Abstract
Background
The presence of osmotic gradients in the development of cerebral edema and the effectiveness of osmotherapy are well recognized. A modification of ventriculostomy catheters described in this paper provides a method of osmotherapy that is not currently available. The Reductive Ventricular Osmotherapy (RVOT) catheter removes free water from ventricular cerebrospinal fluid (CSF) by incorporating hollow fibers that remove water vapor, thereby providing osmotherapy without increasing osmotic load.
Objective
To increase osmolarity in the ventricular CSF through use of RVOT in vivo.
Methods
Twelve Yorkshire swine with contusional injury were randomized to external ventricular drainage (EVD) or RVOT for 12 hours. Magnetic resonance imaging was obtained. Serum, CSF, and brain ultrafiltrate were analyzed. Histology was compared using Fluor-Jade B and H & E.
Results
With RVOT, CSF osmolality increased from 292 ± 2.7 to 345 ± 8.0 mosmol/kg (mean ± SE, p=0.0006), and the apparent diffusion coefficient (ADC) in the injury region increased from 0.735 ±0 .047 to 1.135 ± .063 (p=0.004) over 24 hours. With EVD controls, CSF osmolarity and ADC were not significantly changed. Histologically, all RVOT pigs showed no evidence of neuronal degeneration (Grade 1/4) compared to moderate degeneration (Grade 2.6 +.4/4) seen in EVD treated animals (p=0.02). The difference in intracranial pressure (ICP) by area under the curve approached significance at p = .065 by Mann Whitney test.
Conclusion
RVOT can increase CSF osmolarity in vivo after experimental traumatic brain injury (TBI). In anticipated clinical use, only a slight increase in CSF osmolarity may be required to reduce cerebral edema.
Keywords: Osmotherapy, Hollow Fibers, Cerebral Edema, Reductive Ventricular Osmotherapy, Porcine, Traumatic Brain Injury
INTRODUCTION
The aim of this study was to evaluate the use of RVOT in a swine model of TBI. The central hypothesis is that removal of intracranial free water will provide all the benefits of osmotherapy without delivery of an osmotic agent. Cytotoxic edema is an early, reversible injury1 and the predominate cause of cerebral edema in TBI.2 Considering the relative magnitude of hydrostatic and osmotic pressure, Simard et al.3 concluded that “the driving force” of edema formation is determined “only by osmotic pressure gradients.” Therefore the reversal of the osmotic gradient is a critical need for these patients.
Systemic osmotherapy is used clinically in TBI patients, and has been shown to reduce edema and ICP. It involves intravenous infusion of a hyperosmolar solution of either mannitol or hypertonic saline.4–10 Systemic osmotherapy has limitations, and the choice of agent remains controversial.4–12 Cerebral edema sometimes remains refractory to all currently available treatments, and some patients have poor outcomes even with controlled ICP.13,14
One limitation of systemic osmotherapy may be that maximal levels of systemic osmolarity achieved with systemic 23.4% saline15, even at extremes11 (e.g., 356 mosmol/kg by Rockswold et al., unpublished paper.), will not exceed the level of tissue hyperosmosis documented in clinical studies of TBI. Elevation of tissue osmolality in ischemic injury was demonstrated over 30 years ago by various investigators16–18 as reviewed by Odland and Sutton.19 Kawamata et al.20 found an injured tissue osmolality mean of 371.9 ± 16.1 (SD) mosmol/kg in the central area of the contusion. Tissue hyperosmolarity will cause free water to move from any available source (plasma, CSF) into the tissue based on Starlings formulation.3
On a cellular mechanism of action, cellular swelling occurs at the expense of contracture of the interstitial space as osmotic gradients drive water into the cells. Thus, reduced mass transport within the tissue is another hallmark of cerebral edema. Osmotherapy is probably the only mechanism for reversing cytotoxic edema. Chen and Nicholson21 established that in brain slices exposed to a hyperosmolar solution, cell contraction causes an expansion of the interstitial volume, and consequently improves mass transfer within the tissue.
To avoid systemic complications of systemic osmotherapy and achieve higher levels of osmotic gradient, investigators have studied direct intraventricular infusion of osmotic agents.22–24 These studies have shown a reduction of edema; however, there is concern for side effects, as infusing osmotic agents into the ventricle acutely increases ICP and ventricular osmotic load.
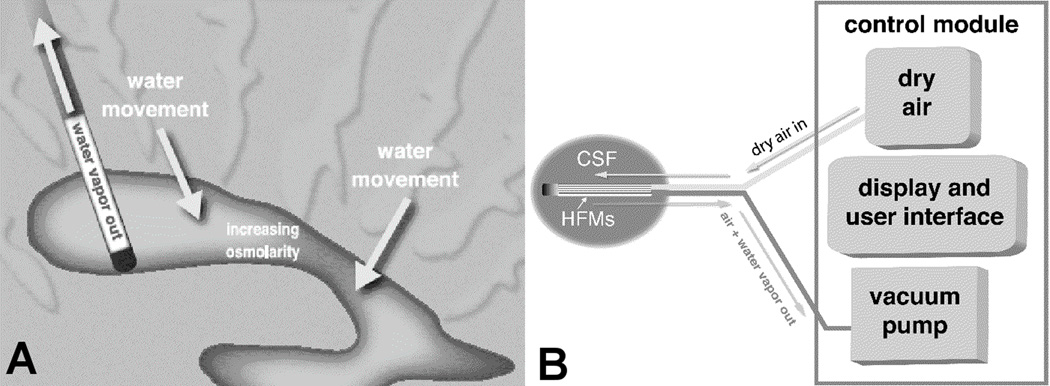
RVOT is a novel therapy that removes only water from ventricular CSF, thereby raising osmolar solute concentrations and producing an osmotic gradient that draws water out of tissue, as illustrated in Figure 1a. A dry air mixture is drawn through an array of hollow fibers which are permeable to water vapor, but not to solute molecules, as shown in Figure 1b. The osmotic gradient favors the movement of interstitial water into the ventricular space, and subsequently out of the ventricle via the catheter and natural drainage. RVOT does not involve infusion of a therapeutic solution, but actually reduces overall volume by removing water. RVOT is the only method of osmotherapy that can increase local osmolarity without infusion of osmotic agents. The authors have demonstrated that RVOT improves mass transport of water in ex vivo studies.25 Following successful studies of RVOT both ex vivo and in vitro, an in vivo study of RVOT was conducted in a swine model of TBI as described below. The objective of this study was to increase osmolarity in the ventricular CSF through use of RVOT in vivo.
Figure 1.
RVOT operation showing: A) Water vapor removal, which increases CSF osmolarity and draws water out of tissue, and B) Device schematic with dry air mixture into catheter inlet and humidified air exiting the catheter.
MATERIALS AND METHODS
RVOT Ventriculostomy Catheter
The RVOT catheter performs two functions: removal of free water through the hollow fibers, and drainage of CSF via a perforated central catheter lumen under the hollow fiber array. The diameter and overall dimensions are similar to those of an EVD catheter (see Figure 2). We designed the catheter with 3.5 mm outer diameter and an exposed membrane length of 2 cm, which is capable of removing 0.15 mL/hour of free water. The dry sweep gas consisted of 95% air and 5% carbon dioxide. To avoid the possibility of overpressurization of the catheter, the sweep gas was drawn through the hollow fibers by a vacuum. The water removal rate was controlled by adjusting the vacuum level and by cycling the sweep gas flow on and off. The function and use of the RVOT catheter has been described in greater detail elsewhere.25
Figure 2.
Photograph of RVOT catheter (top) and Traumacath ventricular drain catheter (bottom).
Large Animal TBI Model
As described previously,26 a survival model of large animal brain injury was developed. A reproducible cortical contusion injury was delivered to exposed dura by a piston impact with controllable velocity, depth, and dwell time. With this model, animals are kept under anesthesia for up to 16 hours, which allows time for surgical preparation and injury, 12 hours of therapy, and one MR scan. Animals are given antibiotics and other medication as needed after resuscitation, and are euthanized at 9 days after injury. The number of animals was based on estimates of expected edema reduction based on the change in ventricular osmolarity in ex vivo studies and modeling.25
Preoperative Preparation and Surgical Procedure
The Animal Care Committee at the VA Medical Center Animal Care Facility, San Francisco approved the experimental protocol. Animals were treated in accordance with the Guide for the Care and Use of Laboratory Animals. Twelve female Yorkshire swine, weight 23–28 kg, were acclimated for at least 5 days prior to surgery. Preoperative Treat Retrieval Test training was performed, typically over the course of 3–5 days. The animal was trained to identify which of three bins containing a food treat could be opened based on the color of the bin.
Prior to the procedure, animals were pre-medicated and intubated. Anesthesia was induced with 1.5–3.5% isoflurane in air, and a respiration rate of 15 breaths/minute was maintained. Arterial oxygen saturation was maintained at >98% and end tidal carbon dioxide at approximately 40 mmHg. Intravenous (IV) Ringer's Lactate Solution was administered at 5 ml/kg/hr. IV Cefazolin (1 gram) antibiotic was given at induction, at anesthesia reversal, and prior to 24 hr MR scans.
A right femoral arterial line was inserted. Blood pressure, heart rate, ECG, oxygen saturation, end tidal carbon dioxide, and rectal temperature were monitored continuously using a Datex Engstrom AS/3 monitoring system. Urine output was measured every 30 minutes. Hourly blood gas and chemistry were performed on the Nova Biomedical pHOx + L, which measures pH, pCO2, pO2, SO2%, Hct, Hb, Na+, K+, Glu, Lac, and Ca++. "Pre-injury" blood osmolality was measured (Wescor Vapro 5520 Osmometer).
With the animal prone, a midline 5-mm burr hole was formed 1 cm posterior to the bregma and 0.5 cm to the right of the midline. A pressure sensor (Codman Microsensor ICP Transducer) for ICP monitoring and a Twin Star Cerebral Ultrafiltration Assay Catheter for collection of interstitial fluid (IF) were placed in the parenchyma via this burr hole. ICP was recorded every 15 minutes throughout the surgical and treatment procedures.
At this time a randomized selection of either a RVOT catheter or control EVD catheter (Integra INS-8420 TraumaCath® Extraventricular Drainage Catheter) was made. A 6-mm burr hole was then created 3 cm anterior to the bregma and 3 mm lateral to the sagittal suture over the right frontal area. The shape of the porcine skull and sinus locations preclude a more anterior entry, and therefore a straight approach along the lateral ventricle axis was not possible. The treatment catheter (RVOT or EVD) was therefore introduced through this burr hole using a curved stylet, which was intended to facilitate orientation of the catheter to be more fully immersed in the ventricular CSF. Return of CSF was considered a sign of ventricular placement. A small amount of CSF (typically 100–500µl) was withdrawn through the catheter lumen for "pre-injury" osmolality and chemistry profiles. A representative sagittal plane MR image is shown in Figure 3, where the outlines of the right ventricle and the RVOT catheter can be seen.
Figure 3.
Sagittal T1 MR scan at approximately 2 hours post-injury showing right ventricle outline and outline of RVOT Catheter passing through the anterior part of the ventricle. The craniotomy at the contusion site can be seen posterior to the catheter.
A 1.5 cm right parietal craniotomy was then created to expose the dura. A LinMot linear motor (Model PS01-37x240) firing device was mounted on a stereotactic frame, and the piston was positioned in contact with the dura. The contusion was created by firing the piston into the dura to a depth of 10 mm at a velocity of 3.5 m/s with a dwell time of 400 milliseconds. The site was then cleaned, the dura repaired (Medtronic Durepair), the bone flap replaced, and the defect covered with mesh plate (Medtronic Timesh). A picture of the surgical field prior to closure of the scalp incision is shown in Figure 4.
Figure 4.
Operative field showing RVOT Catheter (A) entering right frontal area, Timesh covering (B) over contusion in right parietal area, and parenchymal sensors (C) posterior to the contusion.
TBI Treatment and Data Collection
After close monitoring of at least one hour, the animal was transported to the MR Suite for a set of "0 Hr" MR scans using a Siemens Magnetron Symphony 1.5T magnet. The MR sequences included T1 weighted sagittal and coronal images, and coronal T2 FLAIR and T2 spin-echo (SE) sequences. Apparent diffusion coefficient (ADC) maps were generated from the diffusion-weighted imaging (DWI) sequences. The animal was then transported back to the surgery suite. For the duration of the treatment, the animal was placed in a supine position, which facilitated ventilation and airway management.
A "post injury" CSF sample was withdrawn for osmolality and chemistry profile measurements, followed by a 12-hour treatment protocol as follows:
Test pigs (n=6). The RVOT catheter was activated with a vacuum level of 200 mmHg and duty cycle of 50%. Operation was set for 12 hours, with regular measurements of blood chemistry, pressure, urine output, and ICP as described above. The target water removal was 0.15 ml/hr.
Control pigs (n=6). The EVD catheter was connected to a pump set for controlled drainage of bulk CSF for 12 hours, with regular measurements of blood chemistry, pressure, urine output, and ICP as described above. The target bulk fluid removal was 0.15 ml/hr.
Animals were closely monitored throughout the treatment period. Respiration rate was maintained at 15 per minute. During RVOT operation, the sweep gas vacuum, flow rate, exit humidity, water removal rate, and cumulative water removal were electronically recorded.
After treatment, a "post-treatment" CSF sample was withdrawn for osmolality and chemistry profile measurements, anesthesia was reversed, and the pig was allowed to recover. Post-recovery observations of wound condition, attitude, eating, drinking, heart rate, respiration rate, urine output, and pain were recorded, and repeated daily thereafter. A neurological assessment was also done after recovery and daily thereafter, which involved examination of pupils, motor function, tail sensory function, locomotion, and overall coordination. At 8 ±1 hours post-recovery, the animal was again intubated and anesthetized, transported to the MR Suite for a set of "24 Hr" MR scans, and then allowed to recover again. At some time prior to sacrifice, the Treat Retrieval Test was performed to evaluate the animal's recognition of the food-containing bin. Tests were graded on a scale of −1 (opens another bin) to +4 (opens correct bin without approaching either of the other two bins).
The targeted survival time was nine days, which was extended in some cases due to MR scanner scheduling and shortened in some cases because of sudden death or deteriorating health. On the day of sacrifice the animal was intubated and anesthetized, transported to the MR Suite for Final MR scans, and then euthanized. The euthanization procedure included cardiac perfusion with heparinized saline to wash out all blood products, followed by phosphate-buffered 4% paraformaldehyde with picric acid, then full craniectomy and removal of the brain after cessation of cardiac activity. The brains were fixed by immersion in 4% buffered formalin and then cryoprotected in sucrose solution.
Radiological Studies
The MR scans were studied by a staff neuroradiologist to assess differences in right (injured) versus left hemisphere, RVOT versus Control, and 24 Hour and Final (day of sacrifice) versus 0 Hour (immediate post-injury) results. DWI sequences were visually reviewed to evaluate regions of injury, and the corresponding ADC maps were generated. ADC values were measured from each of the three sets of MR scans in six regions of the injured (right) hemisphere and in corresponding regions of the left hemisphere, including the central injury region, occipital white matter, anterior frontal gray matter, and several anterior white matter regions. The area of hemorrhage in the injured tissue was estimated from T2* images. The maximum coronal cross-section area of the injury region was measured from the DWI and FLAIR sequences, which indicate differences in the amount of edema.
Histology
Histological analysis was done by a blinded examiner on 40 µm coronal sections stained with either H&E or Fluoro-Jade B (FJB) for assessment of hemorrhage and neuronal death. FJB is a fluorescent marker with specific affinity for degenerating neurons27. Uptake of the marker is increased with greater degeneration. The relative safety of RVOT and Control therapy was evaluated by comparing histological evidence of hemorrhage using sections stained with H&E. The ordinal method of grading was chosen over counting cells to account for intensity.
The number and fluorescent intensity of neuronal cells stained positively by FJB was assessed in both the contusion region and in a control region in the contralateral hemisphere of each brain. The level of neuronal degeneration was graded from 1 (no degenerating cells) to 4 (severe degeneration). The level of red staining with H&E was assessed in the contusion region and graded from 1 (minimal hemorrhage) to 4 (large areas of hemorrhage).
Statistics
Mean and standard error of all variables were calculated. Values were compared by t-test unless otherwise stated. A p value of .05 was considered significant. Paired t-tests were used to compare pre- and post-treatment CSF chemistries and ADC. A repeated measures ANOVA for unbalanced data was performed on hourly mean ICP measurements. A Wilcoxon rank-sum test was used to compare integrated area under the curve (AUC) values of ICP and histological grades. Excel statistical package and SPSS statistical analysis package (Version 18, IBM Inc.) were used to carry out the analysis.
RESULTS
Baseline Comparison of Experimental and Control Groups
No significant differences were seen in vital signs between the two groups before and during treatment. Group averages are shown in Table 1 for heart rate (HR), mean arterial pressure (MAP), rectal temperature, and arterial pH, pO2, and pCO2. Group differences in pre-test animal conditions and contusion injuries were also evaluated. We considered pig weight, pre-treatment ICP, CSF chemistry, blood chemistry, vital signs, and injury severity as determined by T2 FLAIR images. No significant differences were seen between the two groups. Serum osmolality increased 4.5 mosmol/kg with RVOT and 1.5 mosmol/kg with EVD, but the difference was not significant (p=.39). Both groups developed a metabolic alkalosis, perhaps related to administration of Lactated Ringer’s solution for maintenance fluid. There was no difference in final serum bicarbonate when comparing RVOT and control groups (p = .36).
Table 1.
Average values and Standard Error for vital signs, pH, and respiratory gases at 3 hour intervals for RVOT and EVD control groups.
| Preinjury | 0 HR | 3 HR | 6 HR | 9 HR | END | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SE | Mean | SE | Mean | SE | Mean | SE | Mean | SE | Mean | SE | ||
|
HR, bpm |
RVOT | 117 | 7 | 108 | 5 | 119 | 6 | 115 | 6 | 112 | 4 | 111 | 4 |
| EVD | 108 | 2 | 113 | 11 | 118 | 8 | 115 | 6 | 111 | 5 | 106 | 4 | |
|
MAP, mmHg |
RVOT | 70 | 7 | 70 | 3 | 73 | 4 | 71 | 5 | 71 | 4 | 70 | 3 |
| EVD | 74 | 3 | 74 | 2 | 76 | 3 | 75 | 3 | 73 | 3 | 78 | 3 | |
|
Temp, C |
RVOT | 37.5 | 0.2 | 36.7 | 0.2 | 38.2 | 0.2 | 38.2 | 0.0 | 38.3 | 0.1 | 38.2 | 0.1 |
| EVD | 37.9 | 0.2 | 36.9 | 0.6 | 37.8 | 0.2 | 38.3 | 0.1 | 38.2 | 0.1 | 38.2 | 0.2 | |
| pH | RVOT | 7.503 | 0.018 | 7.548 | 0.016 | 7.530 | 0.010 | 7.535 | 0.011 | 7.523 | 0.009 | 7.529 | 0.009 |
| EVD | 7.516 | 0.019 | 7.560 | 0.012 | 7.549 | 0.009 | 7.550 | 0.010 | 7.568 | 0.009 | 7.548 | 0.010 | |
|
pO2, mmHg |
RVOT | 107.8 | 6.7 | 114.7 | 6.0 | 109.1 | 5.9 | 109.5 | 6.1 | 103.1 | 6.2 | 106.1 | 6.5 |
| EVD | 116.1 | 5.2 | 113.6 | 6.2 | 111.2 | 5.9 | 106.4 | 5.3 | 106.5 | 8.1 | 102.1 | 5.4 | |
|
pCO2, mmHg |
RVOT | 47.8 | 3.1 | 41.0 | 1.5 | 43.2 | 2.1 | 42.2 | 1.7 | 42.2 | 1.5 | 40.3 | 1.7 |
| EVD | 44.0 | 2.0 | 38.3 | 2.1 | 41.6 | 1.4 | 42.8 | 1.4 | 40.2 | 1.2 | 40.5 | 1.4 | |
Animals received 12 hours of treatment except where laboratory scheduling interfered. Actual treatment time for RVOT was 11.7 ± .4 (mean±SE), and for EVD was 11.6 ± .3. RVOT treatment removed an average of 0.15 ml/hr. In two RVOT-treated animals the formation of blood clots on the hollow fiber membranes reduced the rate to 0.09 ml/hr, without affecting the drainage of CSF for CSF chemistry analysis. EVD treatment removed an average of 0.13 ml/hr, which was not significantly different (p=.47). In one animal the EVD bulk drainage was .042 ml/hr for unknown reasons. All animals survived at least one day and had 0 Hr and 24 Hr MR scans; nine animals survived at least eight days (five RVOT and four Controls). Two animals (one RVOT and one EVD Control) died unexpectedly and could not be examined histologically. The last control animal was euthanized immediately after the 24 hour MR scans, but was examined histologically.
CSF and Interstitial Fluid Chemistry
Absolute and relative changes in CSF chemistry are shown in Table 2. CSF osmolality increased with RVOT from 292 ± 2.7 to 345 ± 8.0 mosmol/kg (mean ± SE, p=.0006 in paired t-test) during treatment, whereas with EVD it decreased slightly from 293 ± 2.5 to 287 ± 3.1 mosmol/kg (p =.12). The increase in osmolality with RVOT was almost entirely due to increase in sodium. Sodium increased in the CSF (p=.001), and this increase correlates well with the increase in CSF osmolality (R2=.9). RVOT did not change serum sodium, however (p=.36).
Table 2.
Pre-treatment levels ("pre-treat") and intra-treatment changes (Δ), with their respective standard errors ("SE") in CSF osmole concentrations and apparent diffusion coefficient obtained from MR scans. RVOT causes a significant increase in total osmole concentration and water mobility, with actual reduction in CSF lactate.
| Parameter | RVOT | EVD | ||||||
|---|---|---|---|---|---|---|---|---|
| Pre- treat |
SE | Δ | SE | Pre- treat |
SE | Δ | SE | |
| CSF osmolality, mosmol/kg | 292 | 2.7 | +52.5 | 6.8 | 293 | 2.5 | −6.2 | 3.3 |
| CSF Na+, mmol/L | 140.2 | .82 | +26 | 6.3 | 138.9 | 1.8 | −4.8 | .87 |
| CSF K+, mmol/L | 3.58 | .55 | +.66 | .21 | 4.14 | .70 | +.32 | .77 |
| CSF Ca++, mmol/L | .92 | .03 | +.03 | .03 | .86 | .03 | −.22 | .07 |
| Ca++, mmol/L, adjusted for RVOT concentrating effect | .92 | .03 | −.14 | .03 | N/A | N/A | N/A | N/A |
| CSF lactate, mmol/L | 5.82 | .77 | −.36 | .83 | 5.10 | .93 | +2.14 | 1.67 |
| CSF lactate, mmol/L, adjusted for RVOT concentrating effect | 5.82 | .77 | −1.46 | .81 | N/A | N/A | N/A | N/A |
| CSF glucose, mg/dL | 83 | 16.7 | +12.8 | 5.7 | 57 | 4.2 | −7.2 | 3.2 |
| ADC from DWI Scans | .735 | .047 | +.400 | .079 | .825 | .053 | +.108 | .120 |
The concentrating effect of RVOT, which merely removes pure water from CSF, was assumed to be the same for all osmoles, as has been demonstrated in previous in vitro testing.25 In our analysis of CSF chemistry, we estimated this concentrating effect to equal the percentage increase in total osmolality during treatment. The pretreatment concentration of each osmole was multiplied by the percentage CSF osmolality increase to calculate the effect, and this amount was deducted from the post-treatment concentration to determine an "effective" post-treatment level for each osmole.
The RVOT treatment effect for each osmole was determined as the difference between the effective post-treatment and the absolute pre-treatment concentrations. There was no significant change in CSF or serum potassium. Treatment effects on CSF osmolality are shown in Table 2. The RVOT treatment effect on CSF lactate was significantly different than in EVD therapy (p=.03, Wilcoxon rank-sum test), as CSF lactate actually decreased from 5.8 ± .8 to 4.4 ± .8 mmol/l, adjusting for the concentrating effect of RVOT. In Controls, lactate increased from 5.1 ± .9 to 7.2 ± 1.7 mmol/l. See Table 2. Post-treatment glucose/lactate ratio (p=.04) and calcium (p=.02) were both higher in RVOT treated pigs.
Interstitial fluid (IF) collection was sufficient for analysis in five animals with RVOT treatment and two with EVD treatment. The post-treatment IF lactate after RVOT was 5.8 ± 1.3 mmol/l, versus a ventricular CSF level of 5.5 ± 1.0 mmol/l (unadjusted for concentrating effect of RVOT). Average IF lactate in the two EVD samples was 8.6 mmol/l.
Intracranial Pressure
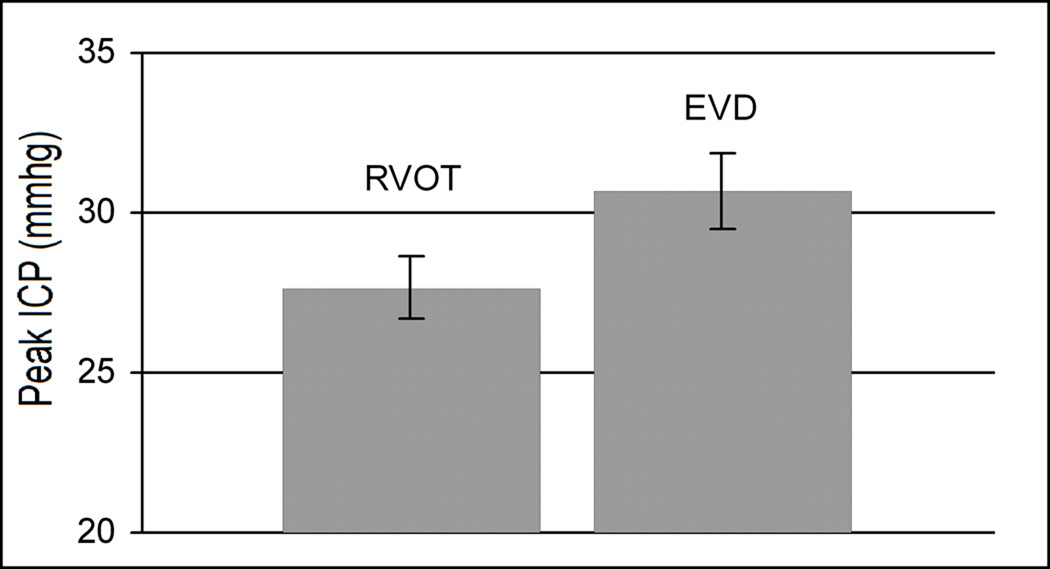
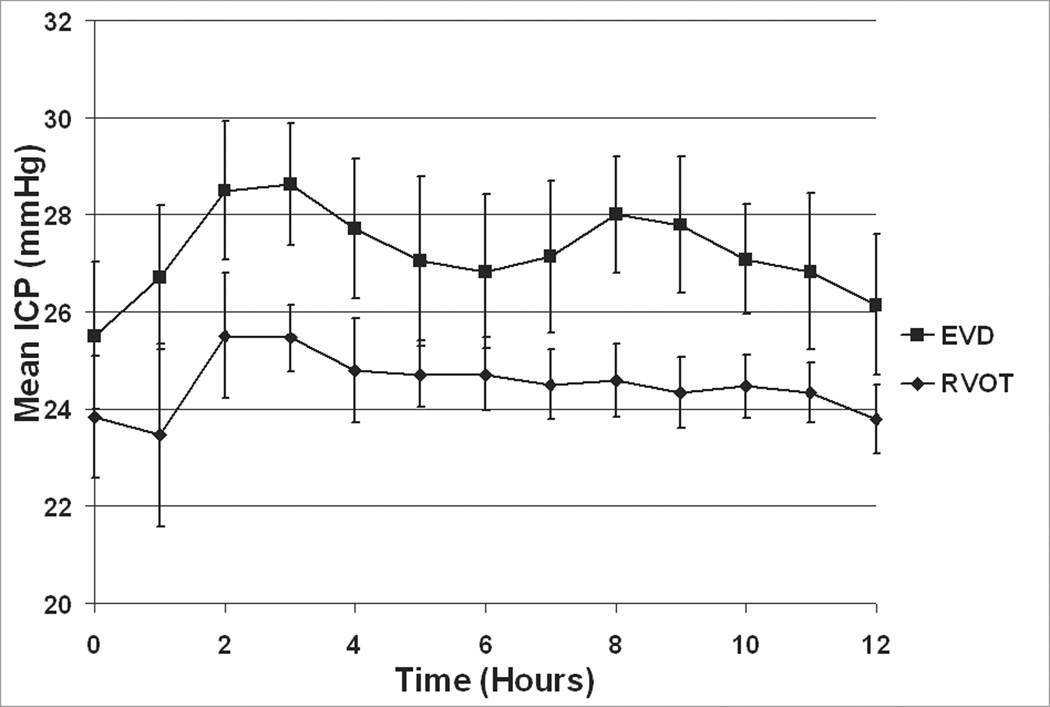
There was no significant difference in ICP between the time of injury up to the start of treatment (p = .42). Peak ICP trended lower during RVOT than EVD treatment (p=.09, as shown in Figure 5). Group averages for ICP just prior to treatment and at mean hourly intervals from 0 hr until the end of treatment are shown in Figure 6. Peak ICP during the last eight hours of treatment was significantly lower in the RVOT group (26 ± .8 mmHg) compared to EVD group (29.8 ± 1.3 mmHg) at p = .04. Although there appeared to be consistent differences between study groups in the mean ICP values over the observation period, the difference did not reach statistical significance in a repeated measures ANOVA (p = 0.11). This is likely due to the relatively small sample size (n = 12 total pigs) and the associated variability of the ICP readings.
Figure 5.
Peak intra-treatment ICP, showing reduced pressure with RVOT treatment.
Figure 6.
Hourly averages of ICP ± SE immediately prior to and during treatment. All data taken with pigs in supine position.
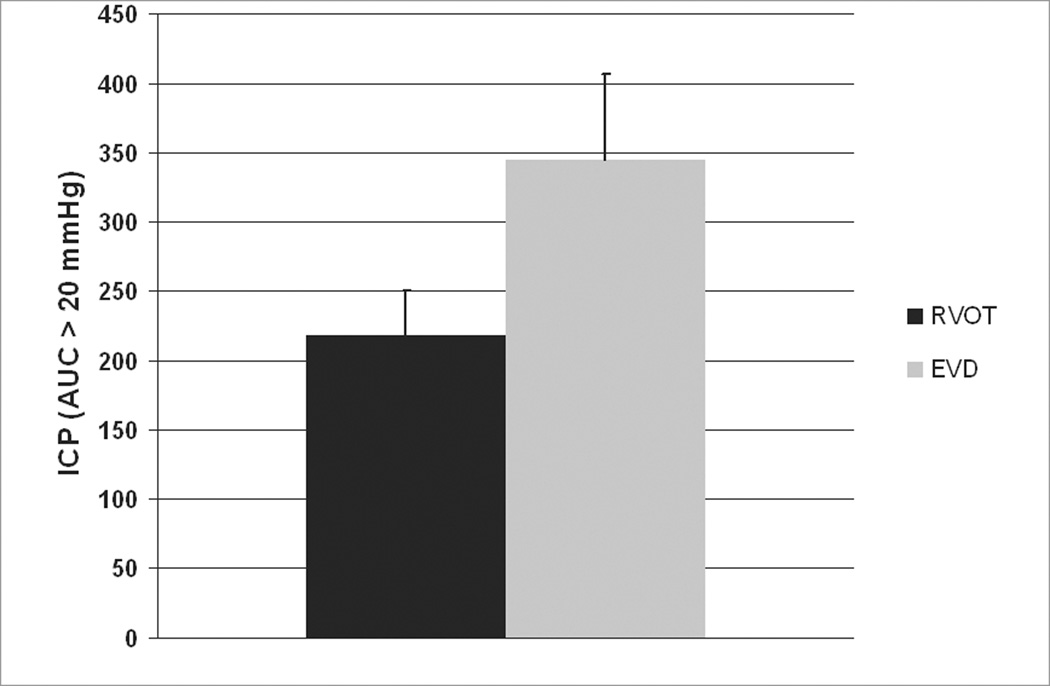
The AUC should provide a better assessment of ICP profiles, and requires fewer assumptions about the distributional properties of ICP than a repeated measures ANOVA of hourly values. The AUC difference of ICP only above a 20 mmHg (Figure 7) was not significant based on a t-test (p = 0.089). However, the difference of AUC of ICP including all values approached significance at p=0.065 when analyzed using a Wilcoxon rank-sum test.
Figure 7.
Area under the curve for ICP greater than 20 mmHg (mmHg X time).
Average fluid removal throughout the treatment period for RVOT treatment was 1.75 ml, versus 1.48 ml for Controls (excluding CSF samples taken for analysis). This difference was not significant (p=.46).
Neurological Assessments
No group differences were seen in neurological assessments. In the Treat Retrieval Test, average score following RVOT treatment was 3.80 (n=5), and following EVD treatment was 3.75 (n=4). Three (one RVOT, two controls) animals died or were euthanized before this test was performed. From a safety perspective, it is important to note that four of five RVOT treated animals made no errors with a perfect score of 4.
Radiological Studies
Edema estimates of the injury region were based on ADC maps from DWI sequences obtained pre-treatment and at 24 hours post-injury. Pre-treatment ADC at the site of the trauma increased from 0.735 ± .047 to 1.135 ± .063 at 24 hours after RVOT treatment (p=.004, paired t-test). In the EVD control group, there was no change over the observation period with EVD in controls, with pretreatment ADC of 0.825 ± .053 and post-treatment ACD of 0.932 ± .10 (p=.41) (see Table 2). The contralateral uninjured hemisphere showed no difference in ADC between RVOT and Controls (p=.58).
MR images showed that the treatment catheter generally intercepted the anterior portion of the right lateral ventricle (see Figure 3). In one case, an EVD catheter did not appear to pass through the ventricle, but fluid removal by that catheter was on target. No correlation was found between the catheter trajectory and either ICP or ADC values for either group. Fluid removal also appeared to be independent of the catheter location.
Histology
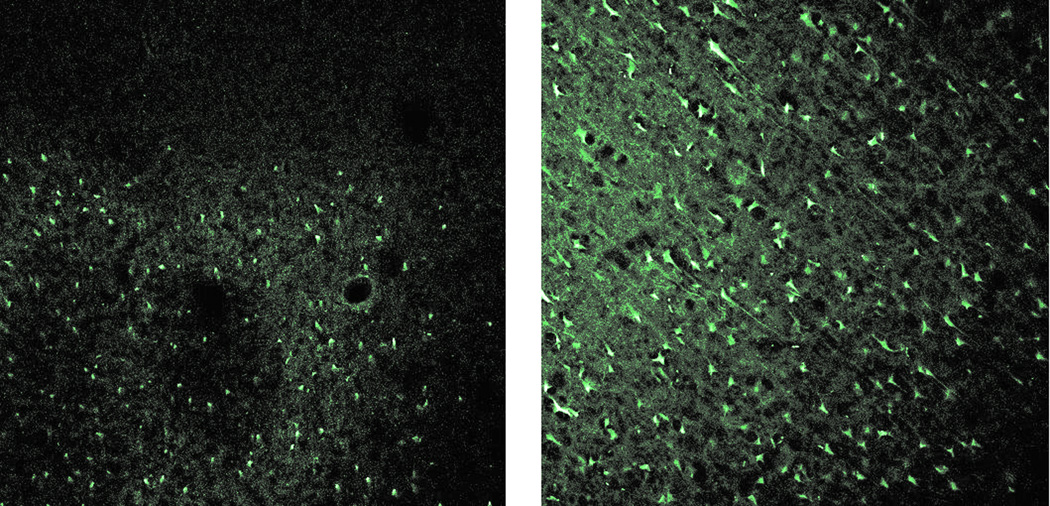
In sections stained with FJB, RVOT pigs had no evidence of neuronal degeneration. In EVD treated animals, the average neuronal degeneration score was 2.6±0.4, which was significantly higher than the grade of 1.0 for all RVOT pigs (p=0.02, Wilcoxon rank-sum test). Figure 8 shows typical FJB stained sections. Some non-neuronal cells are stained in the section from the RVOT treated pig, but few neurons appear. In contrast, a large number of neurons, along with non-neuronal cells, are seen in the EVD treated pig section.
Figure 8.
Photographs of Fluoro-Jade B stained tissue from trauma region after RVOT (Left) and EVD (right) treatment. Degenerating neuronal cells are clearly evident in the EVD section (Grade = 4), but not in the RVOT section (Grade = 1), where only non-neuronal cells are stained. 10X magnification.
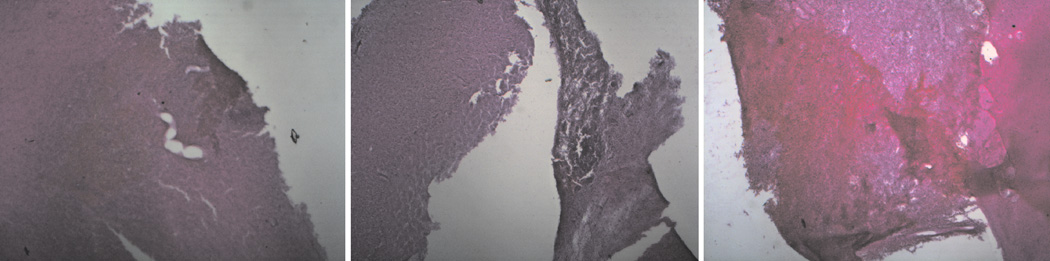
In sections stained with H&E, the average hemorrhage grades were 2.7 for RVOT pigs and 2.5 for EVD pigs, which are not significantly different (p=.78), indicating no effect of RVOT on the degree of hemorrhage. Figure 9 shows typical H&E stained sections with scores between 2 and 4.
Figure 9.
Photographs of hematoxylin & eosin (H&E) stained tissue sections taken from the contusion site demonstrating typical injury severity for graded 2 (left), 3 (middle), and 4 (right). 2.5X magnification.
DISCUSSION
The study results provided a robust demonstration that the RVOT catheter can remove free water from CSF in vivo and thereby increase osmolarity. The study also provided evidence of reduction in cerebral swelling, which resulted in improved metabolic profiles, histology, and measured ADC values in the area of the injury.
RVOT significantly increased ventricular CSF osmolarity. The aim was to choose a high rate of water removal to establish that the effect demonstrated in vitro can be accomplished in vivo. There was no attempt to modulate the rate of water removal based on monitoring CSF osmolarity during the course of treatment, but that can easily be accomplished in clinical studies.
To achieve the target rates of water removal, we theorized that, ideally, the entire hollow fiber portion should be placed within the ventricle. Although ideal placement was not accomplished, there was a very significant rise in CSF osmolarity, showing that partial exposure of the catheter to ventricular CSF may be all that is required. This is especially significant considering the high incidence of collapsed ventricles after severe TBI.
Analysis of metabolites demonstrated that RVOT improved the metabolic profile of the tissues. There was no significant change in CSF or serum potassium, which indicates no cell lysis or ion shifts. RVOT treatment also improved lactate, stabilized calcium, and improved the glucose/lactate ratio. This is evidence that cells were maintained on normal oxidative pathways with RVOT treatment. Elevated lactate has been identified as an early marker of brain injury severity.28 The lactate/pyruvate ratio is thought to be a better metabolic indicator, but pyruvate was not measured in this study.
Further evidence of reduced cellular edema is the finding of higher ADC values in the area of injury with RVOT treatment. Reduced ADC values have been shown to be a sensitive indicator of cytotoxic brain edema.29,30 The findings of lower ADC values in the region of injury in “control” pigs would be most consistent with cytotoxic injury, and hence, the lack of a corresponding decrease in ADC values in the pigs with RVOT would suggest that these pigs suffered relatively less cytotoxic insult. Future studies with measurement of ADC values at greater than 30 days would help to confirm that the relatively higher ADC measured represents a benefit conferred by RVOT.
Histology studies confirmed the viability of cells after RVOT treatment. The Treat Retrieval Test showed no neurological deficit in either RVOT or EVD groups. The fact that RVOT treatment showed no functional defect can be interpreted as evidence of the safety of RVOT, even with high CSF osmolarity achieved during this study. Thus, there did not seem to be any evidence of effect of elevated CSF osmolarity on choroid plexus function, hypersomolar demyelination, or late hydrocephalus.
The finding of significant differences of ADC, specifically in the area of the injury, was not anticipated; however, this may be a very important aspect of RVOT treatment in that RVOT may be most effective in treating the most edematous tissue, even at some distance from the ventricle. Systemic osmotherapy is most effective when there is adequate perfusion of the tissue and a high reflection coefficient. One concern is that the normal, well perfused hemisphere may contract due to systemic therapy, which may actually allow the poorly perfused, injured hemisphere to swell. This effect should not occur with RVOT, which is not dependent on systemic perfusion to be effective.
Improved control of ICP by RVOT was not proven, although there was a favorable trend in all parameters. Changes between pre and post treatment ICP would have been compelling, but because pretreatment ICP trended lower in RVOT animals, extensive analysis or claims are not warranted for this underpowered aspect of the study. Placement of the animals in the supine position caused an immediate rise in measured ICP. Adjusting for the height of the fluid column above the point of measurement, actual ICP readings were estimated to be 10–15 mmHg lower that what was measured, which is consistent with mild injury. With significant injury accompanied by acutely elevated ICP and reduced compliance, the effect of volume reduction by RVOT on ICP may be more robust. The peak ICP population effect size (Cohen’s D) was 1.2, indicating there likely would have been a significant difference in ICP with a larger sample size.
Improved Mass Transport
Reduction of cellular swelling is one of the most challenging aspects of treatment of cerebral edema, and is not accomplished with EVD. This study has confirmed the findings of improved mass transfer with RVOT that was demonstrated in previous ex vivo studies25, and this may be an important and unique benefit of RVOT. An osmotic gradient to reduce cellular swelling may be the key to treatment. It is interesting to note that convective fluid flow also seemed to be improved with RVOT in that measurable interstitial fluid was recovered in 5/6 animals, versus only 2/6 in controls. Also, free water removed with RVOT treatment exceeded the volume of CSF removed with EVD, even though the EVD was pump-driven and not passive.
Clinical Use of RVOT
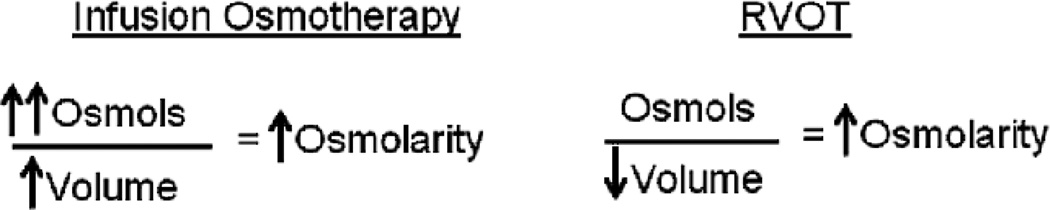
RVOT can increase osmolarity by removing water rather than increasing osmotic load. See Figure 10. With either intravenous or intraventricular osmotherapy, the osmotic agent must be delivered in solution, which increases both the total body osmotic load as well as the fluid volume. RVOT decreases intracranial water, which reduces volume and does not add to osmotic load. A reduction in water volume proportionally increases osmolarity. This has significance for clinical use where ventricular volumes may be reduced or where pretreatment CSF osmolarity may be elevated. CSF osmolarity after TBI with or without systemic osmotherapy has been shown to be as high as 329 mosmols/kg (unpublished data). As demonstrated in vitro, RVOT is more efficient with small volumes or high osmolarity.25 Thus, RVOT may actually be most effective in frequent clinical scenarios of small or collapsed ventricles with elevated CSF osmolarity and reduced CSF turnover.
Figure 10.
Comparison of Infusion Osmotherapy and RVOT. Current methods of osmotherapy involve delivery of an osmotic agent, but the agent must be in a solution, so overall fluid volume is increased. With RVOT, osmolarity is increased by removal of water, so overall fluid volume is decreased. Intracranial infusion specifically is contraindicated in cases of cerebral edema.
While the natural history of CSF osmolarity may involve marked elevation, the objective is to further increase CSF osmolarity only by enough to reverse the gradient of osmotic and hydrostatic pressures that tend to favor movement of water into injured brain tissue. As exemplified by Starlings formulation,3 water movement is influenced by the sum of gradients, so an increase of CSF osmolality of only 1–2 mosmls/kg (approximately 20 to 40 mmHg hydrostatic pressures by van’t Hoff’s law) may be enough to reduce tissue edema. Ideally, the rate of water movement out of the cerebroventricle by RVOT will equal the rate of water movement from edematous brain into the cerebroventricles, so a slightly elevated but steady state of CSF osmolarity can be achieved. The simplest method to control CSF osmolarity is to turn off or restart airflow based on ventricular ICP values. CSF osmolality can be followed directly by assaying drained CSF for osmolality.
Limitations
This was a small sample size. Even so, many variables were statistically different with treatment, and many parameters had clinically relevant Cohen’s D calculations. The difference in CSF osmolarity was very robust, which met our main objective to demonstrate that RVOT can increase osmolarity in vivo after TBI. It is important to note that although many group differences failed to reach statistical significance, every analyzed variable trended toward improved outcome with RVOT. Difficulty in accurate placement of both the RVOT and EVD catheters was noted, even though there seemed to be no effect on fluid removal or outcomes. Accurate freehand placement of EVD drainage catheters has been noted to be challenging clinically.31
Treatment of the Control Animals
We decided the best approach for EVD in controls was to match the RVOT rate of water removal (.15 ml/hr) which was calculated to be sufficient to raise osmolarity. This was determined to be a more relevant "control" treatment than no CSF drainage at all, which probably would have resulted in even higher peak ICP levels. Use of a pump for controlled drainage was preferable to the standard manner of passive drainage of CSF, thereby reducing the variability in fluid drainage volume and allowing a more direct comparison to RVOT. A negative correlation was seen in both RVOT pigs (R2=.69) and Controls (R2=.31) between post-treatment CSF lactate level and total fluid removal. Fluid removal was not strongly correlated with any other outcome measures.
Future Studies
With demonstration of efficacy and safety in this large animal model of TBI, we propose that a human study be considered. Numerous proposed TBI treatments have shown positive preclinical results but were ineffective in clinical trials. The potential benefit of clinical studies outweighs the risk of the dual function RVOT: Because the catheter can be used for free water removal as well as standard EVD function, initial placement of the RVOT catheter and short periods of RVOT treatment will allow initial human studies without placement of both a standard and experimental device. Short periods of treatment and adequate periods of observation off treatment will be possible so that potential adverse effects can be discovered.
CONCLUSION
RVOT resulted in a marked increase in CSF osmolarity after experimental TBI in this controlled preclinical study. We conclude that RVOT has the potential to reduce brain swelling in severe TBI, thus minimizing secondary brain injury and its aftermath. The study established a robust mechanism, showed compelling evidence of efficacy, and demonstrated safety in a large animal model. With demonstration of safety and development of mechanisms to control the water rates, human clinical studies are indicated.
Acknowledgements
The authors wish to acknowledge the contributions of Dr.Mehmet Bilgen who developed our CCI device; Dr.Art Wallace, Anesthesiologist, Dr. David Saloner, Dr. Gabriel Acevedobolton and Dr. Max Wintermark, UCSF/VA, Ms. Nadine E. Walas for timely and valuable research assistance, Ms. Erin Moody for assistance with experimental work and animal care, Dongmin Wang for assistance with histological preparations, Richard G. Holcomb for statistical analysis, and Mary Helen Schmidt for editing and preparation of the manuscript.
Financial Disclosure:
This work was supported by the National Institute of Neurologic Disorders and Stroke (NIH/SBIR Grant # NS43832 to Twin Star Medical, Inc.). All authors received grant support through this NIH grant. Dr. Rick Odland is the Medical Director and Cofounder of Twin Star Medical, Inc., a company that is developing Reductive Ventricular Osmotherapy technology. He has equity in Twin Star Medical and received salary support for this work. John Borgos is an employee of Twin Star Medical and received financial support for this work. Twin Star Medical has provided hourly support for the writing of this paper. Alex McKinney was a consultant on the grant. Gaylan Rockswold was a consultant on the grant and is currently a consultant to Twin Star Medical; he has less than 5% equity in Twin Star Medical Inc.
This manuscript describes a preclinical evaluation of a novel approach to reducing brain edema. The clear findings are that the investigators were able to increase osmolarity within the CSF using the RVOT device. In addition, the investigators found reductions in ICP during the last eight hours of treatment, improved ADC values in peri-ventricular traumatized brain suggesting reduced edema, and reduced Fluoro-Jade B staining of neurons in traumatized brain. This is the first really innovative approach to treatment of TBI that has been proposed in recent years, and the early results appear promising.
There are a number of practical issues that will have to be solved related to optimal positioning of the catheter in patients, and it is not known how well this strategy will work over longer periods of time, but an effective treatment of cerebral edema would be a welcome addition to our treatment regimens.
Claudia Robertson, Houston, Texas
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Previous Presentation:
Portions of this work were presented in poster form at the 26th Annual National Neurotrauma Society Symposium, July 27–30, 2008 (Venugopal et al. A new large animal model of traumatic brain injury), the Second Joint Symposium of the International and National Neurotrauma Societies, September 7–11, 2009 (Venugopal et al. Reductive Ventricular Osmotherapy reduces cellular edema and improves brain metabolism in a swine model of traumatic brain injury), and at the 39th Annual Society for Neuroscience Meeting, October 17–21, 2009 (Venugopal et al. Ventricular osmotherapy reduces brain edema and improves brain metabolism in a swine model of traumatic brain injury).
REFERENCES
- 1.Liang D, Bhatta S, Gerzanich V, Simard JM. Brain oedema in focal ischaemia: molecular pathophysiology and theoretical implications. Neurosurg Focus. 2007;22(5):E2. doi: 10.1016/S1474-4422(07)70055-8. (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marmarou A, Signoretti S, Fatouros PP, Portella G, Aygok GA, Bullock MR. Predominance of cellular edema in traumatic brain swelling in patients with severe head injuries. J Neurosurg. 2006;104:720–730. doi: 10.3171/jns.2006.104.5.720. [DOI] [PubMed] [Google Scholar]
- 3.Simard JM, Kent TA, Chen M, Tarasov KV, Gerzanich V. Brain oedema in focal ischemia: molecular pathophysiology and theoretical implications. Lancet Neurol. 2007;6:258–268. doi: 10.1016/S1474-4422(07)70055-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bullock MR, Povlishock JT, editors. J Neurotrauma. Vol. 24. 2007. Guidelines for the management of severe traumatic brain injury: 3rd Edition. [DOI] [PubMed] [Google Scholar]
- 5.Vialet R, Albanèse J, Thomachot L, et al. Isovolume hypertonic solutes (sodium chloride or mannitol) in the treatment of refractory posttraumatic intracranial hypertension: 2 mL/kg 7.5% saline is more effective than 2 mL/kg 20% mannitol. Crit Care Med. 2003;31(6):1683–1687. doi: 10.1097/01.CCM.0000063268.91710.DF. [DOI] [PubMed] [Google Scholar]
- 6.Battison C, Andrews PJD, Graham C, Petty T. Randomized, controlled trial on the effect of a 20% mannitol solution and a 7.5% saline/6% dextran solution on increased intracranial pressure after brain injury. Crit Care Med. 2005;33:196–202. doi: 10.1097/01.ccm.0000150269.65485.a6. [DOI] [PubMed] [Google Scholar]
- 7.Harutjunyan L, Holz C, Rieger A, Menzel M, Grond S, Soukup J. Efficiency of 7.2% hypertonic saline hydroxyethyl starch 200/0.5 versus mannitol 15% in the treatment of increased intracranial pressure in neurosurgical patients: A randomized clinical trial. Crit Care. 2005;9:R530–R540. doi: 10.1186/cc3767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tyagi R, Donaldson K, Loftus CM, Jallo J. Hypertonic saline: a clinical review. Neurosurg Rev. 2007;30:277–290. doi: 10.1007/s10143-007-0091-7. [DOI] [PubMed] [Google Scholar]
- 9.Francony G, Fauvage B, Falcon D, et al. Equimolar doses of mannitol and hypertonic saline in the treatment of increased intracranial pressure. Crit Care Med. 2008;36:795–800. doi: 10.1097/CCM.0B013E3181643B41. [DOI] [PubMed] [Google Scholar]
- 10.Rockswold GL, Solid CA, Paredes-Andrade E, Rockswold SB, Jancik JT, Quickel RR. Hypertonic saline and its effect on intracranial pressure, cerebral perfusion pressure, and brain tissue oxygen. Neurosurg. 2009;65:1035–1042. doi: 10.1227/01.NEU.0000359533.16214.04. [DOI] [PubMed] [Google Scholar]
- 11.Peterson B, Khanna S, Fisher B, Marshall L. Prolonged hypernatremia controls elevated intracranial pressure in head-injured pediatric patients. Crit Care Med. 2000;28:1136–1143. doi: 10.1097/00003246-200004000-00037. [DOI] [PubMed] [Google Scholar]
- 12.Diringer MN, Zazulia AR. Osmotic Therapy Fact and Fiction. Neurocrit Care. 2004;1:219–233. doi: 10.1385/NCC:1:2:219. [DOI] [PubMed] [Google Scholar]
- 13.van Santbrink H, Maas A, Avezaat CJJ. Continuous monitoring of partial pressure in brain tissue oxygen in patients with severe head injury. Neurosurg. 1996;38(1):21–31. doi: 10.1097/00006123-199601000-00007. [DOI] [PubMed] [Google Scholar]
- 14.Le Roux PD, Newell DW, Lam AM, Grady MS, Winn HR. Cerebral arteriovenous oxygen difference: a predictor of cerebral infarction and outcome in patients with severe head injury. J Neurosurg. 1997;87:1–8. doi: 10.3171/jns.1997.87.1.0001. [DOI] [PubMed] [Google Scholar]
- 15.Kerwin AJ, Schinco MA, Tepas JJ, Renfro WH, Vitarbo EA, Muehlberger M. The use of 23.4% Hypertonic Saline for the Management of Elevated Intracranial Pressure in Patients with Severe Traumatic Brain Injury: A Pilot Study. Journal of Trauma. 2009;67(2):277–282. doi: 10.1097/TA.0b013e3181acc726. [DOI] [PubMed] [Google Scholar]
- 16.Hossman K. In: Dynamics of Brain Edema. Pappius HM, Feindel W, editors. Springer-Verlag; 1976. pp. 219–227. [Google Scholar]
- 17.Bandaranayake NM, Nemoto EM, Stezoski SW. Rat brain osmolality during barbiturate anesthesia and global brain ischemia. Stroke. 1978;9(3):249–254. doi: 10.1161/01.str.9.3.249. [DOI] [PubMed] [Google Scholar]
- 18.Hatashita S, Hoff JT. Role of a hydrostatic pressure gradient in the formation of early ischemic brain edema. J Cereb Blood Flow Metab. 1986;6(5):546–552. doi: 10.1038/jcbfm.1986.100. [DOI] [PubMed] [Google Scholar]
- 19.Odland RM, Sutton RL. Hyperosmosis of cerebral injury. Neurol Res. 1999;21(5):500–508. [PubMed] [Google Scholar]
- 20.Kawamata T, Mori T, Sato S, Katayama Y. Tissue hyperosmolality and brain edema in cerebral contusion. Neurosurg Focus. 2007;22(5):E5. doi: 10.3171/foc.2007.22.5.6. [DOI] [PubMed] [Google Scholar]
- 21.Chen KC, Nicholson C. Changes in brain cell shape create residual extracellular space volume and explain tortuosity behavior during osmotic challenge. Proceedings of Nat'l Academy of Sci of USA. 2000;97(15):8306–8311. doi: 10.1073/pnas.150338197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sutton RS, Quist H, Mathews W, Odland RM. Intraventricular albumin infusion reduces experimental cerebral edema. Abstracts of the 17th Annual National Neurotrauma Society Meeting, Miami, FL, October, 1999. J Neurotrauma. 16(10) [Google Scholar]
- 23.Onal C, Unal F, Turantan MI, et al. The effect of intraventricular albumin in experimental brain oedema. Acta Neurochir (Wien) 1997;139(7):661–669. doi: 10.1007/BF01412002. [DOI] [PubMed] [Google Scholar]
- 24.Is M, Uzan M, Unal F, Kiris T, Tanriverdi T, Mengi M, Kilic N. Intraventricular albumin: an optional agent in experimental post-traumatic brain edema. Neurol Res. 2005;27:67–72. doi: 10.1179/016164105X18296. [DOI] [PubMed] [Google Scholar]
- 25.Odland RM, Panter SS, Rockswold GL. The Effect of Reductive Ventricular Osmotherapy on the Osmolarity of Artificial Cerebral Spinal Fluid and the Water Content of Cerebral Tissue, Ex Vivo. J Neurotrauma. 2011 Jan;28(1):135–142. doi: 10.1089/neu.2010.1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Venugopal S, Coppes V, Moody E, Panter S. A new large animal model of traumatic brain injury. Abstracts from the 26th Annual National Neurotrauma Society Symposium, Orlando, FL. J Neurotrauma. 2008;25:930. [Google Scholar]
- 27.Schmued LC, Hopkins KJ, Fluoro-Jade B. a high affinity fluorescent marker for the localization of neuronal degeneration. Brain Res. 2000 Aug 25;874(2):123–130. doi: 10.1016/s0006-8993(00)02513-0. [DOI] [PubMed] [Google Scholar]
- 28.Makoroff KL, Cecil KM, Care M, Ball WS. Elevated lactate as an early marker of brain injury in inflicted traumatic brain injury. Pediatr Radiol. 2005;35:668–676. doi: 10.1007/s00247-005-1441-7. (2005). [DOI] [PubMed] [Google Scholar]
- 29.Sevick RJ, Kanda F, Mintorovitch J, et al. Cytotoxic brain edema: assessment with diffusion-weighted MR imaging. Radiol. 1992;185:687–690. doi: 10.1148/radiology.185.3.1438745. [DOI] [PubMed] [Google Scholar]
- 30.Barzó P, Marmarou A, Fatouros P, Hayasaki K, Corwin F. Contribution of vasogenic and cellular edema to traumatic brain swelling measured by diffusion-weighted imaging. J Neurosurg. 1997;87:900–907. doi: 10.3171/jns.1997.87.6.0900. [DOI] [PubMed] [Google Scholar]
- 31.Huyette DR, Turnbow BJ, Kaufman C, Vaslow DF, Whiting BB, Oh MY. Accuracy of the freehand pass technique for ventriculostomy catheter placement: retrospective assessment using computed tomography scans. J Neurosurg. 2008;108:88–91. doi: 10.3171/JNS/2008/108/01/0088. [DOI] [PubMed] [Google Scholar]