-344853. Epub 2011/04/30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ballen KK, Spitzer TR, Yeap BY, McAfee S, Dey BR, Attar E, et al. Double unrelated reduced-intensity umbilical cord blood transplantation in adults. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2007;13(1):82–9. doi: 10.1016/j.bbmt.2006.08.041. Epub 2007/01/16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brunstein CG, Barker JN, Weisdorf DJ, DeFor TE, Miller JS, Blazar BR, et al. Umbilical cord blood transplantation after nonmyeloablative conditioning: impact on transplantation outcomes in 110 adults with hematologic disease. Blood. 2007;110(8):3064–70. doi: 10.1182/blood-2007-04-067215. Epub 2007/06/16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.MacMillan ML, Weisdorf DJ, Brunstein CG, Cao Q, DeFor TE, Verneris MR, et al. Acute graft-versus-host disease after unrelated donor umbilical cord blood transplantation: analysis of risk factors. Blood. 2009;113(11):2410–5. doi: 10.1182/blood-2008-07-163238. Epub 2008/11/11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Majhail NS, Brunstein CG, Shanley R, Sandhu K, McClune B, Oran B, et al. Reduced-intensity hematopoietic cell transplantation in older patients with AML/MDS: umbilical cord blood is a feasible option for patients without HLA-matched sibling donors. Bone marrow transplantation. 2011 doi: 10.1038/bmt.2011.114. Epub 2011/05/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Avery S, Shi W, Lubin M, Gonzales AM, Heller G, Castro-Malaspina H, et al. Influence of infused cell dose and HLA match on engraftment after double-unit cord blood allografts. Blood. 2011;117(12):3277–85. doi: 10.1182/blood-2010-08-300491. quiz 478. Epub 2010/12/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hidalgo A, Weiss LA, Frenette PS. Functional selectin ligands mediating human CD34(+) cell interactions with bone marrow endothelium are enhanced postnatally. J Clin Invest. 2002;110(4):559–69. doi: 10.1172/JCI14047. Epub 2002/08/22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sideri A, Neokleous N, De La Grange PB, Guerton B, Le Bousse Kerdilles MC, Uzan G, et al. An overview of the progress on double umbilical cord blood transplantation. Haematologica. 2011;96(8):1213–20. doi: 10.3324/haematol.2010.038836. Epub 2011/05/07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shpall EJ, Bollard CM, Brunstein C. Novel cord blood transplant therapies. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2011;17(1 Suppl):S39–45. doi: 10.1016/j.bbmt.2010.10.004. Epub 2010/10/19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Willemze R, Ruggeri A, Purtill D, Rodrigues CA, Gluckman E, Rocha V. Is there an impact of killer cell immunoglobulin-like receptors and KIR-ligand incompatibilities on outcomes after unrelated cord blood stem cell transplantation? Best practice & research Clinical haematology. 2010;23(2):283–90. doi: 10.1016/j.beha.2010.05.005. Epub 2010/09/15. [DOI] [PubMed] [Google Scholar]
- 18.Delaney M, Cutler CS, Haspel RL, Yeap BY, McAfee SL, Dey BR, et al. High-resolution HLA matching in double-umbilical-cord-blood reduced-intensity transplantation in adults. Transfusion. 2009;49(5):995–1002. doi: 10.1111/j.1537-2995.2008.02077.x. Epub 2009/01/23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mattsson J, Ringden O, Storb R. Graft Failure after Allogeneic Hematopoietic Cell Transplantation. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2008;14(Supplement 1):165–70. doi: 10.1016/j.bbmt.2007.10.025. Epub 2009/02/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eapen M, Rocha V, Sanz G, Scaradavou A, Zhang MJ, Arcese W, et al. Effect of graft source on unrelated donor haemopoietic stem-cell transplantation in adults with acute leukaemia: a retrospective analysis. The lancet oncology. 2010;11(7):653–60. doi: 10.1016/S1470-2045(10)70127-3. Epub 2010/06/19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rodrigues CA, Sanz G, Brunstein CG, Sanz J, Wagner JE, Renaud M, et al. Analysis of risk factors for outcomes after unrelated cord blood transplantation in adults with lymphoid malignancies: a study by the Eurocord-Netcord and lymphoma working party of the European group for blood and marrow transplantation. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2009;27(2):256–63. doi: 10.1200/JCO.2007.15.8865. Epub 2008/12/10. [DOI] [PubMed] [Google Scholar]
- 22.Ooi J, Takahashi S, Tomonari A, Tsukada N, Konuma T, Kato S, et al. Unrelated cord blood transplantation after myeloablative conditioning in adults with ALL. Bone marrow transplantation. 2009;43(6):455–9. doi: 10.1038/bmt.2008.347. Epub 2008/10/29. [DOI] [PubMed] [Google Scholar]
- 23.van Heeckeren WJ, Fanning LR, Meyerson HJ, Fu P, Lazarus HM, Cooper BW, et al. Influence of human leucocyte antigen disparity and graft lymphocytes on allogeneic engraftment and survival after umbilical cord blood transplant in adults. British journal of haematology. 2007;139(3):464–74. doi: 10.1111/j.1365-2141.2007.06824.x. Epub 2007/10/04. [DOI] [PubMed] [Google Scholar]
- 24.Rocha V, Labopin M, Sanz G, Arcese W, Schwerdtfeger R, Bosi A, et al. Transplants of umbilical-cord blood or bone marrow from unrelated donors in adults with acute leukemia. N Engl J Med. 2004;351(22):2276–85. doi: 10.1056/NEJMoa041469. Epub 2004/11/27. [DOI] [PubMed] [Google Scholar]
- 25.Verneris MR, Brunstein CG, Barker J, MacMillan ML, DeFor T, McKenna DH, et al. Relapse risk after umbilical cord blood transplantation: enhanced graft-versus-leukemia effect in recipients of 2 units. Blood. 2009;114(19):4293–9. doi: 10.1182/blood-2009-05-220525. Epub 2009/08/27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brunstein CG, Gutman JA, Weisdorf DJ, Woolfrey AE, Defor TE, Gooley TA, et al. Allogeneic hematopoietic cell transplantation for hematologic malignancy: relative risks and benefits of double umbilical cord blood. Blood. 2010;116(22):4693–9. doi: 10.1182/blood-2010-05-285304. Epub 2010/08/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rubinstein P, Rosenfield RE, Adamson JW, Stevens CE. Stored placental blood for unrelated bone marrow reconstitution. Blood. 1993;81(7):1679–90. Epub 1993/04/01. [PubMed] [Google Scholar]
- 28.Pepe MS, Mori M. Kaplan-Meier, marginal or conditional probability curves in summarizing competing risks failure time data? Statistics in medicine. 1993;12(8):737–51. doi: 10.1002/sim.4780120803. Epub 1993/04/30. [DOI] [PubMed] [Google Scholar]
- 29.Brown L, DaGupta A. Interval estimation for a binomial proportion. Statistical Science. 2001;16:101–33. [Google Scholar]
- 30.Sauter C, Abboud M, Jia X, Heller G, Gonzales AM, Lubin M, et al. Serious Infection Risk and Immune Recovery after Double-Unit Cord Blood Transplantation Without Antithymocyte Globulin. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2011 doi: 10.1016/j.bbmt.2011.02.001. Epub 2011/02/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oran B, Wagner JE, Defor TE, Weisdorf DJ, Brunstein CG. Effect of Conditioning Regimen Intensity on Acute Myeloid Leukemia Outcomes after Umbilical Cord Blood Transplantation. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2011 doi: 10.1016/j.bbmt.2011.01.007. Epub 2011/01/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Robin M, Sanz GF, Ionescu I, Rio B, Sirvent A, Renaud M, et al. Unrelated cord blood transplantation in adults with myelodysplasia or secondary acute myeloblastic leukemia: a survey on behalf of Eurocord and CLWP of EBMT. Leukemia : official journal of the Leukemia Society of America, Leukemia Research Fund, UK. 2011;25(1):75–81. doi: 10.1038/leu.2010.219. Epub 2010/10/01. [DOI] [PubMed] [Google Scholar]
- 33.Baron F, Storb R, Storer BE, Maris MB, Niederwieser D, Shizuru JA, et al. Factors associated with outcomes in allogeneic hematopoietic cell transplantation with nonmyeloablative conditioning after failed myeloablative hematopoietic cell transplantation. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2006;24(25):4150–7. doi: 10.1200/JCO.2006.06.9914. Epub 2006/08/10. [DOI] [PubMed] [Google Scholar]
- 34.Mielcarek M, Storer BE, Sandmaier BM, Sorror ML, Maloney DG, Petersdorf E, et al. Comparable outcomes after nonmyeloablative hematopoietic cell transplantation with unrelated and related donors. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2007;13(12):1499–507. doi: 10.1016/j.bbmt.2007.09.004. Epub 2007/11/21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Giralt S, Logan B, Rizzo D, Zhang MJ, Ballen K, Emmanouilides C, et al. Reduced-intensity conditioning for unrelated donor progenitor cell transplantation: long-term follow-up of the first 285 reported to the national marrow donor program. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2007;13(7):844–52. doi: 10.1016/j.bbmt.2007.03.011. Epub 2007/06/21. [DOI] [PubMed] [Google Scholar]
- 36.Rocha V, Gluckman E. Improving outcomes of cord blood transplantation: HLA matching, cell dose and other graft- and transplantation-related factors. British journal of haematology. 2009;147(2):262–74. doi: 10.1111/j.1365-2141.2009.07883.x. Epub 2009/10/03. [DOI] [PubMed] [Google Scholar]
- 37.Laughlin MJ, Eapen M, Rubinstein P, Wagner JE, Zhang MJ, Champlin RE, et al. Outcomes after transplantation of cord blood or bone marrow from unrelated donors in adults with leukemia. N Engl J Med. 2004;351(22):2265–75. doi: 10.1056/NEJMoa041276. Epub 2004/11/27. [DOI] [PubMed] [Google Scholar]
- 38.Takahashi S, Ooi J, Tomonari A, Konuma T, Tsukada N, Oiwa-Monna M, et al. Comparative single-institute analysis of cord blood transplantation from unrelated donors with bone marrow or peripheral blood stem-cell transplants from related donors in adult patients with hematologic malignancies after myeloablative conditioning regimen. Blood. 2007;109(3):1322–30. doi: 10.1182/blood-2006-04-020172. Epub 2006/10/14. [DOI] [PubMed] [Google Scholar]
- 39.Rocha V, Broxmeyer HE. New approaches for improving engraftment after cord blood transplantation. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2010;16(1 Suppl):S126–32. doi: 10.1016/j.bbmt.2009.11.001. Epub 2009/11/10. [DOI] [PubMed] [Google Scholar]
- 40.Cohen YC, Scaradavou A, Stevens CE, Rubinstein P, Gluckman E, Rocha V, et al. Factors affecting mortality following myeloablative cord blood transplantation in adults: a pooled analysis of three international registries. Bone marrow transplantation. 2011;46(1):70–6. doi: 10.1038/bmt.2010.83. Epub 2010/05/04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wagner JE, Barker JN, DeFor TE, Baker KS, Blazar BR, Eide C, et al. Transplantation of unrelated donor umbilical cord blood in 102 patients with malignant and nonmalignant diseases: influence of CD34 cell dose and HLA disparity on treatment-related mortality and survival. Blood. 2002;100(5):1611–8. doi: 10.1182/blood-2002-01-0294. Epub 2002/08/15. [DOI] [PubMed] [Google Scholar]
- 42.Terakura S, Azuma E, Murata M, Kumamoto T, Hirayama M, Atsuta Y, et al. Hematopoietic engraftment in recipients of unrelated donor umbilical cord blood is affected by the CD34+ and CD8+ cell doses. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2007;13(7):822–30. doi: 10.1016/j.bbmt.2007.03.006. Epub 2007/06/21. [DOI] [PubMed] [Google Scholar]
- 43.Fanning LR, Hegerfeldt Y, Tary-Lehmann M, Lesniewski M, Maciejewski J, Weitzel RP, et al. Allogeneic transplantation of multiple umbilical cord blood units in adults: role of pretransplant-mixed lymphocyte reaction to predict host-vs-graft rejection. Leukemia : official journal of the Leukemia Society of America, Leukemia Research Fund, UK. 2008;22(9):1786–90. doi: 10.1038/leu.2008.55. Epub 2008/03/21. [DOI] [PubMed] [Google Scholar]
- 44.Gutman JA, Turtle CJ, Manley TJ, Heimfeld S, Bernstein ID, Riddell SR, et al. Single-unit dominance after double-unit umbilical cord blood transplantation coincides with a specific CD8+ T-cell response against the nonengrafted unit. Blood. 2010;115(4):757–65. doi: 10.1182/blood-2009-07-228999. Epub 2009/10/14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Narimatsu H, Miyakoshi S, Yamaguchi T, Kami M, Matsumura T, Yuji K, et al. Chronic graft-versus-host disease following umbilical cord blood transplantation: retrospective survey involving 1072 patients in Japan. Blood. 2008;112(6):2579–82. doi: 10.1182/blood-2007-11-118893. Epub 2008/06/19. [DOI] [PubMed] [Google Scholar]