Abstract
The Krebs tricarboxylic acid cycle (TCA) is central to metabolic energy production and is known to be altered in many disease states. Real time molecular imaging of TCA cycle in vivo will be important in understanding the metabolic basis of several diseases. Positron emission tomography (PET) using FDG-glucose (2-[18F]fluoro-2-deoxy-D-glucose) is already being used as a metabolic imaging agent in clinics. However, FDG-glucose does not reveal anything past glucose uptake and phosphorylation. We have developed a new metabolic imaging agent, hyperpolarized diethyl 1-13C 2,3-d2 succinate, that allows for real time in vivo imaging and spectroscopy of the TCA cycle. Diethyl succinate can be hyperpolarized using parahydrogen induced polarization (PHIP) in an aqueous solution with signal enhancement of 5000 compared to Boltzmann polarization. 13C magnetic resonance spectroscopy (MRS) and magnetic resonance imaging (MRI) were achieved in vivo seconds after injection of 10 to 20 μmol of hyperpolarized diethyl succinate into normal mice. The downstream metabolites of hyperpolarized diethyl succinate were identified in vivo as malate, succinate, fumarate and aspartate. The metabolism of diethyl succinate was altered after exposing the animal to 3-nitropropionate, a known irreversible inhibitor of succinate dehydrogenase. Based on our results, hyperpolarized diethyl succinate allows for in real time in vivo MRI and MRS with a high signal to noise ratio and with visualization of multiple steps of the TCA cycle. Hyperpolarization of diethyl succinate and its in vivo applications may reveal an entirely new regime wherein the local status of TCA cycle metabolism is interrogated on the time scale of seconds to minutes with unprecedented chemical specificity and MR sensitivity.
Keywords: parahydrogen, hyperpolarization, 13C MRI, TCA cycle, cancer imaging, PHIP
INTRODUCTION
The Krebs Tricarboxylic Acid Cycle (TCA) and oxidative phosphorylation are central to metabolic energy production. The TCA cycle occurs in the mitochondria of cells and in most cells produces the majority of adenosine triphosphate (>90%). In normal cells, the main energy source for the TCA cycle is pyruvate that is generated from glycolysis of glucose.1 Biochemistry has revealed that many disease states have perturbed TCA cycles. In cancer, TCA cycle succinate dehyrogenase, isocitrate dehydrogenase and fumarate hydratase oncogenes impair the TCA cycle.2 The TCA cycle can have different entry points and especially in cancer a broad range of energy substrates can be used in the TCA cycle (citrate, glutamate/glutamine).3 There is evidence that the TCA cycle is altered in many neurodegenerative diseases (Alzheimer’s, Parkinson’s, Huntington’s, and amyotrophic lateral sclerosis).4–6 Inhibition of succinate dehydrogenase in the brain by the irreversible inhibitor 3-nitropropionate in animals is a model for Huntington’s disease and these animals produce symptoms and lesions similar to observed in the disease.7 Many of the metabolic differences between disease and normal tissue can and could be examined through the use of metabolic imaging agents.
Presently, metabolic imaging is routinely done with positron emission tomography (PET) measurements of the uptake of FDG-glucose (2-[18F]fluoro-2-deoxy-D-glucose) or magnetic resonance spectroscopy (MRS). PET imaging with FDG-glucose only measures the level of uptake of glucose and phosphorylation and reveals nothing about the subsequent metabolism of glucose. In MRS, only the steady state of a tissue/organ’s metabolic profile can be determined and due to low signal to noise ratio (SNR) the length of the exam can be quite long (13C MRS exam can take hours).8 The invention of hyperpolarized molecules has opened the way to real time metabolic imaging in vivo. Hyperpolarization can lead to sensitivity enhancements of >10,000 fold and the polarization (signal enhancement) can be retained on the metabolites of the hyperpolarized molecule.9-12 In addition unlike PET, the process of hyperpolarization is non-radioactive. Hyperpolarization of compounds that can be metabolized in vivo allows for real time metabolic imaging. The mostly widely used methods for hyperpolarization are dynamic nuclear polarization (DNP) and parahydrogen induced polarization (PHIP). The utility of DNP polarized 1-13C pyruvate in metabolism imaging has been explored extensively.13–17 Hyperpolarized 1-13C pyruvate can be used to follow the metabolism of pyruvate to alanine, lactate and bicarbonate but reveals nothing about TCA cycle metabolism. Recently, DNP polarized 2-13C pyruvate was administered to isolated perfused rat hearts and hyperpolarized TCA metabolites glutamate and citrate were detected18 however, when the hyperpolarized compound was used for metabolic imaging in the brain in vivo, only 2-13C lactate was detected.19
In our laboratory, we recently used PHIP method to hyperpolarize succinate, 2-hydroxy ethyl propionate and 2,2,3,3-tetrafluoropropy-1-13C propionate-d2,2,3,3 (TFPP) for in vivo applications.20–22 However, all of these compounds currently have physiological barriers to being used in the clinic. For 13C succinate, the polarization transfer has to be done at acidic pH ≤ 3 or alkaline pH ≥ 9 for optimum hyperpolarization.20 It is known that the dicarboxylic acid succinate is only poorly transported across many biological membranes and in particular barely crosses the mitochondrial membrane to gain access to TCA cycle enzymes involved in metabolism.23,24 2-hydroxy ethyl propionate is toxic and is not metabolized.21 TFPP is not very water soluble and has to be injected in a 20% ethanol aqueous solution.22 We have overcome these hurdles by designing and developing hyperpolarized diethyl succinate. This hyperpolarized compound is better suited for translational research and has potential of being used as a diagnostic imaging agent. We describe the whole development process from synthesis, polarization, and finally in vivo spectroscopy and imaging of the compound and its metabolites in live normal mice.
Diethyl succinate is a neutral molecule, can be used at physiological pH and crosses biological membranes.25–28 There is a significant amount of biochemical research on diethyl succinate illustrating the compound’s ability to be incorporated into cells in tissue culture and to be metabolized by the TCA cycle.25,26 In addition, diethyl succinate is known to be non-toxic29 and is often used in fragrances and flavoring.
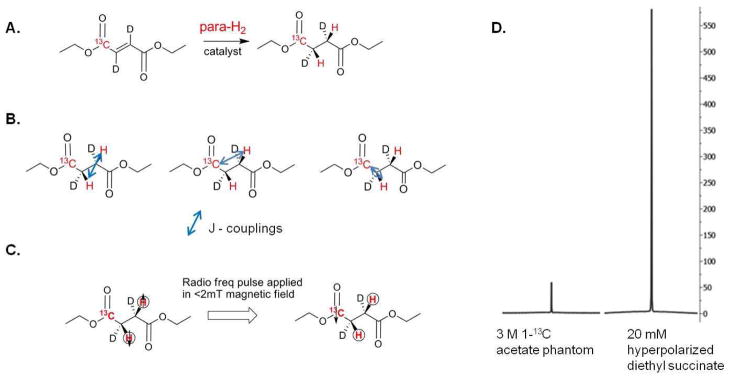
PHIP method uses parahydrogen gas to hydrogenate an unsaturated organic molecule (alkyne or alkene) which is labeled with 15N or 13C.9,10 A catalyst is used to transfer the hydrogens as a unit on the compound without scrambling the spin state.11,21,30,31 With diethyl succinate, we transfer the spin order to the 13C labeled carbonyl using radio frequency pulses.11,32 We generate diethyl 1-13C 2,3-d2-succinate using hydrogenation of diethyl 1-13C 2,3-d2-fumarate (Figure 1). The hydrogenation and polarization transfer are done in our home built PHIP polarizer which is described in detail elsewhere.33 After diethyl succinate is hyperpolarized, the compound is injected into a mouse or is used as a phantom and 13C spectroscopy and imaging is performed. PHIP method of hyperpolarization is significantly faster than the DNP method. Each sample of hyperpolarized diethyl succinate requires 4 seconds of time for ‘polarization transfer’ and an injectable hyperpolarized sample can be generated every three to four minutes (in contrast to the 90 minutes or more required for hyperpolarization of compounds using DNP).33,34 In these experiments diethyl 1-13C 2,3-d2 fumarate was hydrogenated by parahydrogen and rhodium-catalysis to diethyl 1-3C 2,3-d2 succinate and hyperpolarized to 2.1 ± 0.6 % or 5000 - fold signal enhancement compared to Boltzmann polarization before tail-vein injection (i.v.) or intraperitoneal injection (i.p.) into mice.
Figure 1. Hydrogenation and Polarization.
(A) A schematic of the hydrogenation of diethyl 1-13C 2,3-d2 fumarate to diethyl 1-13C 2,3-d2 succinate with parahydrogen (para-H2) using a rhodium bisphosphine catalyst. (B) The coupling constants of diethyl succinate that are needed to create the heteronuclear radio frequency pulse for polarization transfer to 13C atom. (C) A schematic of the polarization transfer from the parahydrogen to the 13C nucleus using a radio frequency pulse applied in a low magnetic field. (D) The magnitude of a 20 mM hyperpolarized diethyl succinate 13C signal compared to that from a 3 M 1-13C acetate phantom. Each spectrum was taken using a single transient. The ratio of the integrated hyperpolarized signal over the value of the acetate signal is used to determine the percentage of polarization. Hyperpolarization of diethyl succinate leads to 5000 fold sensitivity enhancement over Boltzmann polarization.
The biodistribution and metabolic fate of hyperpolarized 13C succinate was followed in a specifically equipped MR scanner to provide images and spectra in the first two minutes after injection. Different hyperpolarized metabolites of diethyl succinate were detected. Metabolism was seen in all thirteen mice injected with hyperpolarized diethyl succinate either by i.v. or i.p. injection. Based on published work and our data, after injection into a mouse hyperpolarized diethyl succinate is entering cells, the ester functionality is hydrolyzed by esterase and hyperpolarized succinate is then metabolized in the TCA cycle. We further verified this result by monitoring metabolism of hyperpolarized diethyl succinate in vivo before and after the animal was treated with 3-nitropropionate, a known irreversible inhibitor of succinate dehydrogenase.35,36 Using 13C True FISP (fast imaging with steady state precession)37 imaging, we can determine the relative location of hyperpolarized diethyl succinate and its hyperpolarized metabolites in vivo.
Based on our results, we feel that hyperpolarized diethyl succinate has the potential of allowing for direct monitoring in real time metabolism in vivo. In addition, unlike DNP hyperpolarized 1-13C pyruvate where only single step metabolism can be visualized,13-17 hyperpolarized diethyl succinate allows metabolic imaging of multiple steps of the TCA cycle. The metabolism of cells is known to be affected in many diseases and direct monitoring of metabolism in vivo has the potential of being an excellent diagnostic tool for early detection of diseased tissue and monitoring the success of therapy.
MATERIALS AND METHODS
Parahydrogen
Commercially available ultra pure hydrogen (Gilmore, South El Monte, USA) was catalytically converted to parahydrogen (para-H2) by slow passage over granular hydrous ferric oxide (IONEX-type O-P catalyst; Molecular Products Inc., Lafayette, CO, USA) at a temperature of 36–55 K. After the gas was converted to parahydrogen it was stored in 7 L aluminum cylinders at room temperature at a pressure of 33 bar. The quality of para-H2 was determined to be >90% by high resolution NMR.34 Each batch was used within two days, during which there was no measurable decrease in the yield.
Synthesis of diethyl 1-13C 2,3-d2 fumarate
The synthetic method for esterification found in reference38 was used with slight changes. In a dry 150 ml round bottom flask with stir bar, 1g (8.44 mmol) of 1-13C fumaric acid (99% 1-13C, 98% 2,3-d2, CDLM-6062-0, Cambridge Isotopes, Andover, MA) was added. Then 60 ml of anhydrous ethanol and 3.2 ml (25.2 mmol) of chlorotrimethylsilane (386529, Sigma-Aldrich, St. Louis, MO) was added via syringe into the reaction. Reaction was stirred at room temperature, overnight and under an inert atmosphere. Reaction was quenched with 20 ml of saturated sodium bicarbonate solution and solid bicarbonate was added until solution became neutral. Solution was filtered to remove excess bicarbonate and ethanol was removed by rotary evaporation. Product was extracted out of aqueous solution using 3 × 25 ml dichloromethane. Organic layers were combined, dried over anhydrous sodium sulfate, filtered, solvent removed by evaporation and 1.10 g (75% yield) of pure product was isolated as a colorless liquid. 1H (CD2Cl2, 500 MHz) δ 4.23 (complex quartet, J = 7.1, 1.5, 1.6 Hz, 4H), 1.29 (t, J = 7.1 Hz, 6H). Proton-decoupled 13C NMR (CD2Cl2, 125 MHz) δ 165.43 (s), 133.6 (sextet J = 25 Hz, t J = 25 Hz), 61.8 (s), 14.43 (s)
PHIP hydrogenation
A full description of the equipment used to develop high levels of 13C polarization using parahydrogen is given elsewhere,33 together with details of quality assurance procedures for achieving these levels of polarization reproducibly and routinely.34
A water soluble rhodium catalyst developed for the purpose of rapid hydrogenation while preserving the desired spin order was freshly prepared by mixing two components.21,31 The bisphosphine ligand, 1,4-bis-[(phenyl-3-propane sulfonate) phosphine] butane disodium salt (Q36333, Isotec, OH, USA), was dissolved in 9:1 water to D2O to yield 8.25 mM concentration followed by removal of oxygen using vacuum and argon purging. The rhodium catalytic moiety was then introduced to the reaction mixture as a solution of bis(norbornadiene)rhodium (I) tetrafluoroborate (catalog number 45-0230, Strem Chemicals, MA) in minimal acetone to yield a concentration of 5.5 mM. The resulting solution was vigorously shaken and acetone was removed under vacuum and argon purging at room temperature.
Diethyl 1-13C 2,3-d2 fumarate was added by injecting the compound neat into the catalyst solution. The syringe was washed twice with catalyst solution by pulling up solution and then injecting into a round bottom flask to remove all of the diethyl fumarate out of the syringe. The solution of diethyl fumarate and catalyst was then filtered through 0.45 μm cellulose acetate syringe filter (VWR, 28145-481). The filtered solution was then quickly taken up in a 30 ml plastic syringe and used for injection of the desired amount of imaging reagent precursor for each experiment (4 mL) into the reaction chamber of the polarizer. Different final concentrations of diethyl 1-13C 2,3-d2 fumarate in catalyst solution were used to optimize polarization transfer pulse sequences, hydrogenation, 13C imaging and 13C spectroscopy. However, we saw the best imaging and spectroscopy using a 20 mM diethyl 1-13C 2,3-d2 fumarate solution in a 9:1 water to D2O solution and it was this concentration of hyperpolarized diethyl 1-13C succinate that was injected into mice unless stated otherwise. Most hydrogenations were done at 60 °C, with 12 bar parahydrogen gas and using 15 bar nitrogen to remove the hyperpolarized compound from the reaction chamber. Hydrogenation was complete using these conditions. Aliquots of hydrogenation reactions were analyzed on Varian 11.7 T NMR instrument and 13C NMR performed. The 13C NMR for the catalyst and diethyl 13C fumarate solution is significantly different before and after hydrogenation. The change in the carbonyl chemical shift from 167.4 ppm (corresponding to diethyl fumarate) to 175.7 ppm (corresponding to diethyl succinate) is easily seen. A resonance at 167.4 ppm was not seen in any of the reactions tested (6 reactions). Aliquots of 0.5 ml of hyperpolarized, hydrogenated 13C reagent was then injected into the mouse via tail vein. In a few experiments, aliquots of 1 ml of hyperpolarized hydrogenated reagent were injected into a mouse’s intraperitoneal cavity.
MR scanner and coils
All MRI imaging and MRS of animals or phantoms was performed in an animal 4.7 T MR scanner (Bruker Avance, Bruker AG, Germany) horizontal bore using a 1H/13C full body mouse volume coil (Doty Scientific, South Carolina, USA).
Animals
Male BALB/c mice purchased from Harlan S/D and on average weighing 25 grams were used. All animal experiments were approved by IACUC of Huntington Medical Research Institutes. The mice were anesthetized by 1.5 % isoflurane gas with 0.8 L/min oxygen per face mask. The lateral tail vein was catheterized with 30-gauge tubing (MVT-1, Braintree Scientific), attached to a two-foot PE50 extension. A warm-water tail bath produced the vasodilation critical to successful cannulation. The sedated mouse was then placed in a heated cradle within the bore of the MR scanner
For experiments that required an intraperitoneal (i.p.) catheter, the anesthetized mouse was subjected to one of two procedures: laparotomy or needle puncture. The laparotomy technique involved a small (2–4 mm) mid-sagittal abdominal incision through skin and peritoneum while the mouse laid supine. The tip of a two foot PE50 catheter was then placed in the intraperitoneal space under direct visualization. Silk suture then anchored the catheter to skin as well as closed the wound. Alternatively, i.p. access was affected by passing the PE50 catheter through an 18 gauge needle after a blind, transcutaneous puncture. The abdominal skin was tented upwards to avoid visceral injury. Once the needle was withdrawn over the catheter, no suture was necessary to prevent leaks. After either procedure, the mouse was carefully pronated and placed in the magnet.
The needle method became the preferred, more refined approach as it utilized less time and material and created a tighter catheter-to-skin junction. The success of this method is, however, highly operator dependent; but in our hands, no abdominal organ injury or other untoward complication occurred.
Measuring Polarization
The polarization of diethyl succinate was measured (25 s to 40 s after polarization) in a plastic syringe using 13C spectroscopy with a single scan pulse and acquire sequence using a pulse angle of 45° in MR scanner. To quantify the degree of hyperpolarization, we used the reference of a single scan spectrum of thermally polarized 3 M 1-13C acetate phantom at 4.7 T using the following formula:
| (1) |
where 1/246,600 corresponds to 13C nuclear polarization at 298 K and at 4.7 T, according to the Boltzmann distribution. The degree of hyperpolarization produced in the PHIP polarizer at time zero was back calculated, using the delivery time and spin-lattice relaxation time T1 of the hyperpolarized agent as follows:
| (2) |
The reported % hyperpolarization refers to %Pt = 0.
1H MRI
Proton anatomic images for mice were obtained with a dual tuned volume coil to allow co-registration of carbon hyperpolarized images with mouse anatomic images. RARE (rapid acquisition with relaxation enhancement)37 tri-pilot was used for placement of the animal and MSME (multi slice multi echo)37 coronal imaging using a range of slice thicknesses (4.5, 7.5 or 15.2 mm) and a field of view (FOV) of either 6 or 7 cm was used for co-registration of carbon hyperpolarized images. Magnetic field homogeneity was adjusted using single voxel proton MRS ((PRESS) point resolved spectroscopy)37 data acquisition approach and the voxel (0.7 × 0.7 × 0.7 cm) of interest was placed just posterior of the kidneys in the animal. The unsuppressed water signal less than 15 Hz half width was routinely obtained. Shimming was essential for peak resolution in 13C MRS experiments.
13C MRS
We have found it optimal for in vivo experiments to perform two to three successive injections of hyperpolarized reagent (13C diethyl succinate), optimizing the use of the rapidly decaying hyperpolarized 13C MR signal in order to obtain information on both anatomic distribution and metabolism of 13C diethyl succinate. Consecutive 13C MRS was acquired using a pulse and acquire approach using a non-selective Gaussian radio frequency pulse for excitation and using usually a pulse angle of 30° unless noted otherwise (bandwidth 25000 Hz and acquisition size 2048) every 7-8 seconds for about 1 minute after injection of hyperpolarized diethyl succinate into the mouse. The spectroscopy was done before or after a 13C FISP image was taken (described below). Assignments of the 13C spectroscopy peaks were determined using experimentally determined chemical shift values of metabolites in a D2O/water solution at known pH (See 13C NMR section). The FID raw data was then processed either in XwinNMR or MestraNova using baseline correction (Berstein Polynomial Fit), line broadening of 5 Hz, manual phasing and referenced to the large diethyl succinate peak at 176.4 ppm.
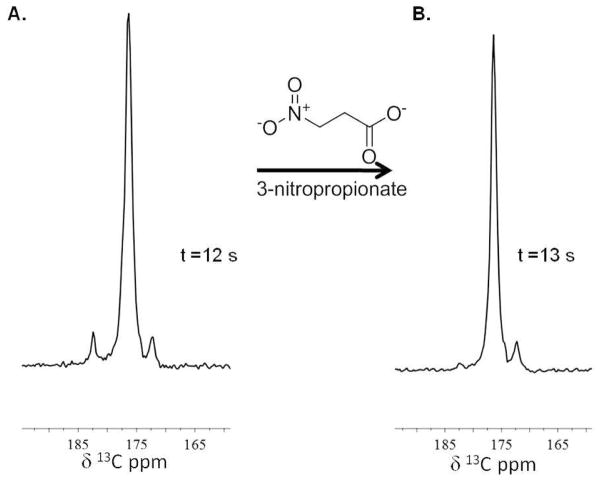
In a few experiments, 13C spectroscopy was performed on animals before and after 3-nitropropionate injection (Figure 4). The mouse was injected with 10 μmol of hyperpolarized diethyl succinate via i.v. injection before and after 200 μl i.p. injection of a basic 5 mg/ml (42 mM) solution of 3-nitropropionate (Sigma-Aldrich) and a 20 minute wait. The aqueous solution of 3-nitropropionate was brought to a pH of 8.5 using a drop of 50% NaOH solution.
Figure 4. 3-nitropropionate inhibition.
(A) and (B) are 13C MRS spectra illustrating diethyl succinate metabolism in an animal before (spectrum A) and after 3-nitropropionate treatment (spectrum B). The mouse was injected with 10 μmol of hyperpolarized diethyl succinate via i.v. injection before and after injection of a 3-nitropropionate solution. The downfield resonance corresponding to succinate is significantly reduced in spectrum B compared to spectrum A.
For T1 experiments, the decay of the polarized carbon signal using a single 10° pulse every 6 seconds for a total of 32 scans and a total of 3.2 minutes was measured on our 4.7 T MR scanner. The decay of the signal is plotted against time and the inverse of the exponential of the curve is the spin lattice relaxation time. The amount of polarization consumed by the observation pulse was not taken into account.
13C MRI
Carbon-13 imaging was done using Bruker TRUE FISP sequence.37 Our imaging sequence used TR = 3.3 ms, TE = 1.6 ms, 4 averages, 32 × 32 matrix and bandwidth 52083 Hz. All imaging was done in MR scanner using a Doty volume coil. Flip angles of 80, 60 or 40 degrees were used in the sequence. Coronal imaging was performed using a FOV of 6 or 7 cm, one to two slices with dimensions 15.2 mm, and the center slice was always selected to be the same as proton images. Images were then converted into false color in Paravision 3.0.2 Software and only pixels above certain intensity were used to remove noise from signal. The image was then overlaid on the proton image. No 13C MRI image was seen if hyperpolarized diethyl succinate was not injected.
13C NMR
To assign 13C resonances observed in vivo, proton-decoupled 13C NMR of about 30 samples containing solutions of different TCA cycle metabolites at concentrations around 30 mM in D2O and water solution with 0.5 % of methanol in the sample as a chemical shift reference was performed on a 11.7 T Varian instrument. pH of samples were adjusted with 356 mM KOH solution and 50 mM phosphate buffer at pH 7.5 and measured using a pH meter. All spectroscopy was performed with 64 to 256 transients, 60° flip angle, relaxation delay of 10 s and all spectra were referenced to methanol peak. The carbonyl assignments for the relevant metabolites and pH of the samples can be seen in Table 1.
Table 1. 13C chemical shifts of TCA cycle metabolites.
The 13C chemical shifts of aqueous solutions of TCA cycle metabolites at given pHs were experimentally determined on a 11.7 T NMR. All samples were doped with methanol and the chemical shifts were referenced to the methanol peak. On the 4.7 T MR scanner, 13C chemical shift of the C-1 of the diethyl succinate referenced to methanol was determined to be 176.4 ppm.
| 13C Chemical Shift (ppm) | Compound | pH |
|---|---|---|
| 183.0 | succinate C1 | 7.1, 7.5 |
| 182.0 | succinate C1 | 5.7 |
| 183.0 | lactate C1 | 6.4 |
| 182.5 | citrate C6 | 7.0, 7.4 |
| 180.5 | citrate C6 | 5.0 |
| 181.8 | glutamate C5 | 7.2 |
| 181.4 | glutamate C5 | 5.3 |
| 181.6 | malate C4 | 7.3 |
| 180.8 | malate C4 | 5.0 |
| 181.4 | isocitrate C1, C5 | 7.0 |
| 180.7 | isocitrate C6 | 7.0 |
| 180.4 | malate C1 | 7.3 |
| 175.8 | malate C1 | 5.0 |
| 179.7 | citrate C1, C5 | 7.0, 7.4 |
| 177.4 | citrate C1, C5 | 5.0 |
| 178.1 | aspartate C4 | 6.0 |
| 174.8 | aspartate C1 | 6.0 |
| 175.8 | diethyl succinate C1 | 7.7, 7.4 |
| 175.9 | diethyl succinate C1 | 5.7 |
| 175.2 | fumarate C1 | 7.3 |
| 174.6 | fumarate C1 | 5.0 |
| 175.1 | glutamate C1 | 7.2 |
| 175.0 | glutamate C1 | 5.3 |
| 135.9 | fumarate C2 | 7.3 |
| 135.8 | fumarate C2 | 5.0 |
RESULTS
Polarization and T1 spin lattice relaxation time
In parahydrogen induced polarization (PHIP) method, the radio frequency pulse used to transfer the spin order from 1H nuclei to 13C is essential to getting high polarization percentages. Changes in the pulse width, amplitude or timing between the proton and carbon radio frequency pulses affect the percent of polarization that is transferred to carbon-13 atom significantly. We generate the radio frequency heteronuclear pulse using the coupling constants for the compound being polarized (Figure 1B) as described by Goldman and Johannesson.32 The coupling constants for succinate at acidic pH were initially used20 to determine the transfer pulse sequence and then manipulated in a similar manner as in reference34 until high polarization was achieved. The polarization of diethyl succinate was measured against a standard 3M 1-13C acetate phantom as described in the methods section using a single transient. The Bo magnetic field in the polarizer was calibrated as previously described.34 We routinely used a Bo field between 2.00 to 2.02 mT and a transfer pulse with a 2:1 peak to peak voltage amplitude ratio of proton to carbon pulse. Polarization percentage increased from 0.19 ± 0.06% to 2.1 ± 0.6 % or 5000 fold signal enhancement compared to Boltzmann polarization after optimization (Figure 1D). Our percent polarization could be considered low compared to the polarization percentages reported in the literature for DNP polarized compounds,13–17 but this could be because of the difference in measurement technique. Regardless of the percent polarization, we can image and perform spectroscopy with hyperpolarized 1-13C diethyl succinate for the same length of time and have comparable signal to noise ratio to DNP polarized compounds.13–17
PHIP polarization on a 13C labeled compound relaxes quickly and the rate is defined by the T1. The spin lattice relaxation time T1 for hyperpolarized diethyl succinate in a syringe and in vivo was measured on a 4.7 T MR scanner by measuring the decay of the polarized carbon signal. We are conservative on our assessment of the apparent T1 and did not take into account the amount of polarization consumed by the observation pulses. The T1 of the labeled carbonyl of a 9:1 water to D2O solution of 20 mM hyperpolarized diethyl succinate is 38 ± 4 s in vivo and 54 ±2 s in the syringe. The T1 is significantly lower when polarization occurs in 100% water (24 s, in vivo). Due to the longer T1 occurring in the 9:1 mixture of water and D2O this solvent mixture was used in all in vivo experiments. The pH of 20 mM diethyl 13C fumarate in 9:1 water to D2O catalyst solution is 8. After hydrogenation, the final pH is 6 arising from the rhodium catalyst degrading into a rhodium hydroxide complex.40 An in vivo T1 of 38s is comparable to other hyperpolarized compounds and gives us more than 3 minutes (5*T1) to image the compound. The final pH of 6 is significantly closer to physiological pH than 2.35 or 11 which was used in our previous succinate polarization experiments.20
In vivo 13C spectroscopy and imaging
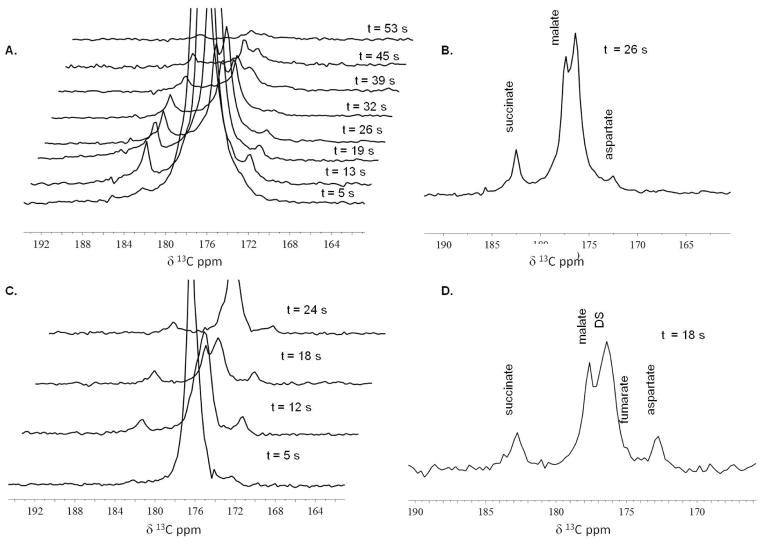
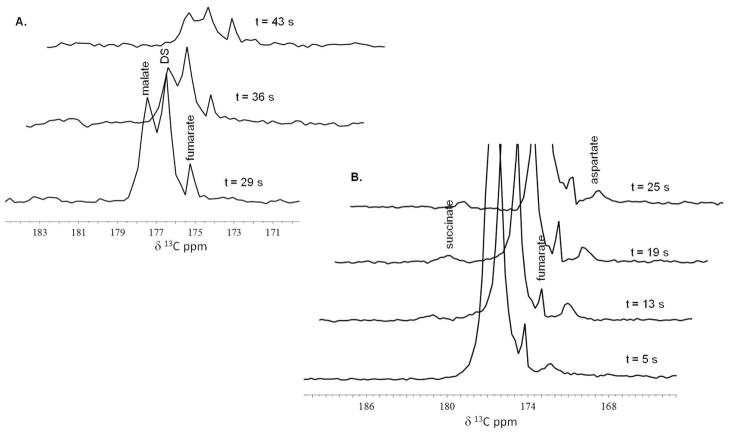
Real time detection of metabolism of diethyl succinate was seen in thirteen mice injected with 10 micromoles (μmol) of hyperpolarized diethyl succinate via tail vein and seen in three mice injected with 20 μmol of hyperpolarized diethyl succinate in the peritoneum. In most cases, it took 40 to 50 seconds after polarization for hyperpolarized diethyl succinate to be administered to the animal and imaging/spectroscopy to begin. Representative 13C MRS exams of diethyl succinate metabolism in mice can be seen in Figure 2 and Figure 3. Metabolites are seen almost instantly and metabolism can easily be monitored. All spectroscopy shown was collected using single 30° pulse and acquire sequence every 5 to 9 seconds. The time values were determined based on the time stamps of the raw data and amount of time the pulse and acquire sequence takes to complete (5 s). The time values correspond to the time elapsed between the injection of the hyperpolarized compound and when the spectroscopy was performed. Metabolic products from hyperpolarized 13C diethyl succinate (the largest peak, labeled DS) were detected within 5 seconds of the injection of the hyperpolarized substance and persisted for approximately 1 minute. All spectra are referenced to the diethyl succinate peak (176.4 ppm) which was referenced in phantom experiments to 13C labeled methanol. In most animals, no signal was seen using 13C spectroscopy with a single transient if hyperpolarized compound was not injected. 13C spectra using a single transient in a few non-injected mice include natural abundance 13C lipid peaks at around 30–35 ppm. To determine the chemical identity of the in vivo metabolites observed, we performed several proton-decoupled 13C NMR experiments on a 11.7 T Varian instrument using methanol (49.50 ppm)41 as a chemical shift reference of samples containing known TCA cycle metabolites in water and D2O at particular pHs. Some of the data can be seen in Table 1. Based on these experiments, we believe that after injection diethyl succinate is metabolized by esterase in the cell and then metabolized to succinate, aspartate, malate and fumarate.
Figure 2. 13C Spectroscopy in vivo (i.v. injection).
(A) and (C) Representative 13C MR time-resolved stackplots as seen in two different mice which received 10 μmol of hyperpolarized diethyl succinate by tail vein injection. All spectroscopy shown was collected using single 30° pulse and acquire sequence every 5 to 9 seconds. The time values correspond to the time elapsed between the injection of the hyperpolarized compound and when the spectroscopy was performed. Metabolic products from hyperpolarized 13C diethyl succinate (the largest peak, labeled DS) were detected within 5 seconds of the injection of the hyperpolarized substance and persisted through 53 seconds. (B) An enlarged view of the region of interest of the 13C MRS acquired t = 26 s as seen in stackplot A. Using the diethyl succinate peak as a reference and set to 176.4 ppm, the three distinct resonances are assigned to malate, succinate and aspartate. (D) An enlarged view of the region of interest of the 13C MRS acquired t = 18 s as seen in stackplot C. The four distinct resonances are assigned to malate, succinate, fumarate and aspartate.
Figure 3. 13C Spectroscopy in vivo (i.p. injection).
(A) and (B) are 13C MR stackplots as seen in two different mice each receiving 20 μmol of hyperpolarized diethyl succinate into the peritoneum. Metabolic products from hyperpolarized 13C diethyl succinate (the largest peak, labeled DS) were detected within 5 seconds of the injection of the hyperpolarized substance (stackplot B) and persisted for about a minute (stackplot A). Using the diethyl succinate peak as a reference, two to three distinct resonances can be detected and are assigned to malate, fumarate, succinate and aspartate.
The pattern of the resonances as well as the tentative assignments by chemical shift, strongly suggests that diethyl 13C succinate hyperpolarized by the PHIP technique is metabolized in vivo and that its metabolites retain a significant fraction of the hyperpolarized 13C nuclei through three or more enzyme catalyzed biochemical reactions. Metabolism of hyperpolarized diethyl succinate was confirmed using 13C MRS in thirteen animals using i.v. injection with 10 μmol of compound and in three mice using i.p. injection with 20 μmol of hyperpolarized diethyl succinate. Metabolism was observed in all animals. As is very frequently the case for in vivo 13C MRS, there are some slight discrepancies between experiments, mostly attributable to differences between one animal and the next. In addition, because of the dynamic state of in vivo metabolism, the peaks and the height of these peaks are highly dependent on when the spectroscopy was done relative to when the compound was injected. In Figure 3A, the spectroscopy was taken from 5 s to 25 s after the injection. While in Figure 3B, the spectroscopy was taken 29 s to 43 s after the injection.
We have not determined the in vivo flux rate of the TCA cycle using our spectroscopy data for a number of reasons. Based on our prior experience of in vivo NMR-metabolic rate calculations, a key requirement is that ‘steady state’ conditions prevail.42,43 Diethyl succinate, which is not an endogenous substance, at concentrations of 10–20 μmol is a substrate rather than a tracer in relation to the intrinsic concentration of succinate around 0.1 μM. We did not achieve steady-state conditions in the experiments described here. In addition, there are a number of other uncontrolled and uncontrollable variables – differing T1’s of substrate and metabolic products, the decay of polarization under the influence of ‘imaging’ radio frequency pulses applied, chemical exchange and other factors, all of which complicate a consistent approach for determining the TCA flux rate. However, knowledge of the precise flux data for diethyl succinate metabolism might not be needed in diagnostic imaging of disease states known to have inhibited or unusual TCA cycle metabolism.
To further explore the metabolism of hyperpolarized diethyl 1-13C 2,3-d2 succinate in a mouse, we performed spectroscopy experiments of diethyl succinate before and after i.p. injection of 3-nitropropionate in four mice. 3-nitropropionate is a known irreversible inhibitor of succinate dehydrogenase.35 The metabolism of hyperpolarized diethyl succinate changes after 3-nitropropionate injection (Figure 4). Figure 4A is the 13C spectroscopy of diethyl succinate metabolism injected by i.v. after 12 s. Figure 4B is the 13C spectroscopy of an i.v. injection of hyperpolarized diethyl succinate in the same animal after being treated with 200 μl of 3-nitropropionate solution and waiting 20 minutes. Based on chemical shifts, the downfield succinate resonance is significantly reduced in the animal after 3-nitropropionate treatment. Three out of the four mice demonstrated a reduction to complete loss of the succinate peak after 3-nitropropionate treatment.
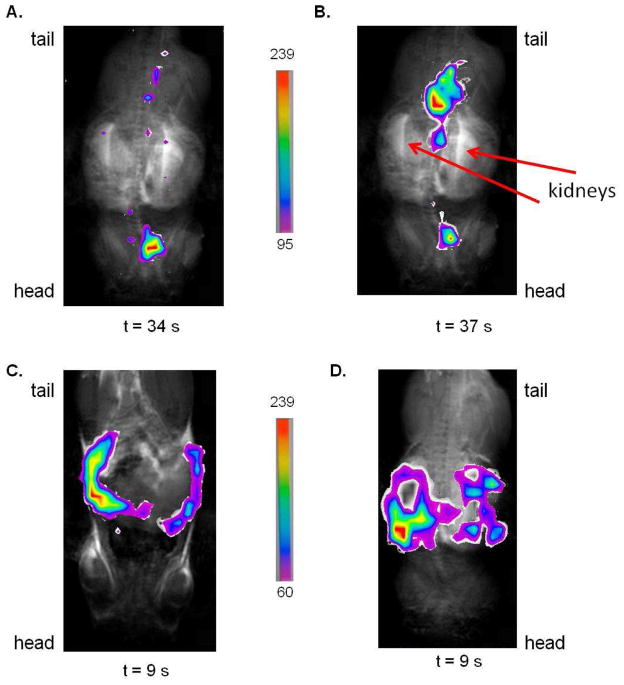
Carbon-13 imaging was accomplished using the Bruker TRUE FISP imaging sequence37 in a volume coil on a 4.7 T MR scanner. Given the gradient rise time of our MR Bruker scanner is 250 microseconds, the slice thickness for the images is 15.2 mm and the field of view (FOV) is either 6 or 7 cm. We can determine the relative location of hyperpolarized diethyl succinate and its metabolites in the animal when injected via tail vein or into the peritoneum using 13C FISP imaging. At the beginning of this work, a flip angle of 80° was used for 13C imaging but, we have been able to image for longer intervals and preserve the signal with flip angles of 60° and 40°. We can image every 9s for up to 1 minute with the TRUE FISP sequence using 60° and 40° flip angles. No 13C image is seen if hyperpolarized compound is not injected. Overlays of 13C FISP images in false color over the proton image of the animal using the same FOV and central slice placement are displayed in Figure 5. Based on the overlays of the images, diethyl succinate when injected i.v. into a mouse seems to quickly go through the cardiovascular system, as seen in Figure 5A and 5B where the FISP image is in the location of the heart, and then we believe the hyperpolarized molecule collects in the bladder and ureters (Figure 5B) based on the FISP images proximity to the kidneys seen in the proton MRI images. With intraperitoneal injection the hyperpolarized compound and its metabolites appear to remain in the peritoneum (Figure 5C and 5D). Other fast imaging sequences, fast imaging employing steady state acquisition (FIESTA)21 or fast low angle shot (FLASH),44 which were not used in this study might give better spatial resolution than the TRUE FISP sequence. In the future, we plan on using different 13C imaging sequences to improve our spatial resolution.
Figure 5. 13C Imaging in vivo.
Images (A), (B), (C), and (D) are overlays of 13C FISP images (60° flip angle) in false color taken of different injections of hyperpolarized diethyl succinate over the proton image of the mouse using the same 6 cm FOV, central slice placement and slice thickness. To remove noise from FISP images, only pixels above a certain threshold were used. The scales corresponding to each set of false color images are seen. The time values below the images is the amount of time in seconds after the injection of the hyperpolarized compound that the 13C image was taken. Image (A) and (B) are 13C FISP images of two different i.v. injections of hyperpolarized diethyl succinate. Based on the location of the 13C image in images (A) and (B), some of the hyperpolarized compound and its metabolites can be found to be localized around the region of the heart. In image (B), the majority of the hyperpolarized signal is found posterior to the kidneys in the region of the bladder and ureters. Images (C) and (D) are overlays of 13C FISP images of two different i.p. injections of hyperpolarized diethyl succinate. Based on the location of the 13C images, the hyperpolarized compound seems to be retained in the peritoneum with i.p. injection in the time window of the image (9 s).
DISCUSSION
In this study, we have been able to synthesize, polarize, and image diethyl 1-13C 2,3-d2 succinate. Diethyl succinate can be hyperpolarized in our polarizer in an aqueous solution with signal enhancement of 5000 compared to Boltzmann polarization and the hyperpolarized solution can be generated at 4 minute intervals with complete conversion to diethyl succinate. The T1 of hyperpolarized labeled carbonyl 13C in diethyl succinate was determined to be 38s in vivo which allows for the signal to be measured for over 3 minutes. 13C MRS and MRI were achieved in vivo using tail vein and intraperitoneal injections of 20 mM hyperpolarized diethyl succinate into normal mice. Metabolism of the compound was seen in all injections. Based on 13C MRS TCA cycle metabolite phantoms, we have assigned the spectral peaks in our in vivo studies of hyperpolarized diethyl succinate to be malate, succinate, fumarate and aspartate (Figure 2 and Figure 3). The metabolism of diethyl succinate was altered after exposing the animal to 3-nitropropionate, a known irreversible inhibitor of succinate dehydrogenase (Figure 4). Based on our results, parahydrogen induced polarized diethyl succinate allows for ultra-fast in vivo MRI and MRS with a high signal to noise ratio and multiple enzyme catalyzed reactions can be visualized.
The reduction of hyperpolarized succinate was seen in multiple studies after inhibition of succinate dehydrogenase by the inhibitor 3-nitropropionate. This is counterintuitive to what one would expect and could be due to several reasons. First, while 3-nitropropionate inhibits succinate dehydrogenase when presented to the purified enzyme, this does not exclude additional effects of the inhibitor in vivo.45 Second, we have no information concerning the behavior of intramitochondrial succinate dehydrogenase in the presence of high excess of a succinate analogue like diethyl succinate. In addition, hyperpolarized metabolic events, which permit the close observation of metabolic regulation in vivo during the first 100 seconds after equilibrium is challenged by substrate or inhibitor, has never before been studied.
In the studies described, the rhodium hydrogenation catalyst was not removed before injection of hyperpolarized diethyl succinate. Based on concentration, 2.75 μmol and 5.5 μmol of catalyst was injected into the mice with every i.v. injection and i.p. injection respectively. The animals seemed to tolerate multiple injections of hyperpolarized diethyl succinate and hydrogenation catalyst. However, we do realize for the technique to be used in patients in clinical studies, sterile methods for removing the catalyst must be developed. We are in the process of attaching the rhodium catalyst to solid support, removing the catalyst after hydrogenation through the use of cationic resins that will remove the cationic catalyst and via fast filtration.
Hyperpolarized diethyl succinate has several advantages compared to DNP hyperpolarized 1-13C pyruvate and PHIP hyperpolarized 1-13C succinate. Using PHIP, hyperpolarization of diethyl succinate can be achieved on a quicker time scale than any DNP hyperpolarized compound, so far reported. We can easily generate 20 different samples of hyperpolarized diethyl succinate in a single day for in vivo imaging experiments. The PHIP method of hyperpolarization allows for multiple injections of hyperpolarized compound in the same animal. All animals in this study were injected 3 to 4 times with hyperpolarized diethyl succinate which allowed us to do over 40 different 13C MRS and MRI experiments. In addition, the 4 minute time interval for hyperpolarization using PHIP allows metabolism to be quickly monitored in the same animal before and after treatment with a compound (for example, 3-nitropropionate). This would allow the monitoring of metabolism in disease animal models before and after drug treatment (ex. chemotherapeutics) in a time scale of seconds to minutes. The T1 of hyperpolarized diethyl succinate is significantly longer (38 s in vivo) than hyperpolarized 1-13C pyruvate (20 to 15 s in vivo), allowing for in vivo studies of longer duration and more physiological concentrations of metabolic imaging reagents.14 In this study, a 20 mM solution of hyperpolarized diethyl succinate is being used with 500 μl to 1 ml injection volumes which is quite comparable to the concentrations used with DNP polarized pyruvate. The lowest concentration of injected hyperpolarized 1-13C pyruvate in a mouse is reported at 10 mM16 however most papers report higher concentrations.13–15 Multiple steps of metabolism can be observed with hyperpolarized diethyl succinate unlike hyperpolarized 1-13C pyruvate.
Hyperpolarized diethyl succinate has several advantages over hyperpolarized succinate, the first physiological PHIP reagent developed in our laboratory.20,30 It is known that the dicarboxylic acid succinate is only poorly transported across many biological membranes and in particular barely crosses the mitochondrial membrane to gain access to TCA cycle enzymes involved in metabolism.23,24 Diethyl succinate is taken up and metabolized in seconds in our in vivo studies. In addition, we can hyperpolarize diethyl succinate under more physiological conditions notably pH close to biological neutrality than compared to the acidic or basic conditions needed to polarize succinate.20 With PHIP polarized diethyl succinate’s long T1, physiological pH, ability to cross biological membranes and high signal to noise ratio with only a 20 mM solution; this compound has the potential of studying metabolism in a variety of diseases.
In the future, we are planning on using hyperpolarized diethyl succinate to study metabolism in cancer and neurodegenerative disease animal models. Rapid visualization of the first minute of the TCA cycle in normal or cancerous tissues may throw new light on the regulation of this pathway previously beyond the limits of detection by conventional in vivo NMR. In addition, we plan on injecting hyperpolarized diethyl succinate in the carotid artery of an animal to determine if the molecule will cross the blood brain barrier (BBB). Hyperpolarized succinate does not cross normal healthy animal BBB30 and, it is known that hyperpolarized ethyl pyruvate crosses the BBB significantly better than hyperpolarized pyruvate.15 Based on the ability of non-charged compounds to cross the BBB, there is a definite possibility that hyperpolarized diethyl succinate will cross the blood brain barrier which would allow for us to compare the metabolism of the compound in normal animals versus neurodegenerative disease animal models.
In summary, we have developed a more flexible hyperpolarized molecular imaging reagent which can be injected at physiological pH, rapidly enter cells and tissues and is metabolized by enzymes in the TCA cycle. Hyperpolarized diethyl succinate has the potential of being used in clinical metabolic imaging and spectroscopy. Furthermore, this molecule has the promise of exploring the metabolic differences in the TCA cycle in both diseased and normal tissue in disease models, providing a real time metabolic fingerprinting of different types of cancers. Early response to targeted cancer therapy and successful outcome can potentially be predicted by the appearance, in otherwise predominantly glycolytic tumors, of the intermediates of a (recovered) TCA cycle. In vivo applications of hyperpolarized diethyl succinate may reveal an entirely new regime wherein the local status of TCA cycle metabolism is interrogated and therapy monitored in diseased models on the time scale of seconds to minutes with unprecedented chemical specificity and MR sensitivity.
Supplementary Material
Acknowledgments
We thank the Caltech NMR facility especially Dr. Scott Ross who helped us with NMR experiments. In addition, we thank Professor Robert Grubbs at Caltech and his laboratory for the use of a fume hood and chemistry discussions. We thank Meng Wei and Dr. Kent Harris for their help with the polarizer. NMZ thanks the James G. Boswell Foundation for their financial support. The work was supported in part by NIH 1R21 CA118509 (PB), NCI 5R01CA122513 (BDR), NIH 1R01NS048589 (BDR) and Tobacco Related Disease Research Program (16KT-0044) (PB).
Footnotes
Supporting Information Available: TCA cycle diagram and table containing all experiments performed. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Garber K. J Natl Cancer Inst. 2004;96:1805–6. doi: 10.1093/jnci/96.24.1805. [DOI] [PubMed] [Google Scholar]
- 2.Bayley JP, Devilee P. Curr Opin Genet Dev. 2010;20:324–9. doi: 10.1016/j.gde.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 3.Ralph SJ, Rodriguez-Enriquez S, Neuzil J, Moreno-Sanchez R. Mol Aspects Med. 2010;31:29–59. doi: 10.1016/j.mam.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 4.Beal MF. Trends Neurosci. 2000;23:298–304. doi: 10.1016/s0166-2236(00)01584-8. [DOI] [PubMed] [Google Scholar]
- 5.Bubber P, Haroutunian V, Fisch G, Blass JP, Gibson GE. Ann Neurol. 2005;57:695–703. doi: 10.1002/ana.20474. [DOI] [PubMed] [Google Scholar]
- 6.Henry PG, Lebon V, Vaufrey F, Brouillet E, Hantraye P, Bloch G. J Neurochem. 2002;82:857–66. doi: 10.1046/j.1471-4159.2002.01006.x. [DOI] [PubMed] [Google Scholar]
- 7.Brouillet E, Guyot MC, Mittoux V, Altairac S, Conde F, Palfi S, Hantraye P. J Neurochem. 1998;70:794–805. doi: 10.1046/j.1471-4159.1998.70020794.x. [DOI] [PubMed] [Google Scholar]
- 8.Ross B, Lin A, Harris K, Bhattacharya P, Schweinsburg B. NMR Biomed. 2003;16:358–69. doi: 10.1002/nbm.852. [DOI] [PubMed] [Google Scholar]
- 9.Bowers CR, Weitekamp DP. Phys Rev Lett. 1986;57:2645–2648. doi: 10.1103/PhysRevLett.57.2645. [DOI] [PubMed] [Google Scholar]
- 10.Bowers CR, Weitekamp DP. Solid State Nucl Magn Reson. 1998;11:123–8. doi: 10.1016/s0926-2040(97)00101-x. [DOI] [PubMed] [Google Scholar]
- 11.Kuhn LT, Bargon J. Top Curr Chem. 2007;276:25–68. [Google Scholar]
- 12.Golman K, Olsson LE, Axelsson O, Mansson S, Karlsson M, Petersson JS. Br J Radiol. 2003;76(Spec No 2):S118–27. doi: 10.1259/bjr/26631666. [DOI] [PubMed] [Google Scholar]
- 13.Arunachalam A, Whitt D, Fish K, Giaquinto R, Piel J, Watkins R, Hancu I. NMR Biomed. 2009;22:867–73. doi: 10.1002/nbm.1401. [DOI] [PubMed] [Google Scholar]
- 14.Golman K, in ‘t Zandt R, Thaning M. Proc Natl Acad Sci U S A. 2006;103:11270–5. doi: 10.1073/pnas.0601319103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hurd RE, Yen YF, Mayer D, Chen A, Wilson D, Kohler S, Bok R, Vigneron D, Kurhanewicz J, Tropp J, Spielman D, Pfefferbaum A. Magn Reson Med. 2010;63:1137–43. doi: 10.1002/mrm.22364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wilson DM, Keshari KR, Larson PE, Chen AP, Hu S, Van Criekinge M, Bok R, Nelson SJ, Macdonald JM, Vigneron DB, Kurhanewicz J. J Magn Reson. 2010;205:141–7. doi: 10.1016/j.jmr.2010.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Golman K, Zandt RI, Lerche M, Pehrson R, Ardenkjaer-Larsen JH. Cancer Res. 2006;66:10855–60. doi: 10.1158/0008-5472.CAN-06-2564. [DOI] [PubMed] [Google Scholar]
- 18.Schroeder MA, Atherton HJ, Ball DR, Cole MA, Heather LC, Griffin JL, Clarke K, Radda GK, Tyler DJ. FASEB J. 2009;23:2529–38. doi: 10.1096/fj.09-129171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marjanska M, Iltis I, Shestov AA, Deelchand DK, Nelson C, Ugurbil K, Henry PG. J Magn Reson. 2010;206:210–8. doi: 10.1016/j.jmr.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chekmenev EY, Hovener J, Norton VA, Harris K, Batchelder LS, Bhattacharya P, Ross BD, Weitekamp DP. J Am Chem Soc. 2008;130:4212–3. doi: 10.1021/ja7101218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bhattacharya P, Harris K, Lin AP, Mansson M, Norton VA, Perman WH, Weitekamp DP, Ross BD. Magn Reson Mater Phys, Biol Med. 2005;18:245–56. doi: 10.1007/s10334-005-0007-x. [DOI] [PubMed] [Google Scholar]
- 22.Bhattacharya P, Chekmenev EY, Reynolds WF, Wagner SR, Zacharias N, Chan HR, Bunger R, Ross BD. NMR in Biomedicine. 2011 April 28; doi: 10.1002/nbm.1717. e-pub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nishiitsutsuji-Uwo JM, Ross BD, Krebs HA. Biochem J. 1967;103:852–62. doi: 10.1042/bj1030852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jans AW, Willem R. Eur J Biochem. 1991;195:97–101. doi: 10.1111/j.1432-1033.1991.tb15680.x. [DOI] [PubMed] [Google Scholar]
- 25.Isaacs JS, Jung YJ, Mole DR, Lee S, Torres-Cabala C, Chung YL, Merino M, Trepel J, Zbar B, Toro J, Ratcliffe PJ, Linehan WM, Neckers L. Cancer Cell. 2005;8:143–53. doi: 10.1016/j.ccr.2005.06.017. [DOI] [PubMed] [Google Scholar]
- 26.Selak MA, Armour SM, MacKenzie ED, Boulahbel H, Watson DG, Mansfield KD, Pan Y, Simon MC, Thompson CB, Gottlieb E. Cancer Cell. 2005;7:77–85. doi: 10.1016/j.ccr.2004.11.022. [DOI] [PubMed] [Google Scholar]
- 27.Ladriere L, Zhang TM, Malaisse WJ. JPEN, J Parenter Enteral Nutr. 1996;20:251–6. doi: 10.1177/0148607196020004251. [DOI] [PubMed] [Google Scholar]
- 28.Malaisse WJ, Zhang TM, Verbruggen I, Willem R. Biochem J. 1996;317(Pt 3):861–3. doi: 10.1042/bj3170861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Diethyl Succinate; MSDS No. 112402 [Online] Sigma-Aldrich; St. Louis, MO: Jul 21, 2010. [accessed March 1, 2011]. http://www.sigmaaldrich.com/catalog/DisplayMSDSContent.do. [Google Scholar]
- 30.Bhattacharya P, Chekmenev EY, Perman WH, Harris KC, Lin AP, Norton VA, Tan CT, Ross BD, Weitekamp DP. J Magn Reson. 2007;186:150–5. doi: 10.1016/j.jmr.2007.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Imamoto T. Pure Appl Chem. 2001;73:373–376. [Google Scholar]
- 32.Goldman M, Johannesson H, Axelsson O, Karlsson M. Magn Reson Imaging. 2005;23:153–7. doi: 10.1016/j.mri.2004.11.031. [DOI] [PubMed] [Google Scholar]
- 33.Hovener JB, Chekmenev EY, Harris KC, Perman WH, Robertson LW, Ross BD, Bhattacharya P. Magn Reson Mater Phys, Biol Med. 2009;22:111–21. doi: 10.1007/s10334-008-0155-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hovener JB, Chekmenev EY, Harris KC, Perman WH, Tran TT, Ross BD, Bhattacharya P. Magn Reson Mater Phys, Biol Med. 2009;22:123–34. doi: 10.1007/s10334-008-0154-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alston TA, Mela L, Bright HJ. Proc Natl Acad Sci U S A. 1977;74:3767–71. doi: 10.1073/pnas.74.9.3767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sun F, Huo X, Zhai Y, Wang A, Xu J, Su D, Bartlam M, Rao Z. Cell. 2005;121:1043–57. doi: 10.1016/j.cell.2005.05.025. [DOI] [PubMed] [Google Scholar]
- 37.Bernstein MA, King KF, Zhou XJ. Handbook of MRI Sequences. Elsevier Academic Press; San Diego: 2004. [Google Scholar]
- 38.Brooks MA, Chan TH. Synthesis-Stuttgart. 1983;3:201–203. [Google Scholar]
- 39.Abe A, Miura I, Furuya H. J Phys Chem. 1987;91:6496–6502. [Google Scholar]
- 40.Evans DA, Miller SJ, Brown JM, Layzell TP, Ramsden JA. Handbook of Reagents for Organic Synthesis: Chiral reagents for asymmetric synthesis. John Wiley and Sons; West Sussex, England: 2003. pp. 76–81. [Google Scholar]
- 41.Gottlieb HE, Kotlyar V, Nudelman A. J Org Chem. 1987;62:7512–7515. doi: 10.1021/jo971176v. [DOI] [PubMed] [Google Scholar]
- 42.Sailasuta N, Tran TT, Harris KC, Ross BD. J Magn Reson. 2010;207:352–5. doi: 10.1016/j.jmr.2010.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sailasuta N, Abulseoud O, Harris KC, Ross BD. J Cereb Blood Flow Metab. 2010;30:950–60. doi: 10.1038/jcbfm.2009.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Golman K, Axelsson O, Johannesson H, Mansson S, Olofsson C, Petersson JS. Magn Reson Med. 2001;46:1–5. doi: 10.1002/mrm.1152. [DOI] [PubMed] [Google Scholar]
- 45.Saad LO, Mirandola SR, Maciel EN, Castilho RF. Neurochem Res. 2006;31:541–8. doi: 10.1007/s11064-006-9054-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.