Abstract
One of the major goals of synthetic biology is the development of non-natural cellular systems. In this work we describe a catalytic biomimetic coupling reaction capable of driving the de novo self-assembly of phospholipid membranes. Our system features a copper catalyzed azide-alkyne cycloaddition that results in the formation of a triazole containing phospholipid analog. Concomitant assembly of membranes occurs spontaneously, not requiring preexisting membranes to house catalysts or precursors. The substitution of efficient synthetic reactions for key biochemical processes may offer a general route toward synthetic biological systems.
Keywords: Synthetic Biology, Bioorthogonal Reactions, Nanotechnology, Phospholipids
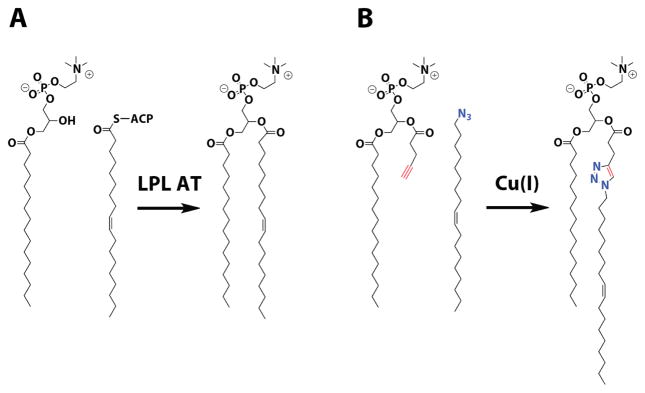
Designing synthetic substitutes for evolved biochemical processes is a strategy towards developing artificial cellular systems.1 Here we describe a catalytic biomimetic coupling reaction capable of driving the de novo self-assembly of phospholipid membranes, organizing structures ubiquitous to all cells. In bacteria and eukaryotes, membrane assembly results from acyl transfer reactions that couple single-chain amphiphiles into membrane-forming phospholipids (Figure 1A). These reactions are catalyzed by enzyme complexes in vivo, which, along with the machinery involved in fatty acid activation, ensure the specificity of phospholipid synthesis.2 We envisioned mimicking this biological system by using a synthetic catalytic coupling reaction. We chose the copper catalyzed azide-alkyne cycloaddition because of its robustness in water, the solvent necessary for bilayer assembly through the hydrophobic effect.3 The reaction also benefits from high selectivity and a nearly nonexistent background in the absence of catalyst, features that are reminiscent of biochemical processes.
Figure 1.
Biomimetic synthesis of phospholipid membranes. A) Native phospholipid synthesis relies on acyl transfer reactions between lysophospholipids and thioester activated fatty acids, which are catalyzed by membrane-associated enzymes. B) This process is mimicked by copper catalyzed cycloaddition between an alkyne lysolipid substitute and oleyl azide. The resulting triazole is an analog of the natural phospholipid POPC. LPL AT, lysophospholipid acyltransferase, ACP, acyl carrier protein.
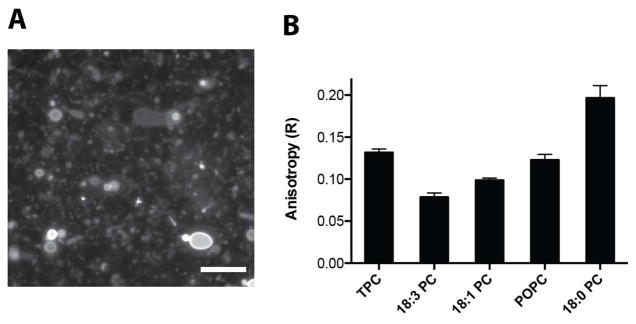
To mimic phospholipid synthesis, we designed substrates to replace the native precursors of the common phospholipid 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC): an oleyl azide in lieu of oleic acid and an alkyne analog of the lysophospholipid 1-palmitoyl-sn-glycero-3-phosphocholine (Figure 1B). The cycloaddition product resembles POPC, with the exception of a triazole linker. As expected, neither the azide nor the alkyne formed membranes in aqueous solution. However the purified triazole product, when hydrated, readily formed large membrane vesicles visible under fluorescence microscopy (Figure 2A). Steady-state anisotropy measurements with the membrane fluidity probe diphenylhexatriene (DPH)4 indicated that the triazole-containing membranes are well ordered with fluidity (Figure 2B) and chain melting temperatures (Figure S1) comparable to native POPC membranes.
Figure 2.
Characterization of triazole phospholipid vesicles. A) Fluorescence microscopy of membrane containing vesicles formed from hydrating a thin film of triazole phospholipid. Membranes were stained using 10 μM Rh-DHPE dye. Scale bar denotes 15 μ. B) Steady-state anisotropy of DPH in membranes formed from triazole phospholipid (TPC) compared to native phosphocholines, with the indicated acyl chains. The unit-less anisotropy ratio (R) is a measure of the acyl packing of the bilayer, with higher values indicating a more-ordered membrane (Methods in Supporting Information).
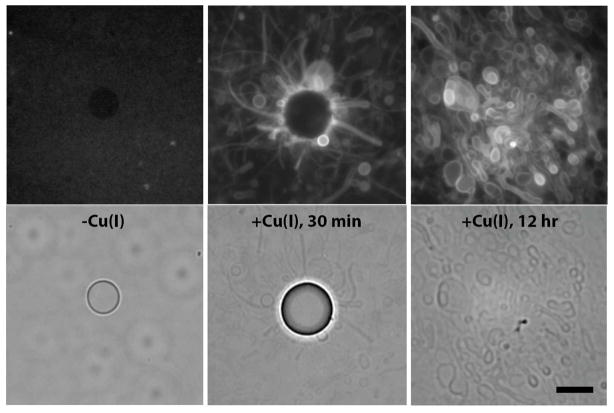
Because of the triazole coupling reaction’s aqueous compatibility, we hypothesized that we could use it to drive spontaneous membrane assembly in situ. Membrane assembly has been observed as a result of pH changes,5 solvent exchanges,6 or application of electric fields7. Here it is driven by covalent bond formation, which both lowers the solubility of the substrates (Figure S2) and switches the aggregate state from micelles to bilayers. After adding catalytic copper to an aqueous emulsion of oleyl azide oil and the alkyne surfactant, we observed the formation of large vesicle structures, both spherical and tubular, on the periphery of the oleyl azide oil droplets (Figure 3). After 24 hours, with no agitation of the solution, the oil droplets were largely consumed and fields of large (> μm) heterogenous vesicles remained. Time-lapse fluorescence microscopy revealed that the vesicles budded off from the oil droplet as tubules (Movie S1), in a manner similar to thin film hydration. Though our initial experiments were carried out in either distilled water or HEPES buffer, we observed similar results in numerous physiologically relevant buffers, a solvent tolerance that is typical of such triazole coupling reactions. To confirm that the resulting structures were membrane compartments, we included a polar fluorophore, 8-hydroxypyrene-1,3,6-trisulfonic acid (HPTS), in the reaction buffer and observed encapsulation with fluorescence microscopy (Figure S3A) and size-exclusion chromatography (Figure S3B). Neither membrane self-assembly nor encapsulation were observed in the absence of copper catalyst.
Figure 3.
Spontaneous vesicle assembly driven by triazole phospholipid synthesis. An aqueous emulsion of oleyl azide (5 mM) and alkyne lysolipid (5 mM) imaged without catalyst (left), 30 minutes after addition of catalyst (0.25 mM) (middle), or 12 hours after addition of catalyst. Top panels are fluorescence micrographs of vesicles using a membrane dye (Rh-DHPE, 2 μM). Bottom row consists of the corresponding phase contrast images. Before addition of the catalyst, only the emulsion oil droplets are visible and phospholipid membranes are not present. Shortly after addition of copper, several vesicle and tubular structures are observed, a large number of which are at the periphery of the azide oil droplets. After 12 hours, the oil droplets are consumed and replaced with large fields of vesicles. Scale bar denotes 10 μ.
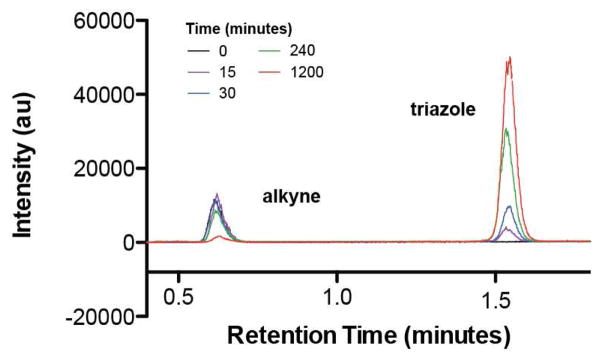
To determine if membrane formation coincided with the synthesis of triazole phospholipid, we analyzed the reaction over time using combined liquid chromatography, mass spectrometry, and light scattering measurements (Figure 4). Addition of copper catalyst led to triazole formation within minutes, and progressed to near completion over a period of 20 hours, correlating well with experimental observation.
Figure 4.
HPLC/ELSD traces monitoring the cycloaddition reaction progress. The time-scale of alkyne lysolipid consumption and triazole phospholipid formation is consistent with that of observed vesicle self-assembly. The alkyne lysolipid and triazole phospholipid retention times were verified by mass spectrometry and use of known standards.
Our observations of vesicle assembly support a model in which the reaction takes place primarily at the interface of the insoluble oleyl azide emulsion droplets and the isotropic alkyne lysolipid analogs, which exist as micelles in the aqueous solvent (Figure 5). However we cannot rule out the possibility that the reaction also occurs in mixed azide/alkyne micelles. Controlling the interface between azide and alkyne, either through use of carrier agents or fluidics, may prove to be a route to controlling membrane assembly in this system.
Figure 5.
Model of membrane assembly. Reactive azide oil droplets (green) interact with alkyne lipid micelles in solution (blue) forming an emulsion. After addition of copper catalyst, the cycloaddition begins to take place, primarily at the interface between the oil droplets and aqueous solution. The reaction results in the formation of phospholipid membranes at the substrate interface. After time, the oil droplets are consumed and replaced with spherical and tubular vesicles composed of phospholipid membranes.
Despite its simplicity, our unnatural approach to phospholipid synthesis shares key characteristics with evolved enzymatic reactions, including: 1) hydrolytically stable substrates, which are unreactive in the absence of catalyst 2) robust and highly specific product formation in the presence of a multi-turnover catalyst and 3) compatibility with aqueous solvents, which leads to organization of the product into bilayer membranes. These features are in contrast to traditional synthetic reactions, which utilize highly reactive substrates and are challenged by reaction specificity and solvent compatibility, with water often acting as a competing nucleophile. Notably, our method provides an advantage over enzymatic systems: natural lipid acyltransferases are primarily membrane bound, requiring pre-existing membranes to function, and are thus unlikely to be useful for de novo membrane assembly. Furthermore, previous attempts to reconstitute acyltransferase enzyme activity using activated natural precursors have met with poor results, likely due to the complexity of the enzymes and inherent difficulties of reconstitution in synthetic systems.8 In contrast, our approach uses highly selective azide/alkyne reactive groups and an exceedingly simple hydrated copper ion as the catalyst. The minimal nature of our approach will likely lend itself to further elaboration, as we envision incorporating this system into a fully synthetic cell. We are also exploring practical applications of triazole membrane assembly, for instance in packaging and delivering therapeutics, improving transfection efficiencies, reconstituting functional membrane proteins, and performing confined biochemical reactions.
Supplementary Material
Acknowledgments
NIH grant K01EB010078
The authors gratefully acknowledge Jack W. Szostak and Na Zhang for helpful discussions. This work was funded in part by NIH grant K01EB010078.
ABBREVIATIONS
- LPL AT
lysophospholipid acyltransferase
- ACP
acyl carrier protein
- POPC
1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine
- DPH
diphenylhexatriene
- RhDHPE
lissamine rhodamine B 1,2-dihexadecanoyl-sn-glycero-3-phosphoethanolamine
- HPTS
8-hydroxypyrene-1,3,6-trisulfonic acid
- HPCL
high performance liquid chromatography
- ELSD
evaporative light scattering detector
Footnotes
Author Contributions
The manuscript was written through contributions of all authors. / All authors have given approval to the final version of the manuscript. /
Supporting Information. Experimental details. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Szostak JW, Bartel DP, Luisi PL. Nature. 2001;409:387–390. doi: 10.1038/35053176. [DOI] [PubMed] [Google Scholar]
- 2.Shindou H, Shimizu T. J Biol Chem. 2009;284:1–5. doi: 10.1074/jbc.R800046200. [DOI] [PubMed] [Google Scholar]
- 3.Rostovtsev VV, Green LG, Fokin VVBSK. Angew Chem Int Ed. 2002;41:2596–2599. doi: 10.1002/1521-3773(20020715)41:14<2596::AID-ANIE2596>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 4.Van Blitterswijk WJ, Van Hoeven RP, Van der Meer BW. Biochim Biophys Acta. 1981;644:323–332. doi: 10.1016/0005-2736(81)90390-4. [DOI] [PubMed] [Google Scholar]
- 5.Walde P, Wick R, Frest M, Mangone A, Luisi PL. J Am Chem Soc. 1994;116:11649–11654. [Google Scholar]
- 6.Pautot S, Frisken BJ, Cheng JX, Xie XS, Weitz DA. Langmuir. 2003;19:10281–10287. [Google Scholar]
- 7.Angelova MI, Dimitrov DS. Faraday Discuss Chem Soc. 1986;81:303–311. [Google Scholar]
- 8.Schmidl PK, Schurtenberger P, Luisi PL. Journal of the American Chemical Society. 1991;113:8127–8130. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.